Journal list menu
Export Citations
Download PDFs
Cover Image
Cover Image, Volume 49, Issue 19
- Pages: i-ii
- First Published: 26 August 2011
The Diels-Alder “click” reaction has emerged as a powerful tool that allows facile assembly of various complex discrete macromolecular constructs. On page 4103, Gurkan Hizal, Umit Tunca, and Amitav Sanyal present well defined macromolecular building blocks incorporating diene and dienophile moieties that can be synthesized using a variety of living polymerization techniques. Oftentimes, macromolecular coupling via the Diels–Alder reaction proceeds under reagent-free conditions. Furthermore, appropriate choice of the diene–dienophile combination enables one to tune the assembly and/or disassembly of these macromolecular constructs over a wide range of temperature.
Inside Cover, Volume 49, Issue 19
- Pages: iii-iv
- First Published: 26 August 2011

Self-assembled films of well-defined olystyrene-b-poly(acryloxypropyltriethoxysilane)-b-polystyrene triblock copolymers are prepared by solvent casting, as presented by Emmanuel Beyou and colleagues On page 4193. An ordered nanostructure of alternating PS and PAPTES lamellae is observed regardless of the copolymer composition. The average d-spacing is estimated from the electron micrographs to be 34.3 nm for the PS272PAPTES130PS268 sample. Cross-linking of the inner PAPTES block is performed through hydrolysiscondensation of the ethoxysilyl groups in acidic medium and the formed hybrid structure is confirmed from the residual weight loss after removing the organic polymer by calcination at 800°C under argon. As shown by the SEM image, the lamellar shape of the PS272PAPTES130PS268 sample is retained after calcination with a dspacing close to that of the corresponding hybrid film.
Highlights
Discrete macromolecular constructs via the Diels–Alder “Click” reaction
- Pages: 4103-4120
- First Published: 11 July 2011
Articles
Bioinspired core-crosslinked micelles from thymine-functionalized amphiphilic block copolymers: Hydrogen bonding and photo-crosslinking study
- Pages: 4121-4128
- First Published: 12 July 2011
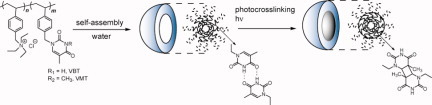
Bioinspired core-bound polymeric micelles, based on hydrogen bonding and photo-crosslinking, of thymine have been prepared from poly(vinylbenzylthymine)-b-poly(vinylbenzyltriethylammonium chloride). Micelle characterization, and critical micelle concentration measurements, and thermostability examination demonstrated that the hydrogen bonding and photo-crosslinking of the attached thymine units stabilizes the micelles.
Polyester from dimethylketene and acetaldehyde: Direct copolymerization and β-lactone ring-opening polymerization
- Pages: 4129-4138
- First Published: 12 July 2011
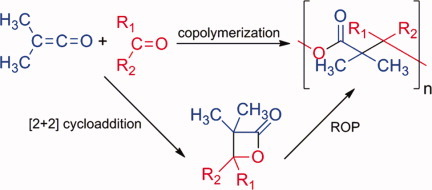
A new aliphatic polyester was synthesized from dimethylketene and acetaldehyde, by direct anionic copolymerization and by ring-opening polymerization of 3,3,4-trimethyl-2-oxetanone previously synthesized by [2+2] cycloaddition. Molecular weights up to 9000 g mol−1, with narrow polydispersity around 1.2, a thermal stability up to 274 °C, a fusion near 139 °C and a crystallinity close to 0.45 were obtained.
Macroinitiator halide effects in galactoglucomannan-mediated single electron transfer-living radical polymerization
- Pages: 4139-4145
- First Published: 18 July 2011
Synthesis and characterization of amphiphilic heterograft copolymers with PAA and PS side chains via “Grafting from” approach
- Pages: 4146-4153
- First Published: 18 July 2011
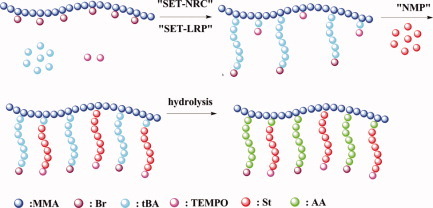
The amphiphilic heterograft copolymers P(MMA-co-BIEM)-g-(PAA/PS) were synthesized successfully by the combination of single -electron -transfer- living radical polymerization (SET-LRP), single- electron -transfer- nitroxide -radical -coupling (SET-NRC), atom transfer radical polymerization (ATRP), and nitroxide-mediated polymerization (NMP) via the “grafting from” approach.
RAFT kinetics revisited: Revival of the RAFT debate
- Pages: 4154-4163
- First Published: 22 July 2011
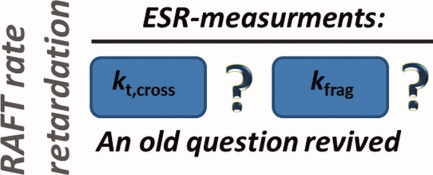
Recently, two ESR-based methods aimed at the determination of equilibrium constants of RAFT equilibria via instationary reaction kinetics yielding diametrically different results. Differences of more than six orders of magnitude are deduced, which cannot be explained on the basis of model-dependencies. Both experimental methods are reviewed, compared, and put into perspective of the RAFT debate, which is already ongoing since a decade raising the question whether retardation in RAFT polymerizations is caused by slow fragmentation or termination of intermediate radicals.
Control of stereospecificity in the radical polymerization of N-methyl-N-(2-pyridyl)acrylamide by conformational switching of the monomer with protonation
- Pages: 4164-4171
- First Published: 22 July 2011
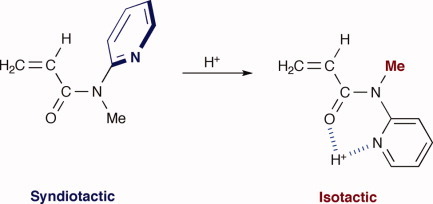
Radical polymerization of N-methyl-N-(2-pyridyl)acrylamide (MPyAAm) in CH2Cl2 was carried out at low temperatures in the presence of trifluoroacetic acid TFA. The stereospecificity was changed from syndiotactic to isotactic with an increase in the amount of added TFA. NMR analysis of the mixtures of MPyAAm and TFA suggested that the stereospecificity of the radical polymerization of MPyAAm was successfully changed by conformational switching of MPyAAm from s-trans to s-cis OCNPy with protonation.
Synthesis, characterization, and computational modeling of benzodithiophene donor–acceptor semiconducting polymers
- Pages: 4172-4179
- First Published: 22 July 2011
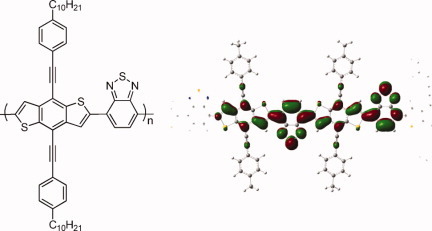
Six donor–acceptor semiconducting polymers containing benzodithiophene with 4-decyl phenylethynyl substituents have been synthesized. Changing the copolymer from donating thiophene and bithiophene to acceptor units of varying strength altered the experimental band gap from 2.3 eV to less than 1 eV. The density functional theory (DFT) calculations were performed for all the synthesized polymers. The good correlation between the experimental calculated band-gaps suggests that the tested density functionals will provide sufficient accuracy for computational pre-screening of novel benzodithiophene donor–acceptor polymers.
Synthesis of a novel macroinimer based on thiophene and poly(ε-caprolactone) and its use in electrochromic device application
- Pages: 4180-4192
- First Published: 22 July 2011
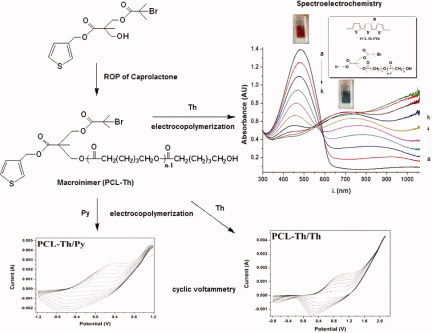
A novel macroinimer consisting of poly(ε-caprolactone), thiophene (Th), and tertiary bromide was prepared and employed used in electrochemical copolymerization with pyrrole (Py) and Th. PCL-Th/PTh copolymer film synthesized on indium tin oxide (ITO)-coated glass slide displayed electrochromic behavior. Optical analyses of the PCL-Th/PTh copolymer film indicated that the copolymer film was suitable to be used as an anodically coloring material for electrochromic device (ECD) applications.
Nanostructured organic–inorganic hybrid films prepared by the sol–gel method from self-assemblies of PS-b-paptes-b-PS triblock copolymers
- Pages: 4193-4203
- First Published: 22 July 2011
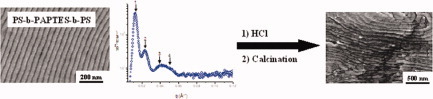
Well- defined polystyrene-b-poly(3-acryloxypropyltriethoxysilane)-b-polystyrene (PS-b-PAPTES-b-PS) triblock copolymers with molecular weights varying from 88,000g.mol−1 to 139,000 g .mol−-1 and various composition distributions were synthesized by nitroxide- mediated polymerization. The small angle X-ray scatteringSAXS and transmission electron microscopyTEM analysis provided evidence for the expansion of the interlamellar distance upon increasing the PAPTES block length, while maintaining constant the PS block size constant. All the acidic-treated triblock copolymers reported in this work showed enhanced mechanical properties after cross-linking of the PAPTES block as evidenced by an increase in the dynamic storage modulus.
Synthesis and characterization of new π-conjugated polymers containing 1,8-naphthyridine in the main chain: Role of the 1,8-naphthyridine unit in π-conjugated polymers
- Pages: 4204-4212
- First Published: 09 August 2011
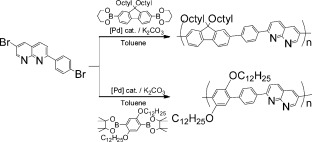
Alternating π-conjugated copolymers of 1,8-naphthyridine-2,6-diyl (1,8-Nap) with 9,9-dioctylfluorene-2,7-diyl (P(Flu-Ph-1,8-Nap)) and 2,5-didodecyloxy-1,4-phenylene (P(ROPh-Ph-1,8-Nap)) were prepared by Pd-catalyzed organometallic polycondensation. P(Flu-Ph-1,8-Nap) showed strong blue photoluminescence at λEM = 440 with a quantum yield of 87% in o-dichlorobenzene. When P(Flu-Ph-1,8-Nap) was treated with 10-camphorsulfonic acid (CSA), the emission peak shifted to λEM = 598 nm.
Synthesis of polybenzoxazine/clay nanocomposites by in situ thermal ring-opening polymerization using intercalated monomer
- Pages: 4213-4220
- First Published: 22 July 2011
Synthesis of multiarmed poly(3-hexyl thiophene) star polymer with microgel core by GRIM and ATRP methods
- Pages: 4221-4226
- First Published: 22 July 2011
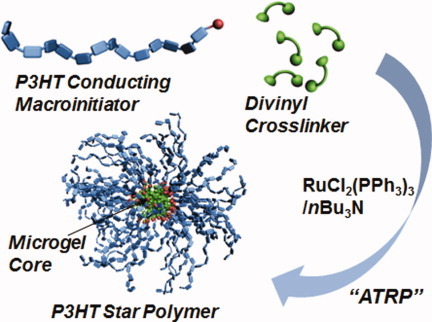
Relatively high molecular weight regioregular poly(3-hexyl thiophene) (rr-P3HT) was synthesized by the crosslinking reaction of the rr-P3HT macroinitiator with ethylene glycol dimethacrylate (EGDMA) crosslinker through Ru-based living radical polymerization. Obtained rr-P3HT star polymers consisted of several tens to hundreds of arms radiating from a microgel core, which strongly depended on the amount of EGDMA. In addition, crystalline structure originating from π–π stacking intermolecular interaction of conventional rr-P3HT was not observed in the star polymers due to their restricted arm chains.
Disproportionating versus nondisproportionating solvent effect in the SET-LRP of methyl acrylate during catalysis with nonactivated and activated cu(0) wire
- Pages: 4227-4240
- First Published: 18 July 2011
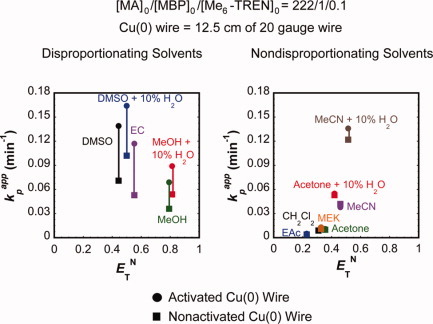
In disproportionating solvents, significant rate enhancement was achieved in the SET-LRP of MA upon switching from the catalysis with nonactivated Cu(0) wire coated with Cu2O to activated Cu(0) wire free of Cu2O. However, the positive rate acceleration was not observed for the SET-LRP of MA in nondisproportionating solvents. The negligible rate change, nonlinear first-order kinetics and progressive loss of bromide functionality provided by the polymerizations in nondisprortionating solvents confirm again that disproportionation is the key step in SET-LRP.
Acid dissolution of copper oxides as a method for the activation of Cu(0) wire catalyst for SET-LRP
- Pages: 4241-4252
- First Published: 18 July 2011
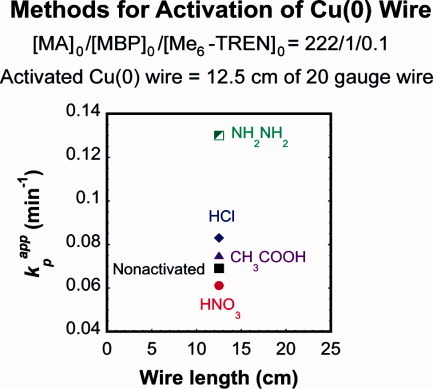
Commercial Cu(0) wire coated with copper oxides was activated via pretreatment with concentrated acids such as HNO3, glacial CH3COOH and HCl. In this process, the oxide layer was removed upon dissolution of copper oxides in acid. SET-LRP of MA catalyzed with Cu(0) wire activated using the acid dissolution method showed comparable kpapp value to that of the nonactivated Cu(0) wire-catalyzed polymerizations. Consistent to the results from hydrazine-activated Cu(0) wire experiment, the polymerizations proceeded with no initial induction period, with predictable molecular weight evolution with conversion, and narrow molecular weight distribution.
Spacer-length-dependent association in polymers with multiple-hydrogen-bonded end groups
- Pages: 4253-4260
- First Published: 26 July 2011
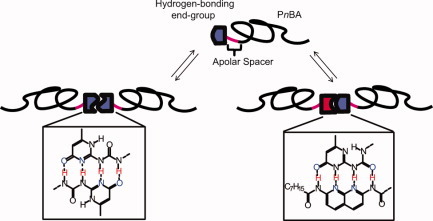
The influence of the aliphatic spacer length on the thermodynamics of end-functionalized hydrogen-bonding polymers based on poly(n-butyl acrylate) is investigated. It is found that short spacers decrease the dimerization constant of the UPy end-functionalized PnBA polymer as a result of competitive intramolecular noncovalent interactions. In contrast, when a longer aliphatic spacer separates the UPy end group from the PnBA polymer, the dimerization and heteroassociation strengths are largely unaffected.
Electron paramagnetic resonance measurement of trapped radical concentrations in frontally polymerized and bulk-polymerized multifunctional (meth)acrylates
- Pages: 4261-4266
- First Published: 22 July 2011

Radicals can be trapped in high concentrations during frontal and bulk polymerization of multifunctional acrylates and methacrylates. The number of radicals varies with functionality of the monomer. Radical concentrations from the frontal polymerization of trimethylolpropane trimethacrylate can be as high as 8 × 10−3 mol/kg.
Silole-containing polymers for high-efficiency polymer solar cells
- Pages: 4267-4274
- First Published: 28 July 2011
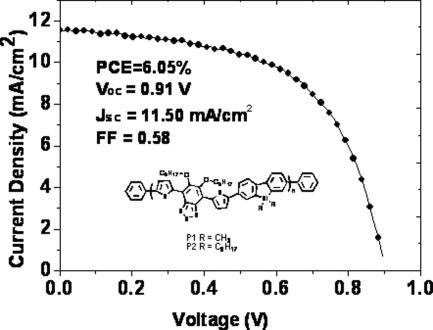
Two silole-containing conjugated polymers used for polymer solar cells, whose absorption spectra cover 300–700 nm, have been designed and synthesized using Suzuki–Miyaura–Schlüter polycondensation. The side chains on the silicon atom showed a marked influence on the performance of devices. Power conversion efficiencies of devices using P1/PC71BM and P2/PC71BM as active layers are 2.72 and 5.08%, respectively. After the device optimization, the best power conversion efficiency of 6.05% (Voc = 0.91 V, Jsc = 11.50 mA cm−2, FF = 0.58) has been obtained for P2 when using TiOx as the optical spacer.
Synthesis of poly(quinoxaline-2,3-diyl)s with alkoxy side chains by aromatizing polymerization of 4,5-dialkoxy-substituted 1,2- diisocyanobenzenes
- Pages: 4275-4282
- First Published: 26 July 2011
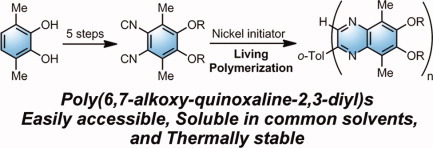
A facile synthesis of poly(quinoxaline-2,3-diyl)s bearing alkoxy side chains was established by using 4,5-dialkoxy-3,6-dimethyl-1,2-diisocyanobenzenes as monomers, which were easily accessible from 3,6-dimethylcatechol. The DSC traces of poly(quinoxaline)s were significantly dependant dependent on their side chains, although all polymers showed similar decomposition temperatures higher than 300 °C from TGA measurement.
Polymer brushes on multiwalled carbon nanotubes by activators regenerated by electron transfer for atom transfer radical polymerization
- Pages: 4283-4291
- First Published: 28 July 2011
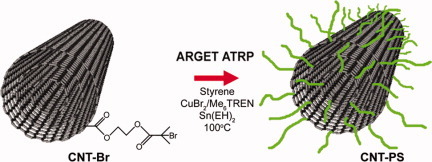
Multiwalled carbon nanotubes are surface functionalized with a tertiary bromide for use as a macroinitiator in activators regenerated by electron transfer in atom-transfer radical polymerization of polystyrene. This produced living polymer brushes of low polydispersity, with conductive properties and was well dispersed in a polymer composite.
Synthesis, molecular, and morphological characterization of initial and modified diblock copolymers with organic acid chloride derivatives
- Pages: 4292-4305
- First Published: 22 July 2011
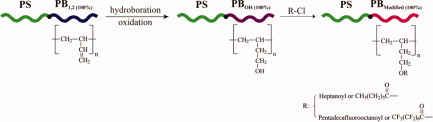
Well-defined PS-b-PB1,2 diblock copolymers with almost 100% of 1,2 geometric isomerism were synthesized to convert the polydiene segments with heptanoyl or/and pentadecafluorooctanoyl chloride through modification reactions to new chains. The importance of this work is the development of low-energy materials by the preparation of surfaces coated with large hydrocarbon groups and halogenated (chlorinated or fluorinated) side-groups, respectively.