Accelerated executive functions decline and gray matter structural changes in middle-aged type 1 diabetes mellitus patients with proliferative retinopathy
合并增殖性视网膜病变的中年1型糖尿病患者的执行功能加速下降与灰质结构改变
Abstract
enBackground
The aim of the present study was to determine trajectories of cognitive and cortical changes over time in middle-aged patients with type 1 diabetes mellitus (T1DM) and proliferative retinopathy.
Methods
Twenty-five patients and 25 controls underwent neuropsychological assessment and neuroimaging twice in a mean (±SD) of 3.56 ± 0.65 and 3.94 ± 0.91 years, respectively (P = 0.098). Cognitive assessment included the domains of general cognitive ability, memory, information processing speed, executive functions, attention, and motor and psychomotor speed. Symmetrized percentage change in local cortical thickness, surface area, and volume was determined using the FreeSurfer 6 vertex-wise general linear model method. Analyses were performed uncorrected and corrected for baseline systolic blood pressure and depressive symptoms.
Results
In patients versus controls, accelerated executive function decline was accompanied by, but not related to, lower left frontal and temporal surface area, left parietal and right frontal thickness, and bilateral frontal and right posterior cingulate volume (family-wise error [FWE]-corrected P < 0.05 for all). In patients, lower executive performance was related to loss of right precuneus surface area (PFWE = 0.005). Higher HbA1c during follow-up was related to executive function decline (r = −0.509, P = 0.016) and loss of left hemisphere surface area (rcorrected analysis = −0.555, P = 0.007).
Conclusions
After 3.5 years of follow-up, middle-aged T1DM patients with proliferative retinopathy, mild focal changes in executive functions, and cortical structure were found, which may indicate accelerated aging.
Abstract
zh摘要
背景
本研究旨在有合并增殖性视网膜病变的中年1型糖尿病(T1DM)患者中测定认知功能与脑皮质随时间变化的轨迹。
方法
本研究共纳入25名患者与25名对照组受试者, 接受2次神经心理评估以及神经影像学检查, 平均随访时间分别为3.56 ± 0.65与3.94 ± 0.91年(P = 0.098)。认知评估包括一般认知能力、记忆、信息处理速度、执行功能、注意力、运动以及精神活动速度。采用FreeSurfer 6 vertex-wise一般线性模型法测定局部皮质厚度、表面积以及体积的对称百分比变化。校正基线收缩压以及抑郁症状, 在校正前及校正后都进行了分析。
结果
与对照组受试者相比, 伴随着左额叶与颞叶表面积、左顶叶与右额叶厚度、双侧额叶与右后扣带回的体积,T1DM患者的执行功能加速下降, 但没有相关性(校正族系误差[family-wise error,FWE]后所有P < 0.05)。患者的执行功能较差与右楔前叶表面积减少相关(PFWE = 0.005)。随访期间HbA1c越高与执行功能越差(r = -0.509,P = 0.016)以及左半球表面积越小相关(r校正后分析 = -0.555,P = 0.007)。
结论
经过3.5年的随访, 发现合并增殖性视网膜病变的中年T1DM患者执行功能以及皮质结构都出现了轻度的局灶性变化, 这可能意味着加速的老龄化。
Introduction
In type 1 diabetes mellitus (T1DM), chronic hyperglycemia is related to the development of microvascular disease, such as retinopathy.1 In addition, T1DM is related to cognitive decrements, primarily in speed-related functions, attention, and executive functions.2 Furthermore, these cognitive decrements have been found to be mirrored by changes in cerebral white matter microstructural integrity,3, 4 and functional connectivity.5, 6 A meta-analysis summarizing 10 voxel-based morphometry (VBM) studies found robust thalamic volume loss in T1DM patients.7 That analysis partially corroborated findings from our own non-VMB study showing bilateral thalamus, putamen, and nucleus accumbens volume loss with spared cortical structure in middle-aged T1DM patients, being especially pronounced in those patients with proliferative retinopathy.8 The effects were strongest in patients who had prevalent proliferative retinopathy, which commonly serves as a marker of chronic hyperglycemic exposure in research.9
The longitudinal trajectories of cognitive changes in adult T1DM are relatively unknown. The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) showed a notable, but mild, decline in speed-related domains in patients with poor glycemic control (i.e. HbA1c >8.8%) over 18 years,10 with patients who had developed proliferative retinopathy being the most vulnerable.11 However, that study lacked a non-diabetes comparison group. A case-control study with a 7-year follow-up period observed declining psychomotor efficiency in patients relative to controls, related to proliferative retinopathy and macroangiopathy.12 The longitudinal trajectories of gray matter changes in adults with T1DM are unknown.
The literature cited above shows that adult T1DM with proliferative retinopathy is characterized by cognitive decrements and gray matter deficits in cross-sectional studies, and that this microvascular complication may also be related to cognitive decline over time. Thus, it could be hypothesized that these patients are also most susceptible to accelerated loss of gray matter structure over time. To this end, we performed follow-up magnetic resonance imaging (MRI) and cognitive assessment in 25 randomly selected T1DM patients with proliferative retinopathy and in 25 matched controls after 3.5–4 years. We hypothesized that accelerated cognitive decline would be mild and limited to speed-related domains and that gray matter structure decreases would be visible in the patient relative to control group. It was hypothesized that these changes were related to poorer glycemic control.
Methods
Participants
This study was approved by the Medical Ethics Committee of the VU University Medical Center and written informed consent was obtained from all subjects at baseline and follow-up. At baseline, 51 patients with proliferative retinopathy, 53 patients without microangiopathy, and 51 healthy controls matched for sex, IQ, and body mass index (BMI) were included in the study.8 Due to funding limitations, we were able to include 50 of the 155 participants. Twenty-five patients with proliferative retinopathy were randomly selected to participate in the longitudinal assessment by drawing their identification number from an envelope. Twenty-five controls were selected and, at group level, matched for age, sex, BMI, and IQ. At baseline, inclusion criteria were age between 18 and 56 years, right handedness, proficiency in Dutch, and, for patients, at least 10 years of diabetes. To be considered for inclusion, a diagnosis of T1DM and the age of diagnosis had to have been reported by the treating physician. Diagnosis was primarily based on clinical presentation (polyuria, polydipsia, weight loss), body habitus, and age of onset. In some patients, antibodies were determined by the treating physician. Exclusion criteria at both time points were (treatment for) psychiatric comorbidity, insufficient visual acuity to perform neuropsychological tests, brain trauma, previous coma unrelated to hypoglycemia, alcohol (men >21 units/week, women >14 units/week) and drug use, use of centrally acting medication, and MRI contraindications. At baseline, controls were not included if they had hypertension.
Power calculation
Including a total of 50 participants from all three groups (i.e. approximately 16 per group), with a power (1 − β) of 0.80, and a two-sided α of 0.05, we would be able to detect a large effect size δ of 0.90 for the repeated measures interaction effect of time with group, which we deemed unfeasible. Including two groups of 25 participants with similar power and two-sided α settings, we were able to detect a medium effect size δ of 0.40 for the repeated measures interaction effect of time with group. Hence, the power calculation supported the inclusion of only two groups instead of three.
Biomedical measurements
Proliferative retinopathy was determined at baseline by fundus photography and rated according to the EURODIAB classification13; albuminuria was defined as a urinary albumin:creatinine ratio >2.5 mg/mmol for men and >3.5 mg/mmol for women using 24-h urine sampling at baseline and morning urine sampling at follow-up. Baseline and follow-up neuropathy status was determined based on the results of an annual check-up that patients receive and is incorporated into the medical records.6 Blood pressure was measured three times at 5-min intervals in the left arm in subjects in a seating position after a 15-min rest. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or the use of antihypertensive drugs. The Center of Epidemiological Studies–Depression scale was used to evaluate depressive symptoms.14 Blood was drawn twice for routine assessments. For patients, all HbA1c values within the follow-up period were obtained from medical records. During the testing days, blood glucose was kept between 4 and 15 mmol/L (72–270 mg/dL). Any hypoglycemic event 24 h prior to the testing day resulted in rescheduling.
Neuropsychological assessment
The neuropsychological assessment was identical at both time points. Estimated IQ was determined at baseline using the Dutch version of the National Adult Reading Test.15 The following tests were used to measure performance in the domains of memory, information processing speed, executive functions, attention, and motor and psychomotor speeds (note, details for each test and baseline performance have been published previously16): the Rey Auditory Verbal Learning Test (parallel version at follow-up), the Wechsler Adult Intelligence Scale, the third revised edition of the Digit Span and Symbol Substitution Test, Stroop Color–Word Test, concept shifting task, simple auditory and visual reaction time tests, computerized visual searching task, the D2-test, the Wisconsin Cart Sorting Test, category word fluency task, tapping test, and letter digit modality test. General cognitive ability was based on the average of all tests. The tests were chosen because they are standard tests for that particular function, well-validated in Dutch, and have been shown to be most sensitive to changes in performance in clinical practice, such as the Rey Auditory Verbal Learning Test, the D2-test, Digit Span, Stroop Color–Word Test, and the Wisconsin Card Sorting Test, and/or because they have been shown to be sensitive to T1DM-related cognitive decrements, including the symbol substitution and concept shifting tests, and computerized reaction time tests.
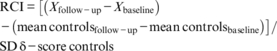
Magnetic resonance imaging protocol
Scanning was performed on a 1.5-Tesla whole-body magnetic resonance system (Siemens Sonata, Erlangen, Germany), using an eight-channel phased-array head coil. Both scanner and imaging protocols were identical at both time points. For the present study, we used the three-dimensional (3D) T1-weighted magnetization-prepared rapid-acquisition gradient echo (T1-MPRAGE; repetition time 2.700 ms, echo time 5.17 ms, inversion time 950 ms, flip angle 8°, 1.0 mm × 1.0 mm × 1.5 mm voxel size), and the 3D fluid attenuated inverse recovery (3D-FLAIR; repetition time 6500 ms, echo time 385 ms, variable flip angle,18 1.3 mm isotropic voxels) at both time points. All scans were corrected for geometric distortions due to gradient non-linearity and excessive neck signal was removed.8
Longitudinal cortical structure analysis
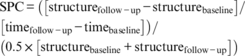
Statistical analyses
The significance of between-group differences at baseline were analyzed using independent-samples Student's t-tests for normally distributed data, Mann–Whitney U-tests for non-normally distributed data, or Chi-squared tests for categorical variables. The significance of differences between T1DM patient and control characteristics during follow-up were assessed using paired-samples Student's t-tests, related-samples Wilcoxon signed-rank tests, and related-samples McNemar tests. All variables were checked for normality using the Shapiro–Wilk test and by visual inspection of the histograms.
Baseline cognitive performance and cortical structure, as well as changes in cognition (RCI) and global whole-brain cortical structure (SPC) were first compared between groups using an analysis of variance (ANOVA) uncorrected for confounding factors. In a second step, baseline SBP and depressive symptoms were added as confounding factors. Furthermore, local between-group differences in cortical structure (SPC) were analyzed using FreeSurfer's vertex-wise general linear modeling (GLM), uncorrected and corrected for baseline SBP and depressive symptoms. Because SPC is not dependent on total intracranial volume, there was no need to add this as a confounding factor. FreeSurfer's GLM is specifically designed to deal with non-parametric surface-based data. For this, the SPC maps were smoothed using a Gaussian kernel with a full-width half-maximum of 15 mm and warped onto the fsaverage template. These vertex-wise statistical maps were thresholded at P < 0.01, and corrected for multiple comparisons using Monte Carlo Z simulation with 10 000 iterations. The family wise error (FWE) cluster-level correction for multiple comparisons threshold was set at P < 0.05. The P-values were further corrected using the Bonferroni method to correct for both hemispheres. In patients, the associations between cognition and cortical structure, HbA1c, and severe hypoglycemic events between baseline and follow-up were determined using univariate correlations.
The vertex-wise analyses were performed using tools provided in FreeSurfer 6; SPSS 20.0 (IBM-SPSS, Chicago, IL, USA) was used for all other analyses.
Unless indicated otherwise, data are presented as the mean ±SD.
Results
Participant characteristics
Three T1DM patients and one non-diabetic control subject refused to participate in the follow-up study. The mean follow-up time was 3.56 ± 0.65 years for patients and 3.94 ± 0.91 years for controls (P = 0.098). At baseline, patients were aged between 32 and 56 years, had higher SBP and HbA1c levels, and reported more depressive symptoms than the controls (all P < 0.05; Table 1), who were aged between 28 and 56 years. There were no changes in diabetic and biomedical profiles of patients over time, except for a slightly higher blood glucose level before neuroimaging, which remained within the required range (P = 0.008; Table 2). In controls, only HbA1c levels increased slightly, although all measurements remained within the normal range (P = 0.001; Table 2).
| T1DM | Controls | P-value | |
|---|---|---|---|
| No. subjects | 25 | 25 | – |
| Age (years) | 46.1 ± 6.3 | 44.3 ± 8.5 | 0.410 |
| Sex | 0.571 | ||
| No. male (%) | 10 (40) | 13 (52) | |
| Female | 15 | 12 | |
| Estimated IQ* | 112.3 ± 12.7 | 109.4 ± 13.1 | 0.433 |
| Depressive symptoms† | 8.5 (0–29) | 4.0 (0–37) | 0.018 |
| BMI (kg/m2) | 26.2 ± 43.9 | 25.1 ± 2.9 | 0.307 |
| SBP (mmHg) | 133.9 ± 14.1 | 126.9 ± 9.4 | 0.045 |
| DBP (mmHg) | 75.4 ± 7.8 | 78.9 ±6.2 | 0.185 |
| HbA1c (%) | 7.9 ± 1.0 | 5.4 ±0.3 | <0.001 |
| HbA1c (mmol/mol) | 63.2 ±10.7 | 34.9 ± 2.7 | <0.001 |
| TC (mmol/L) | 4.4 ± 0.8 | 4.8 ±0.5 | 0.120 |
| Hypertension‡ | 19 (76) | — | — |
| Diabetes onset age (years) | 11.4 ±7.5 | — | — |
| Diabetes duration (years) | 34.7 ± 8.1 | — | — |
| Diabetes early onset§ | 8 (25) | — | — |
| Severe hypoglycemic events¶ | 1 (0–25) | — | — |
| Albuminuria** | 5 (20) | — | — |
| Peripheral neuropathy†† | 10 (40) | — | — |
- Data are presented as the mean ± SD, median (minimum–maximum), or as n (%).
- * Estimated intelligence quotient (IQ) was measured using the Dutch version of the National Adult Reading Test.15
- † Depressive symptoms were assessed using the Center for Epidemiological Studies–Depression scale.14.
- ‡ Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg or above, diastolic blood pressure (DBP) of ≥90 mmHg, or the use of antihypertensive drugs.
- § Early onset age of type 1 diabetes mellitus (T1DM) was defined as an onset before 7 years of age.
- ¶ Severe hypoglycemic events were self-reported during the lifetime and defined as events for which the patient needed assistance from a third person to recuperate as a result of loss of consciousness or seriously deranged functioning, coma, or seizure owing to low glucose levels.
- ** Albuminuria was defined as an albumin: creatinine ratio >2.5 mg/mmol for men and >3.5 mg/mmol for women and assessed with 24-h urine sampling.
- †† Peripheral neuropathy was based on medical records.
- BMI, body mass index; TC, total cholesterol.
| T1DM | Controls | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | P-value | Baseline | Follow-up | P-value | |
| Depressive symptoms* | 8.5 (0–29) | 7.0 (0–22) | 0.367 | 4.0 (0–37) | 4.0 (0–40) | 0.198 |
| HbA1c (%) | 7.9 ± 1.0 | 8.0 ± 1.1 | 0.572 | 5.4 ± 0.3 | 5.5 ± 0.3 | 0.001 |
| HbA1c (mmol/mol) | 63.2 ± 10.7 | 64.2 ± 11.4 | 0.572 | 34.9 ± 2.8 | 36.7 ± 3.4 | 0.001 |
| BMI (kg/m2) | 26.2 ± 4.9 | 26.3 ± 4.8 | 0.739 | 25.1 ± 2.9 | 25.0 ± 3.3 | 0.924 |
| TC (mmol/L) | 4.5 ± 0.8 | 4.7 ± 0.8 | 0.059 | 4.8 ±0.9 | 5.0 ± 1.0 | 0.110 |
| HDL-C (mmol/L) | 1.70 ± 0.50 | 1.84 ± 0.54 | 0.004 | 1.52 ± 0.4 | 1.58 ± 0.5 | 0.303 |
| LDL-C (mmol/L) | 2.32 ± 0.67 | 2.46 ± 0.70 | 0.237 | 2.68 ± 0.9 | 2.86 ± 0.8 | 0.098 |
| TG (mmol/L) | 0.8 (0.3–2.4) | 0.8 (0.3–2.5) | 0.625 | 1.3 (0.7–3.2) | 1.2 (0.5–2.7) | 0.767 |
| SBP (mmHg) | 133.9 ± 14.1 | 133.6 ± 16.3 | 0.943 | 126.9 ± 9.4 | 128.4 ± 12.0 | 0.505 |
| DBP (mmHg) | 75.4 ± 7.8 | 75.2 ± 6.8 | 0.932 | 78.9 ± 6.2 | 78.9 ± 6.4 | 0.965 |
| Hypertension† | 19 (76) | 19 (76) | 0.999 | — | 0 (0) | — |
| Glucose before NPA (mmol/L) | 7.7 ± 3.8 | 8.3 ± 3.4 | 0.559 | — | — | — |
| Glucose before MRI (mmol/L) | 8.2 ± 2.8 | 11.1 ± 4.1 | 0.008 | — | — | — |
| Severe hypoglycemia‡ | 1.0 (0–25) | 0 (0–20) | 0.217 | — | — | — |
| ACR (mg/mmol) | 0.69 (0–33.17) | 0.61 (0–85.49) | 0.223 | — | — | — |
| Albuminuria§ | 5 (20) | 5 (20) | 0.999 | — | — | — |
| Peripheral neuropathy¶ | 10 (40) | 11 (44) | 0.317 | — | — | — |
| Updated HbA1c** | — | 8.0 ± 1.0 | — | — | — | — |
| Updated HbA1c** (mmol/mol) | — | 64.2 ± 11.4 | — | — | — | — |
- Data are presented as the mean ± SD, median (minimum–maximum), or as n (%).
- * Depressive symptoms were assessed using the Center for Epidemiological Studies–Depression scale.14
- † Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or the use of antihypertensive drugs.
- ‡ Severe hypoglycemic events were self-reported during the lifetime and were defined as events for which the patient needed assistance from a third person to recuperate as a result of loss of consciousness or seriously deranged functioning, coma, or seizure owing to low glucose levels.
- § Albuminuria was defined as an albumin: creatinine ratio (ACR) >2.5 mg/mmol for men and >3.5 mg/mmol for women and assessed with 24-h urine sampling.
- ¶ Peripheral neuropathy was based on medical records or, if they were not available, on self-report.
- ** Updated HbA1c is the mean of all HbA1c measurements between baseline and follow-up for the patients. Information for these measurements could not be obtained for three patients.
- BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MRI, magnetic resonance imaging; NPA, neuropsychological assessment; T1DM, type 1 diabetes mellitus; TC, total cholesterol; TG, triglycerides.
Neurocognitive functioning
In the whole group, all variables passed the Shapiro–Wilk test and visual inspection for normality. At baseline, uncorrected for confounding, patients had lower performance on general cognitive ability, information processing and motor speed (all P < 0.05; Table 3). After correction for confounders, motor speed was no longer statistically significant (P = 0.356), whereas psychomotor speed was (P = 0.030). At follow-up uncorrected for confounding, patients showed a lower mean RCI (accelerated decline) in executive functions compared with non-diabetic controls (mean difference −0.318, Cohen's δ = −0.74, P = 0.016), which remained statistically significant after controlling for confounders (P = 0.029). Using insulin pump therapy (n = 13) compared with daily injections (n = 12) at follow-up had no effect on RCI (all P > 0.317). In patients only, the use of antihypertensive medication at follow-up (n = 16) was related to lower mean general cognitive ability (−0.05 ± 0.33 vs 0.21 ± 0.21; P = 0.047) and attention (−0.21 ± 0.72 vs 0.44 ± 0.62; P = 0.020) RCI, but not to executive functions RCI (−0.36 ± 0.62 vs −0.30 ± 0.35; P = 0.708) compared with patients not using antihypertensive medication (n = 9).
| T1DM | Controls | Cohen's δ | P-value | T1DM | Controls | Cohen's δ | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Baseline cognition z-scores | RCI | ||||||||
| General cognitive ability | −0.383 ± 0.450 | 0.013 ± 0.360 | −0.93 | 0.001* | 0.045 ± 0.320 | −0.006 ± 0.190 | 0.20 | 0.496 | |
| Memory | −0.353 ± 0.640 | −0.002 ± 0.630 | −0.56 | 0.058 | 0.160 ± 0.440 | 0.026 ± 0.490 | 0.29 | 0.318 | |
| Information processing speed | −0.762 ± 0.840 | 0.002 ± 0.580 | −1.08 | 0.001* | 0.110 ± 0.660 | −0.019 ± 0.520 | 0.22 | 0.453 | |
| Attention | −0.272 ± 0.930 | 0.001 ± 0.750 | −0.33 | 0.267 | 0.024 ± 0.740 | 0.001 ± 0.790 | 0.03 | 0.915 | |
| Executive functions | 0.056 ± 0.570 | 0.007 ± 0.460 | 0.10 | 0.745 | −0.337 ± 0.530 | −0.019 ± 0.330 | −0.74 | 0.016* | |
| Motor speed | −0.493 ± 0.910 | 0.048 ± 0.790 | −0.65 | 0.031† | 0.144 ± 0.690 | −0.024 ± 0.620 | 0.26 | 0.372 | |
| Psychomotor speed | −0.473 ± 0.800 | 0.021 ± 1.020 | −0.55 | 0.064‡ | 0.169 ± 0.770 | −0.002 ± 1.020 | 0.19 | 0.510 | |
| Baseline cortical structure | SPC (%) | ||||||||
| Surface area (mm2) | |||||||||
| Bilateral | 2507.9 ± 247.1 | 2563.8 ± 333.8 | −0.19 | 0.023†,§ | −0.263 ± 0.190 | −0.114 ± 0.200 | −0.78 | 0.010* | |
| Left hemisphere | 2503.7 ± 241.6 | 2566.7 ± 333.2 | −0.22 | 0.058§ | −0.262 ± 0.250 | −0.117 ± 0.240 | −0.60 | 0.041* | |
| Right hemisphere | 2512.1 ± 251.5 | 2561.0 ± 334.9 | −0.17 | 0.036†,§ | −0.263 ± 0.170 | −0.111 ± 0.220 | −0.79 | 0.009* | |
| Thickness (mm) | |||||||||
| Bilateral | 2.769 ± 0.080 | 2.775 ± 0.100 | −0.09 | 0.830 | −0.209 ± 0.350 | −0.116 ± 0.200 | −0.33 | 0.261 | |
| Left hemisphere | 2.760 ± 0.090 | 2.768 ± 0.100 | −0.09 | 0.735 | −0.177 ± 0.540 | −0.089 ± 0.270 | −0.21 | 0.472 | |
| Right hemisphere | 2.779 ± 0.090 | 2.781 ± 0.110 | −0.02 | 0.930 | −0.241 ± 0.390 | −0.143 ± 0.220 | −0.32 | 0.278 | |
| Volume (mL) | |||||||||
| Bilateral | 505.2 ± 56.3 | 513.3 ± 57.0 | −0.15 | 0.063§ | −0.445 ± 0.510 | −0.233 ± 0.280 | −0.53 | 0.073 | |
| Left hemisphere | 251.7 ± 27.7 | 256.4 ± 27.7 | −0.17 | 0.123§ | −0.422 ± 0.740 | −0.211 ± 0.350 | −0.37 | 0.204 | |
| Right hemisphere | 253.6 ± 28.9 | 256.9 ± 28.6 | −0.12 | 0.084§ | −0.468 ± 0.570 | −0.255 ± 0.300 | −0.48 | 0.107 | |
- Data are presented as the mean ± SD.
- Cohen's δ is used as a measure of effect size. By default, δ ≤ 0.20 is considered small, δ up to 0.70 is considered to indicate a medium effect size, and δ ≥ 0.70 is considered large.24
- * Remained statistically significant after correction for baseline systolic blood pressure (SBP) and depressive symptoms.
- † No longer statistically significant after correction for baseline SBP and depressive symptoms.
- ‡ Statistically significant after correction for baseline SBP and depressive symptoms.
- § These analyses are further corrected for estimated total intracranial volume, because area and volume, but not thickness, depend on head size.
- RCI, reliable change index; SPC, symmetrized percentage change; T1DM, type 1 diabetes mellitus.
Global baseline and longitudinal cortical structural changes
In the whole group, all variables passed the Shapiro–Wilk test and visual inspection for normality. At baseline, uncorrected for confounders, T1DM patients showed a slightly lower whole-brain (mean difference −55.9 mm2, Cohen's δ = −0.19, P = 0.023) and right hemisphere (mean difference −48.9 mm2, Cohen's δ = −0.17, P = 0.036) surface area (Table 3). After correction for confounding, these differences were no longer statistically significant (all P > 0.05). Over time, patients showed an accelerated loss of whole-brain (mean difference −0.149%, Cohen's δ = −0.78, P = 0.010) and left (mean difference −0.145%, Cohen's δ = −0.60, P = 0.041), and right (mean difference −0.152%, Cohen's δ = −0.79, P = 0.009) hemisphere surface area compared with controls, which remained statistically significant after correction for baseline confounding factors (Table 3).
Local longitudinal cortical changes
FreeSurfer 6’s vertex-wise GLM was used, correcting the P-values for multiple comparisons using FWE and Bonferroni for testing two hemispheres. Information about mean SPC values, effect sizes, and the size of the clusters is given in Table 4. All clusters are shown graphically in Fig. 1.
| SPC | Vertex-wise Cohen's δ | Cluster-wise PFWE | Cluster size (mm2) | Anatomical location | |||
|---|---|---|---|---|---|---|---|
| Controls | T1DM | Mean | Maximum | ||||
| Surface area | |||||||
| Uncorrected left cluster 1 | −0.020 ± 0.360 | −0.593 ± 0.510 | −0.85 | −1.14 | 0.002 | 643.60 | Caudal middle frontal, superior frontal, precentral |
| Corrected left cluster 1 | −0.167 ± 0.260 | −0.540 ± 0.480 | −0.90 | −1.26 | 0.013 | 526.03 | Middle temporal, banks of the superior temporal sulcus |
| Corrected left cluster 2 | −0.023 ± 0.340 | −0.584 ± 0.520 | −0.88 | −1.24 | 0.040 | 437.62 | Caudal middle frontal, superior frontal, precentral |
| Thickness | |||||||
| Uncorrected left cluster 1 | 0.070 ± 0.450 | −0.615 ± 0.830 | −0.84 | −1.18 | 0.025 | 652.94 | Superior parietal, inferior parietal |
| Uncorrected left cluster 2 | 0.065 ± 0.430 | −0.476 ± 0.600 | −0.82 | −1.10 | 0.036 | 603.16 | Inferior parietal |
| Uncorrected right cluster 1 | −0.143 ± 0.530 | −0.866 ± 0.790 | −0.83 | −1.11 | 0.004 | 768.02 | Superior frontal, medial orbitofrontal, rostral middle frontal |
| Corrected right cluster 1 | −0.059 ± 0.500 | −0.676 ± 0.600 | −0.90 | −1.21 | 0.019 | 623.29 | Caudal middle frontal, superior frontal, precentral |
| Volume | |||||||
| Uncorrected left cluster 1 | −0.244 ± 0.700 | −1.134 ±1.040 | −0.83 | −0.99 | 0.001 | 1110.04 | Pars orbitalis, rostral middle frontal, lateral orbitofrontal |
| Corrected left cluster 1 | −0.228 ± 0.680 | −1.110 ± 1.040 | −0.90 | −1.09 | 0.003 | 1003.77 | Lateral orbitofrontal, pars orbitalis, rostral middle frontal |
| Uncorrected right cluster 1 | −0.345 ± 0.630 | −1.099 ± 0.770 | −0.80 | −1.01 | 0.0002 | 1331.71 | Superior frontal, rostral middle frontal, rostral anterior cingulate, medial orbitofrontal |
| Uncorrected right cluster 2 | −0.220 ± 0.390 | −0.743 ± 0.560 | −0.77 | −0.99 | 0.035 | 618.59 | Posterior cingulate, isthmus cingulate, paracentral, precuneus |
| Corrected right cluster 1 | −0.337 ± 0.530 | −0.970 ± 0.820 | −0.88 | −1.21 | 0.0002 | 1330.97 | Caudal middle frontal, superior frontal, rostral middle frontal, precentral |
| Correlation RCI right surface area | Vertex-wise β | ||||||
| Mean | Maximum | ||||||
| Uncorrected | 0.53 | 0.61 | 0.005 | 629.57 | Precuneus, superior parietal | ||
| Corrected | 0.54 | 0.61 | 0.031 | 469.78 | Precuneus | ||
| Correlation RCI volume | |||||||
| Uncorrected | −0.56 | −0.70 | 0.049 | 575.85 | Rostral middle frontal | ||
- Data are presented as the mean ± SD.
- Cohen's δ represents the effect size within the clusters on a vertex level. By default, δ ≤ 0.20 is considered small, δ up to 0.70 is considered to indicate a medium effect size, and δ ≥ 0.70 is considered large.24
- The uncorrected clusters represent results without any correction for confounding factors. The corrected results represent significant clusters after correction for systolic blood pressure and depressive symptoms at baseline.
- FWE, family-wise error; RCI, reliable change index; SPC, symmetrized percentage change; T1DM, type 1 diabetes mellitus.
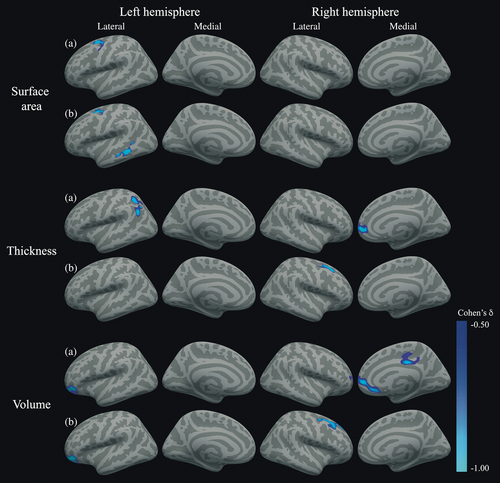
Surface area SPC values were, uncorrected for confounders, lower in patients relative to controls in a cluster consisting of the left caudal middle frontal, superior frontal and precentral regions (PFWE = 0.002), which remained significant after correction for confounders (PFWE = 0.040). After correction, an additional cluster in the left middle temporal and banks of the superior temporal sulcus regions showed significantly lower SPC values in the T1DM group compared with controls (PFWE = 0.013). No right hemisphere differences were found.
Furthermore, T1DM patients showed, in the uncorrected analysis, lower left thickness SPC values in a cluster of the superior parietal lobe extending into the inferior parietal lobe (PFWE = 0.025), and in a second cluster in the inferior parietal lobe (PFWE = 0.036). These clusters did not survive correction for baseline confounding (all PFWE > 0.05). In the right hemisphere, thickness SPC was lower in patients in a superior frontal, medial orbitofrontal, rostral middle frontal, and rostral anterior cingulate cluster (PFWE = 0.004). Although this cluster did not survive correction for confounding factors, a cluster in the caudal middle frontal, superior frontal, and precentral regions reached statistical significance (PFWE = 0.019).
Finally, left hemisphere volume SPC was, uncorrected for confounding, lower in patients than controls in a cluster comprising the pars orbitalis, rostral middle frontal, and lateral orbitofrontal gyri (PFWE = 0.001), which remained statistically significant after correction for confounding factors (PFWE = 0.003). In the right hemisphere, without correction for confounding, there was a cluster of lower SPC in the superior frontal, medial orbitofrontal, rostral middle frontal, and rostral anterior cingulate regions (PFWE < 0.001), as well as in the posterior and isthmus cingulate, paracentral and precuneus regions (PFWE = 0.035). After correction for confounding factors, a cluster in the caudal middle frontal, superior frontal, and precentral regions reached statistical significance (PFWE < 0.001).
Association with executive function decline and cortical structural
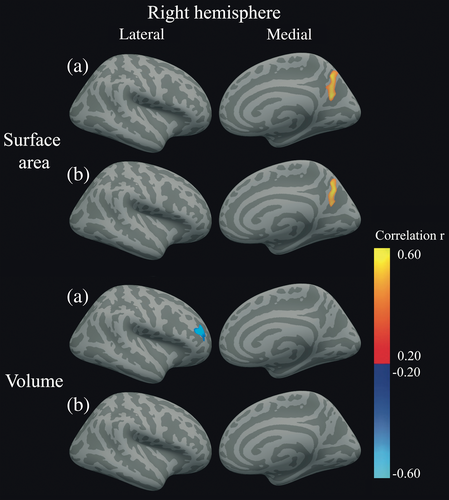
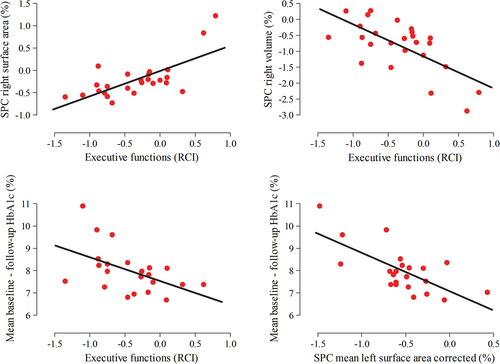
To lower the number of statistical tests, the SPC values of each cluster of surface area, thickness, and volume were averaged for the uncorrected and corrected analyses separately. The subsequent mean values were correlated with the RCI values of executive functions in all T1DM patients. Although the RCI values in the T1DM group were normally distributed, some SPC values were not. Thus, non-parametric Spearman's correlations were used. This did not yield any statistically significant correlations. A vertex-wise correlation analysis with executive functions RCI in T1DM patients showed, uncorrected for confounding, that lower RCI values (worse performance over time) was related to lower right precuneus and superior parietal surface area SPC (loss of surface area over time; PFWE = 0.005; Figs 2 and 3; Table 4) and to higher right rostral middle frontal volume SPC (increase of volume over time; PFWE = 0.049; Figs 2 and 3; Table 4). Only the precuneus and superior parietal cluster survived correction for baseline SBP and depressive symptoms (PFWE = 0.031; Table 4).
Correlation between cognitive and cortical changes and diabetes variables
Univariate Spearman's correlations were determined between executive function RCI, mean cortical SPC as explained above, HbA1c, and severe hypoglycemic events between baseline and follow-up. In all patients, lower executive functions RCI values (declining performance over time) was related to higher mean HbA1c levels between baseline and follow-up (r = −0.509, P = 0.016; Fig. 3). Higher HbA1c levels between baseline and follow-up were also related to lower SPC values of left surface area clusters (loss of surface area over time) in the corrected analyses (r = −0.555, P = 0.007; Fig. 3). There were no correlations with severe hypoglycemic events between baseline and follow-up.
Discussion
In these 25 patients with established proliferative retinopathy, executive functions declined moderately over 3.5 years, which was accompanied by loss of frontal cortical structure in particular. Although these cortical changes were unrelated to decline in executive function performance, loss of right precuneus surface area over time was related to lower executive performance over time. Both cognitive decline and loss of surface area were related to poorer glycemic control.
The moderate decline in executive functions may seem contradictory to the previous longitudinal studies showing declining processing speed.10, 12 However, meta-analyses of cross-sectional studies in T1DM have shown executive function decrements as well.2, 25 Furthermore, executive function decline is seen in normal aging,26 and the T1DM groups included in the previous studies were notably younger than the patient group in the present study and were not selected on the basis of proliferative retinopathy. Thus, the results of the present study may suggest early signs of aging in this selected group of patients. Furthermore, many tests for executive functions depend on timed responses,27 and the speed of information processing at baseline was affected in this group. Lower processing speed may have affected performance on tests of executive functions, which may provide another explanation for executive function decline in this group. Executive function decline was not related to insulin pump therapy or the use of antihypertensive medication use, although the latter was related to lower general cognitive ability and attention performance over time. Thus, there is no medication effect on the results of this particular study. However, for future larger studies, including the potential effect of antihypertensive medication on cognition is advised.
This decline in executive functions was accompanied by accelerated loss of frontal cortical structure, as well by some left temporal surface area, parietal thickness, and right posterior cingulate volume loss. In adult T1DM, this is, to the best of our knowledge, the first longitudinal study on cortical gray matter changes. Cross-sectionally, decreased frontal gray matter volume has been observed in T1DM patients who were, on average, of a similar age as the present group was at follow-up,28 which suggests mild but accelerated brain aging in T1DM. A study in healthy adults showed mild frontal cortical thinning during 3.5 years of follow-up, indicating that frontal thinning is part of normal aging,29 further supporting that frontal thinning could be a sign of early aging in T1DM. It is important to note that the cognitive decline and cortical changes found in the present study do not mirror the magnitude or the pattern of brain changes seen in neurodegenerative diseases.
Although executive functions are classically thought to be a frontal lobe-mediated process,27 more recent studies have shown that executive functions are also related to posterior regions, primarily the precuneus and other parietal regions,27, 30 which may have to do with the complex nature of the functions. In the present study, right precuneus surface area loss was moderately related to loss of executive functions over time. This particular part of the cortex had preserved surface area, but did show lower volume SPC, and was thus affected to a certain extend. Alternatively, the lack of other correlations may be related to the limited sample size or the executive function decline in this group may rely more on white matter integrity or functional connectivity, which was not studied here.
The correlation between higher HbA1c during follow-up, executive function decline, and structural cortical changes supports the notion that chronic hyperglycemic exposure negatively affects the brain in T1DM patients independent of the development of microvascular complications and is in line with previous research.9, 31 The Action to Control Cardiovascular Risk in Diabetes–Memory in Diabetes (ACCORD-MIND) trial in type 2 diabetes mellitus has shown that intensive blood glucose lowering was beneficial for slowing the rate of brain volume loss,32 although the effects on cognition were less convincing.33 Future research is needed to test whether stricter blood glucose control in T1DM will also yield positive results.
A limitation of the present study is the small sample size, which was due to funding limitations. However, with the present sample size we were able to detect differences with a relevant effect size. The results may not be generalizable to T1DM patients without retinopathy, and designing advanced regression models to determine predictors of change over time was not possible. Despite careful matching, SBP and depressive symptoms differed between groups and were thus used as confounding factors in the analyses. Neuropsychological tests are known to be sensitive to practice effects, especially in younger people,17, 27 and this has been observed in T1DM previously.10, 34 This could explain the positive changes in cognition seen on some domains in the present study.
To conclude, mild executive function decline and primarily left frontal cortical structure loss were found in middle-aged T1DM patients with established proliferative retinopathy, related to poorer glycemic control. These results may suggest early and accelerated aging related to chronic hyperglycemia, which keeps exerting its mild negative effects on the brain over time, although more research is needed in larger samples and more diverse groups.
Acknowledgements
The baseline study was funded by Grant 2005.00.006 of the Dutch Diabetes Research Foundation. The follow-up study was funded by a grant from the European Foundation for the Study of Diabetes (EFSD). RIJ received a personal grant from the Netherlands Organization for Scientific Research (NWO) Innovational Research Incentives Scheme Veni (No. 91613082). The sponsors had no role in study design, data collection, analysis, interpretation, or writing of the report.
Disclosure
The authors report no potential conflicts of interest.




