Measurement that matters: A systematic review and modified Delphi of multidisciplinary colorectal cancer quality indicators
Abstract
Aim
To develop a priority set of quality indicators (QIs) for use by colorectal cancer (CRC) multidisciplinary teams (MDTs).
Methods
The review search strategy was executed in four databases from 2009–August 2019. Two reviewers screened abstracts/manuscripts. Candidate QIs and characteristics were extracted using a tailored abstraction tool and assessed for scientific soundness. To prioritize candidate indicators, a modified Delphi consensus process was conducted. Consensus was sought over two rounds; (1) multidisciplinary expert workshops to identify relevance to Australian CRC MDTs, and (2) an online survey to prioritize QIs by clinical importance.
Results
A total of 93 unique QIs were extracted from 118 studies and categorized into domains of care within the CRC patient pathway. Approximately half the QIs involved more than one discipline (52.7%). One-third of QIs related to surgery of primary CRC (31.2%). QIs on supportive care (6%) and neoadjuvant therapy (6%) were limited. In the Delphi Round 1, workshop participants (n = 12) assessed 93 QIs and produced consensus on retaining 49 QIs including six new QIs. In Round 2, survey participants (n = 44) rated QIs and prioritized a final 26 QIs across all domains of care and disciplines with a concordance level > 80%. Participants represented all MDT disciplines, predominantly surgical (32%), radiation (23%) and medical (20%) oncology, and nursing (18%), across six Australian states, with an even spread of experience level.
Conclusion
This study identified a large number of existing CRC QIs and prioritized the most clinically relevant QIs for use by Australian MDTs to measure and monitor their performance.
1 INTRODUCTION
Colorectal cancer (CRC) is one of the main causes of cancer-related death worldwide and is the third most commonly diagnosed cancer in Australia accounting for 11% of Australian cancer cases.1, 2 There were 15,206 cases of CRC diagnosed in 2017 and 5,255 deaths from CRC in 2019.2 The CRC care continuum is complex and involves multiple modalities of treatment delivered by health professionals (HPs) from multiple specialist disciplines including CRC surgery, radiation oncology, medical oncology, radiology, anatomical pathology, nursing, and palliative/supportive care. Multidisciplinary team (MDT)-based care is now widely accepted as a standard of care in cancer.3-7 There are ongoing advances in the various treatment modalities available with rapidly evolving evidence pertaining to their use. Therefore, good communication, collaboration, and coordination among MDT members are needed.6, 8, 9 To ensure consistent delivery of high-quality care, it is important to measure and monitor the delivery of care by MDTs to identify any unwarranted variations.10
An important tool for monitoring care delivery and patient outcomes is a set of clinically relevant, evidence-based quality indicators (QIs).9, 11 QIs are measurable elements of practice performance supported by evidence for assessing the quality of care.12 They can be categorized as structure, process, and outcome measures.13 Whilst variability in outcomes for CRC patients may be attributable to patient factors, significant variation exists in processes and structures of care within healthcare organizations.14 An ideal measurement program should therefore employ multiple QIs to capture a holistic view of patient care delivery. Effective implementation of QI measurement and monitoring can encourage clinical practice reflection, clarify team responsibilities, and focus improvement efforts.15 Defining QIs in a tumor stream can facilitate the creation of benchmarks for standardization of care across healthcare services.9 QI development can be evidence-based and derived from scientific literature or, when scientific evidence is lacking, determined by an expert panel of HPs in a consensus process.16 Ideally, a QI is both selected from research data and supplemented by expert opinion.17
Given the wide-reaching implications of quality measurement, QIs should be developed and selected systematically, in alignment with patient-centered MDT-based care delivery, and engage HPs in their selection.18 However, current cancer care quality measurement efforts remain heavily directed towards meeting the administrative requirements of service providers, and credentialing of services as determined by discipline-specific colleges, accrediting bodies, and government agencies. Furthermore, the utility of QIs has been limited by the availability of and accessibility to quality datasets.19, 20 For these reasons, many of the existing QIs have been selected by consensus and convenience and are discipline focussed not patient-centered MDT focussed. The aim of this study is to identify an evidence-based set of QIs and clinically prioritize them for use in Australian CRC MDTs.
2 METHODS
We conducted a systematic review of the literature describing existing CRC QIs to identify QIs from diagnosis through treatment to survivorship or palliative care and assess the extent to which these QIs have been developed and validated. Following this, a two-step modified Delphi consensus process was undertaken to prioritize the extracted QIs into a final set based on their clinical importance to Australian CRC MDTs.
2.1 Systematic review
The search strategy was guided by the PICO Framework.21 Studies were included if they described the development, selection, validation, or review of CRC QIs from diagnosis, treatment follow-up, and survivorship or palliative care. On August 14, 2019, string terms (Supplementary Material—Search Strategy) were executed in four databases (MEDLINE, EMBASE, CINAHL, and PsychInfo) for the dates January 1, 2009–August 14, 2019. String terms were developed using MeSH and free-text terms referring to key concepts; CRC (e.g., colorectal neoplasms, rectal tumor, and colon carcinoma) and QIs (e.g., quality measures or performance indicators). The search was restricted to studies in English. A citation search and a hand search of the reference lists of relevant papers were also conducted. To retrieve supplementary endorsed and validated QIs in the grey literature, a manual search of the pertinent quality measurement and cancer agency websites and publications was conducted. Citations retrieved from the searches were imported into EndNote for de-duplication, then imported into Covidence for screening.
Two authors (Candice Donnelly and Puma Sundaresan) screened the titles and abstracts against the exclusion criteria (Supplementary Material—Search Strategy) to identify potentially relevant studies. Full-text manuscripts were also screened by two reviewers (Candice Donnelly and Michelle Or), and justifications for inclusion or exclusion were confirmed by a third reviewer (Puma Sundaresan). It was anticipated that the QI literature would include studies of different methodological designs. Therefore, a quality assessment instrument was chosen that had been used in other QI reviews to assess the methodological quality of a range of study types and extracted QIs.22 The quality assessment was conducted by one reviewer (Candice Donnelly). The grading system scored the included manuscripts from the highest quality level (i.e., A1: systematic reviews or two independent prospective cohort studies) to the lowest quality level (i.e., C: non-comparative studies and D: expert opinion)23. This assessment contributed to grading the level of supporting evidence (scientific soundness) for each QI extracted on a scale of “1”, the highest level of evidence to “4”, the lowest level of evidence. Each QI was rated by calculating how many of the graded studies supported the QI combined with the quality of those studies. For example, a QI with a score of “1” was one that had the support of either a study scored as level A1 or two independent studies of level A2.
QIs were extracted by using a tailored template implemented in Microsoft Excel v.16.34. The tailored version included elements of QIs suggested in previous systematic and narrative reviews.24-26 One reviewer (Candice Donnelly) piloted the form on the first 10 articles and only minor refinements were required. Two reviewers (Mathushan Thevaraja and Candice Donnelly) extracted reported QIs from each study and where available characteristics of those QIs including; numerator, denominator, exclusion criteria, study design, period of measurement, and any additional details, where available (i.e., data source, benchmark, risk adjustment and detected differences in care). To synthesize the data, each extracted QI and characteristic were grouped with those addressing the same measurement, then tabulated under a single QI description. Once de-duplicated, the QIs were divided into domains across the continuum of cancer care including diagnosis, staging, clinical aspects of surgery, radiotherapy and chemotherapy, follow-up, and supportive care, aligned with the Cancer Council Australia Colorectal Cancer Guidelines.3 Subsequently, QIs were categorized as structure, process, or outcome measures, according to the Donabedian Model.16 Clinical disciplines contributing to the outcome of each QI were also detailed.
2.2 Delphi consensus process
A modified Delphi technique was used to refine the extracted QIs and prioritize the most clinically important QIs. This consensus approach has been widely used and validated within other quality measurement and health services research.27-29 The consensus was sought over two rounds; a multidisciplinary expert workshop and an online survey. Participants were a purposive sample of multidisciplinary HP experts by organization affiliation. This method of group Delphi can enable participants to provide contextual justifications for deviating judgments.30, 31 Given the candidate QIs were extracted from international literature, workshop participants assessed the QIs for inclusion/exclusion or modification based on relevance to modern Australian CRC care delivery. Participants were encouraged to discuss potential gaps and the development of additional QIs. The QIs retained in this process were included in the second Delphi round.
The second round was conducted by an online survey hosted on REDCap32 open to HP members of CRC MDTs across Australia. Participants were recruited by email invitation through professional networks and public sources of information, and study adverts through professional medical societies. Participants rated QIs from the first round on “clinical importance” on a 4-point Likert scale. Free-text responses were available to provide an opportunity for comments. Clinical importance was defined as “the relevance of this information to clinical decision making and provides the opportunity for the MDT to change practice to improve the quality of care delivered”. The level of concordance was measured by inferential analysis. Each QI that met a percentage agreement of >80% recorded as “important” or “critically important”, was included in the final set of QIs. Descriptive statistics were used to describe participants’ demographic characteristics and subgroup analysis.
3 RESULTS
3.1 Systematic review
The literature search identified 708 records from January 1, 2009, to August 14, 2019 (presented in Figure 1).33 After de-duplication 619 abstracts and titles were screened and 454 records were excluded as they did not have data on the development, selection, validation, or review of CRC QIs or did not meet all of the inclusion criteria. A total of 165 manuscripts were included for full-text review. Group consensus was required for 16/165 (9.7%) of articles to resolve screening disagreements. A total of 118 studies were included. Using the grading system,22 among the included QI studies, the majority were of levels B, C, and D methodological quality; with 85 retrospective comparative studies (72%), six non-comparative studies (5.1%), and 21 expert opinion studies (17.8%), usually based on a consensus process. There were limited A2 studies and none of A1 level; with six prospective cohort studies (5.1%).
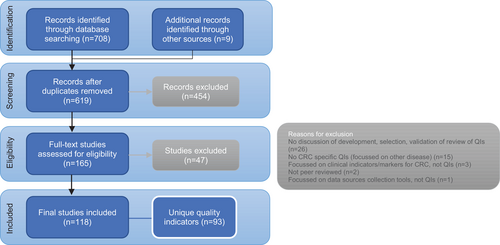
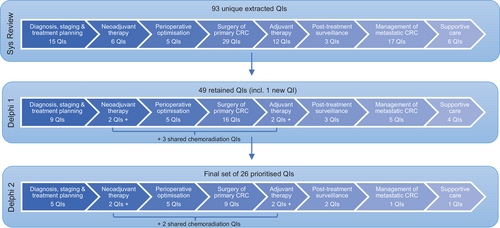
A total of 93 unique QIs and related characteristics were extracted. The number of reported QIs in individual articles varied significantly, with many articles focussed on just one QI and others discussed sets of QIs (range, n = 1–52). The majority of extracted QIs were process indicators (n = 59, 63.4%), followed by outcome indicators (n = 32, 34.4%) and structure indicators (n = 2, 2.2%). The majority of outcome indicators were surgical QIs (26/32, 81.3%). As shown in Figure 2. QIs were disproportionately spread across the Domains of Care. The Domain of Care with the most QIs was “surgical treatment for primary CRC” (n = 29, 31.2%), with the “management of metastatic cancer” domain recording the second most QIs (n = 17, 18.3%) which were also predominantly surgical (n = 8, 47%). The number of QIs measuring supportive care (n = 6, 6.5%) and neoadjuvant therapy (n = 6, 6.5%) was relatively low.
The grey literature search identified seven QI sets endorsed by international quality improvement and cancer organizations, National Health Service Scotland,34 American Society of Clinical Oncology (ASCO)/National Comprehensive Cancer Network,35 ASCO National Initiative on Cancer Care Quality,36 Association of Surgeons of the Netherlands, Dutch Surgical Colorectal Audit,37 Colorectal Surgical Society of Australia and New Zealand,38 Commission on Cancer National Accreditation Program for Rectal Cancer,39 and Queensland Cancer Alliance.40 Only one additional QI that was not identified through the peer-reviewed literature search, ‘mortality after chemotherapy or radiotherapy treatment’ in neoadjuvant and adjuvant therapy34 was identified. All other QIs identified in the grey literature, overlapped with those in the extracted QI set from the peer-reviewed literature.
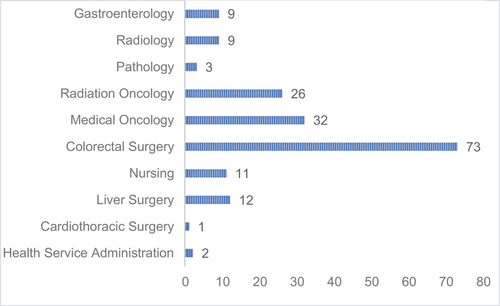
A range of identified disciplines was responsible for contributing to each QI as demonstrated in Figure 3, including; gastroenterology (n = 9), radiology (n = 9), pathology (n = 3), colorectal surgery (n = 73), medical oncology (n = 32), radiation oncology (n = 26) and nursing (n = 11). Approximately half of QIs were multidisciplinary (n = 49, 52.7%), where more than one discipline contributed to its outcome. The evidence supporting each QI was highly variable. Only three QIs were given a scientific soundness score of “1”. These three QIs that have been studied and published extensively included (1) “pre-operative visualization of the colon”, (2) “resected lymph nodes ≥12”, and (3) “post-operative mortality”. Surgical CRC QIs had the highest number of QIs with an evidence score of either “1” or “2”, with 15 QIs supported by a number of retrospective or prospective comparative studies. Management of metastatic CRC QIs accounted for the fewest studies and lowest level of supporting evidence.
3.2 Delphi consensus process
Workshop participants (n = 12) represented a core CRC MDT membership including surgery (n = 3), medical and radiation oncology (n = 4), pathology (n = 1), radiology (n = 1), and nursing (n = 3). Participants assessed the 93 QIs for relevance to modern Australian CRC care delivery. A total of 49 QIs were retained; 24 were excluded due to advances in current clinical practice or technology since publication of those QIs, lack of relevance to Australian clinical practice, basic practice, and not relevant for measurement, or beyond the control of MDT members. Only four QIs were modified to include the latest technique or guideline, and 31 QIs were merged into 11 QIs, for example, workshop participants suggested the combination of specific post-operative complications be grouped into one QI. Some of the QIs that were specific to a treatment or tumor location or grade were modified to be applied more broadly, for example, some QIs for the management of metastatic CRC were extracted from an article that focussed specifically on CRC patients undergoing hepatic resection41 so participants amended the original wording of these QIs to include oligometastatic disease beyond the liver. One new QI was developed to address the delivery and timing of stoma follow-up care.
Participants of the second Delphi round (n = 44) included surgeons (n = 14, 32%), radiation oncologists (n = 10, 23%), medical oncologists (n = 9, 20%), nurses (n = 8, 18%), gastroenterologists (n = 1, 2%), diagnostic radiologist (n = 1, 2%), and an anatomical pathologist (n = 1, 2%). The majority of participants were CRC MDT members from New South Wales (n = 31, 70%), with the rest from Victoria, Northern Territory, Western Australia, South Australia, and Queensland. Participants had an even spread of clinical experience level; <5 years (n = 12, 27%), 5–15 years (n = 17, 39%), and >5 years (n = 15, 34%). There were 26 QIs with a level of concordance >80%. This prioritized set included QIs within each Domain of Care as demonstrated in Figure 2. Table 1 presents the final set of prioritised CRC QIs and their characteristics, categorized into the Domains of Care. Almost all QIs in the final set were from the literature review, with the new indicator identified in Round 1 of the Delphi also prioritized. All three QIs with the highest scientific soundness score were retained in the final set. All disciplines contribute to QIs selected in the priority set. There are a mix of process and outcome QIs in the final set, and only one structure QI. The free text comments included suggestions to re-word QIs or debate the benchmark in timeliness measures. None of the QIs that were prioritized and included in the final set were subjects of debate.
| Indicator description | Numerator | Denominator | Donabedian category | Health professionals | Evidence grading | Related referencesa | Concordance |
|---|---|---|---|---|---|---|---|
| Diagnosis, staging, and treatment planning (five QIs) | |||||||
| Pre-operative visualization of the total colon | Number of CRC patients who had preoperative visualization of total colon consisting of a complete colonoscopy to the caecum or distal ileum (or in case of incomplete colonoscopy, CT colonography as next line) | Number of CRC patients who underwent surgery | Process |
Gastroenterologist Colorectal Surgeon Radiologist |
1 | 26, 34, 37, 42-52 | 93% |
| Imaging prior to definitive treatment | Number of colon cancer patients who had CT (chest, abdomen, and pelvis examinations completed prior to definitive treatment | Number of colon cancer patients who received definitive treatment | Process |
Gastroenterologist Colorectal Surgeon Radiologist |
2 | 26, 34, 37, 39, 43, 45, 48, 51-57 | 100% |
| Number of rectal cancer patients who had high-resolution MRI examination prior to definitive treatment | Number of rectal cancer patients who received definitive treatment | Process/*s |
Colorectal Surgeon Radiation Oncologist Radiologist |
||||
| Staging completed prior to definitive treatment | Number of CRC patients who were staged prior to definitive treatment | Number of CRC patients who received definitive treatment | Process | Radiologist Colorectal Surgeon | 2 | 26, 39, 42, 45, 48-50, 56, 58-63 | 100% |
| A comprehensive and standardized surgical pathology report | Number of CRC patients with a completed surgical pathology report according to the ANZ RCP Colorectal Cancer Structured Reporting Protocol (2016) | Number of CRC patients who underwent surgery | Structure/*p |
Pathologist Gastroenterologist Colorectal Surgeon |
3 | 26, 36, 37, 39, 44, 48, 52, 55-57, 61, 64-67 | 98% |
| Radiation oncology consult for rectal cancer patients | Number of stage II-IV rectal cancer patients who were referred for a consultation with a Radiation Oncologist | Number of stage II-IV rectal cancer patients | Process | Colorectal Surgeon Gastroenterologist | 4 | 36, 49, 56, 61, 68 | 89% |
| Neoadjuvant and adjuvant Therapy (six QIs). *For the purpose of this table, the neo-adjuvant and adjuvant domains were combined as there were two QIs common to both domains. | |||||||
| Neoadjuvant chemoradiotherapy for local regionally advanced rectal cancer patients | Number of local regionally advanced rectal cancer patients who received neoadjuvant chemoradiotherapy | Number of local regionally advanced rectal cancer patients who underwent surgery | Process |
Colorectal Surgeon Medical Oncologist Radiation Oncologist |
2 | 26, 34, 36, 42, 46-48, 50, 52, 55, 60, 63, 69, 70 | 98% |
| Timeliness of neoadjuvant therapy | Number of CRC patients who received neoadjuvant chemoradiotherapy within 6 weeks of diagnosis(54 | Number of CRC patients who received neoadjuvant therapy | Process/*s |
Colorectal Surgeon Medical Oncologist Radiation Oncologist |
3 | 40, 49, 54, 63-65 | 84% |
| Adjuvant chemotherapy for stage II-III CRC patients | Number of stage II-III CRC patients who received adjuvant chemotherapy | Number of stage II-III CRC patients | Process | Colorectal Surgeon Radiation Oncologist | 2 | 26, 36, 47, 48, 50, 52, 58, 63, 69, 71-75 | 84% |
| Timeliness of adjuvant chemotherapy |
Number of stage II-III CRC patients who received adjuvant chemotherapy within 8 weeks26, 36, 43, 49, 50, 54, 56, 64, 65, 76 following surgical resection OR within 4 months following stage III colon cancer diagnosis35 and 9 months following stage II-III rectal cancer diagnosis35 |
Number of stage II-III CRC patients who received adjuvant chemotherapy | Process |
Medical Oncologist Nurse Care Coordinator |
2 | 26, 35, 36, 43, 48-50, 54, 56, 58, 64, 65, 76-78 | 80% |
| Evidence-based regimen of chemotherapy | Number of CRC patients who received a chemotherapy regimen (consistent with published dose/cycles guidelines | Number of CRC who received chemotherapy | Process | Medical Oncologist | 3 | 36, 49, 64, 65 | 95% |
| Mortality following chemotherapy or radiotherapy | Number of CRC patients who died following chemotherapy or radiotherapy who died within 30 and 90 days of treatment | Number of CRC patients who received chemotherapy or radiotherapy | Outcome |
Medical Oncologist Radiation Oncologist |
4 | 34 | 84% |
| Perioperative optimization (two QIs) | |||||||
| Stoma care follow-up appointment | Number of CRC patients who attended a stoma care follow-up appointment within 2 weeks of surgery involving stoma creation | Number of CRC patients who underwent surgery that involved stoma creation | Process | Stoma Nurse Colorectal Surgeon | 3 | *This indicator was developed in Round 1 | 84% |
| Thromboembolic prophylaxis | Number of CRC patients who underwent surgical resection with correct thromboembolic prophylaxis | Number of CRC patients who underwent surgery | Process | Colorectal Surgeon | 2 | 41, 69, 79 | 84% |
| Surgery (nine QIs) | |||||||
| Complete resection (incl. CME/TME) | Number of CRC patients who underwent surgery with complete resection (R0 resection, circumferential resection margin, distal margin, completeness/intact TME plane) | Number of CRC patients who underwent surgical resection | Outcome | Colorectal Surgeon | 2 | 26, 34, 37, 38, 47-51, 55, 58, 60, 61, 69, 70, 78, 80-87 | 95% |
| Adequate lymph node retrieval | Number of CRC patients with ≥1226, 34, 35, 43, 44, 47, 50, 52, 70, 88-94 resected lymph nodes | Number of CRC patients who underwent surgical resection | Process | Colorectal Surgeon | 1 | 26, 34, 35, 37, 38, 42-48, 50, 52, 54, 55, 58-60, 64-66, 70, 71, 74, 75, 77, 88-114 | 89% |
| Intraoperative tumor perforation, puncture, or laceration | Number of CRC cancer patients with intraoperative local tumor perforation, puncture, or laceration resulting in a deviation from the planned resection or involving an additional procedure | Number of CRC patients who underwent surgery | Outcome | Colorectal Surgeon | 2 | 48, 55, 60, 70, 115-117 | 89% |
| Unplanned re-operation | Number of CRC patients who returned to theatre after surgery for an unplanned reoperation (washout of abdomen, small bowel resection, further colorectal resection, drainage of intra-abdominal abscess, division of adhesions, stoma formation or operation on a stoma, and wound complications) within 30 days following surgery34, 118-120 | Number of CRC patients who underwent surgery | Outcome | Colorectal Surgeon | 2 | 34, 38, 45, 48, 51, 55, 56, 79, 118-121 | 82% |
| Unplanned re-admission | Number of CRC patients who were re-hospitalized within 30 days95 of hospital discharge | Number of CRC patients who underwent surgery | Outcome | Colorectal Surgeon | 2 | 95, 118, 121-124 | 80% |
| Post-operative complications | Number of CRC patients who have postoperative complications (i.e., anastomotic leak, infection of the surgical site, abdominal wall dehiscence, deep vein thrombosis, pneumonia, sepsis, hemorrhage or hematoma, blood transfusion, metabolic derangements, urinary dysfunction, bowel dysfunction, fever (>38°C, duration > 2 days, multiple organ failure, neurological/psychiatric complications, cardiac complications, catheter-related bloodstream infection, transfusion reaction) within 30 days following surgery | Number of CRC patients who underwent surgery | Outcome | Colorectal Surgeon | 2 | 34, 37, 38, 45, 48, 51, 52, 55, 56, 59, 60, 66, 69, 70, 74, 79, 111, 116-118, 122, 123, 125-133 | 98% |
| Post-operative mortality | Number of CRC patients who died within 30 days34, 37, 40, 52 following surgery | Number of CRC patients who underwent surgery | Outcome | Colorectal Surgeon | 1 | 26, 34, 37, 38, 44-48, 51, 52, 54, 55, 59, 60, 66, 70, 72, 81, 86, 108, 111, 116, 118, 119, 121, 123, 124, 128, 132, 134-142) | 93% |
| Overall survival | Number of CRC patients who have survived at (t following surgery *t = 1 year143, 2 years143, 5 years40, 41, 48, 54, 56, 60, 86, 143 | Number of CRC patients who underwent surgery | Outcome | Colorectal Surgeon | 2 | 40, 41, 48, 54-56, 60, 86, 125, 142-144 | 84% |
| Recurrence | Number of CRC patients who have had a recurrence within (t following surgery *t = 2 years66, 5 years48, 54-56, 60, 70, 74 | Number of CRC patients who received surgery | Outcome | Colorectal Surgeon | 2 | 48, 54-56, 60, 66, 70, 74, 144 | 89% |
| Post-treatment surveillance (two QIs) | |||||||
| Colonoscopy surveillance | Number of curatively treated CRC patients who received post-operative colonoscopy within 1 year of surgical treatment | Number of CRC patients who received curative treatment | Process | Colorectal Surgeon | 2 | 55, 58, 59, 68 | 80% |
| The number of CRC patients who received postoperative colonoscopy within 6 months after an incomplete preoperative colonoscopy (incl. obstructing lesion at the time of presentation | Number of CRC patients who had an incomplete preoperative colonoscopy | 3 | 48, 50 | ||||
| Documented follow-up history and physical | Number of CRC patients with documented history/physical examination, and postoperative visits every 3 months for 2 years, then every 6 months in years 2–5 (total of 5 years) | Number of CRC patients | Process |
Colorectal Surgeon Radiation Oncologist |
3 | 50 | 91% |
| Metastatic (one QI) | |||||||
| Oligometastatic liver/lung resection or ablative therapy | Number of CRC patients with oligometastatic liver/lung cancer who underwent resection or ablative therapy | Number of CRC patients with oligometastatic liver/lung cancer | Process |
Liver/Colorectal Surgeon Medical Oncologist Cardiothoracic Surgeon Radiation Oncologist |
4 | 145 | 81% |
| Supportive Care (one QI) | |||||||
| Discharge letter details the treatment summary, follow-up, and monitoring | Number of CRC patients with a discharge letter stating details of treatment summary, follow-up, and long-term monitoring, provided to the patient/carer on the day of discharge61 and referring physician within 4 weeks39 | Number of CRC patients | Process | Colorectal Surgeon | 4 | 61 | 80% |
- a References identified in the literature discuss the development, selection, validation, and review of the quality indicator.
- Process/*p.
- Structure/*s.
- Outcome/*o.
- t = specified time period.
4 DISCUSSION
This study identified a large number of existing CRC QIs that have been studied internationally and has prioritized the most clinically relevant QIs for use by Australian MDTs to measure and monitor their performance. Previous literature has described systematic reviews of multidisciplinary CRC QIs conducted in European settings, where one study included a consensus process.25, 26 In addition to updating the literature, the current study has addressed gaps presented in the two previous reviews and has focussed on quality measurement in the Australian MDT setting. Bianchi et al.’s26 review and two-step Delphi consensus process with a 12-person Swiss expert panel aimed to develop QIs for use in a population based setting. The working group in Bianchi et al.’s study proposed new QIs prior to the consensus process. Only 10 QIs identified in Bianchi et al.’s26 paper were also prioritized in the final set of 26 QIs for this study. Keikes et al.’s25 review included 41 studies and found a total sum of 349 QIs across these studies but didn't specify the number of unique indicators. In contrast, our review included 118 studies and found a total sum of 383 QIs, with a total of 93 unique QIs. This highlighted the importance of the second component of this study, in particular, the multidisciplinary involvement in the modified-Delphi. Neither study rated quality indicators and noted an urgent need to develop a validated system for this purpose, whilst our study utilized a grading system to calculate each QI's scientific soundness.
The majority of QIs identified in this systematic review were categorized as process indicators, although many of the QIs identified were multidimensional. It is recognized that many of the process indicators are significantly influenced by structural factors including the availability of the physical facility, equipment, and human resources. For example, “timeliness of CT and/or an MRI for staging”, which is commonly categorized as a process indicator may be influenced by a number of structural indicators including, the availability of staff and MRI machines. The relationships among structural, process, and outcome indicators are important considerations in the accurate interpretation of quality measurement. A significant number of QIs do not have high-quality studies to support their validity and impact on quality improvement, particularly in neoadjuvant therapy, palliative management of metastatic CRC, and supportive care. Many QIs currently used by organizations have been implemented without a high level of evidence. It is possible that these QIs have been selected on the basis of consensus, local practice guidelines, and the convenience and availability of data. However, some studies debated the reliability of extensively endorsed QIs, finding no association between the QI and improved patient outcomes or that QI results are influenced by patient/hospital level factors and should be used with caution.43, 92, 93, 95 This study presents the current state of evidence supporting the use of CRC QIs and highlights important QIs for further feasibility and validity testing.
The current review found, as did Keikes et al.,25 that most QIs were surgical measures. This may be indicative of the number of well-established national and internationally co-ordinated agencies dedicated to collecting analyzing standardized surgical data.37, 38 Another contributing factor may be that assessment of outcomes pertaining to surgery is well-defined and can often be measured immediately following the surgery whilst those pertaining to other therapies may take much longer to determine and may address outcomes that are harder to measure (delayed side effects, quality of life outcomes and patient experience). In areas such as ‘supportive care’ where there are a very small number of QIs, the data required to calculate additional QIs may not be routinely collected by health services or require patient reported data. As electronic medical records evolve, the integration of patient reported outcome measures will be fundamental to capturing and standardizing the reporting of patient health status.146 Further research is needed to identify priority quality of life indicators for patients and how these are most effectively analyzed and translated into quality improvement by local MDTs.
Comparing the core treatment modalities, there was a lack of radiation therapy-specific QIs. This may be due to the non-tumor-specific nature of radiation therapy quality assurance activities that are typically undertaken at a service level. However with the evolution in the roles of radiation therapy (in rectal cancer) and modern technologies, there is an opportunity for disease-specific radiation therapy QIs, particularly in the areas of treatment waiting times, radiation therapy target volume delineation, dose prescription, plan evaluation, and image guidance during treatment delivery. There may also be an opportunity to develop QIs in diagnostic disciplines (radiology, nuclear medicine, and anatomical pathology) to facilitate accurate staging as well as in the areas of informed clinical treatment decisions by patients and HPs, and timely access to treatment. These gaps could be addressed by developing QIs from nationally and internationally published and endorsed clinical guidelines from both cancer organizations and discipline-specific guidelines.
Despite a large number of surgical QIs, over half of QIs in the final set pertain to care delivery by multiple disciplines and HPs thus highlighting the multidisciplinary nature of CRC care, recognizing that an MDT focus on service quality improvement is important. The involvement of HP experts from each discipline delivering CRC care is a strength and should maximize the applicability of the final QI set presented. Future work will focus on the examination of the feasibility and construct validity of the QI as these will be critical to implementation. Furthermore, it will be important to establish and test local benchmarks.
4.1 Limitations
A limitation of extracting QIs from the literature is the time lag from QI development and validation to publication. This may have resulted in missing QIs that describe more recent diagnostic and treatment techniques. Whilst a comprehensive list of quality and safety agencies was purposefully selected for the search, it may not encompass all possible organizations internationally. This study specifically prioritized QIs based on clinical importance and relevance to the Australian MDT setting. Other common measures in Delphi surveys such as ‘feasibility’ were not included in this process. The current feasibility of measuring QIs will be determined in the next project phase with real-world clinical data as opposed to participant opinions of feasibility from different Australian states and data systems. Furthermore, a clinically important QI set may form a basis for which data points should be considered standardization and collection in the future, rather than constructing QIs on the basis of data already collected.
5 CONCLUSION
This study has identified a priority set of evidence-based and clinically important QIs that could be used by CRC MDTs to measure, describe and monitor the quality of care they deliver. Our findings highlight that an MDT-based approach to quality measurement and monitoring addresses the full spectrum of the CRC patient pathway and experience. Future work will focus on the feasibility of implementation and examining the utility of this priority set, which has the potential to facilitate the monitoring of CRC patient care delivery at the tumor stream level, identify potential variation in care, provide standards of care to benchmark against through self-regulation, direct quality improvement activities and embed a culture of quality improvement within the service.
AUTHOR CONTRIBUTIONS
Candice Donnelly, Puma Sundaresan, Kim-Lin Chiew, and Shalini Vinod developed the search strategy. Candice Donnelly, Puma Sundaresan, Michelle Or, and Mathushan Thevaraja completed the components of the systematic review screening and analysis. Candice Donnelly and Puma Sundaresan conducted the consensus process with contributions from Shalini Vinod, Tim Shaw, and Anna Janssen. Candice Donnelly conducted the Delphi analysis. Candice Donnelly drafted the manuscript with contributions from Puma Sundaresan, Michelle Or, James Toh, Shalini Vinod, Anna Janssen, Nimalan Pathma-Nathan, Tim Shaw, and Paul Harnett.
ACKNOWLEDGMENTS
Candice Donnelly is undertaking a PhD and would like to thank her supervision team (Tim Shaw, Shalini Vinod, Paul Harnett, and Anna Janssen) for their guidance. The research team would like to acknowledge the Delphi participants. This work was funded by the Radiation Oncology Network, Western Sydney Local Health District, and the Sydney West Transitional Cancer Research Centre (SWTCRC). The SWTCRC was funded by The Cancer Institute NSW under the Translational Cancer Research Centres (TRC) [15/TRC/1-01]. Funding provided salary support for Candice Donnelly.
Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
Permission to conduct this study was granted by the University of Sydney Human Research Ethics Committee [HREC 2021/778].
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, Candice Donnelly, upon reasonable request.




