Heart failure: A preventable and treatable complication of type 2 diabetes
心力衰竭:可预防与可治疗的2型糖尿病并发症
Chronic heart failure (CHF) is a complex syndrome in which impaired emptying or filling of the heart results in a clinical picture of fatigue, breathless and fluid retention. Chronic heart failure is a common, preventable, and treatable complication of type 2 diabetes (T2D). Diabetes is approximately three to four times more prevalent in patients with heart failure than the general population.1, 2 Not only is T2D a risk factor for the development of CHF, but impaired glucose tolerance may be a consequence of the systemic perturbations of the CHF syndrome.3 Type 2 diabetes is an independent risk factor for progressive heart failure and cardiovascular death,3-6 and patients with T2D have higher New York Heart Association (NYHA) class and symptom burden than non-diabetic patients with similar ejection fractions.7
The most common comorbidities leading to CHF in patients with T2D are coronary artery disease (CAD) and hypertension. Although multivessel CAD is a major cause of CHF in patients with diabetes and a strong adverse predictor of survival,4 the adverse effect of T2D on outcomes in CHF patients is of a similar magnitude in patients with and without CAD.6 This suggests an additional direct effect on the myocardium, a “diabetic cardiomyopathy”. Although several mechanisms have been proposed, and impaired cardiac glucose metabolism is a strong candidate, the underlying pathogenesis is only partially understood and there is no accepted clinical definition.6, 8, 9 Overall, the pathophysiology of heart failure and diabetes overlap; both are associated with insulin resistance, impaired glucose metabolism and the metabolic syndrome.10, 11
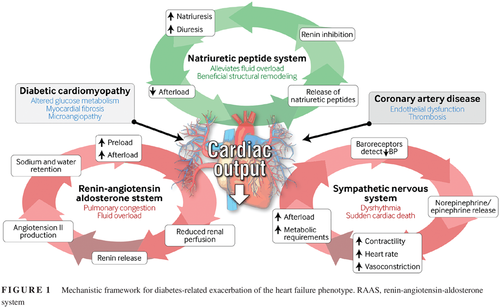
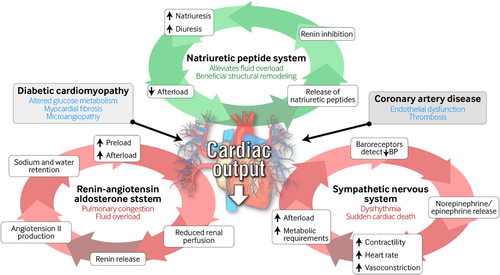
Heart failure is classified based upon the presence of reduced left ventricular function as heart failure with preserved (HFpEF) or reduced (HFrEF) ejection fraction. In both descriptions, reduced cardiac output and a fall in blood pressure are sensed by baroreceptors and renal afferent arterioles. These, in turn, trigger activation of the renin-angiotensin-aldosterone system (RAAS), secretion of antidiuretic hormone (ADH), vasoconstriction, activation of the sympathetic nervous system, and release of epinephrine and norepinephrine. It is these physiological adaptations that result in the clinical syndrome of heart failure.12 Activation of the RAAS leads to secretion of aldosterone and ADH, promoting sodium and water retention. This augments preload, resulting in pulmonary congestion and fluid overload. Vasoconstriction maintains blood pressure to achieve organ perfusion at the expense of excessive afterload, which exacerbates pump failure. Sympathetic stimulation increases heart rate and contractility, initially improving ejection fraction but ultimately increasing the heart's metabolic requirements and predisposing to dysrhythmia and sudden cardiac death. Conversely, natriuretic peptide secretion promotes beneficial structural remodeling, natriuresis, and diuresis and reduces sympathetic tone (Figure 1).
Contemporary therapies aim to limit these physiological adaptations, reduce symptoms and improve survival. Diuretics provide symptomatic relief from fluid retention. Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, and mineralocorticoid receptor antagonists inhibit the RAAS. Neprilysin inhibitors reduce the degradation of natriuretic peptides to promote structural remodeling, vasodilation, and natriuresis. β-Adrenoreceptor antagonists (beta-blockers) counteract sympathetic activation, reduce heart rate and contractility and, largely by their effect on sympathetic activation and reverse remodeling, reduce the risks of dysrhythmia and sudden cardiac death. In those with persistent left ventricular dysfunction and conduction delay, cardiac resynchronization therapy (CRT) improves symptoms and survival, and in selected patients implantable cardiac defibrillators can reduce death due to arrhythmia.12
1 ROLE OF MEDICAL THERAPY
Studies linking diabetes with adverse outcomes, and its effect on therapy in CHF, often predate what would now be considered as contemporary therapy. The now more widespread prescription of beta-blockers and uptake of CRT have contributed to the significant improvements in outcomes over recent years.13 The incidence of sudden cardiac death has diminished over time, but still persists as a major contributor to adverse outcomes, especially in patients with diabetes. In a prospective cohort of 1091 patients, diabetes was associated with higher rates of progressive CHF and all-cause and cardiovascular deaths,6 and in clinical trials,14, 15 although pharmacotherapy and device therapy were effective for both patients with and without diabetes, diabetic patients remain at higher risk. Most recently, we demonstrated that patients with diabetes mellitus are, in fact, more likely to benefit from contemporary heart failure strategies. In a non-randomized prospective cohort of 1797 patients, we described that higher doses of ACE inhibitor and beta-blocker improved outcomes for all patients, but that dose escalation has the greatest effect on mortality in patients with diabetes mellitus, particularly those with the most severely impaired left ventricular function.16 This observation provides support for the direct role of higher doses of beta-blockers in order to achieve the best outcomes in patients with both CHF and diabetes mellitus.16
That beta-blockers seem to have additional benefits for diabetic patients may be due to several mechanisms. First, blockade of β-adrenoceptors is negatively inotropic and chronotropic, thus reducing cardiac metabolic demands. This may be of particular benefit in the context of CAD, which is highly prevalent in diabetic patients. Second, beta-blockers provide prophylaxis against dysrhythmia and sudden cardiac death, which are more common in people with both diabetes mellitus and heart failure.6 Third, beta-blockers promote structural remodeling of the myocardium. We have shown in a prospective study of 628 patients receiving contemporary heart failure therapy that patients with diabetes experienced more adverse structural and functional remodeling such that halting of this more rapid progressive change may be even more important in that group.17 Finally, beta-blockers suppress sympathetic activation, countering the increased basal sympathetic neural outflow in diabetes mellitus.10
Historically there has been reluctance to prescribe beta-blockers to those on insulin or sulfonylureas due to a perceived risk of masking symptoms of hypoglycemia or prolonging these episodes. However, in clinical trials the rates of hypoglycemia were similar between groups, and these theoretical risks were far outweighed by the benefits.18
2 ROLE OF REVASCULARIZATION
The role of coronary artery bypass grafting (CABG) is well established for patients with multivessel CAD with and without diabetes. Clinical trials have shown a mortality benefit following CABG or percutaneous intervention (PCI) for those with multivessel CAD, however those with CHF have often been under-represented. The future revasculization evaluation in patients with diabetes mellitus: optimal management of multivessel disease (FREEDOM) trial demonstrated that, in patients with diabetes, CABG resulted in improved mortality and fewer cardiac events than PCI, but only 3% of these patients had HFrEF.19 The surgical treatment for ischemic heart failure extension study (STICHES) trial showed that CABG was beneficial compared with medical management for patients with HFrEF, but the study was underpowered to detect whether patients with diabetes derived any additional benefit versus non-diabetic patients.20 However, in a large observational study with propensity matching, patients with diabetes and HFrEF treated with CABG had a lower rate of cardiovascular events and death than those with multivessel CAD treated with PCI.21
3 TAKE HOME MESSAGE
Patients with CHF and diabetes die sooner, have worse symptoms and are more frequently hospitalized. Contemporary heart failure therapies seem to confer additional benefit in those patients with diabetes. Despite this, there is often a reluctance to prescribe doses achieved in clinical trials, particularly when prescribed by non-cardiologists.22 That incremental improvements in mortality reduction and hospitalization are observed with increasing doses should provide patients and healthcare professionals confidence in providing these life-saving medications.
慢性心力衰竭(Chronic heart failure,CHF)是一种复杂的综合征,患者心脏排空或者充盈受损会导致临床上出现疲劳、呼吸困难与液体潴留。慢性心力衰竭是2型糖尿病(T2D)患者常见的、可预防并且可治疗的并发症。在心力衰竭患者中糖尿病的患病率大约是普通人群的3到4倍1,2。T2D不但是导致CHF的危险因素,而且糖耐量受损也可能是CHF综合征造成全身影响后的结果3。2型糖尿病是进展性心力衰竭以及心血管死亡的独立危险因素3-6,并且与射血分数相似的非糖尿病患者相比,T2D患者心衰的纽约心脏病协会(NYHA)分级更高并且症状负担更重7。
在T2D患者中最常见的可导致CHF的合并症是冠状动脉疾病(CAD)与高血压。尽管在糖尿病患者中CHF的一个主要原因是合并多支血管病变CAD,并且这也是一个强有力的不利于生存率的预测因素4,T2D对CHF患者有不良影响,对于合并或者不合并CAD的患者来说影响程度都相似6。这意味着糖尿病对心肌具有额外的直接影响,亦称为“糖尿病心肌病”。尽管目前已经提出了几种作用机制,但是受损心肌葡萄糖代谢紊乱是一个重要的影响因素。目前对其潜在的发病机制只了解了一部分,但是其临床意义还没有得到公认6,8,9。总的来说,心力衰竭与糖尿病的病理生理学机制相互重叠;两者都与胰岛素抵抗、糖代谢受损以及代谢综合征有关10,11。
根据左心室功能是否出现下降将心力衰竭分类为射血分数正常(heart failure with preserved ejection,HFpEF)或者降低(heart failure with reduced ejection,HFrEF)的心力衰竭。在这两种心力衰竭分类中,压力感受器与肾传入小动脉都能感受到心输出量减少与血压下降。这些因素将依次触发肾素-醛固酮系统(reninangiotensin-aldosterone system,RAAS)的激活、抗利尿激素(antidiuretic hormone,ADH)的分泌、血管收缩、交感神经系统的激活以及肾上腺素与去甲肾上腺素的释放。正是这些生理适应作用导致了心力衰竭的临床综合征12。RAAS激活后可导致醛固酮与ADH的分泌,促进水钠滞留。这会增加前负荷,导致肺充血与体液负荷超重。血管收缩后可以维持血压保证器官灌注,而代价是过度的后负荷,这会加剧泵衰竭。刺激交感神经后可增加心率与收缩力,最初可改善射血分数,但是最终可增加心脏的代谢需求,而且容易出现心律失常与心源性猝死。相反,尿钠肽的分泌可以促进有益的结构重塑、尿钠增多与利尿,并且降低交感神经张力。
现代疗法旨在限制这些生理适应性,减轻症状,提高生存率。利尿剂可以缓解液体滞留的症状。血管紧张素转换酶抑制剂、血管紧张素II受体阻滞剂以及盐皮质激素受体拮抗剂可以抑制RAAS。脑啡肽酶抑制剂可以减少尿钠肽的降解,促进结构重塑、血管扩张与尿钠增多。β-肾上腺素受体拮抗剂(β受体阻滞剂)可抑制交感神经激活,降低心率与收缩力,主要是通过影响交感神经激活与逆转重塑,减少心律失常与心源性猝死的风险。在存在持续性左心室功能障碍与传导延迟的患者中,心脏再同步化治疗(cardiac resynchronization therapy,CRT)可以改善症状提高生存率,在一些选定的患者中,植入心脏除颤器可以减少心律失常所导致的死亡12。
1 药物治疗的作用
调查糖尿病与不良结果的关系以及对CHF治疗影响的研究往往要早于目前认可施行的现代治疗方法。最近几年以来,随着β受体阻滞剂与CRT的广泛使用治疗效果得到了显著的改善13。心源性猝死的发生率随着时间的推移在逐步地降低,但仍然是主要不良结果之一,尤其是在糖尿病患者中。在一项纳入了1091名患者的前瞻性队列研究中,发现糖尿病组的进展性CHF、全因与心血管死亡发生率都较高6,并且在临床试验14,15中,虽然药物治疗与器械治疗对糖尿病患者以及非糖尿病患者都有效,但是糖尿病患者仍然处于较高的风险之中。最近,我们证实了糖尿病患者实际上更有可能从现代心力衰竭治疗策略中获益。在一项纳入了1797名患者的非随机前瞻性队列研究中,我们发现更高剂量的ACE抑制剂与β受体阻滞剂可以改善所有患者的预后,并且剂量增大后对糖尿病患者的死亡率影响最大,尤其是左心室功能受损最严重的糖尿病患者16。这一观察结果为直接使用更高剂量的β受体阻滞剂提供了支持,以期在合并CHF的糖尿病患者中达到最佳的效果16。
β受体阻滞剂对于糖尿病患者来说似乎具有额外的益处,其作用机制可能有以下几种。首先,阻断β-肾上腺素受体具有负性变力与变时作用,从而降低心脏的代谢需求。这对于CAD来说可能具有特别的好处,而CAD在糖尿病患者中非常普遍。其次,β受体阻滞剂可以预防心律失常与心源性猝死,而这在合并心力衰竭的糖尿病患者中更为常见6。第三,β受体阻滞剂可以促进心肌结构重塑。我们已经在一项纳入了628名接受现代心力衰竭治疗方案患者的前瞻性研究中证实,糖尿病患者经历了更为不利的结构与功能重塑,因此在这个患者组中阻止这种更快速的进展性变化可能更为重要17。最后,β受体阻滞剂可以抑制交感神经激活,对抗糖尿病患者基础交感神经兴奋10。
历史上医生都不愿意给那些使用胰岛素或磺酰脲类药物的患者处方β受体阻滞剂,因为一直认为β受体阻滞剂有掩盖低血糖症状或者延长低血糖发作时间的风险。然而,在临床试验中两组之间的低血糖发生率却相似,并且获益远大于这些理论上的低血糖风险18。
2 血运重建的作用
对于合并多支血管病变的CAD患者来说,无论他们是否合并糖尿病,冠状动脉旁路移植术(coronary artery bypass grafting,CABG)的疗效都很明确。临床试验已经表明,那些合并多支血管病变的CAD患者使用CABG或者经皮介入治疗(coronary artery bypass grafting,PCI)后可以减少死亡率,然而,合并CHF的患者代表性却不足。糖尿病患者未来血运重建评估:多支血管病变的最佳治疗(The future revasculization evaluation in patients with diabetes mellitus: optimal management of multivessel disease,FREEDOM)试验证实,在糖尿病患者中,CABG组的死亡率以及心脏事件与PCI组相比都有所减少,并且在这些患者中只有3%合并HFrEF19。外科治疗缺血性心力衰竭的扩展研究(The surgical treatment for ischemic heart failure extension study,STICHES)试验表明,对于HFrEF患者来说使用CABG治疗优于药物治疗,但是这项研究的效力不足,无法判定糖尿病患者与非糖尿病患者相比是否能够获得一些额外的益处20。然而,在一项倾向评分匹配的大型观察研究中,合并HFrEF的糖尿病患者接受CABG治疗后的心血管事件发生率以及死亡率都要低于接受PCI治疗的多支血管病变的CAD患者21。
3 关键信息
合并CHF的糖尿病患者死亡更快,症状更重,住院率也更高。现代心力衰竭治疗策略似乎能够给那些糖尿病患者带来额外的益处。尽管如此,临床医生往往不愿意处方在临床试验中已经证实有益的较大剂量的治疗药物,尤其是非心脏病专家所开具的处方22。在临床试验中已经观察到,随着这些救命药物剂量的增加,能够进一步降低患者的死亡率以及住院率,因此患者与医疗保健专业人员都应该有这个信心来增加药物剂量。
ACKNOWLEDGEMENTS
No funding was received for the completion of this manuscript. MTK receives support from the British Heart Foundation and KKW holds an National Institute for Health Research Clinical Scientist Award.
DISCLOSURE
The authors declare no conflicts of interest relevant to this manuscript.