Post-Prostatectomy Magnetic Resonance-Guided Radiotherapy on a 1.5 Tesla Magnetic Resonance Integrated Linear Accelerator: Feasibility, Toxicity, and Preliminary Clinical Outcomes
Funding: The authors received no specific funding for this work.
ABSTRACT
Introduction
This study aimed to prospectively investigate magnetic resonance (MR)-guided radiotherapy (MRgRT) for post-prostatectomy prostate cancer and report preliminary clinical outcomes.
Methods
All included patients underwent salvage or adjuvant adaptive MRgRT on a 1.5T MR integrated linear accelerator (MR-LINAC). Gastrointestinal and genitourinary toxicities were assessed. The primary endpoint was the progression-free survival (PFS) rate estimated by Kaplan-Meier (KM) survival analysis. A progression event was defined as the first occurrence of biochemical failure, radiological progression, or death. Secondary endpoints were biochemical failure-free survival (bFFS) rate, radiological PFS (rPFS) rate, and ≥G2 adverse events.
Results
Thirty post-prostatectomy patients were enrolled and followed (median follow-up: 32.0 months; 3.0–48.1 months). Three patients had biochemical failure during follow-up. One patient developed pelvic node metastases. All patients were alive. The estimated PFS rates were 96.4% (95% confidence interval [95%CI]: 89.8%–100.0%) at 2 years and 78.8% (95%CI: 61.3%–100%) at 3 years. The estimated bFFS rates were 96.4% (95%CI: 89.8%–100%) /86.6%(95%CI: 73.4%–100%) at 2/3 years, respectively. The corresponding rPFS rates were 100% at 2 years and 92.3% (95%CI: 78.9%–100%) at 3 years, respectively. There was only one acute G2 GI adverse event (1/30, 3.33%) of abdominal pain occurred. Two late G2 events (one rectal bleeding and one urinary frequency) were scored (2/30, 6.67%). No ≥G3 events were observed.
Conclusion
Our findings suggest the feasibility, excellent patient tolerance, and encouraging efficacy of post-prostatectomy MRgRT, extending our knowledge of the clinical outcomes of MRgRT and serving as a benchmark for future investigation.
1 Introduction
Prostate cancer (PC) stands as a prevailing health concern, representing one of the most common malignancies affecting men worldwide [1]. Radical prostatectomy, a primary intervention in localized PC, offers a potentially curative strategy. However, biochemical recurrence (BCR) is observed in a substantial proportion of PC (20%–40%) patients within 5–10 years post-prostatectomy, exhibited by a persistently high level or delayed rise of prostate-specific antigen (PSA) [2]. A number of clinicopathological factors include but are not limited to pre-prostatectomy PSA level, PSA doubling time (PSA-DT), an interval of surgery, PSA rising pathological T and N stage, and surgical margin status [3]. For these individuals, adjuvant or salvage therapies, such as post-prostatectomy radiotherapy (RT), play a pivotal role in reducing the risk of disease progression and improving long-term outcomes [4].
Post-prostatectomy PC is still potentially curable with adjuvant radiotherapy (ART) or salvage radiotherapy (SRT), combined with or without other systemic treatment [5, 6]. A wealth of clinical studies and data suggest that SRT can improve biochemical control rates in post-prostatectomy PC patients. There is also clinical data supporting the use of early SRT at lower PSA levels (<0.5 ng/mL) to achieve optimal biochemical control [4]. The RADICALS-RT study compared the efficacy and safety of ART versus an observation policy with SRT for PSA progression and concluded that ART increased the risk of urinary morbidity whereas observation with SRT should be the standard after radical prostatectomy [7]. Several recent lines of level I evidence, based on phase 3 clinical trials or systematic review, showed that early SRT is as at least effective as ART [4, 8, 9]. The recent NRG Oncology/RTOG 0534 SPPORT phase 3 trial further demonstrated that the addition of androgen deprivation therapy (ADT) and pelvic lymph node radiotherapy (PLNRT) to prostate bed SRT resulted in meaningful reductions in progression (biochemical and radiological) after prostatectomy in PC patients [10]. More recently, the phase 2 randomized SALV-ENZA trial found that SRT plus androgen receptor pathway inhibition (ARPI) treatment using enzalutamide (but without ADT) for 6 months in PSA-recurrent high-risk PC after prostatectomy was safe and delayed PSA progression compared to SRT alone [11].
Historically, RT treatment planning heavily relied on computed tomography (CT) imaging, presenting inherent limitations in accurately delineating the prostatic bed due to suboptimal soft tissue resolution. These limitations often result in suboptimal targeting and potential under-treatment of residual disease, possibly compromising therapeutic efficacy.
In recent years, the integration of magnetic resonance imaging (MRI), at either low field 0.35T [12] or high field 1.5T [13], with a linear accelerator (MR-LINAC) has emerged as a compelling adjunct in guiding post-prostatectomy RT. The superior soft tissue contrast afforded by MRI offers unparalleled visualization of the prostatic bed and adjacent structures, enabling precise delineation of target volumes and organs-at-risk (OARs) [14-17]. This advancement has the potential to enhance treatment accuracy and reduce normal tissue toxicity, thereby potentially improving patient outcomes and quality of life.
There is increasing interest and ongoing investigation into the feasibility, toxicity profile, and clinical efficacy of post-prostatectomy MR-guided RT (MRgRT). Recently, Wegener et al. prospectively examined 16 patients treated with SRT on a 1.5T MR-LINAC and reported the workflow, feasibility, and acute toxicity during treatment and 3 months later [18].
Our study aimed to further explore and consolidate the feasibility and safety of this evolving MRgRT approach in post-prostatectomy patients. We also examined preliminary clinical outcomes, including both toxicity profiles and oncological outcomes (progression-free survival [PFS] and biochemical failure-free survival [bFFS] rates), to provide insights into the potential advantages and challenges associated with this innovative MRgRT technique.
2 Material and Methods
2.1 Patient Selection
This was a single-center, prospective observational study, approved by our hospital research ethics committee (REC-2021-28). All post-prostatectomy PC patients who were to be treated on a 1.5T MR-LINAC were eligible for enrollment and provided written consent.
Eligible patients had a persistently detectable PSA or an initially undetectable (<0.05 ng/mL) with rising PSA following radical prostatectomy as the primary treatment for PC, or the presence of risk factors (e.g., close or positive surgical margin, Gleason score [GS] ≥ 8). Patients with and without lymphadenectomy were all eligible regardless of pathological evidence of lymph node involvement (N0/Nx/N1). Pre-MRgRT prostate-specific membrane antigen positron emission tomography (PSMA-PET) to detect local recurrence, and pelvic and distant metastases was optional. Local macroscopic recurrence and regional nodal and/or bone metastases in the pelvis were allowed.
Other eligibility criteria included patients with pT2 or above disease, pathological GS of 7–9, age ≥18 years, no MRI contraindication, no history of other cancers, and an Eastern Cooperative Oncology Group (ECOG) Performance Status < 2.
Exclusion criteria were as follows: age <18 years, a history of cancer other than PC, absolute MRI contraindication (e.g., claustrophobia and metal implants), previous RT or hormonal therapy or chemotherapy for PC, a history of inflammatory bowel disease or other notable comorbidities, distant nodal/bone or visceral metastases detected by pre-MRgRT PSMA-PET, ECOG performance status ≥2, or follow-up duration <3 months.
2.2 Simulation and Planning
No patients received hydrogel spacer insertion for MRgRT. All patients received CT and MR simulation scans in the treatment position on the same day without a rectal balloon. Before the simulation, patients were instructed to empty their rectum. An MRI rectal screening was conducted to check the rectal status. Fleet enema was used if the diameter of the rectum was larger than 3 cm. For bladder preparation, patients were asked to void and then drink 250 mL water before simulation scans. An ultrasound bladder scan was used to measure bladder volume. The bladder volume was controlled within a range of 130–160 mL as the reference.
The MRI simulation was performed on a 1.5T MRI simulator with a three-dimensional T2-weighted turbo spin echo (3D-T2W-TSE) sequence with identical imaging parameters to daily online MRI on the MR-LINAC and other sequences, as per clinical purposes [19, 20].
All included patients underwent MR-guided prostate bed RT (PBRT), either in a salvage or adjuvant setting, on a 1.5T MR-LINAC. Intensity-modulated RT (IMRT) plans were generated using Monaco v.5.40 (Elekta, Stockholm, Sweden), in which the Monte Carlo algorithm was used to account for the presence of the magnetic field. The standard PBRT consensus clinical target volume (CTV) was used to administer PBRT [21]. The addition of PLNRT was prescribed at the discretion of the radiation oncologist in consideration of high-risk factors. The PLNRT nodal CTV included the obturator, external iliac, proximal internal iliac, presacral, and common iliac nodes, estimated using the vascular structures up to the level of L5–S1 (between the inferior aspect of the fifth lumbar and superior aspect of the first sacral vertebrae) [22]. Planning target volume (PTV) margins were 8 mm for the prostate bed (exception: 5 mm in posterior) and 5 mm for lymphatics/nodal/bone, in accordance with consensus guidelines. A total dose of 66.5–70 Gy was delivered to the PBRT PTV at 2.0 Gy per fraction daily at five fractions per week (35 fractions in total). Various OARs including the rectum, bladder, sigmoid, bowel, penis, penile bulb, femoral heads, sacral plexus, and cauda equina were contoured by radiation dosimetrists following institutional contouring guidelines. Concurrent doses of 70–77 Gy to PSMA-PET-detected local recurrent/residual tumors, 50–56 Gy to lymphatics, and 60–77 Gy to nodal/bone metastases were also delivered. Figure 1 shows an example of a typical MRgRT plan on a patient without nodal/bone metastases. ADT with a luteinizing hormone-releasing hormone agonist or antagonist was generally planned for a short duration of 3–6 months. For patients who presented high-risk factors of pT3b disease or PSMA-PET-detected nodal/bone metastasis, or a pre-RT baseline PSA > 0.5 ng/mL, ADT of 24 months or longer was planned.
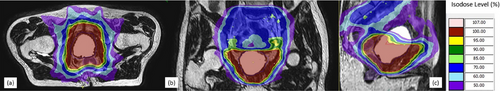
2.3 Treatment Delivery and Adaptation
MRgRT was performed as step-and-shoot IMRT with 7 MV photons. At each fraction, bowel and bladder preparation was the same as on the simulation day. The bladder volume was kept within 20% of the reference volume. This controlled bladder volume provided a stable filling rate. A daily MRI scan using a 3D-T2W-TSE sequence was acquired to obtain on-the-date anatomy information and ensure all targets and OARs are in place, including bladder and rectum status [23]. Online plan adaptation was conducted based on the acquired daily MRI using either adapt-to-position (ATP) or adapt-to-shape (ATS). In general, ATP was prioritized to maximize workflow efficiency by a virtual couch shift without the need for recontouring. The necessity of ATS was determined according to our institutional criteria and the attending oncologist's experience [24]. When ATS was adopted, the attending oncologist adapted the contours of the targets and OARs via manual contouring and/or deformable registration, if necessary. Plan re-optimization on the adjusted contours was applied to achieve all planned dosimetric criteria. Optimal target coverage was prioritized to constrain doses to OARs. An online patient-specific quality assurance was then performed, followed by a second MRI scan to ensure patient position consistency. Immediately after positional confirmation, the dose was delivered as per the adopted plan. No motion monitoring or gating was conducted during dose delivery.
2.4 Patient Follow-up and Outcome Measurements
PSA assessments were planned at the completion date of MRgRT, 1-month, 3-month, and then every 6 months unless the PSA was ≥0.2 ng/mL, in which case PSA was obtained at 3-month intervals. PSMA-PET was planned during follow-up when PSA was ≥0.2 ng/mL or as clinically indicated.
Gastrointestinal (GI) and genitourinary (GU) functions were assessed at the baseline, per week during the RT course, 1-month, 3-month, and then every 6-month. Toxicities, including but not limited to GU and GI adverse events, were assessed using the Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Acute toxicity was defined as the occurrence of adverse events from the start date to 3 months after the completion date of MRgRT. Adverse events occurring >3 months after the completion date of MRgRT were classified as late toxicity.
The primary endpoint was the progression-free survival (PFS) rate at 2 years. A progression event was defined as the first occurrence of biochemical failure (PSA ≥0.2 ng/mL and then a confirmatory PSA ≥0.2 ng/mL as per the American Urological Association AUA definition), radiological progression (local failure of the irradiated macroscopic recurrence in the prostate bed or irradiated node/bone metastases, or new regional/distant metastasis detected by post-RT PSMA-PET), or death from any cause. Secondary endpoints were bFFS rate (standard or alternative: PSA ≥0.4 ng/mL followed by another increase or the start of second salvage therapy [7, 25], radiological PFS (rPFS) rate, and ≥G2 adverse events.
2.5 Statistical Analysis
Each patient's follow-up duration was defined as the time interval (in months) between the first MRgRT treatment fraction and the progression event date or the last clinical follow-up visit. The median follow-up duration of the entire cohort was calculated using the reverse Kaplan-Meier (KM) method. Descriptive statistics was used to summarize continuous data, presented as medians with ranges. The categorical patient characteristics, safety, and toxicity were categorized using frequencies and percentages. KM survival curves of PFS, bFFS, and rPFS were estimated and plotted [26]. The log-rank test [27] was used to evaluate whether stratification factors (including pre-RT PSA levels>0.2 ng/mL, intervals between prostatectomy and rising PSA date >12 months, with or without pelvic lymph node dissection, pathological GS > 7, pathological T stage ≥ T3a, pathological N stage N0/Nx v.s. N1, PSMA-PET detected local recurrence in prostate bed or metastases, undetectable (<0.05 ng/mL) or persistent post-prostatectomy PSA, short or long ADT duration) resulting in significant differences in 2-year PFS rate. The significant level was set at 0.05, and the Bonferroni correction was applied for multiple statistical tests. All statistical analyses were performed using R v1.2 (RStudio, Boston, MA, USA).
3 Results
3.1 Patient Baseline Characteristics, Planning, and MRgRT Treatment Delivery
From April 2020 to April 2023, thirty post-prostatectomy patients (SRT, n = 29; ART, n = 1) were enrolled in this study after excluding two patients who were followed for less than three months. Patient characteristics are shown in Table 1.
| Patient characteristics | Patient number (%), median (range) |
|---|---|
| Age at MRgSRT (years) | 67 (54–81) |
| Interval between prostatectomy and MRgSRT (months) | 19.5 (2.6–114.1) |
| Local recurrence in prostate bed (by PSMA-PET) | 9 (30.0%) |
| Nodal/bone recurrence (by PSMA-PET) before MRgSRT | 3 (10.0%) |
| Post-prostatectomy PSA | |
| Undetectable (<0.05 ng/mL) | 24 (80.0%) |
| Detectable | 2 (6.7%) |
| Persistent | 4 (13.3%) |
| Pre-MRgSRT PSA level (ng/mL) | 0.18 (0.01–1.65) |
| Androgen deprivation therapy (ADT) | |
| No ADT | 1 (3.3%) |
| Short (≤6 months) | 18 (60.0%) |
| Long (≥12 months) | 11 (36.7%) |
| Pathological T stage (prostatectomy) | |
| pT2b | 2 (6.7%) |
| pT2c | 17 (56.7%) |
| pT3a | 5 (16.7%) |
| pT3b | 5 (16.7%) |
| pT4 | 1 (3.3%) |
| Pathological N stage N1/N0/Nx | |
| pN0 | 16 (53.3%) |
| pN1 | 4 (13.3%) |
| pNx | 10 (33.3%) |
| ISUP Grade Group | |
| Group 1 (GS ≤6) | 0 (0.0%) |
| Group 2 (GS 3+4) | 10 (33.3%) |
| Group 3 (GS 4+3) | 7 (23.3%) |
| Group 4 (GS 8) | 4 (13.3%) |
| Group 5 (GS >8) | 9 (30.0%) |
| American Joint Committee on Cancer (AJCC) Prostate Cancer Stage at Prostatectomy | |
| IIB | 6 (20.0%) |
| IIC | 7 (23.3%) |
| IIIA | 3 (10.0%) |
| IIIB | 3 (10.0%) |
| IIIC | 7 (23.3%) |
| IVA | 4 (13.3%) |
| Prostatectomy Margin | |
| Positive | 12 (40.0%) |
| Close | 3 (10.0%) |
| Negative | 15 (50.0%) |
Among these 30 patients, nine patients had local macroscopic recurrence in the prostate bed detected by PSMA-PET before MRgRT and one patient had residual uptake in the PSMA-PET images after his prostatectomy, yielding 10 CTVlocal in the treatment planning besides CTVbed. Five patients were detected with oligometastatic recurrent disease (defined by ≤5 metastatic lesions in PSMA-PET images) by PSMA-PET, yielding seven CTVbone and six CTVLN.
All fractions were successfully delivered in the form of step-and-shoot IMRT using 11–15 beam angles. No interruption occurred during any fraction. The fraction duration ranged from 22 to 46 min with a median of 38 min. Of the total fractions, 96% and 4% of total fractions were adapted in the form of ATP and ATS, respectively. An example of ATP and ATS in a patient is illustrated in Figure 2.
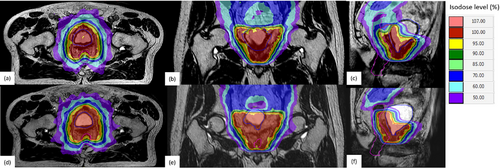
3.2 Patient Follow-up and Clinical Outcome
The median follow-up duration was 32.0 months (range: 3.0–48.1 months). Regarding biochemical outcome, all patients had their PSA levels below 0.05 ng/mL within 1-month after MRgRT. During the follow-up, three patients (bFFS rate: 27/30, 90.0%) had a biochemical failure at 18.4, 27.4, and 29.0 months, respectively, based on the AUA definition of two consecutive PSA ≥0.2 ng/mL. Using the alternative biochemical failure definition of PSA ≥0.4 ng/mL followed by another increase, only one patient (alternative bFFS rate: 29/30, 96.7%) had a biochemical failure at 29.0 months. According to the KM analysis, the log-rank test showed that all stratification factors were not significant at a 5% significance level (shown in Table 2). As none of the comparisons showed statistically significant differences between the groups, Bonferroni correction was not further performed.
| Group 1 (Patient number) | Group 2 (Patient number) | Group 3 if applicable (Patient number) | p-value | |
|---|---|---|---|---|
| Pre-RT PSA levels >0.2 ng/mL | Yes: n = 15 | No: n = 15 | – | 0.99 |
| Intervals between prostatectomy and rising PSA date >12 months | Yes: n = 11 | No: n = 19 | – | 0.53 |
| PSMA-PET detected local recurrence in the prostate bed | Yes: n = 9 | No: n = 21 | – | 0.78 |
| PSMA-PET detected metastases | Yes: n = 3 | No: n = 27 | – | 0.47 |
| Post-OP PSA (undetectable/detectable/persistent) | Detectable: n = 2 | Persistent: n = 4 | Undetectable: n = 24 | 0.52 |
| ADT duration [no/short/long] | Long: n = 11 | Short: n = 18 | No ADT: n = 1 | 0.79 |
| Pathological T stage ≥ T3a | Yes: n = 11 | No: n = 19 | – | 0.41 |
| Pathological N stage | N1: n = 4 | N0/Nx: n = 26 | – | 0.32 |
| Pathological GS >7 | Yes: n = 13 | No: n = 17 | – | 0.35 |
| Pelvis lymph node dissection | Yes: n = 12 | No: n = 18 | – | 0.16 |
Five patients (5/30, 16.7%) received at least one post-MRgRT PSMA-PET scan during follow-up. Regarding radiological progression, three patients who had biochemical failure showed no local failure or new metastasis on their post-RT PSMA-PET images. Another patient showed complete remission of two irradiated bone metastases. However, one patient was found to have new para-aortic lymph node metastases after 32 months of MRgRT using PSMA-PET when his post-RT PSA elevated to 0.13 ng/mL. None of these five patients developed distant metastases during the follow-up. All patients were alive at this point.
Based on KM survival analysis, the estimated PFS rates were 96.4% (95% confidence interval [95%CI]: 89.8%–100.0%) at 24 months and 78.8% (95%CI: 61.3%–100%) at 36 months. The estimated bFFS rates were 96.4% (95%CI: 89.8%–100%)/86.6%(95%CI: 73.4%–100%) (AUA definition) and 100% (95%CI: Not available)/94.1% (95%CI: 83.6%–100%) (alternative definition) at 2-year and 3-year, respectively. The corresponding rPFS rates were 100% at 24 months and 92.3% (95%CI: 78.9%–100%) at 36 months, respectively. Figure 3 illustrates the KM survival curves of PFS, bPFS, and rPFS.
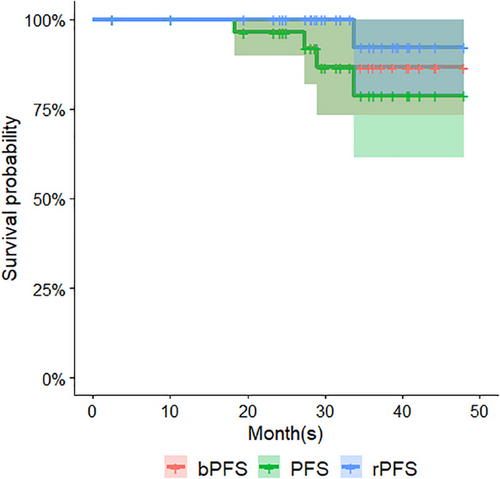
Clinician-reported outcome measurements of GI and GU toxicities are summarized in Table 3. Fourteen and twenty-two patients presented acute G1 GI (mostly diarrhea and rectal hemorrhage) and GU (mostly urinary frequency and urinary tract pain) toxicities, respectively, accounting for 46.7% and 73.3% of total patients. There was only one G2 GI adverse event of abdominal pain that occurred at fraction 20 in one patient. Regarding late toxicities, 20 patients (66.7%) presented late G1 GU adverse events, some of which occurred alongside fatigue and hormonal symptoms such as flushing and sweating. Three patients (10%) presented late G1 GI adverse events during the follow-up. There were two late G2 events (one rectal bleeding and one urinary frequency) were scored in two patients (6.67%), but no G3 or above events were scored during the entire follow-up. There were no acute or late blood or bone marrow adverse events throughout the follow-ups. Most adverse events were resolved at subsequent follow-ups.
| Follow-up phase | Intra-treatment | Acute | Late | |||
|---|---|---|---|---|---|---|
| Toxicity grade (CTCAE v. 5.0) | G1 | G2 | G1 | G2 | G1 | G2 |
| GI Toxicity | ||||||
| Abdominal pain | 0 | 0 | 2 | 1 | 0 | 0 |
| Bloating | 1 | 0 | 0 | 0 | 1 | 0 |
| Constipation | 0 | 0 | 0 | 0 | 1 | 0 |
| Diarrhea | 0 | 0 | 6 | 0 | 0 | 0 |
| Fecal incontinence | 0 | 0 | 0 | 0 | 1 | 0 |
| Nausea | 0 | 0 | 0 | 0 | 0 | 0 |
| Proctitis | 0 | 0 | 1 | 0 | 0 | 0 |
| Rectal hemorrhage | 0 | 0 | 5 | 0 | 0 | 1 |
| Rectal pain | 0 | 0 | 0 | 0 | 0 | 0 |
| GU Toxicity | ||||||
| Urinary frequency | 8 | 0 | 18 | 0 | 9 | 1 |
| Urinary incontinence | 12 | 0 | 1 | 0 | 9 | 0 |
| Urinary retention | 0 | 0 | 0 | 0 | 0 | 0 |
| Urinary tract pain | 0 | 0 | 3 | 0 | 1 | 0 |
| Urinary urgency | 0 | 0 | 0 | 0 | 0 | 0 |
4 Discussion
To our knowledge, our report represents the first prospective clinical study of MRgRT, mostly MR-guided SRT (MRgSRT), in post-prostatectomy PC patients (n = 30), featuring a relatively long median follow-up of ∼2 years, and data on preliminary outcomes. The present study further demonstrates the feasibility and efficacy of MRgRT in routine clinical practice, and extends our knowledge of the clinical outcomes of MRgRT, supporting the further investigation of this innovative treatment in the selected post-prostatectomy PC patients.
Regarding the treatment procedure, our approach is most analogous to that utilized in the NRG Oncology/RTOG 0534 SPPORT trial group 3 [10] by combining conventionally fractionated PBRT with PLNRT and ADT, but with a substantial difference in adaptive MRI guidance and major differences in patient selection and outcome measurements. First, in the SPPORT trial, SRT was triggered only after PSA elevated to >0.1 ng/mL, while ∼17% (4/29) of our patients who presented adverse risk factors received their post-prostatectomy MRgSRT before their PSA level rose to 0.1 ng/mL. Additionally, patients with pathological lymph node metastasis were not included in [10]. Our study accepted long-term ADT of ≥24 months while the SPPORT trial only had a short ADT duration of 4–6 months. It is unclear whether pre-RT PSMA-PET was utilized to detect regional or distant metastasis in the SPPORT trial. It is also noteworthy that the SPPORT trial adopted the Phoenix definition of PSA nadir +2 ng/mL to define biochemical failure rather than the AUA definition of ≥0.2 ng/mL in our study. With these differences, the SPPORT trial reported that the 5-year rates of freedom from either biochemical or clinical progression in group 3 patients were 87.4% (95%CI: 84.7%–90.2%), significantly better than the rates of 70.9% (95%CI: 67.0%–74.9%) in group 1 and 81.3% (95%CI: 78.0%–84.6%) in group 2. In our study, the estimated 2-year PFS was 96.4% (95%CI: 89.8%-100.0%) based on the AUA BCR definition of ≥0.2 ng/mL, and 100.0% (95%CI: NA) based on the alternative definition of ≥0.4 ng/mL.
In the phase 2 STREAM trial [28], which had a similar clinical endpoint with comparable follow-up, the 2-year PFS was 65% (95%CI: 47%–78%) and the 3-year PFS was 53% in a cohort of 37 post-prostatectomy PC patients who received SRT combined with both ADT and ARPI (enzalutamide). Eleven (29%) men experienced G3 toxicities, and there were no G4–5 or unexpected toxicities. The reported 2-year PFS and toxicity profiles were poorer than those in our study. Although both studies allowed pN1 patients, the STREAM trial did not include radiologically metastatic disease, as did ours. However, the proportions of ≥ pT3 (36.7% vs. 79%) and pN1 (13.3% vs. 21.1%) diseases, and the median pre-RT PSA level (0.18 ng/mL vs. 0.4 ng/mL) in our study were substantially lower than those in STREAM [28]. Therefore, the apparently better 2-year PFS and toxicity profiles in our patient cohort should be interpreted with caution. Since the study by Wegener et al. [18] did not report primary oncological outcomes of MRgSRT except for 3-month acute toxicities, we cannot compare our results with any other MRgSRT study as a reference.
Clinical outcomes are dependent on the definition of endpoints and are impacted by the use of PSMA-PET and other advanced imaging techniques, which also hinder fair comparisons across different studies. If we had adopted the Phoenix definition of PSA nadir +2 ng/mL (as in the SPPORT trial) and had not conducted post-RT PSMA-PET, the 2-year PFS rate in our study would been 100%. If the alternative biochemical failure definition of 0.4 ng/mL had been applied, the biochemical failure rate at 2 years would be reduced to 1/30 (3.3%) rather than 10%. One strength of our study is that pre-RT PSMA-PET data were available in 90% (27/30) of our patients, much higher than in other relevant studies. This helped us to obtain an accurate baseline metastatic status of the patients for MRgRT treatment planning and increased the reliability of the reported PFS rate by excluding patients whose baseline local recurrence or metastases had not been detected prior to the PSA elevation or failure after the completion of MRgRT.
Regarding the toxicity profile, the rates of acute G1 GU and GI toxicity were similar, while the rate of acute G2 GI toxicity was lower in our study than in the Wegener et al. 1.5T MRgRT study (1/30 vs. 2/16) [18]. There was no acute G2 GU toxicity in our results, against the rate of 1/16 in [18]. Compared to the reported acute and late ≥G2 adverse event rates in the SPPORT trial group 3 [10], our results seemed to be better (acute ≥G2 GI: 3.3% (ours) vs. 7%; acute ≥G2 GU: 0 vs. 12%; late ≥G2 GI: 3.3% vs. 9%; late ≥G2 GU: 3.3% vs. 40%). These lower late ≥ G2 adverse event rates may be attributable to the use of MR-guided on-line adaptation in our study. However, the much shorter follow-up duration of ∼32 months in our study compared with the median 8.2-year follow-up in the SPPORT trial must be highlighted. Longer follow-up is warranted to evaluate the durability of the remission.
Limitations of our study include the single-center study design, heterogeneous patient characteristics, lack of control group, lack of patient-reported outcomes, a small sample size, and a short follow-up duration. The very few progression events might hamper the statistical significance of the log-rank test for the influence of stratification factors on PFS. Meanwhile, a 2-year PFS might not be an optimal surrogate for overall survival. Although metastasis-free survival may be a more appropriate surrogate, the lack of mandatory post-RT PSMA-PET scanning for every patient hindered thorough analysis. For the same reason, the rPFS in this study should be interpreted with caution due to the low post-MRgRT PSMA-PET rate. Additionally, the PTV margin for the prostate bed might be further optimized [29]. Despite these limitations, our results support the safety and efficacy of adaptive MRgRT (PBRT, PLNRT, and local recurrence and metastasis boosting) along with ADT, and provide useful benchmarks and safety data to support the further exploration of this innovative MRgRT approach. Future research may utilize hypofractionated or ultra-hypofractionated MRgRT to improve the cost-effectiveness of the approach [30, 31]. Automated online adaptation workflow based on artificial intelligence techniques and advanced motion management should be further developed to improve workflow efficiency and clinical outcomes. Randomized clinical trials are warranted for further validation (Supporting Information).
5 Conclusion
The present study successfully demonstrated the utilization of conventionally fractionated adaptive MRgRT in a cohort of 30 post-prostatectomy PC patients and reported safety, toxicity profiles, and preliminary clinical outcomes. Our findings suggest the feasibility, excellent patient tolerance, and encouraging efficacy of post-prostatectomy MRgRT, extending our knowledge of the clinical outcomes of this innovative treatment and serving as a benchmark for future investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Research data are not shared.




