Longer leukocyte telomere length increases cardiovascular mortality in type 2 diabetes patients
白细胞端粒长度增长会增加2型糖尿病患者的心血管死亡率
Ziwei Chen, Yao Shen, and Jing He have contributed equally to this work.
Abstract
enAims
Leukocyte telomere length (LTL), as a biomarker of biological aging, is associated with the prevalence and complications of diabetes. This study aims to investigate the associations between LTL and all-cause and cause-specific mortality in patients with type 2 diabetes.
Methods
All participants with baseline LTL records were included from the National Health and Nutrition Examination Survey 1999–2002. Death status and its causes were ascertained for National Death Index based on International Classification of Diseases, Tenth Revision code. Cox proportional hazards regression models were established to estimate the hazard ratios (HRs) of LTL associating with all-cause and cause-specific mortality.
Results
The study enrolled 804 diabetic patients with the mean follow-up of 14.9 ± 2.59 years. There were 367 (45.6%) all-cause deaths, 80 (10.0%) cardiovascular deaths, and 42 (5.2%) cancer-related deaths. Longer LTL was associated with reduced all-cause mortality, whereas this association disappeared after adjusting for other variables. Compared with the lowest tertiles of LTL, the multivariable-adjusted hazard ratio of cardiovascular mortality was 2.11 (95% confidence interval [CI] 1.31–3.39; p < .05) in the highest tertiles. In terms of cancer mortality, the highest tertile was negatively correlated with the risk of cancer mortality (HR 0.58 [95% CI 0.37, 0.91], p < .05).
Conclusion
In conclusion, LTL was independently associated with the risk of cardiovascular mortality in patients with type 2 diabetes and was negatively correlated with the risk of cancer mortality. Telomere length may be a predictor of cardiovascular mortality in diabetes.
摘要
zh目的:白细胞端粒长度(LTL)作为生物学衰老的生物标志物, 与糖尿病的患病率和并发症相关。本研究旨在探讨2型糖尿病患者LTL与全因死亡率、原因特异性死亡率之间的关联。
方法:纳入1999-2002年全国健康与营养调查(NHANES)中有基线LTL记录的研究对象。根据国际疾病分类10(ICD-10)编码, 使用国家死亡索引(National Death Index)对研究对象的死亡状况及其死因进行确认。建立Cox比例风险回归模型评估LTL与全因死亡率及原因特异性死亡率的关联风险比。
结果:纳入804例糖尿病患者, 平均随访14.9±2.59年。其中全因死亡367例(45.6%), 心血管疾病死亡80例(10.0%), 癌症相关死亡42例(5.2%)。较长的LTL与降低全因死亡率相关, 而在调整其他变量后, 这种相关性消失。与LTL的最低分位数相比, 心血管死亡率的多变量校正风险比和95%可信区间为2.11 (1.31-3.39;P<0.05)。在癌症死亡率方面, 最高三分位数与癌症死亡风险呈负相关(HR 0.58 [0.37,0.91], P<0.05)。
结论:综上所述, LTL与2型糖尿病患者心血管死亡风险独立相关, 与恶性肿瘤死亡风险呈负相关。端粒长度可能是糖尿病患者心血管死亡率的预测因子。
1 INTRODUCTION
Diabetes is a leading cause of morbidity and mortality worldwide.1, 2 Patients with diabetes have 2 ~ 4 times risk to develop cardiovascular diseases (CVDs).3 An international registry found that the risk of cardiovascular death was greater in patients with diabetes compared with nondiabetes (16.5% versus 13.1%; adjusted hazard ratio [HR] 1.27).4 In addition, diabetes was associated with a greater risk of heart failure, nonfatal myocardial infarction, and stroke.5 Besides, the diabetes status was involved in aging, oxidative stress, and chronic inflammation, promoting shortening of telomeres.
Telomeres are the TTAGGG repetitive DNA sequences at the ends of eukaryotic chromosomes and are responsible for maintaining the genome integrity during cell division.6 Excess oxidative stress and proinflammatory mediators like tumor necrosis factor-alpha, interferon-gamma, interleukin-6 and interleukin-10 were two mechanisms contributing to age-dependent leukocyte telomere length (LTL) shortening.7, 8 Highly shortened telomeres can cause cellular senescence and subsequent arrest in the cell cycle.9 Epidemiological studies have shown that shorter LTL was associated with a number of age-related disorders such as CVDs, neurodegenerative diseases, and cancers.10-12 A community-based cohort study found that shorter LTL was associated with increased overall cardiovascular, respiratory, digestive, and musculoskeletal mortality but not cancer-related mortality.13 The telomere system played an important role in the pathogenesis and progression of diabetes mellitus as well as in its vascular complications.14 However, the prognostic effect of telomere length on cardiovascular mortality or cancer mortality remains unclear in diabetes patients.
Hence, this study was designed to evaluate the relationship between LTL and all-cause and cause-specific mortality in type 2 diabetes by using data from the National Health and Nutrition Examination Survey (NHANES).
2 METHODS
2.1 Study population
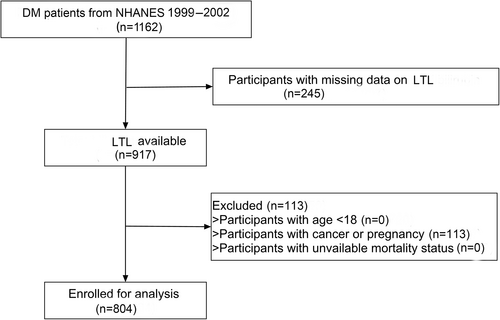
Our study included individuals from the NHANES. A total of 1162 patients with type 2 diabetes were included from NHANES cycle of 1999–2002. After excluding those with missing LTL records (n = 245) and with cancer or pregnancy (n = 113), a total of 804 participants were enrolled in our study. Figure 1 depicted the selection process. The protocol was approved by the Ethics Review Board of National Center for Health Statistics (Protocol # 98–12).
2.2 Leukocyte telomere length measurement
Genomic DNA was extracted from the buffy coat in the peripheral blood sample using QIAamp 96 DNA Blood Kits (Qiagen, Valencia, CA) according to the manufacturer's protocol. Relative telomere length was measured by comparing the ratio of telomere repeat number (T) to a single copy gene number for albumin (S) in the experimental sample to a standardized reference sample value using multiplexed quantitative polymerase chain reaction method. All experimental samples were assayed in duplicate, and the average value of the two replicates was used for the final analysis for each subject. The mean of the T/S ratio values was calculated, and the largest and the smallest T/S ratio values were marked as potential outliers. If the absolute value of the log of the ratio between the recalculated mean (excluding the potential outliers) to the value of the potential outlier was >0.4, then the value was marked as an outlier. The interassay coefficient of variability for LTL was 4%.
2.3 Covariates collection
Information on sex, age, race, education level, smoking status, alcohol habit, prior comorbid illness, and medication use were obtained from standardized questionnaires. Body mass index (BMI, kg/m2) was calculated as weight divided by height squared. Race was classified as non-Hispanic white, non-Hispanic black, Mexican American, or other. Education levels were categorized into less than high school, high school or equivalent, and college or above. Family income-to-poverty ratio was classified as 0–1.0, 1.0–3.0, or >3.0, indicating a low, middle, and high income status, respectively. Smoking habits were defined as current, past and never. Previous history of diseases (hypertension and CVD) and medications (aspirin, hypoglycemic drugs, and lipid-lowering drugs) were determined from self-reported questionnaires.
2.4 End points
The primary outcome was all-cause mortality and secondary outcomes included death from CVD, diabetes, or malignant neoplasms. Mortality status was obtained by linkage to the National Death Index by 31 December 2015. Causes of death included malignant tumors, CVDs, respiratory diseases, Alzheimer's disease, diabetes, nephropathy-related diseases, accidental death, and other causes. CVDs were defined as International Classification of Diseases, Tenth Revision (ICD-10) codes I00-I09, I11, I13, or I20-I51. Malignant neoplasm was defined as ICD-10 codes C00-C97 (Supplementary Table S1).
2.5 Statistical analysis
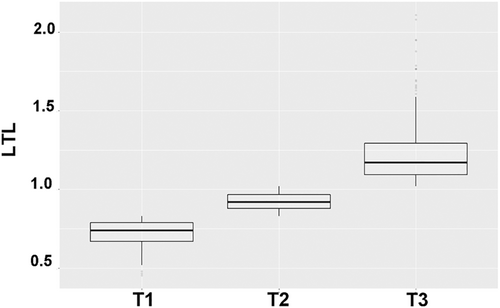
Descriptive statistics were presented according to the levels of LTL (T1: <0.83, T2: 0.83–1.02, T3: >1.02; Figure 2) and group differences were explored by one-way analysis of variance and chi-square tests. First, we explored the association of LTL tertiles with overall survival using Kaplan–Meier analysis. Associations between levels of LTL and the risk of all-cause mortality was estimated by classical Cox regression models. We fitted Cox model by specifying the event of interest, and by censoring for competing events. Multiple imputation based on predictive mean matching was performed for covariates with missing values using package mice form R (Supplementary Table S2). All analysis were performed using R version 3.6.
3 RESULTS
This study included 804 participants with 14.9 ± 2.59 year of follow-up. The baseline characteristics of the study population according to LTL tertiles (T1: <0.83, T2: 0.83–1.02, T3: >1.02) were shown in the Table 1. Compared with the lowest group, participants in the highest group were more likely to be younger, non-Hispanic black, and have higher BMI.
| Variable | All (n = 804) | T1 (n = 268) | T2 (n = 269) | T3 (n = 267) | p value |
|---|---|---|---|---|---|
| LTL | 0.96 (0.11) | 0.73 (0.08) | 0.92 (0.05) | 1.23 (0.19) | <.001 |
| Male sex (%) | 435 (54.1) | 143 (53.4) | 151 (56.1) | 141 (52.8) | .709 |
| Age, years | 61.3 (13.9) | 67.0 (11.9) | 61.4 (12.4) | 55.4 (14.80) | <.001 |
| Race (%) | |||||
| Non-Hispanic white | 294 (36.6) | 119 (44.4) | 97 (36.1) | 78 (29.2) | .001 |
| Non-Hispanic black | 193 (24.0) | 48 (17.9) | 67 (24.9) | 78 (29.2) | |
| Mexican American | 240 (29.9) | 85 (31.7) | 78 (29.0) | 77 (28.8) | |
| Others | 77 (9.6) | 16 (6.0) | 27 (10.0) | 34 (12.7) | |
| Education (%) | .283 | ||||
| Less than high school | 416 (51.7) | 152 (56.7) | 134 (49.8) | 130 (48.7) | |
| High school or equivalent | 161 (20.0) | 52 (19.4) | 52 (19.4) | 57 (21.3) | |
| College or above | 227 (28.2) | 64 (23.9) | 83 (31.0) | 80 (30.0) | |
| BMI, kg/m2 | 31.5 (6.8) | 30.4 (6.1) | 32.0 (7.1) | 32.2 (7.3) | .003 |
| IPR (%) | .01 | ||||
| <1.0 | 129 (16.1) | 116 (13.8) | 113 (14.9) | 95 (12.2) | |
| 1.0–3.0 | 361 (45.2) | 356 (42.3) | 283 (37.2) | 298 (38.3) | |
| >3.0 | 309 (38.7) | 369 (43.9) | 364 (47.9) | 385 (49.5) | |
| Smoking (%) | .515 | ||||
| Current | 186 (23.1) | 60 (22.4) | 61 (22.7) | 65 (24.3) | |
| Past | 55 (6.8) | 13 (4.9) | 20 (7.4) | 22 (8.2) | |
| Never | 563 (70.0) | 195 (72.8) | 188 (69.9) | 180 (67.4) | |
| Activity (%) | |||||
| Vigorous | 296 (36.8) | 74 (27.6) | 117 (43.5) | 105 (39.3) | .002 |
| Moderate | 437 (54.4) | 168 (62.7) | 133 (49.4) | 136 (50.9) | |
| Inactive | 71 (8.8) | 26 (9.7) | 19 (7.1) | 26 (9.7) | |
| Past history (%) | |||||
| Hypertension | 332 (41.3) | 124 (46.3) | 102 (37.9) | 106 (39.7) | .118 |
| CVD | 161 (20.0) | 64 (23.9) | 54 (20.1) | 43 (16.1) | .080 |
| Prior medication (%) | |||||
| Aspirin drug | 527 (65.5) | 174 (64.9) | 169 (62.8) | 184 (68.9) | .322 |
| Hypoglycemic drug | 520 (64.7) | 183 (68.3) | 159 (59.1) | 178 (66.7) | .06 |
| Lipid-lowering drug | 570 (70.9) | 205 (76.5) | 195 (72.5) | 170 (63.7) | .004 |
| LDL-C, mg/dL | 119.4 (33.7) | 112.7 (29.6) | 126.6 (35.1) | 119.0 (34.8) | <.001 |
- Note: Data are presented as mean (SD) or n (%). T1: <0.83, T2: 0.83–1.02, T3: >1.02.
- Abbreviations: BMI, body mass index; CVD, cardiovascular diseases; IPR, income-to-poverty ratio; LDL-C, low-density lipoprotein cholesterol; LTL, leukocyte telomere length.
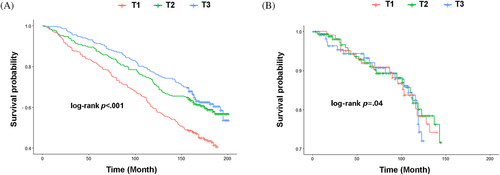
A total of 367 deaths occurred among 804 participants (45.6%). Kaplan–Meier survival curve showed lower LTL was associated with increased all-cause mortality (p < .001; Figure 3A) and cardiovascular mortality (p = .04; Figure 3B).
As shown in Table 2, compared with the lowest tertile, the HR for all-cause mortality was 0.72 (95% confidence interval [CI] [0.56, 0.93], p < 0.05) for individuals with the highest tertile in an adjusted model. However, the significance disappeared after adjusting for gender, age, race, education, BMI, smoking, activity, hypertension, CVD, aspirin, hypoglycemic drugs, lipid-lowering drugs, and low-density lipoprotein cholesterol (LDL-C). Surprisingly, the highest LTL group increased the risk of cardiovascular mortality (HR 1.62 [95% CI 1.02, 1.72], p < .05) in an unadjusted model, even in a fully adjusted model. (HR 2.11 [95% CI 1.31, 3.39], p < .05). In terms of cancer mortality, the highest tertile was negatively correlated with the risk of cancer mortality (HR 0.58 [95% CI 0.37, 0.91], p < .05) after adjusting for gender, age, race, education, BMI, smoking, activity, hypertension, CVD, aspirin, hypoglycemic drugs, lipid-lowering drugs, and LDL-C.
| All-cause and cause-specific mortality | Cases | N | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| All causes | |||||
| T1 | 156 | 268 | Ref | Ref | Ref |
| T2 | 109 | 269 | 0.69 [0.54, 0.88]** | 0.86 [0.67, 1.10] | 0.92 [0.70, 1.20] |
| T3 | 102 | 267 | 0.72 [0.56, 0.93]* | 1.20 [0.92, 1.55] | 1.15 [0.88, 1.50] |
| Cardiovascular | |||||
| T1 | 27 | 268 | |||
| T2 | 26 | 269 | 0.99 [0.59, 1.66] | 1.33 [0.78, 2.26] | 1.40 [0.88, 2.24] |
| T3 | 27 | 267 | 1.62 [1.02, 1.72]* | 1.86 [1.10, 3.17]* | 2.11 [1.31, 3.39]* |
| Malignant neoplasms | |||||
| T1 | 17 | 268 | |||
| T2 | 13 | 269 | 0.84 [0.42, 1.71] | 0.85 [0.42, 1.73] | 0.92 [0.41, 1.38] |
| T3 | 12 | 267 | 0.72 [0.35, 1.51] | 0.75 [0.36, 1.57] | 0.58 [0.37, 0.91]* |
- Note: Model 1 was unadjusted. Model 2 was adjusted for age and gender. Model 3 was adjusted for gender, age, race, education, body mass index, smoking, activity, hypertension, cardiovascular disease, aspirin, hypoglycemic drugs, lipid-lowering drugs, and low-density lipoprotein cholesterol.
- Abbreviations: HR, hazard ratio; CI, confidence interval.
- * p < .05;
- ** p < .01.
4 DISCUSSION
In our study, we found that longer leukocyte telomere length increased the risk of cardiovascular mortality in patients with type 2 diabetes. However, leukocyte telomere length was not associated with all-cause mortality in diabetic patients. Telomere length may be a predictor of cardiovascular mortality in diabetes.
Previous study reported that shorter LTL was associated with a higher risk of mortality in the general population, which could be explained by increased systemic inflammation and oxidative stress.15 It was understandable that our univariate survival analysis also suggested that lower LTL was associated with increased all-cause mortality. However, after adjusting many covariates, the association disappeared in our study, which suggested that other factors could mediate their association. In the general population, LTL was negatively16 or U-shaped17 associated with the risk of all-cause mortality. On contrary to the previous studies, we did not’ observe a significant association between LTL and all-cause mortality in diabetic patients. We speculated that telomeres have already shortened substantially among diabetes subjects,18 and given the presence of other major risk factors for death, the independent effect of telomere length on mortality may be reversed. Besides, Chen et al found that shortened relative leukocyte telomere length was associated with an increased risk of all-cause mortality in type 2 diabetes.19 The difference may be due to (a) different population (US population in our study and Asian population in the previous study); (b) a lower level of LTL in our study (~0.96) compared with the previous study (~4.6), which could be attributed to the different methods used for the measurement of LTL; and (c) a high all-cause mortality in our study (45.6%), although it was not implausible, given the starting ages of the subjects and the long follow-up time.
Surprisingly, we found that longer LTL increased the risk of cardiovascular mortality in patients with diabetes. Although a report found shortening of telomeres would protect against cardiovascular mortality in elderly men,20 our results contrasted with other previous reports that associated shorter telomeres with cardiovascular mortality.21, 22 Chen et al also found that shortened leukocyte telomere length was associated with prevalent and incident cardiovascular complications in type 2 diabetes.23 There may be a competing risk against cardiovascular mortality in diabetic patients and it was stronger in shorter LTL subgroups. The relation should be interpreted with some caution until more studies are designed to explore this association. In consistent with previous reports, we found an inverse correlation between LTL and cancer mortality.
Our study has some limitations. First, some covariates were self-reported. Second, we used mean LTL measured in leukocyte cells, which are a heterogeneous mix of cells with varying telomere length. It is possible that the association may vary depending on cell type used to measure telomere length. Third, data on LTL was collected only once at baseline, and it was unclear whether longitudinal changes over time could affect the association with mortality. Finally, heart diseases or other variables may not be present at baseline.
5 CONCLUSION
We found that leukocyte telomere length was independently associated with the risk of cardiovascular mortality, not all-cause mortality, in patients with type 2 diabetes. Telomere length may be a predictor of cardiovascular mortality in diabetes.
AUTHOR CONTRIBUTIONS
Xiao Mingbing and Wu Xiaohui designed the study; Chen Ziwei, Shen Yao, and He Jing wrote the main manuscript; Shen Yao and Zhu Weida prepared the figures and tables.
ACKNOWLEDGEMENTS
This study was supported by the Municipal Natural Science Foundation of Nantong (Nos. JC22022012).
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
All authors have nothing to disclose regarding conflict of interest with respect to this manuscript.
ETHICS STATEMENT
The protocol was approved by the Ethics Review Board of National Center for Health Statistics (Protocol # 98–12).
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author Mingbing Xiao upon request.




