Immune checkpoint inhibitor-related myocarditis in thymic epithelial tumors: Recent progress and perspectives
Jianqiong Yin and Zhuoran Yao have contributed equally to this work.
Abstract
Thymic epithelial tumors (TETs) are rare anterior mediastinal malignancies originating in the thymus with poor outcomes, and standard platinum-based chemotherapy shows limited efficacy for treating metastatic or recurrent disease. In this setting, further improved novel treatment strategies are needed. Immune checkpoint inhibitors (ICIs) are widely applied in clinical practice for cancer therapy and early results of clinical trials have brought notable objective responses and lasting survival benefits to patients with TETs. However, the incidences of immune-related adverse events (irAEs), especially cardiac adverse events, are higher than those of other tumor types. Myocarditis is a rapidly progressive and life-threatening irAE in patients treated with ICIs, thereby hindering the further utilization of ICI in TETs patients. Therefore, this article aims to review the results of case series and clinical trials that evaluated ICIs for the treatment of TETs and to provide an overview of the clinical features of fatal ICI-related myocarditis in TETs. Furthermore, we approach insights into the immunobiology of thymic tumors and focus on revealing the mechanisms of cardiotoxicity in patients with TETs, hoping to provide several valuable insights for maximizing the therapeutic potential of ICIs in TETs.
Graphical Abstract
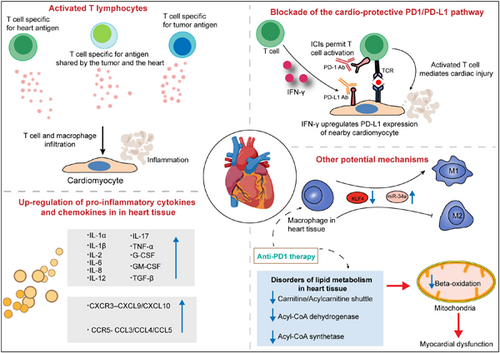
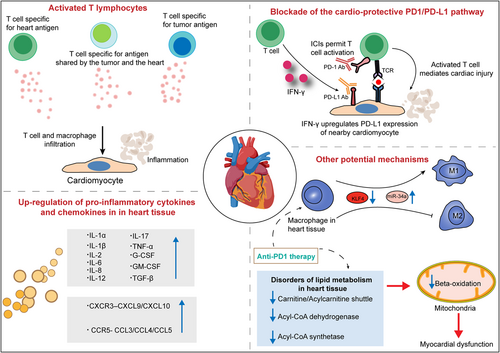
Potential mechanisms driving myocarditis associated with immune checkpoint inhibitor (ICI) therapy. Insights from studies of ICI-related cardiotoxicity suggest that various mechanisms contribute to the development of myocarditis, which includes activated T lymphocytes, pro-inflammatory cytokine storms in heart tissues, overexpressed chemokine axes, polarization of macrophages and disorders of lipid metabolism in heart tissues and so on.
1 INTRODUCTION
Thymic epithelial tumors (TETs), including thymic carcinoma and thymoma, are rare anterior mediastinal malignancies originating from thymic epithelial cells. The incidence of TETs is approximately 1.3–3.2 cases per million worldwide and is higher in China than in European and American countries.1 According to the World Health Organization (WHO) classification, TETs are classified as thymomas (WHO types A, AB, B1, B2, and B3) or thymic carcinomas (WHO type C) based on histological features.2
Surgery is the only curative treatment to achieve long-term survival for TET patients.3 For patients with early stage and localized disease, the standard of care is complete resection supplemented by postoperative treatment with adjuvant intent (radiation with or without chemotherapy). However, treatment is often challenging for clinicians because a subset of cases is often diagnosed with adjacent invasion or distant metastasis. For unresectable patients with advanced stage, metastasis, or relapsing disease, platinum-based multiagent chemotherapy is administered. Nevertheless, the clinical efficacy of chemotherapy is confirmed only in retrospective studies or small phase II trials, and the results revealed limited benefits to the outcome and efficacy, especially in refractory disease. Targeted therapies, such as receptor tyrosine kinase (c-KIT) inhibitors, epidermal growth factor receptor inhibitors, angiogenesis inhibitors, and insulin-like growth factor-1 receptor inhibitors have been evaluated as alternative therapeutic options for TETs.4, 5 However, targeted therapies have shown modest benefits in relapsed TETs.6, 7 Given the unsatisfactory responses to chemotherapy or targeted therapy in clinical settings, further improved novel treatment strategies are needed. The emergence of immunotherapy has ushered in a new era of cancer therapy. Immune checkpoint inhibitors (ICIs) such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1), and programmed death ligand 1 (PD-L1) inhibitors have become the most widely used forms of immunotherapy in the clinic and have brought obvious objective responses and lasting benefits to patients with a variety of cancers.8-10 Recent studies have reported that thymic epithelial cells express PD-L1 at a frequency of up to 70%.11 The high expression of PD-L1 in TETs suggests that anti-PD-1/PD-L1 agents could be a novel treatment in TETs.
Nevertheless, the clinical benefits have been accompanied by the development of immune-related adverse events (irAEs), particularly for TETs, which have been shown to have a higher incidence of irAEs than other tumor types, especially cardiac adverse effects.12 Myocarditis is a rapidly progressive and life-threatening irAE in patients treated with ICIs13 and was reported to have the highest fatality rate (52 [39.7%] of 131 reported cases).14 Furthermore, the myocardium possesses limited regenerative capacity to repair damage mediated by effector T cells. Therefore, in this article, we reviewed the results of case series and clinical trials that evaluated ICIs for the treatment of TETs and provided an overview of the clinical features of fatal ICI-related myocarditis in TETs. We discuss the underlying mechanisms, potential biomarkers, and current management strategies of ICI-related myocarditis, hoping to provide several valuable insights for maximizing the therapeutic potential of ICIs in TETs.
2 ICIS FOR TREATMENT OF TETS
The emergence of immunotherapy has revolutionized cancer treatments. Immunotherapy modalities include cancer vaccines, ICIs (anti-PD-1, anti-PD-L1, and anti-CTLA-4), and adoptive cell therapy, of which ICIs are the most widely used options. PD-1 and CTLA-4 are immunosuppressive molecules mainly expressed in immune cells such as T cells. PD-1 inhibits the activation of T cells by binding with PD-L1 ligand expressed on tumor cells. CTLA-4 exerts immunosuppressive effect by binding with B7-1 and B7-2 on antigen-presenting cells. ICIs activate T cells by blocking the PD-1–PD-L1 axis or CTLA-4 pathway, thus increasing antitumor immunity.15 The early results of clinical trials have confirmed the clinical activity of immunotherapy for TETs, but it has also been found that the incidence of irAEs, especially cardiac and neuromuscular adverse events, was higher than that of other tumor types, which leads to the practical application of immunotherapy being limited to studies in clinical trials and has not become the standard of care for patients with TETs. Therefore, larger clinical trials are urgently required to evaluate the therapeutic value and safety of these agents.16
2.1 PD-L1 expression of TETs
Although ICIs have achieved significant success in clinical application, the efficacy and responsiveness vary greatly between different tumor types and individual patients, which implies the importance of finding predictive biomarkers to maximize the therapeutic benefits.17 The expression of PD-L1 has become a widely explored predictive biomarker for predicting the response to anti-PD-(L)1 immunotherapies, and the overexpression of PD-L1 is significantly related to a better response in patients with a series of cancer types.18 The positive expression of PD-L1 is found in approximately 70% of thymic carcinomas and 20% of thymomas (types A, AB, and B).19 PD-L1 expression in more than 50% of tumor cells is defined as high PD-L1 expression, and in 23%–92% of thymoma cases, along with 36%–80% of thymic carcinomas, high expression of PD-L1 is observed.20-25 These studies implied that TETs demonstrated relatively abundant PD-L1 expression, which supports the potential clinical efficacy of ICIs in TETs. Giaccone et al.26 reported that patients with high PD-L1 expression had longer survival than patients with low or no PD-L1 expression. In summary, high PD-L1 expression in tumor cells seems to provide a rationale for the use of ICIs in TETs, and high PD-L1 expression appears to be associated with a better response.
2.2 Clinical trials evaluating ICIs for therapy of TETs
Recently, there have been multiple studies evaluating the efficacy of ICI therapies for advanced or relapsing TETs. Three phase II studies (two about pembrolizumab, one about nivolumab) and one phase I study about avelumab were discussed11, 26-28 (Table 1). These studies suggested that both PD-1 blockade (pembrolizumab, nivolumab) and PD-L1 blockade (avelumab) are active for patients with TETs. In addition, higher expression of PD-L1 was linked with better clinical activity to treatment with ICIs, both in thymic carcinomas and thymoma. However, a high incidence of grade 3–4 irAEs, especially musculoskeletal, neuromuscular, and cardiac irAEs, occurred with the application of ICIs in TETs, which are usually less common in other cancer types. Although the development of irAEs is related to better clinical outcomes after treatment with ICIs,29 the mechanisms of these serious adverse events, such as myocarditis, remain poorly understood. In addition, several clinical trials with ICIs alone or in combination with other agents to treat TETs are ongoing (Table 2). In light of this, further research should aim to identify those who are more likely to benefit from immunotherapy without the occurrence of serious irAEs such as myocarditis. Additional studies are also urgently needed to provide higher levels of evidence for immunotherapy in TETs.
| Author | Phase | TET in trial | Trial drug | N | ORR (%) | mPFS (mo) | mOS (mo) | irAEs G ≥ 3 (%) | Impact of high PD-L1 expression to response with ICI therapy | Incidence of myocarditis (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cho et al. | II | TC | Pembrolizumab | 26 | 19.2 | 6.1 | 14.5 | 15.4 | Good response | No | [11] |
| Cho et al. | II | T | Pembrolizumab | 7 | 28.6 | 6.1 | NR | 71.4 | Good response | 9.1 | [11] |
| Giaccone et al. | II | TC | Pembrolizumab | 40 | 22.5 | 4.2 | 24.9 | 15 | Good response | 5 | [26] |
| Rajan et al. | I | T | Avelumab | 7 | 57 | NA | NA | 38 | NA | NA | [27] |
| Katsuya et al. | II | TC | Nivolumaba | 13 | 0 | 3.8 | NR | 13 | NA | No | [28] |
- Abbreviations: G ≥ 3, Grade ≥ 3; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; mo, month; mOS, median overall survival; mPFS, median progression-free survival; N, number; NA, not available; No, none; NR, not reached; ORR, overall response rate; T, thymoma; TC, thymic carcinoma; TET, thymic epithelial tumor.
- a Study was closed prematurely and no responses were found.
| Trial (NCT) | Phase | Tumor type | Experimental drug | Estimated enrolment | Targeted outcomes of trial |
|---|---|---|---|---|---|
| NCT04925947 | II | TC | KN046 (PD-L1/CTLA4 bispecific single domain Fc protein antibody) | 29 | RR, Safety |
| NCT04710628 | II | TC, Type B3 T | Pembrolizumab and Lenvatinib | 43 | PFS |
| NCT04554524 | IV | TC, T | Pembrolizumab and Chemotherapy | 40 | ORR |
| NCT04469725 | II | TC | KN046 (PD-L1/CTLA4 bispecific single domain Fc protein antibody) | 66 | ORR |
| NCT04417660 | II | TC, T | Bintrafusp Alfa (M7824) | 38 | RR |
| NCT04321330 | II | TC | Atezolizumab | 34 | ORR |
| NCT03583086 | I/II | TC | Nivolumab and Vorolanib (VEGFR/PDGFR dual kinase inhibitor X-82) | 177 | Safety, ORR |
| NCT03463460 | II | TC | Pembrolizumab and Sunitinib | 40 | RR |
| NCT03295227 | I | TC, T | Pembrolizumab | 30 | DLT |
| NCT03134118 | II | TC, Type B3 T | Nivolumab | 55 | 6-month PFS |
| NCT03076554 | II | TC, T | Avelumab | 55 | Safety, RR |
| NCT02364076 | II | TC | Pembrolizumab and Epacadostat | 45 | RR |
| NCT04234113 | I/Ib | TC | Pembrolizumab and SO-C101 (IL-15/IL-15R α) | 96 | DLT, TRAE, SAE |
| NCT03858582 | II | TC, T | Pembrolizumab (neoadjuvant concomitant with CT and adjuvant) | 40 | MPR |
- Abbreviations: CT, chemotherapy; DLT, dose-limiting toxicity; MPR, major pathology response; NCT, National Clinical Trial; ORR, objective response rate; PFS, progression-free survival; RR, response rate; SAEs, serious adverse events; T, thymoma; TC, thymic carcinoma; TETs, thymic epithelial tumors; TRAE, treatment-related adverse events.
- Source: clinicaltrials. gov; last accessed: August 5, 2022.
3 CARDIAC IRAES ASSOCIATED WITH ICIS IN TETS
ICI-related cardiac toxicity is rapidly progressive and life-threatening despite corticosteroid therapy, in which myocarditis is one of the most commonly occurring cardiotoxicities in ICI treatment across various cancer types.30 Besides myocarditis, ICI-related cardiac toxicity also includes pericardial disease, congestive heart failure, conduction abnormalities, acute coronary syndrome, and Takotsubo-like syndrome. Although ICI-related cardiac toxicity is rare, it can be life-threatening.31 The incidence of ICI-induced myocarditis in non-TET patients is <1% for most cancers, but the fatality rate is the highest among all ICI-related adverse events (39.7%).14 Forty-six percent of patients with myocarditis after immunotherapy can even develop severe cardiovascular sequelae.32 The incidence of ICI-related myocarditis in patients with TETs is dramatically higher than that in patients with other types of cancers (5%–9.1% vs. 0.06%–1%), but the small trial sample size and potential differences in detection methods may not provide a complete understanding.11, 26, 33 Due to the high morbidity of immune-related myocarditis in TETs, a more thorough understanding of the mechanisms and clinical characteristics of immune-related myocarditis is urgently needed.
3.1 Incidence, risk factors, and timeline of ICI-related myocarditis in TETs
ICI-related myocarditis is more common in patients with TETs than in patients with other cancer types, with an incidence of 5%–9.1% according to the results from case reports and limited clinical studies.11, 26 Patients with thymoma seem to be more likely to develop ICI-related myocarditis than thymic carcinoma. Because patients with autoimmune disease are usually excluded from multiple clinical trials, the incidence of ICI-related myocarditis may be much higher in the real world. In addition to clinical trials, there are also several case series that have already described the development of fatal ICI-related myocarditis in TETs12, 34-38 (Table 3). Furthermore, the incidence of myocarditis was even higher upon combination therapy with anti-PD(L)1/anti-CTLA4 than monotherapy.32 There is a lack of well-defined descriptions with respect to the risk factors for ICI-related myocarditis, but patients with old age, male sex, pre-existing cardiac disease, obstructive sleep apnea, diabetes mellitus, higher body mass index, and anti-PD1/anti-CTLA4 combined therapy appear to be at a greater risk.32, 39 Moreover, the presence of other irAEs, such as myasthenia gravis (MG), peripheral myositis, and hepatitis, leads to an increased risk of ICI-related myocarditis, which suggests that the risk of myocarditis is related to immunological characteristics.40 The time to onset of ICI-related myocarditis is variable, and cases have been observed anywhere from 1 to 240 days from the beginning of therapy.41 Mahmood et al.32 reported that the median time to onset of clinical myocarditis was 34 days (interquartile range 21–75 days), and 81% of patients developed myocarditis within 3 months of starting treatment according to the data from a multicenter registry collecting 35 patients. According to the analysis of VigiBase (the World Health Organization's database with individual case safety reports), a median time to onset of 27 days (range, 5–155 days) was revealed among 101 cases of severe myocarditis, and 76% of cases occurred within 6 weeks of initiating treatment.42 Unfortunately, the time to onset of ICI-related myocarditis restricted to TETs remains poorly comprehensively analyzed in view of the relative rarity of TETs and limited case reports and small-sample retrospective analyses.
| Author | Patient (age/gender) | Cancer type | Drug | PD-L1 expression (%) | Onset days after the first dose | Clinical manifestations | Histology | Treatment | Outcome | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Szuchan et al. | 70/F | TC | Pembrolizumab | 70 | 21 days | Dyspnea, orthopnoea, weakness, right bundle branch block with elevated troponin, complete heart block | Mild myocyte hypertrophy(30–40 mm) | IV methylprednisolone switched to oral prednisone | Recovered | [12] |
| Abundant lymphocytosis | SC enoxaparin | |||||||||
| Lymphocytes and macrophages infiltrated in the myocardium | Oral aspirin | |||||||||
| Chen et al. | 43/M | Type B3 T | Nivolumab | NA | 10 days | Chest pain, dyspnea, fatigue, right bundle branch block, thickened interventricular septum (12 mm), low-normal ejection function of 49% | Myocyte necrosis | IV immunoglobulin | Death | [34] |
| Interstitial edema | High-dose IV methylprednisolone to reduce the dose gradually | |||||||||
| Lymphocytic infiltrates (predominantly T lymphocytes) | ||||||||||
| Hyun et al. | 45/F | Type B2 T | Pembrolizumab | NA | 15 days | Dyspnea on exertion, severe orthopnea, complete heart block with elevated cardiac markers (CK, CK-MB, BNP, troponin I), right and left bundle branch blocks, asystole, ventricular tachycardia | No biopsy | High-dose IV methylprednisolone | Death | [35] |
| Shen et al. | 53/F | Type B3 T | Pembrolizumab | 80 | 35 days | Chest congestion, bradycardia at night, complete right bundle branch block and T-wave inversion, elevated cardiac markers (CK, CK-MB, troponin T) | No biopsy | IV methylprednisolone pulse therapy | Recovered | [36] |
| Konstantina et al. | 58/F | Mixed B2/B3 thymoma | Pembrolizumab | >50 | 9 days | Thoracic pain, shortness of breath, extensive anterior myocardial infarction with high troponin level (>50), acute heart failure with impaired EF (<30%), diffuse hypokinesia of the heart | No biopsy | Beta-blocker, antiarrhythmics, mycophenolate mofetil | Death | [37] |
| Konstantina et al. | 30/F | Type B3 T | Pembrolizumab | 60 | 3 days | Acute chest pain, ischemic changes in ECG leads II, III and aVR, elevated CPK and troponin I | No biopsy | Oral prednisolone | Death | [37] |
| Liu et al. | 42/M | Type B3 T | Sintilimab | 99 | 16 days | Chest tightness, suffocation, pericardial effusion, elevated cardiac markers (CK, CK-MB, LDH) | No biopsy | Methylprednisolone | Death | [38] |
| Liu et al. | 52/M | Type B3 T | Tislelizumab | 55 | 20 days | Chest tightness, suffocation, complete right branch block, elevated cardiac markers (CK, CK-MB, LDH, BNP) | No biopsy | Methylprednisolone | Recovered | [38] |
- Abbreviations: BNP, brain natriuretic peptide; CK, creatine kinase; CK-MB, creatine kinase-MB; CPK, creatine phosphokinase; ECG, electrocardiogram; EF, ejection fraction; F, female; IV, intravenous injection; LDH, lactate dehydrogenase; M, man; SC, subcutaneous; T, thymoma; TC, thymic carcinoma; TETs, thymic epithelial tumors.
3.2 Overview of clinical manifestations and diagnosis
ICI-related myocarditis is graded from grade 2 to grade 4 according to the symptoms ranging from mild to serious, as suggested by the Common Terminology Criteria for Adverse Events Version 5, and ICI-related myocarditis in patients with TETs is generally grade 3 or even more serious (5%–9.1%)11, 19, 26, 43, 44 (Figure 1). Myocarditis accounts for more than 90% of reported cardiotoxicity in TETs in case reports or clinical trials. Furthermore, in TETs, myocarditis has been reported to be accompanied by the occurrence of other organ irAEs, especially those associated with skeletal muscles, such as myositis and MG.1 The clinical manifestation of myocarditis is highly variable and nonspecific and may manifest as nonspecific cardiovascular symptoms caused by ICI-related myocarditis, ranging from dyspnea, fatigue, chest pain, chest congestion, bradycardia, palpitation, syncope, dizziness, left ventricular systolic dysfunction, arrhythmia, myocardial infarction, and heart failure, which sometimes overlap with the common symptoms observed in primary cancer.45, 46

Due to a lack of consistency in the clinical performance of ICI-related myocarditis, the diagnosis is currently challenging and lacks specific guidelines. In patients with suspected cardiotoxicity, the diagnosis of ICI-related myocarditis usually requires a combination of multiple imaging, laboratory tests, and histopathological evidence.47 Imaging tests typically include electrocardiogram,33, 48-50 echocardiogram, and cardiac magnetic resonance imaging,51-53 whereas laboratory tests mainly include a series of serum biomarkers, such as troponin (cardiac troponin I [cTnI] or troponin T [cTnT]), brain natriuretic peptide (BNP), N-terminal (NT) pro-brain natriuretic peptide, and creatine kinase (CK).54-56 Nevertheless, only an endomyocardial biopsy (EMB) is the gold standard for the diagnosis of ICI-related myocarditis.57 Bonaca et al.58 proposed an approach to characterize the degree of certainty and severity of ICI-related myocarditis according to a combined result of multiple imaging, laboratory tests and histopathological evidence, which divided the diagnosis into three categories: definite myocarditis, probable myocarditis and possible myocarditis.
3.3 The mechanisms of ICI-related myocarditis in TETs
3.3.1 Failure of central and peripheral immune tolerance to the heart
The thymus is an immune organ located in the anterior region of the mediastinum that is mainly composed of thymocytes, lymphocytes and epithelial mesenchymes and plays a vital role in the development of central immune tolerance and the production of functional T cells.59 The thymus is responsible for normal T cell maturation, in which cell progenitors undergo maturation through interactions with cortical and medullary thymic epithelial cells. During the cell progenitors' migration through the thymic cortex and corticomedullary junction, thymocytes that survive after interaction with major histocompatibility complex class II (MHC II) expressed on thymic epithelial cells undergo “positive selection” and enter the thymic medulla. In addition, functional T cell receptors are generated after a series of phenotypic modifications.60 Next, in the thymic medulla, T cells (autoreactive T cells) that interact with tissue-specific self-antigens (TSAs) with a high affinity undergo apoptosis, which is called the “negative selection” process.61 Thymocytes with an intermediate affinity for TSAs differentiate into Foxp3 + T regulatory cells (Tregs). Medulla mainly includes medullary thymic epithelial cells (mTECs) and dendritic cells. mTECs express various TSAs, and the expression of TSAs is tightly controlled by autoimmune regulator genes (AIREs) and forebrain-expressed zinc finger 2 (Fezf2).62, 63 AIRE plays a pivotal role in T cell negative selection and the formation of Tregs and is highly expressed in mTECs as a transcription factor.64, 65 mTECs expressing AIRE renew rapidly and eventually suffer cell death (apoptosis), releasing TSAs into thymic dendritic cells, and then dendritic cells present antigens to developing thymocytes, resulting in T cells that react with TSAs experiencing apoptosis, thus ultimately mediating the development of immune tolerance.66, 67 Therefore, the process of T cell maturation requires normal thymic architecture (cortex and medulla), expression of major histocompatibility complex (MHC) class and AIRE.63, 68 The key role of the thymus in maintaining central autoimmune tolerance explains the higher incidence of irAEs in patients with TETs compared with other cancer types because TETs are the most common neoplasm arising from the thymus. Although the thymic function decreases with age, the continuous thymogenesis in the residual thymus tissue still plays an important role in the production of new CD8+ T cells. In addition, research shows that the remaining thymic activity can continue and affect T cell responses after the seventh decade of life, so that age-associated thymic degeneration will not cause clinical adverse events in healthy individuals. These results indicate that the thymus still plays an important role in central immune tolerance in adults, even the elderly. In TETs, normal thymic architecture is disrupted, and thymic epithelial cells possess downregulated MHC II complex and AIRE expression.69 Moreover, the application of ICIs may lead to the loss of central immune tolerance of the thymus by changing the mechanism of thymic epithelial cell death.19 Consequently, the downregulated expression of AIRE in mTECs leads to the absence or low expression of some TSAs; for example, α-myosin heavy-chain (α-MyHC) is not expressed in the thymus, and even healthy individuals have relatively high frequencies of α-MyHC-specific CD4+ T cells in peripheral blood.70 T cells that fail to interact with absent TSAs, including myocardial antigen-specific naïve T cells, will escape into the circulation (Figure 2). It was found that cardiac-myosin-specific autoimmune CD4+ and CD8+ T cells increased significantly in the hearts of mice with ICI-related myocarditis.71 And cardiac-myosin-specific CD8+ cytotoxic T cells were found in the heart of patients with ICI-related myocarditis.72
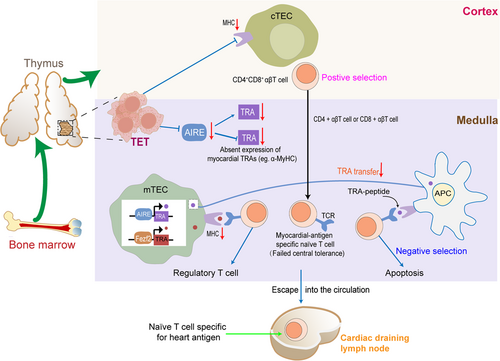
Failure of central immune tolerance to the heart. T cell progenitors located in the bone marrow leave the bone marrow and migrate to the thymus. In the thymus, T cell progenitors undergo maturation by interacting with cortical and mTECs. However, the occurrence of TETs in the thymus leads to the destruction of normal thymic architecture and downregulated expression of the MHC II and AIRE. Consequently, the downregulated expression of AIRE in mTECs leads to the absence of the expression of some TSAs including myocardial TRAs, such as α-MyHC. T cells that fail to interact with absent TRAs, including myocardial antigen-specific T cells, will escape the negative selection of the thymus and mature into naïve T cells. These autoreactive naïve T cells specific to heart antigens will be released into the periphery and recirculate through cardiac draining lymph nodes. α-MyHC, α-myosin heavy-chain; AIRE, autoimmune regulator gene; MHC, major histocompatibility complex; mTEC, medullary thymic epithelial cell; TETs, thymic epithelial tumors; TRAs, tissue-restricted antigens; TSAs, tissue-specific self-antigens.
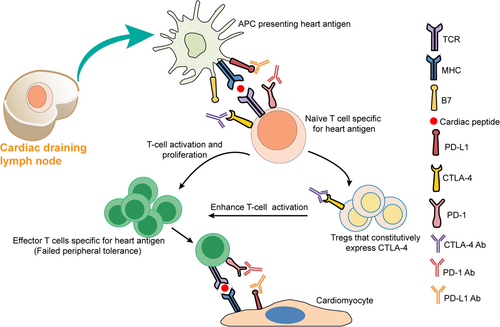
Some autoreactive T cells that escape into the circulation may further escape impaired “peripheral tolerance” mechanisms caused by various factors, such as PD-1/PD-L1 pathway blockade by PD-1/PD-L1 inhibitors, reduced threshold of T-cell activation due to anti-CTLA-4 administration and upregulated costimulators on DCs induced by injury to heart tissues, which ultimately result in an increased risk of irAEs related to effector T cell activation.73 Moreover, CTLA-4 blockade may deplete Tregs that constitutively express CTLA-4 through antibody-dependent cell-mediated cytotoxicity (ADCC) and cellular phagocytosis (ADCP) activity, resulting in impaired suppressive function of Tregs and enhanced activation of cardiac antigen-specific T cells.74-76 The application of ICIs breaks peripheral immune tolerance, which may cause cardiac antigen-specific naïve T cells to be activated and differentiate into amplified clones of effector T cells, further mediating myocardial damage77 (Figure 3). Although the exact mechanisms underlying the development of irAEs such as myocarditis in TETs remain unclear, one possible explanation is that the damage to the thymus induced by tumor growth diminishes its ability to maintain central self-tolerance. In addition, it is possible that the application of ICIs in TETs could enhance the loss of peripheral immune regulation, resulting in treatment with ICIs being related to a high rate of irAEs in TET patients.78 Ji et al.79 performed RNA-sequencing (RNAseq) analysis on heart tissues from monkeys receiving combined therapy with ipilimumab/nivolumab and found significantly upregulated genes related to the activation and migration of T cells and antigen presentation in the heart, which also suggests impaired peripheral immune tolerance to the heart.
3.3.2 Blockade of the cardioprotective PD1/PD-L1 pathway and infiltration of immune cells in the heart
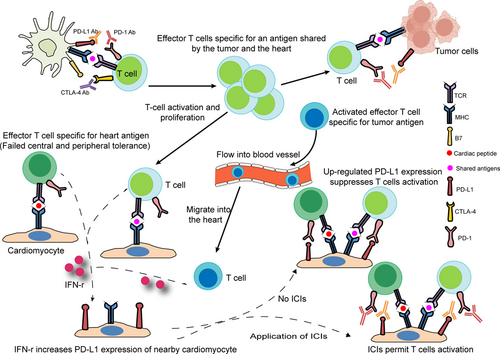
The PD-1/PD-L1 pathway has been reported to directly cause T cell exhaustion and promote the formation of an immunosuppressive microenvironment, eventually contributing to autoimmunity prevention. High expression and excessive activity of this pathway suppresses the function of T cells and immunity, especially in a wide variety of human cancers.80, 81 Similar to tumor cells, cardiomyocytes might also employ the PD-1/PD-L1 pathway to protect the myocardium from T cell injury under physiological conditions. Several studies have identified the constitutive expression of PD-L1 in human and murine myocytes.82-84 Moreover, a high level of PD-L1 is reported in normal human organs such as skeletal muscle, placenta and lungs, as well as in the heart.85 Grabie et al.86 also showed that the upregulation of PD-L1 in the myocardium is obvious in patients with idiopathic lymphocytic myocarditis and in cancer patients treated with ICIs. A profoundly upregulated expression of PD-L1 on cardiac endothelial cells from tumor-bearing mice receiving anti-PD1 was found in a recent study.40 In a report of a patient with type B3 thymoma who developed fatal myocarditis during nivolumab therapy, myocardial and muscle biopsies revealed strongly expressed PD-L1 within the impaired myocardial regions.34 In addition, under inflammatory conditions triggered by several cytokines, especially IFN-γ, PD-L1 protein expression is induced in a subset of human and mouse endothelial cells, including cardiac endothelial cells.87, 88 In contrast to the pro-inflammatory effects of IFN-γ in the majority of tissues, IFN-γ has been shown to mediate anti-inflammatory effects in cardiac inflammation.89-91 The present data indicate that the expression of PD-L1 induced by IFN-γ in cardiac endothelial cells is another significant mechanism to protect the heart from excessive inflammation. Various mouse models of T cell-dependent myocarditis exist. Grabie et al.92 have shown that during cytotoxic T lymphocyte-induced myocarditis, increased expression of PD-L1 in the heart is dependent on T cell-derived IFN-γ, and IFN-γ signal blockade significantly aggravates cardiac injury. Moreover, the genetic deletion of PD-L1/PD-L2 or treatment with PD-L1 blocking antibody can mediate more severe cardiac inflammation accompanied by higher levels of blood cTnI and increased numbers of polymorphonuclear leukocytes (PMNs), which suggests that PD-L1 plays a critical role in protecting the myocardium from injury and the vital role of T cell–derived IFN-γ in this regulatory pathway. Lethal autoimmune myocarditis occurred in lupus-susceptible (MRL) mice in which PD-L1 was deleted.93 In another study, the role of the PD-1/PD-L pathway in the development of murine acute myocarditis caused by coxsackievirus B3 was investigated. The study showed that in vivo PD-L1 blocking antibodies could significantly increase myocardial inflammation as well as the expression of IFN-γ, FasL, CD40 L, perforin, and viral genomes in myocardial tissue in mice, which suggests that PD-L1 plays a key role in suppressing cardiac infection.94 Nishimura et al.60 reported that disruption of the gene encoding PD-1 in BALB/c mice caused the development of dilated cardiomyopathy, which indicates that PD-1 may be an important factor contributing to the prevention of autoimmune diseases. Similarly, MRL mice developed fatal myocarditis and produced antibodies specific for cardiac myosin due to PD-1 deficiency.95 PD-1–deficient mice displayed increased inflammation, enhanced serum markers of myocardial damage, and increased infiltration of inflammatory cells, which also implied the vital role of PD-1 in protecting against inflammation and myocyte damage.96 Based on previous observations, the PD-1/PD-L1 pathway plays an important role in regulating immune homeostasis within the myocardium. PD1 signaling suppresses autoreactive T cells in heart tissues by maintaining T cells in an anergic state.97 Johnson et al.13 also found increased expression of PD-L1 in the injured myocardium, but not skeletal muscle cells or tumor cells, in two patients with fulminant ICI-related myocarditis. In the same study, the authors showed that two autopsied patients with fulminant ICI-related myocarditis possess identical CD4+ and CD8+ T cell clones in the myocardium and in tumors. Furthermore, high levels of muscle-specific antigens such as desmin and troponin were observed in tumors from both patients. These findings imply that the occurrence of myocarditis is mediated through several possible mechanisms, including T cells targeting an antigen shared by the tumor and the heart or the same T cell receptor targeting a tumor antigen and a different but homologous muscle antigen. To deliver sufficient oxygen and nutrients to myocytes, the myocardium develops a dense microvascular network, which gives effector T cells a chance to migrate to the uninflamed heart.86 The histopathological analysis of EMB reveals that the myocardium of patients with fulminant ICI-related myocarditis mainly infiltrates CD4+, CD8+ T lymphocytes, and CD68+ macrophages.98
In summary, treatment with anti-PD-1 and anti-PD-L1 antibodies in various human tumors can obviously activate the immune system, resulting in an increase in T cells specific for tumor antigens as well as T cells that target shared antigens by the tumor and the heart. Subsequently, activated T cells observed in the blood of cancer patients can migrate into inflamed myocardium, through the dense microvascular network.86 While PD-L1 expression in the myocardium is subsequently upregulated to curtail T cell-mediated inflammation by suppressing T cell activation and resistance to the killing of antigen-specific effector T cells, ICIs abrogate its immunosuppressive function, resulting in the occurrence of myocarditis (Figure 4).
3.3.3 Regulation of pro-inflammatory cytokines and chemokines
Cytokines, a class of small molecular proteins approximately 5–20 kDa in size, are mainly synthesized and secreted by various immune cells. Multiple cytokines possess strong inflammatory activities, such as stimulating the recruitment of immune cells, regulating innate immunity and adaptive immunity, differentiation and effector functions, and participating in inflammation. Cytokines are also thought to be involved in the pathophysiological mechanisms of irAEs.99, 100 It has been reported that some cytokines are involved in myocarditis, cardiovascular disease, and heart failure.101, 102 However, few studies have reported whether these circulating cytokines have a role in the development of ICI-related cardiotoxicity. The application of ICIs excessively activates the immune response, which may trigger inflammatory storms to attack the heart and then cause the occurrence of myocarditis. Indeed, a study reported that the levels of inflammatory cytokines, such as tumor necrosis factor (TNF), interleukin-2 receptor (IL-2R), and IL-6 significantly increased in a patient diagnosed with grade 3 ICI-related myocarditis, which revealed that the cytokine response may be involved in the development of ICI-related myocarditis.103 Tsuruda et al.104 reported increased levels of several cytokines, such as IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor (GM-CSF), in different stages in three patients who developed cardiac complications after ICI treatment. In addition, IL-8 was the most predominant cytokine among the three patients. Quagliariello et al. showed that nivolumab or ipilimumab significantly increased the levels of pro-inflammatory cytokines in human cardiomyocytes, including IL-1α, IL-1β, IL-6, IL-8, IL-12, IL17-α, TNF-α, G-CSF, GM-CSF, and transforming growth factor-β. In contrast, the levels of IL-4 and IL-10 with anti-inflammatory properties were significantly decreased. The authors speculated that ICIs may induce pro-inflammatory cytokine storms in heart tissues attributed to cytotoxic effects mediated by the NLRP3/IL-1β and MyD88 pathways.105 NLRP3 (inflammasome) is involved in Toll-like receptor type 4 activation and strictly participates in heart failure, metabolic endotoxemia, and viral myocarditis caused by anthracycline drugs. MyD88 (Myddosome), a complex of proteins, is involved in inflammation, arthritis, severe gout, myocarditis, and heart failure through the activation of activating protein-1 and NF-kB.105 In another study that aimed to reveal the pathomechanism of ICI-related myocarditis by characterizing transcriptional changes, Finke et al.106 found that GBP5, a class of inflammasome-regulating proteins responsible for assembling the NLRP3 inflammasome, was significantly upregulated in cardiomyocytes of ICI-related myocarditis patients.
C–X–C motif chemokine receptor 3 (CXCR3) can mediate the activation, differentiation, and recruitment of CD4+/CD8+ T cells.107-109 C–C chemokine receptor type 5 (CCR5) can recruit CD8+T cells by responding to chemokine ligands such as C–C chemokine ligand 3 (CCL3) and CCL4.110 Increased serum levels of C–X–C motif chemokine ligand 10 (CXCL10) were observed in a metastatic melanoma patient with ICI-related myocarditis. CXCL10, as a chemokine ligand of CXCR3, can recruit and traffic activated T cells both to sites of the tumor microenvironment and to active inflammation sites.111 The expression of the CXCR3 chemokine axis (CXCL9, CXCL11, and their receptor CXCR3) was overexpressed in ICI-related myocarditis heart tissues.112 In a monkey model characterized by multiple organ toxicities, including ICI-related myocarditis, gene expression analysis showed that the expression of multiple chemokine receptors increased in monkey heart tissues, including CXCR3–CXCL9/CXCL10 and CCR5/CCL5 chemokine axis molecules.79 In summary, the increased expression of chemokines in the heart can promote T cells to traffic into heart tissues, which may ultimately mediate the development of myocarditis by excessive infiltration of T lymphocytes in the heart.
3.3.4 Other potential mechanisms
Several studies have reported that interpatient heterogeneity of the gut microbiome plays a role in the development of irAEs, particularly gastrointestinal toxicity such as colitis.113-115 However, it remains to be investigated whether the gut microbiota is involved in the pathogenesis of ICI-related myocarditis. After treatment with ICIs, leukotriene B4, which can cause atherosclerosis and fibrosis, and inflammatory prostaglandin precursors are overexpressed in cardiomyocytes, suggesting that leukotrienes play a role in ICI-induced cardiotoxicity. Although the exact mechanism of leukotriene overexpression is not totally understood, researchers speculated that lymphocytes and macrophages infiltrating myocardial tissue may increase phospholipid oxidation by producing reactive oxygen species, consequently activating the arachidonate-leukotriene pathway.105 Xia et al.116 found that a PD-1 inhibitor exerted its effect in mediating inflammation of the murine heart by polarizing macrophages into the M1 pro-inflammatory phenotype by upregulating the expression of miR-34a and downregulating the expression of Krüppel-like factor 4 (KLF4). MicroRNA-34a (miR-34a), an endogenous, small, noncoding RNA, is abundant in mammalian cardiac tissues and can mediate the development of immune-related myocarditis by modulating macrophage polarization.117, 118 KLF4, a DNA-binding transcriptional regulator and a target of miR-34a, may extenuate ICI-induced cardiotoxicity by suppressing M1 macrophage polarization in heart tissues.119 In a recent study, broad changes in lipid metabolism in the heart were found during the early anti-PD1 therapy stage in an established murine melanoma model, which included significantly enriched substrates for beta-oxidation, reduced carnitine/acylcarnitine carrier protein, acyl-CoA dehydrogenase, and acyl-CoA synthetase. Disorders of lipid metabolism in heart tissues can affect the production of cellular energy, cause left ventricular dysfunction, and even cause myocarditis.40 Taken together, the above results indicate that the mechanisms of ICI-related myocarditis are linked with various possible factors, and there are still multiple challenges about the specific mechanisms of ICI-related myocarditis (Figure 5).
3.4 Current approaches to the management of ICI-related myocarditis
The main treatments for ICI-related myocarditis currently include ICI cessation, systemic glucocorticoid administration, additional immunosuppressive strategies, and conventional cardiac treatment.120-123 The main conventional cardiac therapy is antiheart failure, such as the use of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, and defibrillation according to different presentations.124, 125 Oncologists and cardiologists need to collaborate closely to manage ICI-related myocarditis, especially with regard to the timing of discontinuing immunotherapy. Considering the high mortality of ICI-related myocarditis, most of the guidelines strongly recommend immediate discontinuation of ICI therapy for all grades of myocarditis. Once myocarditis is strongly suspected or definitely diagnosed, glucocorticoids should be rapidly initiated as the first-line therapy. The data from two registries suggest that high-dose steroids (1–2 mg/kg prednisone) are associated with a better treatment response.32, 126 The duration of administration depends on the clinical response and troponin levels, which usually last for 3–6 months because of the high risk of death.14, 121 If steroids fail, further optimal subsequent therapy should be considered. Other potential treatment options include antithymocyte globulin, intravenous immunoglobulin, infliximab, mycophenolate, alemtuzumab, abatacept, and plasmapheresis.123 The application of these agents is mostly recommended based on results from reported case series, and the efficacy in ICI-related myocarditis is still not fully clear. The immunosuppressive mechanisms of these drugs are different; for example, abatacept, as a CTLA-4 agonist, may lead to the normal immune response being rapidly inactivated, and alemtuzumab, as a monoclonal antibody, can destroy peripheral immune cells by binding to CD52 on some immune cells.127, 128 Moreover, infliximab is associated with an increased risk of worsening heart failure, so it must be given extreme caution, especially in patients with heart failure.129
4 POTENTIAL PREDICTIVE BIOMARKERS OF ICI-RELATED MYOCARDITIS
Currently, predictive biomarkers of ICI-related myocarditis mainly include serum biomarkers. Significantly increased levels of troponin have been observed in patients with ICI-related myocarditis.130 The degree of troponin elevation is positively correlated with the degree of myocardial injury. B-type natriuretic peptide (BNP) is a marker of wall stress, which plays an important role in predicting the diagnosis and prognosis of myocarditis but may be nonspecifically elevated in patients with cancer.131, 132 Mahmood et al.32 conducted a retrospective case–control study involving 35 patients with ICI-related myocarditis. They found that troponin was elevated in 94% of patients with ICI-related myocarditis, and 66% had elevated NT pro-BNP. It has been reported that CK-MB on presentation is a reliable biomarker for predicting the progression and mortality of ICI-related myocarditis.133 Furthermore, Tsuruda et al. observed a significantly increased level of IL-8 in three patients with ICI-related myocarditis, indicating that IL-8 may be a predictive biomarker for the severity of ICI-related myocarditis.104 To date, research on biomarkers that predict ICI-related myocarditis is still very scarce.46 Thus, more large-scale clinical trials are needed to explore biomarkers that can effectively predict the development, diagnosis, and prognosis of ICI-related myocarditis.
5 SUMMARIES AND FUTURE EXPECTATIONS
The development of ICI therapy has ushered in a new era of cancer therapy. Multiple clinical trials have confirmed the clinical benefits of ICI therapy for advanced or relapsing TETs. However, a higher incidence of irAEs, especially rapidly progressive and life-threatening myocarditis, has been observed in TETs than in other tumor types. Insights from studies of the pathophysiology of ICI-related cardiotoxicity suggest that various mechanisms contribute to the development of myocarditis (Figure 5). However, the mechanisms of ICI-related myocarditis are not entirely clear. Many questions remain: (a) How does the unique biology of the thymus affect the central immune tolerance of myocardial antigen-specific T cells? (b) How do heart self-antigens trigger an immune response? (c) What role do immune checkpoints play in cardiac immune homeostasis? (d) What types of activated immune cells can infiltrate the myocardium, and how do these cells migrate to the heart? (d) What immune cell types, pro-inflammatory cytokines, and chemokines are involved in the pathogenesis of ICI-related myocarditis? (e) How does ICI-induced disorder of metabolism in heart tissue affect cardiac function?
Further research is crucially needed to understand the underlying mechanistic pathways of ICI-related myocarditis in TETs. Furthermore, genetic, environmental, clinical, and immunological risk factors, as well as available novel biomarkers for predicting ICI-related myocarditis, also need to be clearly elucidated to achieve early detection and diagnosis of ICI-related myocarditis and obtain a better prognosis. Myocardial biopsy is often difficult to obtain, and the diagnostic protocols of ICI-related myocarditis still need improvement according to more extensive clinical data. It is still necessary to conduct in-depth research on the mechanism of immune-related myocarditis and further explore molecular, gene, and metabolic-related mechanism pathways. Future studies should also focus on exploring potential new intervention targets, such as the intervention of the signaling pathway between the immune system and the cardiovascular system, to block the interference of the immune system activated by ICI treatment with the normal cardiovascular system. For ICI therapy of TETs, how to balance the antitumor effect of ICIs and the high risk of immune-related myocarditis in patients with TETs still needs to be further explored in the future. More clinical trials are still needed to evaluate the efficacy and safety of combinations of ICIs with other anticancer therapies for TETs, including chemotherapy, radiotherapy, and targeted therapy. In addition, novel ICIs targeting other molecules, such as LAG-3, TIM-3, TIGHT, VISTA, and dual targets, also need to be further explored for their efficacy and safety for treating TETs.
AUTHOR CONTRIBUTIONS
Jianqiong Yin and Zhuoran Yao wrote the manuscript and prepared the figures and tables. Jing Pan provided language editing. Lu Gan revised and polished the manuscript. Jianxin Xue conceived the idea for this review article. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21003), National Natural Science Foundation of China (No. 81872478), and the Outstanding Youth Talent Foundation for Science and Technology of Sichuan Province (2022JDJQ0056).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.