Abnormalities in thrombotic pathways in diabetes: A tale of two platelets
糖尿病患者血栓形成途径异常:两种血小板的故事
Acute coronary syndromes and thrombosis
Ischemic cardiovascular disease associated with type 2 diabetes mellitus (T2DM) is characterized by the presence of relatively unstable coronary artery plaques with increased atheroma and intraplaque thrombus. The rupture of a plaque promotes the release of procoagulant material that stimulates the generation of an occlusive platelet-rich fibrin clot and, ultimately, the development of an acute coronary syndrome (ACS). The recognition of the importance of thrombus formation in the pathophysiology of cardiovascular disease led to a revolution in management that has culminated in markedly improved outcomes in both ACS and secondary prevention of cardiovascular disease.
Mechanisms in thrombosis
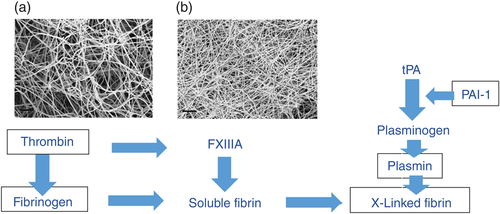
A major part of the problem facing diabetologists and cardiologists is that interactions between the metabolic milieu of T2DM and the bewildering array of inflammatory thrombotic molecules seem to underpin the cardiovascular phenotype. In simple terms, one can think of these processes as the fluid (circulating molecules) and cellular (platelet, endothelium, macrophages) phases that regulate clot formation. The fluid phase consists of the coagulation cascade, which, when activated, generates thrombin, which cleaves fibrinogen and activates Factor XIII to produce a fibrin mesh (Fig. 1), which binds activated platelets. This early phase of activation produces the platelet-rich arterial thrombus that is characteristic of arterial occlusion. Latterly, activation of the fibrinolytic system regulates lysis and remodeling of the thrombus (Fig. 1) that physiologically, has an important role in wound healing, but in the peculiar circumstances of coronary artery occlusion is nearly always “too little, too late”.
Interactions between diabetes on thrombosis
In the presence of T2DM, all the mechanisms described in the previous paragraph are abnormal. The principal drivers for these abnormalities are the combined effects of hyperglycemia and glycation and insulin resistance. In diabetes, increased thrombin generation related to increased activation of coagulation, the presence of underlying vascular disease, and hypoglycemia activates platelets, endothelial cells, and macrophages and promotes clot formation.1-3 Glycation of fibrinogen in poorly controlled diabetes accentuates these effects by producing a densely packed fibrin clot that is also resistant to the effects of clot lysis.4 Work from our laboratory has demonstrated that glycation of plasminogen reduces the catalytic activity of generated plasmin, further limiting the ability to lyse an established thrombus.5 Finally, in the presence of insulin resistance, elevated levels of plasminogen activator inhibitor-1 further inhibit plasmin activity to effectively block clot lysis.6 Putting this together, in the presence of diabetes there is increased generation of a tightly packed thrombus that is itself resistant to lysis, whereas the lytic processes are themselves inhibited. A bad combination.
The platelet and diabetes
It gets worse. The platelet in diabetes is increasingly activated due to a number of influences. First, loss of endothelial cell nitric oxide generation in the presence of insulin resistance leads to increased vasoconstriction and platelet activation.7 Insulin resistance and hyperglycemia also have direct effects on the platelet, all combining to generate increased platelet activation.8 Understanding this concatenation of events has led to the use of both anticoagulants and antiplatelet agents in the management of ACS, and antiplatelet agents in combination after ACS for secondary prevention. Although this approach has markedly reduced mortality in subjects with and without diabetes, there remains a currently unbridgeable gap in outcome between subjects with and without diabetes in which diabetes populations always fare worse. This gap is known as residual risk and, at the time of writing, the cause of this has not been clearly identified. A recent important paper from Monash University (Melbourne, Vic., Australia) has reported on an immature fraction of platelets, called reticulated platelets, and their regulation by glycaemia.9 The reticulated platelets are large immature platelets that are intrinsically activated to a greater extent and have been shown to be resistant to the actions of antiplatelet agents.10, 11 In hematology, these platelets are known to be increased in thrombocytopenic states, presumably to counter the increased bleeding risk associated with such conditions. It has been known for many years that diabetes is associated with an increase in this platelet fraction, but it has been largely ignored, perhaps considered of little importance. Recent evidence, in addition to indicating these platelets are resistant to inhibition, has also reported that this fraction is related to poorer outcomes after ACS.12 Colleagues from Monash University have brought this topic to center stage in diabetes by demonstrating that suboptimal diabetes control increases the production of reticulated platelets and by reporting the mechanisms underpinning these effects and the potential reversibility by improved glycemic control.9 The authors of that study reported that increased glycemia stimulates hepatic Kuppfer cells to release interleukin-6, which stimulates thrombopoietin production to increase the secretion of bone marrow-derived reticulated platelets, an effect reversed by improving glycemic control.9 The available data indicates that an increased reticulated platelet count is associated with resistance to antiplatelet agents, increased cardiovascular risk, and, in diabetes, an effect that may be reversed by improving glycemic control. In addition to the abnormalities in clot formation and lysis described above, diabetes patients have alterations in platelet type and function, all of which promote the development of occlusive arterial disease and are resistant to the normal regulatory processes that limit clot formation.
Conclusion
Further understanding of these processes and in particular the importance of reticulated platelets in the clinical setting in T2DM may provide the opportunity to develop novel strategies to manage cardiovascular risk in both the acute setting and as secondary prevention. The Holy Grail of narrowing the residual risk in T2DM may lie in clarifying some of these issues.
Acknowledgements
The author's work on thrombotic and fibrinolytic abnormalities in diabetes was funded by grants from the British Heart Foundation, Diabetes UK, and the National Institute for Health Research (NIHR) UK.
Disclosure
PJG is co-Chair of the European Guidelines for Cardiovascular Disease Prevention and Management in Diabetes. The views expressed herein are the author's own and not those of the guideline committee. PJG has given lectures and been on advisory boards for Novo Nordisk, Amgen, Janssen Pharmaceuticals, Bayer, and Astra Zeneca. He is Editor-in-Chief of the International Journal of Diabetes and Cardiovascular Disease, and diabetes advisor for Synexus.
急性冠状动脉综合征与血栓形成
与2型糖尿病(T2DM)相关的缺血性心血管疾病的特征是存在冠状动脉斑块相对不稳定, 并且粥样斑块以及斑块内的血栓都是增多的。斑块破裂后可导致促凝物质的释放, 促进闭塞性的富含血小板纤维蛋白凝块的生成, 并且最终导致急性冠状动脉综合征(acute coronary syndrome,ACS)的发生。认识到血栓形成在心血管疾病病理生理中的重要性后, 导致针对ACS以及心血管疾病二级预防的管理模式出现了革命性的改变, 最终显著地改善了患者的预后。
血栓形成机制
糖尿病专家与心脏病专家面临着一个问题, 其主要部分就是T2DM代谢环境与一连串的炎性血栓分子之间的相互作用看起来似乎是心血管表型的基础。简单地说, 人们可以将这些作用过程当做是可以调节凝血块形成的流体(循环分子)与细胞相(血小板、内皮细胞、巨噬细胞)。流体相由凝血级联反应组成, 当它们被激活后, 会产生凝血酶, 后者可以切割纤维蛋白原, 并且激活XIII因子产生纤维蛋白网(图1),最终就会结合活化的血小板。在激活过程的早期阶段会产生富含血小板的动脉血栓, 而这就是动脉闭塞的特征。到了晚期, 激活纤溶系统后可以调节血栓的溶解与重塑(图1),在生理上, 它对伤口的愈合具有重要的作用, 但是在冠状动脉闭塞这种特殊的情况下, 纤溶系统的激活几乎总是“太少、太晚”。
糖尿病与血栓形成之间的相互作用
在存在T2DM的情况下, 前面段落中描述的所有机制都会出现异常。这些异常的主要驱动因素是高血糖、糖基化以及胰岛素抵抗的联合作用。在糖尿病患者中, 凝血酶生成的增加与凝血激活的增加以及存在潜在的血管疾病都有关, 并且低血糖还能够激活血小板、内皮细胞与巨噬细胞, 促进凝血块的形成1-3。在控制不佳的糖尿病患者中, 纤维蛋白原糖基化后通过生成的致密纤维蛋白凝血块加重了这些效应, 并且后者还能拮抗凝血块溶解的影响4。我们实验室的工作成果表明, 纤溶酶原糖基化后催化生成纤溶酶的活性降低了, 而这进一步限制了溶解已形成血栓的能力5。最后, 在存在胰岛素抵抗的情况下, 升高的纤溶酶原激活物抑制剂-1水平可以进一步抑制纤溶酶的活性, 最终有效地阻断了凝血块的溶解6。将这些机制结合起来, 在存在糖尿病的情况下, 更多地产生了一种可以自我拮抗溶解的致密血栓, 然而溶解过程却受到了自我抑制。这是一种糟糕的组合。
血小板与糖尿病
情况变得更糟了。糖尿病患者越来越多的血小板由于受到了多种因素的影响被激活了。首先, 在存在胰岛素抵抗的情况下内皮细胞一氧化氮生成缺失可导致血管收缩以及血小板激活增加7。胰岛素抵抗以及高血糖对血小板也有直接的影响, 所有的因素结合在一起都会导致激活的血小板生成增多8。因为我们了解这一事件的前后关联, 所以在ACS的管理中要应用抗凝剂与抗血小板药物治疗, 并且在发生ACS后要联用抗血小板药物进行二级预防。尽管使用这种方法治疗后已经显著降低了糖尿病患者以及非糖尿病患者的死亡率, 但是在糖尿病与非糖尿病受试者者之间的结果仍然存在着不可逾越的鸿沟, 糖尿病人群的预后情况总是更糟。这种差距被称为残余风险, 在撰写本文时, 这一原因尚未十分明确。最近Monash大学(Melbourne, Vic., Australia)报道了一篇重要的关于血小板未成熟部分的论文, 将之称为网状血小板, 它们受到了血糖的调节9。网状血小板是大型的未成熟血小板, 其本质上可以被更大程度地激活, 并且目前已经被证明具有拮抗抗血小板药物的作用10,11。在血液学中, 目前已经发现在血小板减少的状态下它反而有所增加, 这大概是为了拮抗与这种情况相关的出血风险增加。众所周知, 糖尿病与这部分血小板增加有关, 但是既往它被大部分人忽略了, 也许认为它没有什么重要的地方。最近的证据表明, 这些血小板除了拮抗抑制作用, 还有人报告这部分血小板与ACS后的不良结果有关12。来自Monash大学的同事们已经将这个话题的重要性提升到了糖尿病的中心阶段, 他们证实了糖尿病控制未达最佳标准可导致网状血小板的生成增多, 他们还报道了支持这些效应的机制以及通过改善血糖控制有可能使得这些效应产生可逆变化9。据该项研究的作者报告, 升高的血糖可刺激肝脏Kuppfer细胞释放白细胞介素-6,后者可刺激促血小板生成素的生成, 从而增加骨髓来源网状血小板的分泌, 通过改善血糖控制可以逆转这种效应9。目前可用的数据表明, 糖尿病患者的网状血小板计数增加与患者对抗血小板药物的耐药性以及患者的心血管风险增加有关, 而通过改善血糖控制可以逆转这种效应。除了上述的凝血块形成与溶解的异常之外, 糖尿病患者的血小板类型与功能也出现了变化, 所有的这些因素都会促进闭塞性动脉疾病的发展, 并且对限制凝血块形成的正常调节过程具有拮抗作用。
结论
在T2DM的临床环境中要进一步了解以上过程, 特别是网状血小板的重要性, 这可能有助于我们研究新的策略来管理心血管风险, 尤其是对于心血管疾病的急性发作以及二级预防。减少T2DM残余风险的难点可能在于要澄清其中的一些问题。
图1 血栓前机制与纤溶作用之间的相互作用。这张示意图描绘了凝血级联反应的下半部分。由受损细胞释放的组织因子激活凝血因子VII导致“张力酶”复合体形成, 后者可以裂解凝血酶原最终产生凝血酶。凝血酶具有多种功能, 包括激活血小板, 但是在凝血级联反应中它可以切割纤维蛋白原, 激活XIII因子, 并引发交联纤维蛋白的形成, 而交联纤维蛋白在动脉循环中可以结合血小板并形成血栓, 最终导致闭塞性动脉疾病。纤溶系统起到溶解与重塑凝血块的作用, 而凝血块在组织的修复中有着重要的作用。在该系统中, 组织纤溶酶原激活剂(tPA)将纤溶酶原切割后形成了纤溶酶, 后者可以分解纤维蛋白。该系统在多处都受到了抑制, 特别是纤溶酶原激活物抑制剂-1(PAI-1)可作用于tPA,并且抗纤溶酶(未标出)还可以直接抑制纤溶酶。方框中的蛋白质是受到糖尿病影响的蛋白质, 就如同本文中所讨论的那样。(a)是来自一名非糖尿病受试者的血浆在体外形成的纤维蛋白电子显微照片,(b)则来自一名控制不佳的糖尿病患者。在(b)中的显微照片显示了一种纤维蛋白结构, 它是一种具有抗纤溶作用的紧密堆积的薄层纤维。FXIIIA,凝血因子XIII。




