Rationale for glycemic control to improve cardiovascular outcome: Lessons from east and west
控制血糖以改善心血管结局的理论基础:来自东方和西方的经验教训
It is clear that there is a strong relationship between diabetes and cardiovascular disease (CVD). Both for men and for women, diabetes confers the highest lifetime risk for coronary arterial disease (CAD) as a single independent risk factor.1 In a metaanalysis of 102 prospective studies of more than 500 000 persons, there was a doubling of risk both of CAD and stroke associated with diabetes, adjusted for age, cigarette use, obesity, hypertension, and sex.2 Further analysis from this group of a larger cohort of nearly 700 000 persons showed that diabetes, a history of myocardial infarction (MI), and a history of stroke were associated with similar mortality. The presence of any two of these factors led to a doubling of risk, and the presence of all three to a doubling again for mortality risk.3 Although the Swedish National Diabetes Register showed progressive reduction in CVD from 1998 through 2014 among persons both with and without diabetes, the gap in CVD death and hospitalization rates between the groups persisted throughout the period.4
Diabetes is characterized by persistent elevation in blood glucose levels. As such, it is reasonable to postulate that greater glycemic exposure is associated with greater levels of risk of complications. Indeed, in a cohort of persons representative of the US population varying from euglycemia to prediabetes to diabetes with progressively higher HbA1c levels followed from 1998 through 2011, CVD mortality increased by ~25% with HbA1c 8%-8.9% compared with HbA1c of 7%-8% and by another 25% with HbA1c of 9% or more.5 In the Swedish National Diabetes Register diabetes cohort of more than 250 000 persons, mortality, MI, stroke, and heart failure increased progressively with rising HbA1c with marked increase as HbA1c rose from 7% to 9%.6 In a further study by this group using an expanded cohort of more than 400 000 persons with diabetes, over a 7-year period, the stroke rates in persons with HbA1c 7.1%-8%, 8.1%-9%, 9.1%-10%, and >10% were 1.27-, 1.68-, 1.89-, and 2.14-fold greater than that in matched persons without diabetes.7
Is there then evidence from trials showing that CVD outcomes improve with glycemic control in persons with diabetes? The evidence here is complex. In a metaanalysis of trials of 3-6-year duration of persons with diabetes and underlying CVD or CVD risk factors, there was significant reduction in rates of MI but not for stroke8 and mortality.9 However, in persons receiving more vs less intensive glycemic treatment beginning earlier in the course of diabetes, there was a legacy effect after ~10-20 years of follow-up with reduction in MI, mortality, and CVD rates both in type 210 and in type 1 diabetes.11 In an analysis of the Veterans Administration Diabetes Trial comparing more vs less intensive glycemic control where participants were divided by the presence of CVD based on coronary artery calcium (CAC) measurement, those with negative scores (CAC score ≤ 100) showed lower rates of CVD but not those with positive CAC scores.12 In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial where higher mortality rates were reported with intensive glycemic treatment, the increase in mortality occurred only in participants with higher baseline HbA1c13 or higher mean on-trial HbA1c.14 Taken together, these trials' evidence suggested that the CV benefits of intensive glycemic treatment, while minimizing risk of hypoglycemia, were most evident when implemented early, and certainly before development of atherosclerotic cardiovascular disease. In contrast, for those with higher HbA1c and long disease duration, intensive glycemic treatment, particularly with agents associated with high risk of hypoglycemia, might not show benefit (Figure 1). Indeed, in the ACCORD trial15 and similar studies,16 severe hypoglycemia was a particularly significant mortality risk factor.
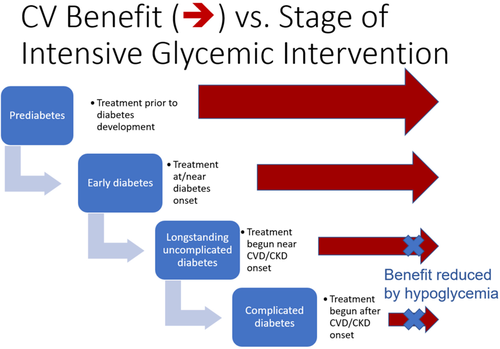
These observations may be particularly important in people who develop type 2 diabetes at younger age and face many decades of hyperglycemia. In the Hong Kong Diabetes Register, total hyperglycemic exposure is more than 3-fold greater in patients diagnosed before the age of 40, who had 3-6-fold higher risks of CVD, kidney disease, and infections compared with those diagnosed after the age of 40.17, 18 In these young patients, it was estimated that intensive control of all cardiometabolic risk factors to near normal targets as evaluated in the J-DOIT3 (Japan Diabetes Optimal Integrated Treatment study for three major risk factors of cardiovascular diseases) randomized study (HbA1c < 6.2%, systolic blood pressure < 120 mmHg, low-density lipoprotein-cholesterol < 2 mmol/L, triglyceride < 1.3 mmol/L, waist circumference < 85 cm [men] or 80 cm [women], and cessation of smoking) could reduce the lifetime burden of hospitalization by 30%, whereas by delaying the onset of diabetes, these diseases' burden can be reduced by 50%-70%.
Against this background, in the Da Qing Diabetes Prevention Study of persons with impaired glucose tolerance, early lifestyle intervention prevented diabetes in approximately half of the participants, leading to lowering of CVD and microvascular disease and mortality in the 30-year post-trial follow-up period.19, 20 Globally, there are 463 million people with diabetes with a similar number having prediabetes. Given this disease burden, an integrated population- and system-based approach is needed for early diagnosis, prevention, and treatment.21 In Hong Kong, a territory-wide diabetes risk assessment and management program using a team approach aimed at gathering data regularly and systematically to stratify risk, empower self-management, and inform early decision-making, together with public health measures, contributed to the 50%-70% reduction in mortality, cardiovascular complications and acute infections from 2000-2016 in 0.5 million people with diabetes.22-25
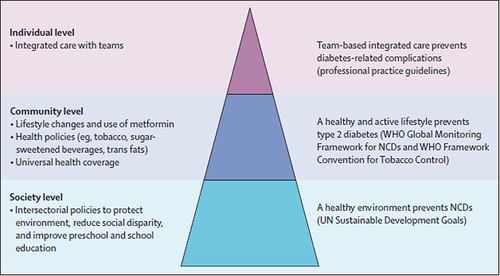
In the Lancet Commission Report on Diabetes, global experts curated evidence and estimated the impacts of a society-community-individualized approach in reducing the burden of diabetes and CVD. Using China as an example, with nearly 100 million persons with diabetes, experts estimated that the use of team-based integrated care aimed at increasing diagnosis rate to 50% with 70% treatment rates for reducing blood glucose, blood pressure, and blood low-density lipoprotein-cholesterol levels could potentially prevent 800 000 CVD events in 3 years. By using policies aimed at creating a health-enabling environment with universal education and social protection together with a community-based approach of using nonphysicians and technology to assist physicians to diagnose, treat, and prevent diabetes early, we can prevent tens of millions of diabetes and CVD events over a 20-year horizon (Figure 2).22 Together with the latest evidence regarding the greater efficacy of glucagon like peptide 1 receptor agonist (GLP-1RA) and sodium glucose transporter 2 (SGLT2) inhibitors in reducing CVD and renal outcomes in Asian populations,26 it is anticipated that by closing the gaps in professional knowledge, strengthening the healthcare system, and ensuring access to care, education, and medications, we will be able to translate these decades of evidence to practice that will benefit hundreds of millions of persons with or at risk of developing diabetes and CVD over the coming decades.27
糖尿病与心血管疾病(CVD)有很强的关系。无论对男性还是女性,糖尿病都是冠心病(CAD)终身风险最高的独立危险因素。在一项对102项前瞻性研究进行的meta分析中,对超过50万人进行了研究,校正年龄、吸烟、肥胖、高血压和性别后,与糖尿病相关的冠心病(CAD)和中风的风险都增加了一倍。对这组近70万人的进一步分析表明,糖尿病、心肌梗死(MI)史和卒中史死亡率相似。这三个因素中的任何两个的存在都会导致风险翻倍,并且这三个因素都存在会导致死亡风险再次翻一番。尽管瑞典国家糖尿病登记显示,从1998年到2014年患有糖尿病和不患有糖尿病的人CVD发生逐渐减少,但是两组的心血管疾病死亡率和住院率的差距一直存在。
糖尿病的特征是血糖水平持续升高。因此,我们有理由假设,较高的血糖暴露水平与较高的并发症风险水平相关。事实上,从1998年到2011年,在代表美国人群的一组人群中,从正常血糖到糖尿病前期,再到糖化血红蛋白水平逐渐升高的糖尿病,心血管疾病死亡率随着糖化血红蛋白水平的升高而增加,糖化血红蛋白水平为8%-8.9%相对7%-8%时死亡率增加25%,糖化血红蛋白为9%或更高则进一步增加了25%。在瑞典国家糖尿病登记超过25万人的糖尿病队列中,死亡率、心肌梗死、卒中和心力衰竭的可能性随着糖化血红蛋白升高而升高,特别是在糖化血红蛋白从7%升高至9%。在一项对40万人超过7年时间的研究中,HbA1c为7.1%-8%、8.1%-9%、9.1%-10%和>10%的患者卒中发生率分别是没有糖尿病的匹配者的1.27、1.68、1.89和2.14倍。
那么,是否有来自临床试验的证据表明糖尿病患者的血糖控制改善了心血管疾病的结局?已有的证据很复杂。在对糖尿病患者的3-6年病程和潜在的心血管或心血管危险因素进行的临床试验的meta分析中,MI的发生率显著降低,但卒中和死亡率没有显著降低。然而,在糖尿病病程早期开始接受高强度和低强度血糖治疗的人中,经过10-20年的随访,2型糖尿病和1型糖尿病患者的MI、死亡率和心血管疾病发生率都有所降低,这是一种遗留效应。在退伍军人管理局糖尿病试验的一项分析中,比较了高强度和低强度的血糖控制,其中参与者根据冠状动脉钙化(CAC)的情况进行了心血管疾病的划分,CAC为阴性的发生CVD的概率更低。在控制糖尿病心血管风险行动(ACCORD)试验中,高血糖治疗报告的死亡率较高,只有基线糖化血红蛋白(HbA1c)较高或试验中平均HbA1c较高的参与者死亡率增加。综上所述,这些试验的证据表明,强化血糖治疗在将低血糖风险降至最低的同时,对心血管疾病的益处在早期实施时最为明显。相比之下,对于HbA1c较高和病程较长的患者来说,强化降糖治疗,特别是使用与低血糖风险高相关的药物,可能确实没有显示出好处。在ACCORD试验和类似研究中,严重低血糖是一个特别重要的死亡风险因素。
这些观察结果可能对那些在年轻时患上2型糖尿病并面临数十年高血糖的人特别重要。在香港糖尿病登记处,40岁之前确诊的患者的总高血糖暴露时间是40岁之后确诊的患者的3倍多,他们心血管疾病、肾脏疾病和感染的风险是40岁以后确诊的患者的3-6倍。这些年轻患者根据J-DOIT3(日本糖尿病三大主要风险因素的最佳综合治疗研究)随机研究的评估,强化控制所有心脏代谢危险因素到接近正常目标(三酰甘油<1.3 mmol/L、腰围<85 cm[男性]或80 cm[女性]、戒烟)可使终生住院负担减轻30%,而延缓糖尿病发病可使这些疾病的负担减轻50%~70%。
在此背景下,在大庆糖耐量受损人群糖尿病预防研究中,早期生活方式干预在大约一半的参与者中预防了糖尿病,导致试验后30年随访期内心血管和微血管疾病的减少和死亡率的降低。全球有4.63亿糖尿病患者,他们中有类似比例的糖尿病前期患者。考虑到这种疾病负担,需要一种基于人群和系统的综合方法来进行早期诊断、预防和治疗。在香港,一个采用团队方法的全港性糖尿病风险评估和管理计划,旨在定期和系统地收集数据,对风险进行分层,赋予自我管理能力,为早期决策提供信息,并采取公共卫生措施,从2000年到2016年,50万糖尿病患者的死亡率、心血管并发症和急性感染减少了50%-70%。
在Lancet委员会关于糖尿病的报告中,全球专家整理了证据,并估计了社会-社区-个性化方法在减轻糖尿病和心血管疾病负担方面的影响。以拥有近1亿糖尿病患者的中国为例,专家估计,以团队为基础的综合护理旨在将诊断率提高到50%,治疗率达到70%,以降低血糖、血压和血液低密度脂蛋白-胆固醇水平,可能在3年内预防80万起心血管事件。通过使用旨在创造普及教育和社会保护的有利健康环境的政策,以及以社区为基础的方法,协助医生及早诊断、治疗和预防糖尿病,我们可以在20年内预防数千万糖尿病和心血管事件。结合最新证据表明,胰高血糖素样肽1受体激动剂(GLP-1RA)和钠葡萄糖转运蛋白2(SGLT-2)抑制剂在降低亚洲人群的心血管疾病和肾脏预后方面效果更好,我们能够将这几十年的证据转化为实践,这将在未来几十年使数亿患有糖尿病和心血管疾病的患者或有患糖尿病和心血管疾病风险的患者受益。




