Population pharmacokinetics of doxorubicin: A systematic review
Abstract
Because of the high interindividual pharmacokinetic variability, several population pharmacokinetic (PopPK) models of doxorubicin (DOX) were developed to characterize factors influencing such variability. However, significant predictors for DOX pharmacokinetics identified using PopPK models varied across studies. Thus, this review aims to summarize PopPK models of DOX and its metabolites (if any) as well as significant covariates influencing DOX (and its metabolites) pharmacokinetic variability. A systematic search from PubMed, CINAHL Complete, Science Direct, and SCOPUS databases identified 503 studies. Of these, 16 studies met the inclusion criteria and were included in this review. DOX pharmacokinetics was described with two- or three-compartment models. Most studies found a significant increase in DOX clearance with an increase in body surface area from the median value of 1.8 m2. Moreover, this review identified that while a 10-year increase in patient age resulted in a decrease in DOX clearance in adults and the elderly, younger children had lower DOX clearance compared to older children. Further, low DOX exposure was observed in pregnant women, and thus dosage adjustment is required. Concerning model applicability, predictive performance assessment of these published models should be performed before implementing such models in clinical practice.
1 INTRODUCTION
Doxorubicin (DOX) exhibits a broad-spectrum antitumor activity against various types of malignancies, including leukemias, sarcomas, breast cancer, small cell lung cancer, and ovarian cancer.1 The exact mechanisms of action underlying its cytotoxicity have not been elucidated; however, the probable modes of action included (1) a formation of free radicals, (2) DNA intercalation, (3) interaction with cellular membranes, and (4) provocation of DNA breaks.1, 2
The oral bioavailability of DOX is low, which is mainly due to the intestinal P-glycoprotein efflux transporter.3 DOX plasma protein binding is approximately 50%–85%.1 The apparent volume of distribution (VD) is quite large, ranging from 20 to 30 L/kg, suggesting an extensive tissue uptake.1 The drug undergoes extensive metabolism producing several metabolites, and doxorubicinol (DOXOL) is the major metabolite formed. DOXOL is cytotoxic but is less potent than the parent compound, while aglycone metabolites including doxorubicinone, doxorubicinolone, 7-deoxydoxorubicinone, and 7-deoxyrubicinolone do not exhibit a cytotoxic effect.1, 4 Though aglycone metabolites are not cytotoxic, they are associated with cardiotoxicity.2, 4, 5 DOX and its metabolites are mainly excreted in bile,1, 2, 6 with only 5%–12% of the drug is renally excreted.6
DOX exhibits linear pharmacokinetics within a dose range of 20–60 mg/m2.7 Its concentration–time profiles are described by both biphasic8-10 and triphasic7, 9 disposition depending on the duration of infusion. The use of DOX is limited by its myelosuppression and cardiomyopathy, which exhibit a dose-dependent manner.1, 5, 6, 11 Moreover, DOX manifests high interindividual variability (IIV) and interoccasion variability (IOV),12, 13 with more than 10-fold of the area under a concentration–time curve (AUC) variation among individuals.6 Additionally, studies have shown age-dependent DOX pharmacokinetics.14-16 Given these pharmacokinetic variations, several population pharmacokinetic (PopPK) models of DOX have been developed to characterize such variability and to investigate potential influences of various covariates.6, 13, 14, 17-25 These models, however, were developed in different patient populations with different dosage regimens. Further, model structures are different across studies, for example, some studies developed parent–metabolite models for DOX and DOXOL,14, 17, 24 while some studies developed exclusive DOX PopPK models.16, 26 Thus, this review aims to describe PopPK models of DOX and its metabolites (if any) and to summarize significant covariates influencing DOX (and its metabolites) pharmacokinetic variability.
2 METHODS
2.1 Search strategy and study selection
We systematically searched four databases (PubMed, CINAHL Complete, Science Direct, and SCOPUS) from the date of their inceptions to July 2021. The following search terms were employed (“doxorubicin” OR “Adriamycin”) AND (“population pharmacokinetic*” OR “pharmacokinetic model*” OR “nonlinear mixed effect model*” OR “NONMEM” OR “interindividual variability” OR “intersubject variability” OR “residual variability” OR “intrasubject variability”). These search terms were created based on the PICO framework. Additional studies were identified through the reference list screen.
The retrieved articles were stored in a citation manager (EndNote 20, Thomson Reuters, New York, NY, USA). Nonrelevant studies were excluded following title and abstract screen, which was independently performed by JM and KT. Subsequently, full-text articles were screened and selected by the same reviewers based on the following inclusion criteria: (1) PopPK studies conducted in humans who used doxorubicin and (2) studies employing a nonlinear mixed effects approach. Studies published in non-English or non-Thai language and those with insufficient information on model development methodology were excluded. Moreover, studies with DOX formulations in the form of copolymer or liposome were also excluded. Any disagreements were resolved by consensus of all authors. This review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline.
2.2 Data extraction
JM and KT independently extracted the data using the predesigned data abstraction form. The extracted information included (1) physiologic and pathological characteristics such as body size, age, sex, and laboratory values; (2) pharmacokinetic data that included dosage regimens, concurrent medications, sampling strategy, and analytical assays; and (3) model development methodology encompassing fixed and random effect models, investigated and retained covariates, and model qualification approach. The sampling strategy was categorized into extensive sampling when the number of samples per patient was greater than six; otherwise, it fell into a sparse sampling approach. For the fixed effect parameters, information on both DOX and DOXOL was extracted. Concerning the random effect models, types of relationships and magnitudes of variability (both IIV and residual variability; RV) were excerpt. Model qualification was categorized into basic and advanced internal and external evaluation.27 Any discordance on data extraction was resolved by all authors.
3 RESULTS
3.1 Study identification
A PRISMA diagram of study identification is shown in Figure 1. In brief, a total of 503 articles were identified from database searching, and of these 97 duplicate articles were removed. Subsequently, 376 articles were excluded by title and abstract screen, and the remaining 30 full-text articles were assessed according to the inclusion and exclusion criteria. Finally, 16 studies were included in this review.
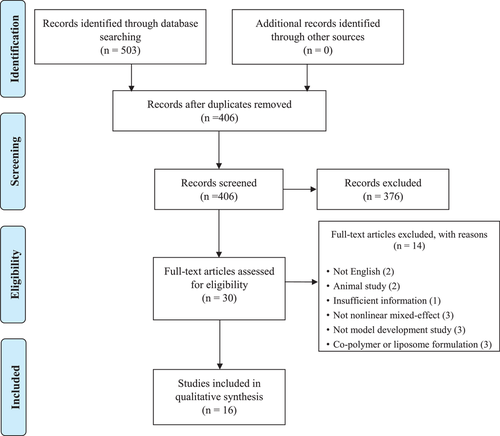
3.2 Physiologic, pathological characteristics, and pharmacokinetic data
DOX PopPK studies were conducted in patients with various types of cancer such as small cell lung cancer,23 breast cancer,14, 15, 26, 28 lymphoma,13, 18, 24, 29 synovial sarcoma,29 osteosarcoma,18, 29, 30 Ewing sarcoma,30 leukemia,16, 18, 29 neuroblastoma,18, 29 hepatoblastoma,18, 29 Wilm's tumor,18 and AIDS-related Kaposi sarcoma.2 Most studies were performed in adults (and elderly),2, 14, 15, 17, 23, 24, 28 while four studies consisted of children and adults,16, 18, 19, 30 and one study consisted of children, adults, and elderly.22 In addition, two studies included pregnant women in the models.21, 26 In all studies, DOX was administered by intravenous infusion, with the dose ranging from 20 to 120 mg/m2, and the infusion duration of 5 min to 72.5 h. Most studies employed an extensive sampling strategy.2, 15, 17, 18, 21, 26, 28-30 Four studies used a sparse sampling approach,13, 14, 22, 24 whereas two studies utilized a mixed approach.16, 23 Concerning a bioassay, all studies quantitated DOX (and its metabolites) using liquid chromatography, except for one study in which capillary electrophoresis was used.20 A summary of population characteristics and pharmacokinetic data is presented in Table 1.
| No. | Author | Cancer type | N | Male (%) | Mean age (range) | Mean BW (range) | BSA (range) | DOX Dose (mg/m2) [range] | Infusion duration | Concomitant | Sampling strategy | Assay | %CV | LOQ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Freyer et al.23 | Small cell lung cancer | 24 |
57a (45-70) |
69a (54-93) |
1.79a (1.54-2.10) |
50 | 15 min | Etoposide, ifosfamide, methylprednisolone, ondansetron | Mixed (extensive and sparse) | HPLC | <15% | 2.5 ng/ml | |
| 2 | Callies et al.17 | Metastatic or locally advanced cancer who failed conventional therapy | 36 | 18 (50) |
57a (27–73) |
82.7a (43.1–137) |
1.99a (1.37–2.69) |
45 60, 75 |
DOX: 0.5 h Zosuquidar: 48 h continuous inf |
Zosuquidar 40, 80, 160, 320, 640, 960, and 1280 mg/m2 | Extensive | HPLC | 7.09% | 0.5 ng/ml |
| 3 | Joerger et al.2 | AIDs-related Kaposi sarcoma | 7 |
7 (100) |
43.4 (29–53) |
67.2 (61–77) |
20 | 30 min | Bleomycin, vincristine | Extensive | HPLC | <5% |
DOX and DOXOL: 1 ng/ml Aglycones: 0.5 ng/ml |
|
| 4 | Joerger et al.14 | Breast cancer | 59 | 0 (0) |
56a (49–63) |
70a (62–80) |
1.81a (1.68–1.91) |
60 | 15 | Cyclophosphamide | Sparse | HPLC | 1.8–2.4 nmol/L | |
| 5 | Wilde et al.24 | Hodgkin's lymphoma | 30 | 21 (70) |
33.6 (17–59) |
74.0 (53.5–99) |
1.91 (1.58–2.26) |
25, 35 | 30 min | Bleomycin, etoposide, cyclophosphamide, vincristine, procarbazine, prednisolone | Sparse | Capillary electrophoresis | ||
| 6 | Thompson et al.29 | Hodgkin disease, acute lymphoblastic leukemia, Burkitt's lymphoma, non-Hodgkin lymphoma, osteosarcoma, neuroblastoma, synovial sarcoma, hepatoblastoma | 22 | 16 (72.73) |
15a (3.3–21.5) |
51.5a (12.4–80) |
BMI: 19.7a (13.2–30) |
20–60 | 0.08–6 h | None | Extensive | HPLC | 7% | 2 ng/ml |
| 7 | Kumar et al.22 | Various types (majority: breast cancer) | 28 | 11 (32.29) |
39.05 ± 15.57 (6–73) |
52.19 ± 12.24 (15–86) |
62.41 ± 24.33 mg (20–120 mg) |
1–2 h | Have concomitant but NR | Sparse | HPLC | |||
| 8 | Kontny et al.6 | Use dataset from published studies14, 17, 24, 29 | ||||||||||||
| 9 | van Hasselt et al.26 | Breast cancer | Non-preg: 59 (from Joerger et al.14) | 0 (0) | ||||||||||
| Preg: 14 Postpartum: 4 | 0 (0) |
GA: 28.6a (14.7–34.3) |
50–60 | 0.5 h | Extensive | NR | ||||||||
| 10 | Wong et al.15 | Breast cancer | 84 | 0 (0) | 50.4 ± 10.1 | BMI: 24.5 ± 4.7 | 1.57 ± 0.15 | 75 | NR | Docetaxel | Sparse | HPLC | ||
| 11 | Voller et al.16 | Solid tumors and leukemia | 94 | 46 (48.94) |
5.32a (0.2–17.7) |
19.3a (3.6–88.1) |
0.77a (0.23–2.05) |
25a (2.4–57) |
3.92a (0.25–24) |
NR | Mix | HPLC | ||
| 12 | Liang et al.28 | Breast cancer | 17 | 0 (0) |
45a (28–58) |
65.3a (54.5–140.4) |
1.71a (1.6–2.5) |
60 every 14 daysa 4 cycles (54–65) |
15 min (5–60 min) |
Cyclophosphamide, enalapril | Extensive | HPLC | DOX: <10% DOXOL: <11% | 1 ng/ml |
| 13 | Perez-Blanco et al.13 | Non-Hodgkin's lymphoma | 45 | 23 (51.11) |
71 ± 12 (43–110) |
1.8 ± 0.2 (1.3–2.3) |
51 ± 7 (25–71) |
0.5 ± 0.2 (0.2–1.3 h) |
Rituximab, cyclophosphamide, vincristine, prednisolone | Sparse | Ultrahigh HPLC | <10% |
DOX: 8 ng/ml DOXOL: 3 ng/ml |
|
| 14 | Kunarajah t al.18 | Hodgkin, hepatoblastoma, non-Hodgkin, Wilms' tumor, T-all, Pre-B All, synovial sarcoma, neuroblastoma, osteosarcoma | 17 | 11 (64.71) |
7a (3.4–14.7) |
31.1 (11–88.6) |
1.01 (0.55–1.91) |
30a (25–75) |
1a (0.75–72.5 h) |
cyclophosphamide | Sparse | LC | DOX: 10.6% DOXOL: 20.1% | 4.7 ng/ml |
| 15 | Liu et al.30 | Hodgkin Lymphoma, Ewing sarcoma, osteosarcoma | 66 | 39 (59.09) |
22.2a (6.7–48.8) |
75.5a (25.5–121.) |
1.9a (0.93–2.5) |
(25–75) | 48 h infusion and short infusion | Various chemotherapy regimens | Extensive | LC | DOX: 10.6% DOXOL: 20.1% | 4.7 ng/ml |
| 16 | Janssen et al.21 | Non-preg: 61 (from Joerger et al, 2007) | ||||||||||||
| Preg: 22 | 0 (0) | Days postpartum Med: 46.5 (28–61) | NR | NR | 25, 50, 60 | 0.5 | HPLC | |||||||
- a Median value.
- Abbreviations: BW, bodyweight; BMI, body mass index; BSA, body surface area; CV, coefficient of variation; DOX, doxorubicin; DOXOL, doxorubicinol; HPLC, high-performance liquid chromatography; LOQ, limit of quantitation; non-preg, nonpregnancy; NR, not reported; Preg, pregnancy.
3.3 Pharmacokinetic models, covariates, and model qualifications
All DOX PopPK studies included in this review were conducted with NONMEM® software, and most of them developed a parent–metabolite model.2, 6, 13-18, 24, 28-30 First-order conditional estimation with interaction was the method most frequently employed,6, 16-18, 21, 26, 28, 30 while some studies used the first-order method2, 22 or first-order conditional method.14, 24 In terms of disposition, 11 studies described DOX pharmacokinetics using a three-compartment models6, 13, 16-18, 21, 23, 26, 28-30; however, five studies employed a two-compartment model structure.2, 14, 15, 22, 24 The estimated Vc ranged from 3.87 to 51.4 L. Concerning DOX elimination, all studies reported linear elimination with the population CLDOX ranging from 21.7 to 62.4 L/h, excluding the one with substantially low value of 1.43 L/h,22 while the CLDOXOL exhibited a wider range of 11.1–92.7 L/h. For studies estimating apparent DOXOL clearance (CLDOXOL/fm), the values ranged from 106 to 143 L/h.
An exponential relationship was used to explain the IIV in most studies,6, 13, 15-17, 21-24, 26, 28-30 while a proportional model was used in two studies.2, 14 Of these, the magnitudes of the IIV on DOX clearance (CLDOX) and central volume of distribution (Vc) ranged from 8.33% to 46.80% and 10.5% to 183%, respectively. Further, IOV was explored in five studies,6, 16, 17, 23, 28 mainly on CLDOX and Vc. Regarding the RV for DOX, most studies used a proportional relationship (n = 11),2, 6, 13-17, 21, 24, 26, 30 whilst a combined proportional and additive relationship was used in the rest of the studies.18, 22, 23, 28, 29 As for the DOXOL, most studies used the same RV relationship as DOX, except two studies in which different types of relationship were employed.17, 28 The magnitude of RV for the proportional relationship ranged from 8.73% to 45.8%, while the standard deviation of the additive relationship ranged from 0.04 to 64 ng/ml. Table 2 summarizes the software, estimation methods, structural models, IIV, IOV, and RV used in the model development process.
| No. | Author | Model | Software | Estimation method | Interindividual variability | Interoccasion variability | Residual variability |
|---|---|---|---|---|---|---|---|
| 1 | Freyer et al.23 | 3-CMT with linear elimination | NONMEM 5 | Proportional | Combined | ||
| 2 | Callies et al.17 | 3-CMT for DOX and 2 pathways for DOXOL formation | NONMEM 5 | FOCEI |
DOX: Exponential DOXOL: Exponential |
DOX: Exponential DOXOL: Exponential |
DOX: Proportional DOXOL: Combined |
| 3 | Joerger et al.2 | 2-CMT with first-order elimination for DOX linked to 4 metabolites CMT (DOXOL, Doxorubicinone, 7-deoxydoxorubicinolone, 7-deoxydoxorubicinone) | NONMEM 5 | FO |
DOX: Proportional DOXOL: Proportional |
DOX: Proportional DOXOL: Proportional (additive of the log-transformed) | |
| 4 | Joerger et al.14 | 2-CMT with first-order elimination (DOX) linked to DOXOL metabolite | NONMEM 5 | FOCE |
DOX: Proportional DOXOL: Proportional |
DOX: Proportional DOXOL: Proportional (additive of the log-transformed) | |
| 5 | Wilde et al.24 | 2-CMT for DOX and 1-CMT for DOXOL with first-order elimination | NONMEM 5 | FOCE |
DOX: Exponential DOXOL: Exponential |
DOX: Proportional DOXOL: Proportional | |
| 6 | Thompson et al.29 | 3-CMT for DOX and 1-CMT for DOXOL | NONMEM 6 |
DOX: Exponential DOXOL: Exponential |
DOX: Combined DOXOL: Combined |
||
| 7 | Kumar et al.22 | 2-CMT | NONMEM 5 | FO | Exponential | Combined | |
| 8 | Kontny et al.6 | 3-CMT for DOX and 1-CMT for DOXOL | NONMEM 7.2 | FOCEI | Exponential | Exponential | Proportional |
| 9 | van Hasselt et al.26 | 3-CMT linear model | NONMEM 7 | FOCEI | Exponential | Exponential | Proportional |
| 10 | Wong et al.15 | 2-CMT for DOX and 1 sequential CMT for DOXOL | NONMEM 7 | Exponential | Proportional | ||
| 11 | Voller et al.16 | 3-CMT linear model | NONMEM 7.2 | FOCEI | Exponential | Proportional | |
| 12 | Liang et al.28 |
PK: 3-CMT for DOX and 1-CMT for DOXOL PD: indirect model with linear stimulatory effect (5 transit CMT) |
NONMEM 7 | Exponential | Exponential | Combined | |
| 13 | Perez-Blanco et al.13 | 3-CMT for DOX and 2-CMT for DOXOL with first-order distribution and elimination | NONMEM 7.3 | Exponential | Proportional | ||
| 14 | Kunarajah et al.18 |
3-CMT for DOX and 1-CMT for DOXOL with first-order metabolism to DOXOL A turn-over effect model for a time-course of cTnI |
NONMEM 7.3 | FOCEI | Exponential | Combined | |
| 15 | Liu et al.30 | A linked 3-CMT for DOX and 1-CMT for DOXOL | NONMEM 7.2 | FOCEI | Exponential | Proportional | |
| 16 | Janssen et al.21 | A linear 3-CMT with first-order elimination | NONMEM 7.3 | FOCEI | Exponential | Proportional |
- Abbreviations: DOX, doxorubicin; DOXOL, doxorubicinol; FO, first-order; FOCE, first-order conditional estimation; FOCEI, first-order conditional estimation with interaction.
Several potential covariates influencing DOX pharmacokinetics were investigated (Table 3) including body size (e.g., weight, body surface area [BSA], height, body mass index [BMI], body fat, lean body mass),2, 6, 13-18, 21-24, 28-30 age,6, 13-16, 18, 22-24, 28, 30 sex,6, 13, 16-18, 21-24, 30 DOX dose,17, 24, 28 liver function (alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkali phosphatase [ALP]),2, 13-17, 22, 23, 28 renal function (serum creatinine [SCr], creatinine clearance [CLCR]),2, 14-17, 23, 24, 28 and laboratory values such as hematocrit (HCT),16 leukocyte, neutrophil, and platelet count,13 genetic polymorphisms,16 albumin (ALB),14-17 total protein (TP),23 total bilirubin (TBIL),2, 14, 15, 23, 28 free bilirubin (BIL),13, 16, 17 and lactate dehydrogenase (LDH).23 Moreover, additional covariates like disease stage, for example, presence or absence of metastatic, tumor entity, Eastern Co-operation Oncology Group (ECOG) performance status,13-15, 23 comedications,2, 14, 17, 28 center,6, 16 and pregnancy status21, 26 were also tested. Of these, BSA was the most frequently identified covariate in the final models,6, 14, 16, 18, 24, 29, 30 followed by age.14-16, 18, 21, 24 Only one study found a significant effect of AST on CLDOX and a significant effect of CLCR on doxorubicinol clearance (CLDOXOL).14
| No. | Author | Parameter | WT | BSA | BMI | LBW | HT | Age | Sex | TBIL/BIL | TP | ALB | LDH | DOX Dose | ALT | AST | ALP | SCr | CLCR | Hematology | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Freyer et al.23 | X | X | X | X | X | TBIL | X | X | X | X | X | X | Disease state | |||||||
| 2 | Callies et al.17 | CL | X | BIL | X | X | Zosuquidar | ||||||||||||||
| V2 | X | X | Zosuquidar | ||||||||||||||||||
| CLDOXOL/fm | X | BIL | X | X | Zosuquidar | ||||||||||||||||
| VDOXOL/fm | X | X | Zosuquidar | ||||||||||||||||||
| 3 | Joerger et al.2 | X | TBIL | X | X | X | Comedication | ||||||||||||||
| 4 | Joerger et al.14 | CLDOX, | X | X | X | TBIL | X | X | X | Performance status according to WHO criteria, presence of liver metastases, comedication with enzyme inducing AEDs | |||||||||||
| CLDOXOL/fm | X | ||||||||||||||||||||
| 5 | Wilde et al.24 | CLDOX, VDOX, VDOXOL, | X | CLDOX | VDOX | X | VDOXOL | X | X | X | Cpeak, infusion rate, infusion duration, no. of cycle BEACOPP scheme variant | ||||||||||
| 6 | Thompson et al.29 | CLDOX | CLDOX | X | Body fat on CLDOX | ||||||||||||||||
| 7 | Kumar et al.22 | CL, V | X | X | X | X | X | ||||||||||||||
| 8 | Kontny et al.6 | X | X (all parameters) | X | X | X | X | Analytical center | |||||||||||||
| 9 | van Hasselt et al.26 | CL | Pregnancy | ||||||||||||||||||
| V1 | Pregnancy | ||||||||||||||||||||
| V2 | Pregnancy | ||||||||||||||||||||
| V3 | Pregnancy | ||||||||||||||||||||
| 10 | Wong et al.15 | X | X | X | X | CL | TBIL | X | X | X | X | X | Race, baseline clinical tumor stage, baseline clinical nodal stage, presence or absence of metastatic, intra-abdominal fat volume, total abdominal fat volume, total muscle volume, intra-abdominal: total abdominal fat ratio | ||||||||
| 11 | Voller et al.16 | X | All parameters | X | X | X | CL | X | BIL | X | X | X | X | X | HCT | Daily volume of liquid IV at time of DOX administration, tumor entity, country, genetic polymorphism | |||||
| 12 | Liang et al.28 | X | X | X | X | X | TBIL | X | X | X | X | X | baseline left ventricular ejection fraction, the use of enalapril | ||||||||
| 13 | Perez-Blanco et al.13 | CLDOX, CLDOXOL | X | X | X | X | X | X | X | BIL | X | X | X | leucocyte, neutrophil, platelet count | ECOG status, IPI | ||||||
| 14 | Kunarajah et al.18 | PK parameters | X | All parameters | X | CL | X | ||||||||||||||
| E | INFUSION rate of DOX | ||||||||||||||||||||
| Cbase,cTnI | Prior cumulative anthracycline dose amount | ||||||||||||||||||||
| 15 | Liu et al.30 | X | All parameters | X | X | X | X | X | Disease group (osteosarcoma, Ewing sarcoma, Hodgkin lymphoma) | ||||||||||||
| 16 | Janssen et al.21 | CL | X | X | Pregnancy | ||||||||||||||||
| V1 | X | X | Pregnancy |
- Note: Bold represents significant covariate.
- Abbreviations: AEDs, antiepileptic drugs; ALB, albumin; ALT, alanine transaminase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; BIL, free bilirubin; BMI, body mass index; BSA, body surface area; Cbase,cTnI, baseline cardiac troponin I concentration; CL, clearance; CLCR, creatinine; clearance; DOX, doxorubicin; E, cardiotoxicity exposure effect; ECOG, Eastern Cooperative Oncology Group; fm, fraction of DOX metabolized to DOXOL; HT, height; IPI, International Prognostic Index; IV, intravenous; TBIL, total bilirubin; LBW, lean body weight; LDH, lactate dehydrogenase; PK, pharmacokinetics; SCr, serum creatinine; TP, total protein; V, volume of distribution; WT, weight.
Most studies assessed model qualification using both basic and advanced internal approaches,2, 13-16, 18, 21, 26, 28, 30 while three studies solely used a basic internal strategy.17, 22, 23 Only one study employed all evaluation strategy, that is, basic and advanced internal as well as external approaches.6 Model validation techniques along with final PopPK models of each study are outlined in Table 4.
| No. | Author, Year | Final Model | Inter-individual variability | Inter-occasion variability | Residual variability | Model Evaluation |
|---|---|---|---|---|---|---|
| 1 | Freyer et al, 200123 | CL (L/h) = 54 | 17.20% | 16% |
|
Basic internal |
| V (L) = 9.3 | 19.20% | |||||
| 2 | Callies et al, 200317 |
|
20.5% | 8.36% |
|
Basic internal |
| C2 = 26.2% when zosuquidar ≥ 500 mg | 13.6%, | 18.5% | ||||
| V3 (L) = 104 | ||||||
| Q2 (L/h) = 85.8 | 10.3% | 50.1% | ||||
| Q3 (L/h) = 35.6 | ||||||
|
41.6% | 28.3% | ||||
|
47.7% | 33.6% | ||||
| 3 | Joerger et al, 20052 | CL (L/h) = 61.8 | 13.3% |
|
Basic and advanced internal | |
| Q (L/h) = 112 | 25.5% | |||||
| V1 (L) = 23.3 | 51.9% | |||||
| V2 (L) = 1130 | 19.7% | |||||
| fm3/V3 = 0.00933; doxorubicinone | NE | |||||
| fm4/V4 = 0.00344; 7-deoxydoxorubicinone | 67.8% | |||||
| fm5/V5 = 0.00733; doxorubicinol | 20.8% | |||||
| K30 (1/h) = 22.2; doxorubicinone | 44.8% | |||||
| K40 (1/h) = 7.22; 7-deoxydoxorubicinone | 98.9% | |||||
| K56 (1/h) = 2.18; doxorubicinol | NE | |||||
| K60 (1/h) = 62.2; 7-deoxydoxorubicinone | 91.8% | |||||
| 4 | Joerger et al, 200714 | CLDOX (L/h) = 47.6*(BSA/1.8)1.4*(AST/21)-0.24*(AGE/56)-0.54 | 24.6% |
|
Basic and advanced internal | |
| CLDOXOL/fm (L/h) = 108*(CLCR/80)0.54 | 29.4% | |||||
| Q (L/h) = 60.3 | 20.7% | |||||
| V1 (L/h) =12.3 | 11.8% | |||||
| V2 (L/h) = 421 | 25.0% | |||||
| V3/fm (L) = 1580 | 51.3% | |||||
| 5 | Wilde et al, 200724 | CLDOX (L/min) = 0.962 |
|
|||
| Q (L/min) = 1.22 | ||||||
| V1 (L) = 18.4 | 183% | |||||
| V2 (L) = 789.7 | 46% | |||||
| CLDOXOL (L/min) = 0.579 | ||||||
| QDOXOL (L/min) = 0.32; conversion rate from DOXOL to DOX | ||||||
| VDOXOL (L) = 477.1 | ||||||
| 6 | Thompson et al, 200929 | CL (L/h/m2) = 25.1 | ||||
| Q1 (L/h/m2) = 24.8 | ||||||
| Q2 (L/h/m2) = 6.59 | ||||||
| V1 (L/m2) = 6.96 | ||||||
| V2 (L/m2) = 557 | ||||||
| V3 (L/m2) = 16.5 | ||||||
| CLDOXOL/fm (L/h/m2) = 50.2 | ||||||
| VDOXOL/fm (L/m2) = 1100 | ||||||
| 7 | Kumar et al, 200922 | CL (L/h) = 1.43 | 46.50% |
|
Basic internal | |
| V (L) = 51.4 | 36.10% | |||||
| K12 (1/h) = 0.271 | ||||||
| K21 (1/h) = 0.689 | ||||||
| 8 | Kontny et al, 20136 | CLDOX (L/h/1.8m2) = 53.3*(1+(BSA-1.8 m2)*0.643) | 31% | 13% |
|
Basic and advanced internal and external |
| VDOX (L/h/1.8 m2) = 17.7*(1+(BSA-1.8 m2)*0.643) | 19% | 21% | ||||
| Q2 (L/h/1.8 m2) = 58.7*(1+(BSA-1.8 m2)*0.643) | 31% | |||||
| V2 (L/1.8 m2) = 1830*(1+(BSA-1.8 m2)*0.643) | 20% | |||||
| Q3 (L/h/1.8 m2) = 21.8*(1+(BSA-1.8 m2)*0.643) | 29% | |||||
| V3 (L/h1.8 m2) = 71.6*(1+(BSA-1.8 m2)*0.643) | ||||||
| CLDOXOL (1/h/1.8 m2) = 44*(1+(BSA-1.8 m2)*0.643) | 50% | |||||
| VDOXOL (L/1.8 m2) = 1150*(1+(BSA-1.8 m2)*0.643) | 57% | |||||
| 9 | van Hasselt et al, 201426 | CL (L/h) = 42.8 | 46.80% |
|
Basic and advanced internal | |
|
37.50% | |||||
| V2 (L) = 23 | 29.60% | |||||
|
69.90% | |||||
| Q1 (L/h) = 11.8 | NR | |||||
| Q2 (L/h) = 38.3 | NR | |||||
| 10 | Wong et al, 201415 | CL (L/h) = 53.5*(age/50)-0.393 | 15.40% |
|
Basic and advanced internal | |
| V1 (L) = 25.6 | 15.80% | |||||
| V2 (L) = 446 | 14.30% | |||||
| Q (L/h) = 55.9 | ||||||
| CLDOXOL (L/h) = 92.7 | 23.80% | |||||
| VDOXOL (L) = 1700 | 35.40% | |||||
| 11 | Voller et al, 201516 | CL (L/h/m2) = 24.1*(1+(BSA-0.77 m2)*1.30)*(1+(age/5.32)0.286) | 30.70% |
|
Basic and advanced internal | |
| V1 (L/m2) = 9.34*(1+(BSA-0.77 m2)*1.30) | 26.7% | 124% | ||||
| Q2 (L/h/m2) = 26.8*(1+(BSA-0.77 m2)*1.30) | 35.20% | |||||
| V2 (L/m2) = 560*(1+(BSA-0.77 m2)*1.30) | ||||||
| Q3 (L/h/m2) = 12.1*(1+(BSA-0.77 m2)*1.30) | ||||||
| V3 (L/m2) = 27.8*(1+(BSA-0.77 m2)*1.30) | ||||||
| CLDOXOL (L/h/m2) = 42.5*(1+(BSA-0.77 m2)*1.30) | 43% | |||||
| VDOXOL (L/m2) = 760*(1+(BSA-0.77 m2)*1.30) | 48% | |||||
| 12 | Liang et al, 201628 | CLDOX (L/h) = 54.2 | 8.33%; | 3.86% |
|
Basic and advanced internal |
| V1,DOX (L) = 16.9 | 10.50% | |||||
| Q2,DOX (L/h) = 66.1 | 15.40% | |||||
| V2,DOX (L) = 1650 | 26.70% | |||||
| Q3,DOX (L/h) = 23.4 | ||||||
| V3,DOX (L) = 61.8 | ||||||
| CLDOXOL/fm (L/h) = 106 | 18.50% | |||||
| V1,DOXOL/fm (L) =1880 | 9.39% | |||||
| 13 | Perez-Blanco et al, 201613 | CL (L/h) = 62.4 | 23% |
|
Basic and advanced internal | |
| V1 (L) = 17.7 (fixed) | NA | |||||
| Q2 (L/h) = 50.7 | 64.1% (fixed) | |||||
| V2 (L) = 1830 (fixed) | NA | |||||
| Q3 (L/h) = 28.4 | 28.2% (fixed) | |||||
| V3 (L) = 71 (fixed) | NA | |||||
| V4 (L) = 79.8 (fixed) | NA | |||||
| CLDOXOL (L/h) = 26.8 | 47.2% (fixed) | |||||
| Fm = 0.22 | 41.70% | |||||
| V5 (L) = 653 (fixed) | NA | |||||
| Q5 (L/h) = 424 | 58.90% | |||||
| 14 | Kunarajah t al, 201718 |
CL (L/h/1.8 m2) = 58.7*[1+(BSA-1.8)*0.465*[1+(age/8.4)0.736] V1 (L/1.8 m2) = 32.2*[1+(BSA-1.8)*0.465] Q2 (L/h/1.8 m2) = 35.8*[1+(BSA-1.8)*0.465] V2 (L/1.8 m2) = 3810*[1+(BSA-1.8)*0.465] fixed |
18.60% |
|
Basic and advanced internal | |
| Q3 (L/h/1.8 m2) = 65.1*[1+(BSA-1.8)*0.465] fixed | 22.30% | |||||
| V3 (L/1.8 m2) = 705*[1+(BSA-1.8)*0.465] fixed | 15.50% | |||||
| CLDOXOL (L/h/1.8 m2) = 19.9*[1+(BSA-1.8)*0.465] | 32.40% | |||||
| VDOXOL (L/1.8 m2) = 508 *[1+(BSA-1.8)*0.465] | ||||||
| QDOXOL (L/h/1.8 m2) = 32.1*[1+(BSA-1.8)*0.465] fixed | 33.20% | |||||
| PD model | ||||||
|
||||||
|
0.03% | |||||
| 15 | Liu et al, 201830 | CLDOX (L/h/1.8 m2) = 39*[1+(BSA-1.8)*0.643] | 39.70% |
|
Basic and advanced internal | |
| V1 (L/1.8 m2) = 14.6*[1+(BSA-1.8)*0.643] | ||||||
| Q2 (L/h/1.8 m2) = 51.4*[1+(BSA-1.8)*0.643] | 44.80% | |||||
| V2 (L/1.8 m2) = 1550.3*[1+(BSA-1.8)*0.643] | ||||||
| Q3 (L/h/1.8 m2) = 24.7*[1+(BSA-1.8)*0.643] | ||||||
| V3 (L/1.8 m2) = 69.4*[1+(BSA-1.8)*0.643] | ||||||
| Q4 (L/h/1.8 m2) = 8.57*[1+(BSA-1.8)*0.643] fixed | ||||||
| V4 (L/1.8 m2) = 368.9*[1+(BSA-1.8)*0.643] | 58.80% | |||||
| CLDOXOL (L/h/1.8 m2) = 19.9*[1+(BSA-1.8)*0.643] | 34.90% | |||||
| fm: 22% (fixed) | ||||||
| 16 | Janssen et al, 202121 |
|
38.20% |
|
Basic and advanced internal | |
|
35.60% | |||||
| Q1 (L/h) = 11.4 | ||||||
| V2 (L) = 25.4 | ||||||
| Q2 (L/h) = 38.2 | ||||||
| V3 (L) = 756 | 71% | |||||
- Add: additive, AST: aspartate aminotransferase, BSA: body surface area, C: concentration, CL: clearance, CLCR: creatinine clearance, cTnI: cardiac troponin I, CbasecTnI: baseline cardiac troponin I concentration, DOX: doxorubicin, DOXOL: doxorubicinol, E: cardiotoxicity exposure effect, Emax: maximum effect, EC50: combined plasma concentration producing half-maximal effect, fm: fraction of DOX metabolized to DOXOL, K: transfer rate constant, PCAMT: prior cumulative anthracyclines doses received by the patient, Prop: proportional, Q: inter-compartmental clearance, SD: standard deviation, V: volume of distribution, kin: zero-order release rate constant into plasma of cTnI, kdeg: first-order rate constant of loss from the plasma compartment
4 DISCUSSION
During the past few decades, PopPKs along with Bayesian estimation play an important role in drug individualization since it provides acceptable pharmacokinetic parameter estimates with a limited sample. Moreover, it enables investigators to explore potential covariates influencing drug pharmacokinetics. To date, several DOX PopPK models have been developed with different model structures and significant covariates to aid individualize drug dosing. These models and covariates are discussed below.
Callies et al.17 investigated the effect of a potent P-gp inhibitor (zosuquidar trihydrochloride), with a Ki of 59 nM, on the pharmacokinetics of DOX and DOXOL. They found that low dose (<500 mg) zosuquidar trihydrochloride did not significantly affect the pharmacokinetics of DOX, while high dose (≥500 mg) resulted in the decrease in CLDOX, the peripheral volume of distribution (Vp), CLDOXOL/fm, and DOXOL apparent volume of distribution (Vm/fm) by 25%, 26%, 48%, and 73%, respectively, and in turn increase in the exposures of DOX and DOXOL by 1.33- and 2-fold, respectively. Though zosuquidar trihydrochloride has been discontinued from the development, the results of this study can be applied to future pharmacokinetic interaction studies.
DOX is metabolized by various cells, but mainly in liver cells, kidney cells, and red cells.1 Thus, impaired liver function can decrease CLDOX. Supporting this, Joerger et al.14 reported a decrease in CLDOX as AST increased. Moreover, they found an increase in CLDOXOL with higher CLCR. Though DOX and its metabolites are chiefly excreted in the bile, approximately 0.7%–23% are renally excreted. Hence, the influence of CLCR on CLDOXOL is justifiable.
Supporting the current practice of BSA-guided DOX dosing, most studies found a significant effect of BSA on DOX pharmacokinetics, though the magnitudes of its influence were slightly different. Joerger et al.14 identified the BSA effect on CLDOX with an exponent of 1.4, while Kunarajah et al.,18 Liu et al.,30 Thompson et al.,29 Wilde et al.,24 and Kontny et al.6 reported a linear relationship between BSA and CLDOX, with the magnitudes ranging from 45% to 64.3% for each 1 m2 increase in BSA from the median value of 1.8 m2. One study did not report the magnitude of the BSA effect.29 Further, a greater effect of BSA on DOX pharmacokinetic parameters was found by Voller et al.,16 with the magnitude of 1.3 for each 1 m2 increase in BSA from the median value of 0.77 m2. The higher magnitude of the BSA effect in this study could be explained by the population characteristics that consisted exclusively of children. In addition to the BSA effect, Thompson et al.29 described that individuals with body fat higher than 30% exhibited lower CLDOXOL but not CLDOX, which posts a caveat in clinical practice, given DOXOL can play a role in cardiotoxicity.31
Joerger et al.14 and Wong et al.15 reported a negative impact of age on CLDOX with the exponents of (–0.54) and (–0.393), respectively, corresponds to a 9% and 7% decrease in CLDOX for a 10-year increase in patient age. This effect is expected given the deterioration of elimination organs with advancing age. On the other hand, Voller et al.16 characterized a lower CLDOX in children aged <3 years compared to older children. The magnitude of the age effect in this study was 0.286, suggesting an approximately 6% increase in CLDOX for a 10-year increase in patient age. However, this effect levels off as children approaching 18 years. Based on this result, DOX dosage adjustment based on patients’ age should be applied in addition to the BSA in clinical practice when DOX is to be prescribed in younger children. Interestingly, Wilde et al.24 found a negative effect of age on DOXOL VD, with a 2% decrease in DOXOL VD per year. The concrete explanation for this effect could not be elucidated. However, this effect is rather small and may not be of clinical significance.
Two studies21, 26 investigated the effect of pregnancy on the pharmacokinetics of DOX. While Van Hasselt et al.26 did not find a significant effect of pregnancy on CLDOX, Janssen et al.21 reported a 1.14-fold increase in CLDOX in pregnant patients. Evidence has shown that the clearances of drugs metabolized by cytochrome P450 (CYP)2A6, CYP2C9, CYP2D6, and CYP3A4 increase during pregnancy.31-34 Since more than one CYP3A enzymes play a role in DOX metabolism,35 increased activity of these enzymes during pregnancy results in an increase in CLDOX. In a study by Van Hasselt et al.,26 the effect of pregnancy on CLDOX was not statistical significant, and this could probably be due to the large IIV of the small number of pregnant women (n = 14). Furthermore, Janssen et al.21 and van Hasselt et al.26 found a significant effect of pregnancy on DOX Vc with the magnitudes of 1.08 and 1.23, respectively. Impact of pregnancy on DOX Vp was also specified with the degree of 1.32.26 An increase in DOX Vc and Vp in pregnant women is not surprising since plasma volume and total body water in all body fluid compartments are expanded during pregnancy.36 Moreover, an increase in body fat by approximately 4 kg was also reported.36 Further, a decrease of plasma protein binding of drugs to both albumin and alpha-1 acid glycoprotein during pregnancy has been identified,36-38 which can contribute to an increase in DOX Vp since DOX binds approximately 50%–85% to plasma protein.1 Altogether, the influence of pregnancy on DOX exposures should be underscored, and dosage adaptations should be reckoned.
Kunarajah et al.18 explored the relationship between cardiac troponin I (cTnI), one of the markers of cardiotoxicity, and DOX pharmacokinetics using a turn-over effect model and found linear relationships between increased DOX infusion rate and prior anthracycline dosing with baseline cTnI concentration. However, the effect of an increase in DOX infusion rate was not significant. This study indicated a 0.31% increase in baseline cTnI with every 1 mg/m2 increase in prior anthracycline therapy, confirming prior knowledge as to cardiotoxicity induced by cumulative anthracycline exposure. The results of this study emphasize careful monitoring of anthracycline use and can be applied in predicting long-term cardiotoxicity risk.
5 CONCLUSIONS
This review identified that DOX PopPK models were characterized using two- or three-compartment structure and DOX pharmacokinetic variability can be explained by BSA and age. Additionally, AST and CLCR also influenced CLDOX and CLDOXOL, respectively. Moreover, pregnant women exhibited lower DOX exposure that may require dosage adjustment. However, since most models were not externally assessed, predictive performance of such models should be executed before implementing these models in clinical practice.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
JM and KT conceptualized the idea of and designed the study. JM performed study supervision. JM and KT performed study screening and data extraction. JM prepared the manuscript. NL and RA provided critical review and comment. JM, KT, NL, and RA approved the final version of the manuscript.




