The landscape of TIGIT target and clinical application in diseases
Shu Rui and Xiangyu Kong contributed equally to this study.
Abstract
Immune checkpoint blockade has dramatically altered the concept of cancer therapeutics over the past few years. Beyond the existing classical pathways, novel immune checkpoints, such as T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif (ITIM) structural domain (TIGIT), have also emerged in recent years and have promising therapeutic potential. Recent researches have provided ample evidence that TIGIT is extensively involved in various cancerous and noncancerous diseases such as chronic inflammation, autoimmune diseases, abnormal pregnancy status, and most recently coronavirus disease 2019. In contrast to the programmed cell death receptor 1 pathway which primarily affects T-cell function, targeting TIGIT pathway regulates multiple types of immunocytes but has fewer immune-related adverse events. Owing to its unique advantages and extensive involvement in diseases, extensive clinical trials blockade TIGIT or combine it with other targets are ongoing, and numerous phase II clinical trials have already seen promising results. In this review, we summarized the existing research on TIGIT in various diseases and discussed the perspective and challenges related to targeting this molecular for therapy, with an attempt to provide directions for subsequent studies.
Graphical Abstract
T-cell immunoglobulin and ITIM structural domain (TIGIT), a novel immune checkpoint, is widely expressed in a variety of immune cells. Recently studies have shown that TIGIT is extensively involved in various cancerous and noncancerous diseases such as chronic inflammation, autoimmune diseases, and abnormal pregnancy status. Thus, targeting TIGIT could be an effective immunotherapy option in the future.
1 INTRODUCTION
Immune system has the ability to recognize tumor cells and limit their growth, while tumors are capable to escape immune surveillance. Tumors activate a plethora of immunosuppressive mechanisms including antigen presentation impairing, negative costimulatory signals activating, and immune tolerance inducing. Also, tumor-induced immunosuppressive factors paralyze T cells to reprogram the tumor microenvironment (TME). At the same time, tumor cells also activate the immune checkpoint pathway to evade immune surveillance.1, 2 The immune checkpoints interact with their ligands to inhibit the function of a variety of immune cells such as natural killer (NK) cells and T cells, thereby suppressing antitumor immunity.
Despite the tumor, immune system also plays a critical role in noncancerous diseases. In autoimmune diseases, self-reactive T cells mediate autoimmune disease. When these T cells escape central and peripheral tolerance mechanisms, autoimmune disease arises.3, 4 In viral infections, the ongoing chronic stimulation makes T cells enter a state of dysfunction, which is also known as T cell exhaustion. Exhausted T cells highly upregulated coinhibitory receptor expression levels, lost proliferative capacity, and displayed a defective pro-inflammatory cytokine response.5
Over the past few years, immunotherapy has emerged as a promising treatment for cancer. To date, clinical benefit has been shown for many human solid tumors targeting immune checkpoints such as programmed cell death receptor 1 (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). However, durable response to these therapies is restricted to a subset of patients, and initial and acquired resistance remains a challenge.6 Therefore, new therapeutic approaches are urgently needed for patients who do not respond to or who acquired resistance to the existing immune checkpoint blockade therapies.7 TIGIT, a novel coinhibitory molecule, was discovered in 2009.8 Since its discovery more than a decade ago, TIGIT has been studied extensively in various malignant tumors. Also, the TIGIT pathway was associated with multiple immune-related processes owing to its role in regulating immune response, such as autoimmune diseases, viral infections, maternal-fetal exchange, and pregnancy-related diseases, and we have even observed changes in its expression on different lymphocytes in coronavirus disease-2019 (COVID-19).
To solve the dilemma faced by existing immunotherapy, many clinical trials targeting novel immune checkpoints are underway, trying to complement the existing treatments and find new approaches to the therapies. To date, the results of numerous preclinical experiments and published clinical trials suggested that TIGIT would be a suitable target for cancer and other diseases. Furthermore, because TIGIT is widely expressed on different types of immune cells, it may play a role in a variety of physiological or pathological processes mediated by immune cells. As research on autoimmune-related diseases, chronic infections, and pregnancy immune-related diseases develop, the potential value of targeting TIGIT to treat these diseases may be of greater interest and anticipation. This review systematically describes the structure, pathways, mechanisms of action, and relevant findings of TIGIT in different physiological or pathological processes, summarizes the therapeutic advantages of TIGIT as an immune checkpoint, and outlines some of the clinical studies currently underway and possible future research directions.
2 THE CHARACTERISTICS OF TIGIT
2.1 Structure, expression, and ligands
TIGIT is a transmembrane glycoprotein consisting of an extracellular Ig variable region, a type 1 transmembrane region, a cytoplasmic tail containing an immunoreceptor tyrosine-based inhibitory motif (ITIM), and an Ig tail tyrosine (ITT)-like motif.8-13 It is highly expressed on various types of immune cells (Table 1).8 Numerous studies have proven that TIGIT and several other molecules act together to form an immune regulatory axis and lead to stimulatory or inhibitory signals, despite some mechanisms of the pathway that has been not fully understood yet. To date, the exact involvement of CD155, CD112, Nectin-4, CD226, TIGIT, CD96, CD112R, CD113, CD111, and the bacterial ligand Fap2 in this regulatory axis has been identified (Figure 1), which we will describe in detail below.
| Class | TIGIT | CD96 | CD226 | CD112R |
|---|---|---|---|---|
| Expression | ||||
| CD4+ T cell | + | + | + | − |
| CD8+ T cell | Dysfunctional T cells | + | + | + |
| NK cells | + | + | + | + |
| Dendritic cells | − | − | − | − |
| Monocytes/macrophages | − | − | + | − |
| Memory B cells | + | |||
| Signaling motifs | ITT-like, ITIM | ITIM, YXXM | ITT-like | ITIM |
| Ligands | CD112, CD155, CD113, nectin-4, Fap2 protein | CD155, CD111 | CD112, CD155 | CD112 |
- Abbreviations: ITIM, immune receptor tyrosine-based inhibitory motif; ITT, Ig tail tyrosine; TIGIT, T-cell immunoglobulin and ITIM structural domain.
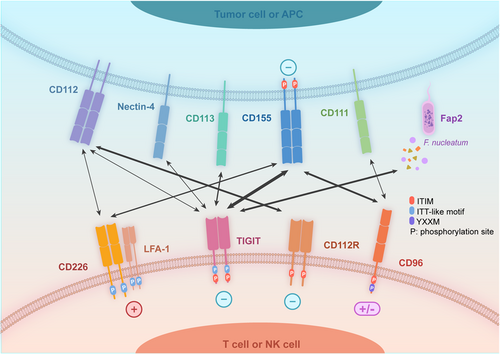
TIGIT binds to CD155 and CD112 to deliver inhibitory signals, with a higher affinity to the former.14-17 CD155 is lowly expressed in normal human cells such as dendritic cells (DCs), endothelial cells, but highly in tumors such as lung cancer, melanoma, and so on. In 2020, Adi Reches et al. first reported that Nectin-4 could bind specifically to TIGIT and inhibit the activity of NK cells.18 Besides, CD113 is also a ligand for TIGIT but their interactions are unclear8 currently.
CD226, which expresses on T cells, monocytes, and NK cells,19 can compete with TIGIT and bind to CD155 and CD112 to transmit the costimulatory signals11, 13, 20 by enhancing the cytotoxicity of NK cells21 and synergizing with lymphocyte function-associated antigen-1 (LFA-1) to regulate T-cell functions.22 Besides, CD96 (also known as TACTILE) can also bind to CD155 with a higher affinity than CD226 but weaker than TIGIT.8, 23 Compared to TIGIT, CD96 possesses a more complicated extracellular structure including three Ig-like structural domains.24 A YXXM motif of CD96 indicated a potential ability for activation,24 which is confirmed by several studies.25, 26 Furthermore, CD112R was also a novel immune checkpoint on human T cells27 and NK cells,28 it binds to CD112 and plays a nonredundant synergistic role with TIGIT in immunosuppression.29
Combining the existing studies, we conclude that TIGIT, CD96, CD112R, Nectin-4, and CD226 could bind to the same ligands but opposite functions.30 Compared with the well-defined classical CD28/CTLA4-CD80/CD86 pathway31 which only regulates T-cell activation, CD226/CD96/TIGIT/CD112R/Nectin-4/CD155/CD112 pathway can affect not only T cell but also NK cell functions,30 suggesting the regulatory ability in both innate and acquired immunity.
2.2 Mechanisms of action
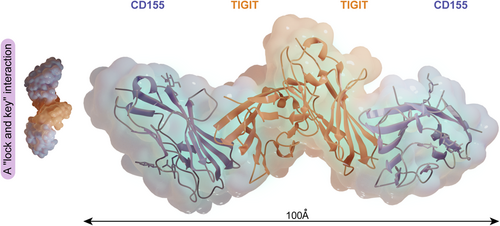
Analysis of the crystal structure revealed that the TIGIT/CD155 interface displayed a “lock and key” interaction. A heterotetrameric structure consisting of a TIGIT homodimer and a CD155 homodimer is essential for inhibitory signal generation (Figure 2).32 Both TIGIT and CD155 contain an ITIM in the cytoplasmic tail,8 suggesting the ability to transmit inhibitory signals in the corresponding cells. The current uncovered mechanisms of TIGIT/CD155-mediated coinhibitory signaling included: direct intracellular signaling, reduction of costimulatory signaling, and cell-extrinsic signaling cascades (Figure 3).
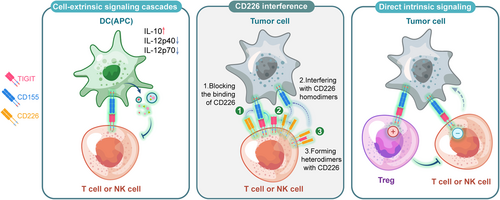
2.2.1 Direct intracellular signaling
Numerous studies have confirmed that the TIGIT pathway impedes NK and T cells function directly.
In NK cells, the phosphorylation of Tyr225 on the ITT-like motif through the TIGIT/CD155 binding may be the primary mechanism, while the ITIM motif meditation is the minor.33, 34 Phosphorylation of the ITT-like motif resulted in the recruitment of SHIP1 through bridging molecule Grb2, which further blocked PI3K and MAPK signaling, leading to reduced cytotoxicity and cytokine secretion.33 Additionally, it could also recruit SHIP1 through bridging molecule β-arrestin2, impairing the auto-ubiquitination of tumor necrosis factor receptor-associated factor 6 (TRAF6) to eliminate NF-κB activation, then causing a reduction of IFN-γ production.5 Both above suggested that SHIP1 is a key molecule in NK cells after the TIGIT/CD155 binding, which was also validated by SHIP1 silencing experiment.5 TIGIT can also directly induce T cell suppression by blocking T cell activation, proliferation, and acquisition of effector functions.35 Nevertheless, unlike the classical coinhibitory receptor PD-1 which interferes with signaling cascade downstream of the TCR in T cells, previous studies do not provide a clear view of TIGIT-mediated signaling cascade.35, 36 The mechanism of TIGIT/CD155 in T cells is still vague, probably by downregulating the expression of TCR alpha chains to disrupt the TCR complex, besides, reducing the TCR induced phosphorylation of ERK and production of IFNγ may also be involved.9, 35, 37
2.2.2 Reduction of CD226 costimulatory signaling
This effect is mainly composed of three ways. First, TIGIT has a higher binding affinity to CD155 compared with CD226, so it can inhibit CD226 costimulatory signals in a dose-dependent manner. Second, it can interfere with CD226 to disrupt its homodimerization. Thirdly, TIGIT can bind to CD226 to form heterodimers and these three ways may work separately or together to reduce the CD226 costimulatory signals in different diseases.
2.2.3 Cell-extrinsic signaling cascades
Confined in DCs, the ITIM of CD155 was phosphorylated after linkage to TIGIT, then the ITIM domains recruited SHP-2, resulting in activation of the Erk/MAPK signaling cascade.38 This modulated the response of CD155-expressing antigen-presenting cells (APCs), bringing as a result of a shift to a tolerogenic phenotype in mature DCs, which create more anti-inflammatory cytokine (IL-10 etc.) but less pro-inflammatory cytokine (IL-12p40 and IL-12p708 etc.).
Notably, there is more than one mode of involvement of TIGIT in a pathological process, and different modes located on different cells work together to regulate the immune responses and thus influence the progression of diseases, as elaborated below.
3 TIGIT IN MALIGNANT TUMORS
Tumor cells constantly interact with their microenvironment. TME promotes tumor angiogenesis and induces immune tolerance by releasing cell signaling molecules, while tumor-infiltrating lymphocytes (TILs) can influence cancer cell growth and development.39 A brief summary of the immune process in tumors is briefly stated as follows: innate immune NK cells attack tumor cells, DCs capture antigens from dying cancer cells, activated DCs migrate to lymph nodes to infiltrate T cells, activated T cells migrate back to the tumor and recognize peptide-MHCs to kill tumor cells, and the cycle can continue again.40
Nevertheless, TILs are exposed to a variety of immunosuppressive mechanisms, including negative regulatory pathways and upregulation of immune checkpoints on TILs, rendering them functionally inert or “depleted.”41, 42 Current research suggests that immune checkpoint blockade (ICB) therapy reactivates an effective antitumor immune response by infiltrating immune cells in the tumor and that the composition of the TME affects the response to ICB therapy.43
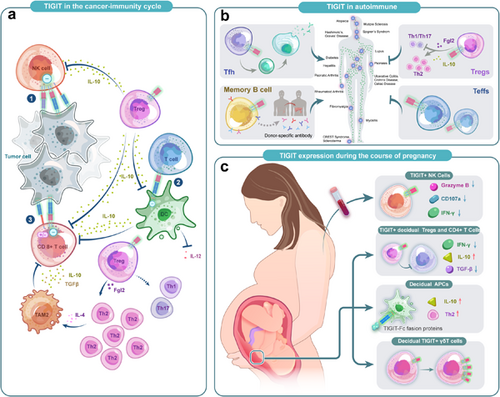
Recent researches have provided ample evidence that expression of TIGIT upregulates in TILs.44 It has been observed in NK cells, CD8+ T cells, and regulatory T cells (Tregs) of both mice and human.45-49 TIGIT may play a role in all steps of the cancer immune cycle (Figure 4).
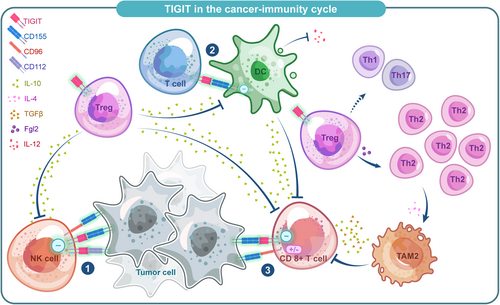
3.1 NK cells
The expression of TIGIT was upregulated on NK cells in animal models as well as various human malignancies and may be associated with a poor prognosis.34, 50 In two tumor-bearing mouse models, TIGIT was associated with NK cell exhaustion characterized by less production of IFN-γ, and blocking TIGIT prevented the depletion and enhanced innate immunity through an NK cell-dependent manner.5, 50 Besides, in patients with multiple cancers such as chronic myelogenous leukemia, myelodysplastic syndromes, soft tissue sarcoma, and osteosarcoma, TIGIT expression was significantly upregulated in peripheral blood NK cells51-53 and associated with a worse prognosis, suggesting that targeting TIGIT is an effective therapeutic strategy to enhance innate antitumor immunity and complement existing treatments.
Notably, several research groups have found that CD96, which also binds to CD155, acted as a suppressor in both mouse and human NK cells. However, limited studies also observed that CD96 could enhance NK cell functions in human malignancies,25, 26, 54 possibly owing to the coexistence of activating and inhibitory sequences of CD96 in humans.
3.2 Tumor-infiltrated CD8+ T cells
High expression of TIGIT was also seen on CD8+ TILs in both animal models and patients with solid tumors such as nonsmall cell lung cancer (NSCLC), melanoma, papillary and anaplastic thyroid cancer.13, 53, 55-60 By reducing proliferation and production of effective cytokines, the antitumor response was diminished. In some nonsolid tumors such as lymphoblastic leukemia and follicular lymphoma patients,37, 61 results as previously described were also observed. High endogenous expression of TIGIT on CD8+ TILs reduced secretion of IFN-γ and IL-17 and suggested that TIGIT blockade could restore the effector function of CD8+ T cells. This was also confirmed by the fact that in acute myeloid leukemia patients, siRNA knockdown of TIGIT could result in a significant increase in TNF-α and INF-γ and reduced sensitivity in CD8+ T cells to apoptosis.62
Additionally, several researchers have found a decreased expression of CD226 along with the expression of TIGIT in CD8+ T cells,55, 62 thus a reduction of the CD226−mediated costimulatory signals. Also, TIGIT expression in Tregs and APCs could alter the TME, thereby indirectly inhibiting the antitumor response of CD8+ TILs. Similar to NK cells, CD8+ TILs were recently found accessible to both costimulatory and coinhibitory signals from CD96,63, 64 which needs further research to explain the mechanism.
3.3 Treg cells
A high proportion of Tregs was found in the TME in different types of tumors, which was characterized by highly upregulated TIGIT expression.45-48 Compared with TIGIT− Tregs, TIGIT+ Tregs upregulated several Treg-associated markers which are essential to the differentiation of naive T cells to Tregs phenotype. Several studies have verified that TIGIT+ Tregs selectively inhibit Th1/Th17 rather than Th2 immune responses via FGL2,12, 65, 66 leading to polarization of type 2 tumor-associated macrophages (TAM2),12 which deprived nutrients in the TME and suppressed T cell functions. Meanwhile, TIGIT+ Tregs can also reduce the ability of CD155 to bind CD226 on CD8+ Teffs and NK cells,67 thus affecting the Teffs and NK cell-mediated antitumor responses. Notably, it was recently found that a higher ratio of TIGIT/CD226 on Tregs was significantly associated with poor clinical outcomes toward ICB therapy.68 Further researches should be carried out to illuminate the potential value of TIGIT/CD226 ratios in predicting ICB therapy clinical response.17
3.4 DCs
The binding of TIGIT to DCs can induce phosphorylation of the inhibitory ITIM motif of CD155 on DCs, triggering a signaling cascade that promotes a tolerogenic phenotype of DCs characterized by increased interleukin (IL)-10 secretion.8 As an immunosuppressive cytokine, IL-10 directly acts on T cells to suppress their proliferation and production of pro-inflammatory cytokines such as IFN-γ.8 Besides, DCs were also regulated by TIGIT+ Tregs to have a tolerogenic phenotype. This mechanism plays a synergistic effect with direct TIGIT/CD155 signaling in DCs to form a positive feedback loop amplifying a suppressive DC phenotype, inhibiting the generation of effector T cell responses.67
3.5 Bacterium-dependent mechanism of immune escape in tumor
Unexpectedly, TIGIT has been reported to be involved in a bacterium-dependent tumor-immune evasion mechanism.69 Fusobacterium nucleatum, an intrinsic oral bacterium, is a common anaerobic Gram-negative bacterium in the TME and negatively correlates with T cell density in colorectal cancer tissues, suggesting a possible role of this bacterium in tumorigenesis.70 Gur et al. found that the functions of TIGIT−expressing NK cells and T cells could be inhibited when the Fap2 protein of F. nucleatum is bound to TIGIT, revealing a new bacterium-dependent mechanism of immunosuppression.69
To sum up, TIGIT may involve multiple immune components of the antitumor immune responses, and its expression on various types of immune cells determines the involvement in innate and adaptive immunity. It suggested targeting TIGIT is a promising therapeutic strategy for cancers.
4 UNIQUE ADVANTAGES OF TIGIT BLOCKADE AND CLINIC TRIALS
The approved anti-PD-1 or anti-CTLA-4 therapies exhibit significant side effects, like autoimmune syndromes.71, 72 The next generation of immune therapeutic targets, like TIGIT, represent a broader specturum of immune cell-regulation, tissue-specificity, and lower toxicity (Figure 5).
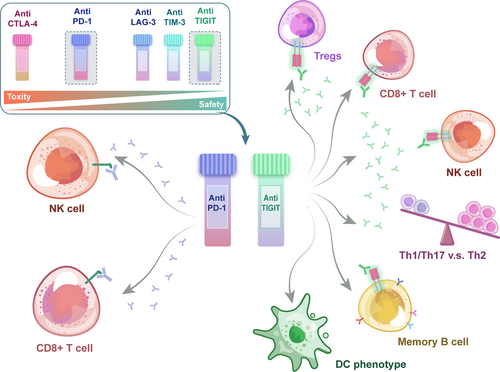
4.1 Broader specturum of cell-regulation
Kurtulus et al. found that TIGIT deficiency in Tregs was sufficient to retard tumor growth and could increase IFN-γ, TNF-α, and IL-2 productions by CD8+ TILs in an experimental model,45 indicating that TIGIT expression in Tregs may perform a more prominent function in suppressing antitumor immune responses compared with it in CD8+ T cells. One possible explanation is that both CD96 and TIGIT are expressed on CD8+ Teffs, and the absence of TIGIT on the cells may also be replaced by CD96, whereas in Tregs, the lack of CD96 expression leads to a more nonredundant regulatory role of TIGIT.67 Someone suggested that Tregs are of utmost significance in the initial phases of tumor development, as they initiate an immunosuppressive landscape during tumor development.73 Some researchers have found that Tregs promoted dysfunctional phenotype of CD8+ TILs by modifying the TME.74, 75 However, TIGIT expression on CD8+ T cells may have a more pronounced effect in the established tumors. Kurtulus et al.45 found TIGIT+ CD8+ TILs were not only poor mediators of tumor clearance, but also contributed to immune suppression locally in the TME.
Meanwhile, two recent studies have provided evidence for TIGIT expression in NK cells-mediated tumor metastasis.76, 77 Besides, recently additionally several studies on TIGIT in B cells also suggest its possible involvement in some B cell-mediated immune processes.78-80 As elaborated, in contrast to the PD-1 pathway, the involvement of TIGIT in NK cells and B cells makes it possible to regulate innate immunity, adaptive immunity, and humoral immune, which functions as a nonredundant complement to PD-1 inhibition.
4.2 Higher tissue specificity and safety
CTLA-4 and PD-1 stand for first line of immune checkpoints and are primarily responsible for maintaining self-tolerance and restriction of T cells, the absence of which may lead to severe autoimmune reactions, whereas the deficiency of TIGIT and several other molecules does not, as their suppressive capabilities are just apparent in a susceptible background or actively induced diseases. Notably, compared to others, a unique regulatory role of TIGIT is to alter the balance between type 1/17 and type 2 immunity.81 Furthermore, TIGIT seems to be expressed more frequently in TME cells than that in peripheral, theoretically providing well-targeted treatment to reduce systemic autoimmune toxicity.82 For example, TIGIT−/−35 or CD96−/−83 mice have not shown significant spontaneous development of immunopathology, which is distinct from ICB targeting CTLA-4 and PD-1. Nevertheless, it has also been shown that TIGIT−/− mice are more sensitive to the induction of experimental autoimmune encephalomyelitis (EAE)35 and TIGIT blockade could increase the development of experimental arthritis,9 suggesting the results of animal experiments need to be interpreted cautiously.
4.3 Coblockade of TIGIT and other immune checkpoints and clinic trials
TILs usually upregulate TIGIT expression together with PD-1, TIM-3, and LAG-3,84 and coblockade of TIGIT and other checkpoints such as PD-1,55 PD-L1,13, 85 and TIM-345 have been shown to specifically enhance T cell function. While every single blockade did not significantly hinder colorectal tumor growth in mice, TIGIT and PD-1 inhibition together synergistically enhanced the proliferation and the antitumor function of CD8+ TILs and prolonged overall survival.13, 50, 86
Several recent studies have shed light on the mechanism of dual blockade of the PD-1 and TIGIT coinhibitory receptors in antitumor therapy. In preclinical tumor models, Banta et al. found that PD-1 inhibited phosphorylation of both CD226 and CD28 on CD8+ T cells via its intracellular effectors such as SHP-2 phosphatase, which was recruited by the PD-1 ITIM-containing intracellular domain, whereas TIGIT restricting CD226 costimulation by blocking interaction with CD155.87 Therefore, coblockade of TIGIT and PD-1 is required to completely restore CD226 signaling, resulting in optimal antitumor CD8+ T-cell responses.87 Other researchers have established hepatocellular carcinoma (HCC) mouse models and found that the use of PD1 mAb simultaneously increased the expression of immune checkpoint molecules, including TIGIT, which may thereby lead to a depleted state of CD8+ T cells and ultimately to resistance to PD1 mAb. Moreover, high levels of TIGIT expression in peripheral blood T cells of HCC patients unresponsive to PD1 mAb have also been observed. Targeted blockade of TIGIT may sensitize HCC to PD1 mAb, considering that TIGIT is associated with PD1 mAb resistance88 and coblockade an optimal therapy. Recently, a preclinical study found that CD155/TIGIT axis played an important role in mediating immune evasion of pancreatic cancer, and coblockade of TIGIT/PD-1 coupled with CD40 agonist produced a strong antitumor response.89 Based on the results of some animal models, clinical trials on the combination of PD-1/PD-L1 and TIGIT blockade are being widely conducted in recent years. Moreover, recently a phase I trial about a recombinant humanized TIGIT×PD-L1 bispecific antibody in advanced solid tumors was started (NCT05102214) and we are looking forward to its findings.
Additionally, a prerequisite for PD-1/TIGIT coblockade for tumor therapy may be the high expression of CD226 on lymphocytes. Previous studies found that PD-1/TIGIT coblockade was abolished when CD226 was blocked, indicating that ICB targeting TIGIT may function mainly via CD226 costimulatory signals.13, 55, 90 Jin et al.91 verified that CD226hi CD8+ T cells are required in a blockade of TIGIT pathway. Moreover, they also found that CD226+ CD8+ T cells in patients with pancreatic carcinoma might potentiate the effects of TIGIT or PD-1 blockade.91 Nevertheless, CD8+ TILs tend to decrease the expression of CD226 in tumors, partly limiting the effectiveness of coblockade of PD-1 and TIGIT.55, 62 So we expected the combination therapy (TIGIT blocker + CD226 agoist) in clinical trials.
Given the emerging advantages of TIGIT and ample preclinical evidence, many pharmaceutical agencies have set up clinical trials of anti-TIGIT mAbs as single agents or in combination in multiple types of carcinomas in recent years, the current (by 2022) drugs entering clinical trials include 18 drugs, with a total of 109 programs, and 17 of them have entered Phase III clinical trials in various solid tumors (Table 2). Here, we summarized the related clinical trials.
| Target | Drug (manufacturer) | Drug type | NCT number | Cancer types | Phase | Combinations |
|---|---|---|---|---|---|---|
| TIGIT | BMS-986207 (Bristol Myers Squibb) | TIGIT blocking human IgG1 mAb | NCT04150965 | Multiple myeloma with relapse | I/II | BMS-986207 or Relatimab (anti-LAG-3) or Elotuzumab (anti-SLAMF7) + Potomalidimide + Dexamethasone |
| NCT04570839 | Advanced solid tumor | I/II | BMS-986207 + COM701 (anti-PVRIG) + Nivolumab (anti-PD-1) |
|||
| NCT02913313 | Broad solid tumor | I/II | BMS-986207 + Nivolumab (anti-PD-1) or BMS-986207 + Nivolumab (anti-PD-1) + Ipilimumab (anti-CTLA-4) or BMS-986207 alone |
|||
| NCT05005273 | Nonsmall cell lung cancer | II | BMS-986207 or placebo + Nivolumab (anti-PD-1) + Ipilimumab |
|||
Ociperlimab, BGB-A1217 (BeiGene) |
TIGIT blocking humanized IgG1 mAb | NCT04047862 | Metastatic solid tumors | I | Ociperlimab + Tislelizumab (anti-PD-1) | |
| NCT04693234 | Cervical cancer | II | Ociperlimab + Tislelizumab (anti-PD-1) or Tislelizumab alone |
|||
| NCT04866017 | Nonsmall cell lung cancer | III | Ociperlimab + Tislelizumab (anti-PD-1) + Chemotherapy or combinations without Ociperlimab | |||
| NCT04732494 | Esophageal squamous cell carcinoma | II | Tislelizumab (anti-PD-1) + Ociperlimab or Placebo |
|||
| NCT04952597 | Limited stage small cell lung cancer | II | Ociperlimab + Tislelizumab (anti-PD-1) + Chemoradiotherapy or Tislelizumab (anti-PD-1) + Chemoradiotherapy or Chemoradiotherapy alone |
|||
| NCT04746924 | Nonsmall cell lung cancer | III | Ociperlimab + Tislelizumab (anti-PD-1) or Pembrolizumab (anti-PD-1) + Placebo or Tislelizumab + Placebo |
|||
| NCT05014815 | Nonsmall cell lung cancer | II | Ociperlimab or Placebo + Tislelizumab (anti-PD-1) + Chemotherapy | |||
| NCT04948697 | Advanced hepatocellular carcinoma | II | Ociperlimab + Tislelizumab (anti-PD-1) + BAT1706 or BGB-A1217 + BAT1706 |
|||
| Advanced biliary tract carcinoma | II | Ociperlimab + Tislelizumab (anti-PD-1) + Chemotherapy gemcitabine | ||||
| NCT05267054 | Large B-cell lymphoma | I/II | Ociperlimab + Tislelizumab (anti-PD-1) or Ociperlimab + Rituximab |
|||
| NCT05023109 | Biliary tract carcinoma | II | Ociperlimab + Tislelizumab + Chemotherapy gemcitabine | |||
| NCT05577702 | Nonsmall cell lung cancer | II | Tislelizumab + _Ociperlimab or Tislelizumab + LBL-007 or Tislelizumab alone |
|||
| Tiragolumab, MTIG7192A (Genentech) | TIGIT blocking human IgG1 mAb | NCT05483400 | Head and neck neoplasms, MSI-H cancer, and melanoma | II | Tiragolumab + Atezolizumab (anti-PD-L1) | |
| NCT03563716 | Locally advanced or metastatic nonsmall cell lung cancer | II | Tiragolumab or placebo + Atezolizumab | |||
| NCT04665856 | Untreated extensive-stage small lung cell cancer | III | Tiragolumab or placebo + Atezolizumab (anti-PD-L1) + Etoposide + Carboplatin |
|||
| NCT05315713 | Non-Hodgkin lymphoma and follicular lymphoma | I/II | Mosunetuzumab SC + Tiragolumab + Atezolizumab + Tocilizumab Or Mosunetuzumab SC + Tiragolumab + Tocilizumab |
|||
| NCT04294810 | Untreated locally advanced, unresectable, or metastatic PD-L1-selected nonsmall cell lung cancer | III | Tiragolumab or placebo + Atezolizumab (anti-PD-L1) |
|||
| NCT05394337 | Metastatic malignancy | I | Atezolizumab or Tiragolumab |
|||
| NCT03708224 | Squamous cell carcinoma of the head and neck | II | Atezolizumab + Tiragolumab or combinations without Tiragolumab |
|||
| NCT05459129 | Squamous cell carcinoma of the head and neck | I/II | Atezolizumab Or Atezolizumab + Tiragolumab Or Atezolizumab + Tiragolumab + iSBRT Or Atezolizumab + Tiragolumab + Carboplatin + Paclitaxel |
|||
| NCT04929223 | Metastatic colorectal cancer | I | Atezolizumab + Tiragolumab or Atezolizumab + Tiragolumab + Bevacizumab or combinations without Tiragolumab |
|||
| NCT04486352 | Endometrial cancer | I/II | Atezolizumab + Tiragolumab or combinations without Tiragolumab |
|||
| NCT05251948 | Gastric or gastroesophageal junction carcinoma | I/II | Atezolizumab + Capecitabine + Oxaliplatin + Tiragolumab or Atezolizumab + Capecitabine + Oxaliplatin |
|||
| NCT05116202 | Morpheus-melanoma | I/II | Tiragolumab + RO7247669 or Tiragolumab + Atezolizumab or combinations without Tiragolumab |
|||
| NCT03281369 | Locally advanced unresectable or metastatic gastro-esophageal junction cancer or esophageal cancer | I/II | Tiragolumab + Atezolizumab (anti-PD-L1) or Tiragolumab + Atezolizumab (anti-PD-L1) + Cisplatin + 5-Fluorouracil or combinations without Tiragolumab |
|||
| NCT04300647 | PD-L1-positive cervical cancer | II/III | Tiragolumab + Atezolizumab (anti-PD-L1) or Atezolizumab (anti-PD-L1) alone |
|||
| NCT05034055 | Metastatic nonsmall cell lung cancer | II | Atezolizumab (anti-PD-L1) + Tiragolumab | |||
| NCT04665843 | PD-L1 positive squamous cell carcinoma of the head and neck | II | Tiragolumab or placebo + Atezolizumab (anti-PD-L1) | |||
| NCT04958811 | Nonsquamous nonsmall cell lung cancer | II | Tiragolumab + Atezolizumab (anti-PD-L1) + Bevacizumab |
|||
| NCT04308785 | Limited stage small cell lung cancer | II | Tiragolumab or placebo + Atezolizumab (anti-PD-L1) |
|||
| NCT04513925 | Locally advanced, unresectable nonsmall cell lung cancer | III | Atezolizumab (anti-PD-L1) + Tiragolumab or Durvalumab |
|||
| NCT04543617 | Esophageal squamous cell carcinoma | III | Tiragolumab + Atezolizumab (anti-PD-L1) or Atezolizumab (anti-PD-L1) + Atezolizumab Matching Placebo or Tiragolumab Matching Placebo + Atezolizumab Matching Placebo |
|||
| NCT05060003 | Stage II Melanoma | II | Tiragolumab + Atezolizumab (anti-PD-L1) or Atezolizumab alone |
|||
| NCT04256421 | Untreated extensive-stage small cell lung cancer | III | Tiragolumab or Tiragolumab Matching Placebo + Atezolizumab (anti-PD-L1) + Carboplatin + Etoposide | |||
| NCT04540211 | Unresectable locally advanced, unresectable recurrent, or metastatic esophageal carcinoma | III | Atezolizumab (anti-PD-L1) + Tiragolumab + Paclitaxel + Cisplatin Or Paclitaxel + Cisplatin + Atezolizumab Matching Placebo + Tiragolumab Matching Placebo |
|||
| NCT04619797 | Untreated advanced nonsquamous nonsmall cell lung cancer | II | Tiragolumab + Atezolizumab (anti-PD-L1) + Pemetrexed + Carboplatin + Cisplatin or Pemetrexed + Carboplatin + Cisplatin + Tiragolumab Matching Placebo + Pembrolizumab (anti-PD-1) |
|||
| NCT05286801 | SMARCB1 or SMARCA4 deficient tumors | I/II | Atezolizumab + Tiragolumab | |||
| NCT04584112 | Triple-negative breast cancer | I | Tiragolumab + Atezolizumab (anti-PD-L1) + Nab-paclitaxel or Tiragolumab + Atezolizumab (anti-PD-L1) + Nab-paclitaxel + Carboplatin + Doxorubicin + Cyclophosphamide + Granulocyte colony-stimulating factor (G-CSF) + Granulocyte-macrophage colony-stimulating factor (GM-CSF) or Tiragolumab + Atezolizumab + Nab-paclitaxel + Doxorubicin + Cyclophosphamide + Granulocyte colony-stimulating factor (G-CSF) + Granulocyte-macrophage colony-stimulating factor (GM-CSF) |
|||
| NCT04933227 | HER2 negative unresectable, recurrent or metastatic gastric cancer or adenocarcinoma of gastroesophageal junction | II | Atezolizumab (anti-PD-L1) + Tiragolumab + Oxaliplatin + Capecitabine | |||
| NCT04045028 | Relapsed or refractory multiple myeloma or with relapsed or refractory B-cell nonhodgkin lymphoma | I | Tiragolumab or Tiragolumab + Daratumumab/rHuPH20 or Tiragolumab + Rituximab |
|||
| NCT04832854 | Previously untreated locally advanced resectable stage II, IIIA, or select IIIB nonsmall cell lung cancer | II | Atezolizumab (anti-PD-L1) + Tiragolumab + Carboplatin + Cisplatin + Pemetrexed + Gemcitabine + Paclitaxel | |||
| NCT02794571 | Advanced/metastatic tumors | I | Tiragolumab or Atezolizumab (anti-PD-L1) + Tiragolumab or Atezolizumab + Tiragolumab + Carboplatin+ Cisplatin + Pemetrexed or Atezolizumab + Tiragolumab + Carboplatin + Paclitaxel or Atezolizumab + Tiragolumab + Carboplatin + Cisplatin + Etoposide or Atezolizumab + Tiragolumab + Capecitabine or Atezolizumab + Tiragolumab + Bevacizumab or Tiragolumab + Pembrolizumab |
|||
| NCT03554083 | High-risk stage III melanoma | II | Tiragolumab + Atezolizumab (anti-PD-L1) or combinations without Tiragolumab |
|||
| NCT03977467 | Nonsmall cell lung cancer and solid tumor | II | Tiragolumab + Atezolizumab or Atezolizumab + chemotherapy |
|||
| NCT03869190 | Urothelial carcinoma and bladder cancer | I/II | Tiragolumab + Atezolizumab (anti-PD-L1) or combinations without Tiragolumab |
|||
| NCT04524871 | Advanced liver cancers | I/II | Tiragolumab + Atezolizumab (anti-PD-L1) + Bevacizumab or combinations without Tiragolumab |
|||
| NCT03193190 | Metastatic pancreatic ductal adenocarcinoma | I/II | Tiragolumab + Atezolizumab (anti-PD-L1) + Nab-Paclitaxel + Gemcitabine or combinations without Tiragolumab |
|||
| NCT04632992 | Advanced unresectable or metastatic solid malignancy | II | Tiragolumab + Atezolizumab (anti-PD-L1) or combinations without Tiragolumab |
|||
| NCT05009069 | Rectal neoplasms and rectal cancer |
II | Tiragolumab + Atezolizumab + Fluorouracil + Capecitabine + Radiotherapy + radiotherapy or Atezolizumab + Fluorouracil + Capecitabine + Radiotherapy + radiotherapy |
|||
| NCT05259319 | Metastatic solid tumors | I | Tiragolumab + Capecitabine + Oxaliplatin + Atezolizumab or Capecitabine + Oxaliplatin + Atezolizumab |
|||
Domvanalimab, AB154 (Arcus Biosciences) |
TIGIT blocking humanized IgG1 mAb | NCT03628677 | Advanced solid malignancies | I | Domvanalimab + Zimberelimab (anti-PD-1) or Zimberelimab alone |
|
| NCT04262856 | PD-L1 positive, locally advanced, or metastatic nonsmall cell lung cancer | II | Domvanalimab + Zimberelimab (anti-PD-1) or Domvanalimab + Zimberelimab + Etrumadenant (anti-A2a/bR antagonist) or Zimberelimab alone |
|||
| NCT05419479 | Pancreatic cancer | I/II | Domvanalimab + Zimberelimab (anti-PD-1) + APX005M or Folfiri |
|||
| NCT04736173 | PD-L1 positive nonsmall cell lung cancer | III | Domvanalimab + Zimberelimab (anti-PD-1) or combinations without Domvanalimab |
|||
| NCT03547973 | Metastatic urothelial cancer | II | Sacituzumab Govitecan-hziy + Zimberelimab + Domvanalimab or combinations without Domvanalimab |
|||
| NCT05502237 | Nonsmall cell lung cancer | III | Zimberelimab + Domvanalimab + Carboplatin + Cisplatin + Paclitaxel + Nab-paclitaxel + Pemetrexed or combinations without Domvanalimab |
|||
| NCT05211895 | Nonsmall cell lung cancer | III | Domvanalimab or placebo + Durvalumab | |||
| NCT05568095 | Advanced upper gastrointestinal tract adenocarcinoma | III | Zimberelimab + Domvanalimab + Oxaliplatin + Leucovorin + Fluorouracil + Capecitabine or combinations without Domvanalimab |
|||
| NCT04791839 | Nonsmall cell lung cancer | II | Domvanalimab + Zimberelimab (anti-PD-1) + + Etrumadenant (anti-A2a/bR antagonist) | |||
| NCT05130177 | Melanoma | II | Zimberelimab + Domvanalimab | |||
| NCT05329766 | Gastrointestinal tract malignancies | II | Zimberelimab or Domvanalimab or Fluorouracil or Leucovorin or Oxaliplatin |
|||
ASP8374 (Astella Pharma Global Development) |
TIGIT blocking human IgG4 mAb | NCT03260322 | Advanced tumors | I | ASP8374 + Pembrolizumab (anti-PD-1) or ASP8374 alone |
|
| NCT04826393 | Recurrent glioblastoma | I | ASP8374 + Cemiplimab or ASP8374 + Cemiplimab + 89Zr-Df-IAB22M2C |
|||
| NCT03945253 | Advanced solid tumors | I | ASP8374 | |||
| Vibostolimab, MK-7684 (Merck Sharp & Dohme) | TIGIT blocking humanized IgG1 mAb | NCT04303169 | Melanoma | I/II | Vibostolimab + Pembrolizumab (anti-PD-1) or Pembrolizumab (anti-PD-1) + V937 or Pembrolizumab (anti-PD-1) alone |
|
| NCT04305041 | PD-1 refractory melanoma | I/II | Vibostolimab or Lenvatinib + Pembrolizumab (anti-PD-1) + Quavonlimab (anti-CTLA-4) |
|||
| NCT05005442 | Relapsed/refractory hematological malignancies | II | Pembrolizumab (anti-PD-1)/vibostolima coformuation | |||
| NCT04725188 | Metastatic nonsmall cell lung cancer | II | Pembrolizumab (anti-PD-1)/Vibostolimab coformuation + Docetaxel or Pembrolizumab (anti-PD-1)/Vibostolimab coformuation or Docetaxel + Placebo |
|||
| NCT04738487 | PD-L1 positive metastatic nonsmall cell lung cancer | III | Pembrolizumab (anti-PD-1)/Vibostolimab or Pembrolizumab (anti-PD-1) |
|||
| NCT05007106 | Multiple solid tumors | II | Pembrolizumab (anti-PD-1)/Vibostolimab Coformulation or Pembrolizumab (anti-PD-1) or Pembrolizumab(anti-PD-1)/Vibostolimab Coformulation + Lenvatinib or Pembrolizumab (anti-PD-1)/Vibostolimab Coformulation + 5-Fluorouracil + Cisplatin or Pembrolizumab(anti-PD-1)/Vibostolimab Coformulation + Paclitaxel |
|||
| NCT02964013 | Advanced solid tumors | I | Vibostolimabor Vibostolimab + Pembrolizumab(anti-PD-1) + or Vibostolimab + Pembrolizumab(anti-PD-1) + pemetrexed + carboplatin or Pembrolizumab(anti-PD-1)/vibostolimabor Vibostolimab + Pembrolizumab(anti-PD-1) + carboplatin + cisplatin + etoposide |
|||
| NCT04165070 | Nonsmall cell lung | II | Pembrolizumab(anti-PD-1) + Carboplatin + Paclitaxel + Vibostolimab or combinations without Vibostolimab |
|||
| NCT02861573 | Metastatic castration-resistant prostate cancer | I/II | Pembrolizumab (anti-PD-1)/Vibostolimab coformulation or combinations without Vibostolimab |
|||
| NCT04305054 | Melanoma | I/II | Pembrolizumab (anti-PD-1) + Vibostolimab or combinations without Vibostolimab |
|||
| NCT05298423 | Stage III nonsmall cell lung cancer | III | Pembrolizumab/Vibostolimab + Cisplatin + Pemetrexed + Eoposide + Carboplatin + Paclitaxel + thoracic radiotherapy or combinations without Vibostolimab |
|||
| NCT05224141 | Nonsmall cell lung cancer | III | Pembrolizumab/Vibostolimab + Saline placebo Setoposide + Cisplatin + Carboplatin or combinations without Vibostolimab |
|||
| NCT05226598 | Nonsmall cell lung cancer | III | Pembrolizumab/Vibostolimab + Carboplatin + Cisplatin + Paclitaxel + Nab-paclitaxel + Pemetrexed or combinations without vibostolimab | |||
| NCT02625961 | Nonmuscle invasive bladder cancer | II | Pembrolizumab or Pembrolizumab/Vibostolimab or Favezelimab/Pembrolizumab |
|||
| NCT04895722 | Colorectal cancer | II | Pembrolizumab/Vibostolimab or combinations without vibostolimab |
|||
| NCT04626479 | Renal cell carcinoma | I/II | Pembrolizumab/Vibostolimab + Belzutifan or combinations without vibostolimab |
|||
IBI-939 (Innovent) |
TIGIT blocking human IgG1 mAb | NCT04353830 | Advanced malignancies | I | IBI-939 + Sintilimab or IBI-939 alone |
|
| NCT04672356 | Advanced lung cancer | I | BI-939 + Sintilimab | |||
| NCT04672369 | Advanced lung cancer | I | BI-939 + Sintilimab | |||
Etigilimab, OMP-313M32 (Mereo BioPharma) |
TIGIT blocking human IgG1 mAb | NCT05026606 | Platinum-resistant recurrent clear cell ovarian, primary peritoneal, or fallopian tube cancer | II | Etigilimab + Nivolumab (anti-PD-1) | |
| NCT04761198 | Locally advanced or metastatic tumors. | I/II | Etigilimab dosing + Nivolumab (anti-PD-1) | |||
COM902 (Compugen) |
TIGIT blocking human IgG1 mAb | NCT04354246 | Advanced malignancies | I | COM902 + COM701 or COM902 alone |
|
M6223 (Merck KGaA) |
TIGIT blocking human IgG1 mAb | NCT04457778 | Metastatic solid tumors | I | Bintrafusp alfa + M6223 or M6223 alone |
|
| NCT05327530 | Locally advanced or metastatic urothelial carcinoma | II | Avelumab + M6223 or Avelumab or Avelumab + Sacituzumab Govitecan or Avelumab + NKTR-255 |
|||
EOS884448 (iTeos Therapeutics) |
TIGIT blocking human IgG1 mAb | NCT04335253 | Advanced cancer | I | EOS884448 | |
| NCT05060432 | Advanced solid tumors | I/II | EOS884448 + pembrolizumab (anti-PD-1) or EOS884448 + inupadenant |
|||
| NCT05565378 | Nonsmall cell lung caner | II | Dostarlimab + EOS884448 or Pembrolizumab or Dostarlimab |
|||
| NCT04446351 | Neoplasms | I | Dostarlimab + EOS884448 Or Dostarlimab + EOS884448 + GSK6097608 or other combinations without EOS884448 |
|||
| NCT03739710 | Neoplasms | I | Dostarlimab + EOS884448 Or Dostarlimab + EOS884448 + GSK6097608 or other combinations without EOS884448 |
|||
| NCT05289492 | Multiple myeloma | I/II | EOS884448 alone or EOS884448 + Iberdomide or EOS884448 + Iberdomide + Dexamethasone |
|||
| SEA-TGT (Seagen) | TIGIT blocking human IgG1 mAb | NCT04254107 | Advanced cancer | I | SEA-TGT + sasanlimab or SEA-TGT alone |
|
| NCT04585815 | Nonsmall cell lung cancer | I/II | Sasanlimab Prefillled syringe + Encorafenib + Binimetinib or Sasanlimab + Axitinib + SEA-TGT |
|||
BAT6021 (Bio-Thera Solutions) |
TIGIT blocking human IgG1 mAb | NCT05073484 | Solid tumors | I | BAT6021 + BAT1308 (anti-PD-1) or BAT6021 alone |
|
| NCT05120375 | Solid tumors | I | BAT6021 | |||
| PD-L1 and TIGIT | HLX301 (Shanghai Henlius Biotech) |
Anti-TIGIT/Anti-PD-1 bispecific antibody | NCT05102214 | Locally advanced or metastatic solid tumors | I/II | HLX301 |
| NCT05390528 | Advanced tumors, lymphoma, and metastatic tumors | I/II | HLX301 | |||
| HB0036 | Anti-TIGIT/Anti-PD-1 bispecific antibody | NCT05417321 | Advanced solid tumor nonsmall cell lung cancer |
HB0036 | ||
AZD2936 (AstraZeneca) |
Anti-TIGIT/Anti-PD-1 bispecific antibody | NCT04995523 | Nonsmall cell lung cancer | I/II | AZD2936 | |
| NCT03819465 | Nonsmall cell lung cancer | I | AZD2936 Or AZD2936 + Pemetrexed+ Carboplatin + Cisplatin or combinations without AZD2936 |
|||
| CD112R | COM701 (Compugen) | CD112R/PVRIG inhibitor | NCT03667716 | Advanced solid tumors | I | COM701 + Nivolumab (anti-PD-1) or COM701 alone |
| NCT04570839 | Advanced solid tumors | I/II | COM701 + BMS-986207 + Nivolumab (anti-PD-1) | |||
| CD96 | GSK6097608 (GlaxoSmithKline) |
CD96 inhibitor | NCT04446351 | Neoplasms | I | GSK6097608 + Dostarlimab or GSK6097608 alone |
CD226 (Terminated) |
LY3435151 (Eli Lilly and Company) | CD226 agonist | NCT04099277 | Advanced solid tumors | Ia/Ib | LY3435151 + Pembrolizumab (anti-PD-1) or LY3435151 alone |
- Note: The ongoing clinical trials for TIGIT, CD112R, and CD96 published on ClinicalTrials.gov.
- Abbreviations: IgG, immunoglobulin; ITIM, immune receptor tyrosine-based inhibitory motif; mAb, monoclonal antibody; PD-1, programmed cell death receptor 1; PD-L1, programmed cell death-ligand 1; TIGIT, T-cell immunoglobulin and ITIM structural domain.
5 TIGIT IN NONCANCEROUS DISEASES
Suppressive signaling is essential for immune homeostasis and self-tolerance. Recently, abnormal expression of TIGIT has also been found in several nontumor diseases, including autoimmune diseases, infection-related and pregnancy-related diseases, and have seen therapeutic potential.
5.1 Autoimmune disease and transplant status
The central role of coinhibitory receptors in maintaining immune system homeostasis makes it possible that their loss may lead to spontaneous, severe autoimmunity (Figure 6). These days, increasing studies have illustrated the function immune checkpoint played in autoimmune diseases, and its upregulation may contribute to the prevention and management of autoimmune diseases.
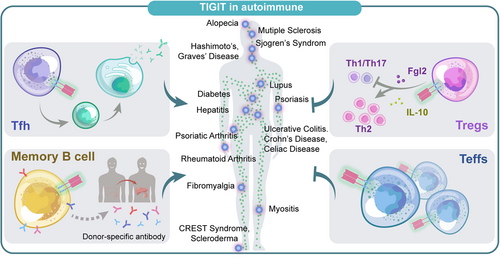
5.1.1 TIGIT expression in autoimmune diseases
As existing research have confirmed that abnormalities in function or number of Tregs are a feature of autoimmune diseases, to date, numerous animal models and human studies have verified the upregulation of TIGIT+ Tregs in various autoimmune diseases, such as NOD mouse islets, type1 diabetes (T1D) patients,92, 93 thyroiditis,94, 95 aplastic anemia (AA),96 multiple sclerosis (MS)97 and noninfectious uveitis,98 and may be proportional to disease severity.
TIGIT expression also changes in non-Tregs with autoimmune diseases. Animal studies have shown that TIGIT attenuates disease in collagen-induced arthritis (CIA) model by suppressing CD4+ Teffs responses, which was also confirmed by graft-versus-host disease (GvHD) model,9 suggesting that autoimmune or alloimmune diseases may be beneficial from the suppressive properties that trigger TIGIT. In patients with MS,99 psoriasis100 and AA,96 the proportion of CD4+ T cells expressing TIGIT is significantly lower than in healthy controls and negatively correlated with disease severity. Nevertheless, the upregulation of TIGIT expression can also be seen in some autoimmune disease processes, which is contrary to our previous knowledge. Luo et al. observed that TIGIT was highly expressed on CD3+CD4+ T cells in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) and positively correlated with the activity of diseases.101, 102 Maybe this can be explained that at a certain point of disease progression, TIGIT expression on specific cells may increase in an attempt to attenuate the inflammatory response, trying to control the disease state. Therefore, we suppose that depleting or reducing this subset of cells with upregulated TIGIT expression may indicate disease progression.
5.1.2 TIGIT in humoral immunity
Interestingly, the involvement of TIGIT in humoral immunity has been recently observed in autoimmune diseases and allotransplantation. A team working with experimental autoimmune encephalomyelitis (EAE) found that TIGIT was expressed in Tim-1+ B cells and essential for inhibition of inflammation and immune tolerance induction.78 Besides, another study found that in kidney and liver allograft patients, the absence or reduction of TIGIT+ memory B cells is positively related to the production of donor-specific antibodies and Tfhs responses.79 Godefroy et al. identified a subpopulation of TIGIT-expressing human circulating follicular helper cells which could promote B-cell activation and differentiation by enhanced IL-4 and IL-21 secretion, thus inducing B-cell differentiation into plasma cells and promoting IgG production.
In summary, abnormal expression of TIGIT in different disease types, stages, and cell subsets contributed to autoimmune disorders and transplant status.79, 103 All these findings open the way to identify new diagnostic and therapeutic strategies and may modulate the process of vaccination, autoimmunity, and immunodeficiency,79, 80, 103 although more evidence is needed to support the clinical trials.
5.2 Chronic infection
Persistent antigen exposure and dysfunctional Teffs are essential features of chronic infection.4 When the infection antigens persist, the immune system will initiate a number of suppressive pathways to inhibit the response and prevent tissue damage from immune-mediated, a process known as “depletion.”104 In fact, T cells express various immune checkpoints in chronic infections, including CTLA-4, PD-1, TIGIT, TIM-3, and LAG3.105-109 Limited therapies and a significant population of patients have triggered studies of the coinhibitory receptors in chronic infection diseases110-115 such as human immunodeficiency virus (HIV), hepatitis B virus (HBV) and so on.116
5.2.1 TIGIT in LCMV, HIV, HTLV-1, and viral hepatitis
In a mouse model, it was clarified that exhausted CD8+ T cells of chronic lymphocytic choriomeningitis virus (LCMV) infection could coexpress TIGIT and several other inhibitory receptors such as PD-1, T cell immunoglobulin-3 (TIM-3), and Lymphocyte activation gene-3 (LAG-3), and dual blockade of TIGIT and PD-L1 specifically enhanced the effector function of CD8+ T cell, leading to more effective viral clearance than treatment alone.13 The same result could be seen in other virus infections. During HIV infection and human type 1 T-cell leukemia virus (HTLV-1) infection, TIGIT has been reported to be upregulated and correlated with poorer progression.85, 117
However, a prospective study found that in patients with slow plus acute liver failure (ACLF), expression of TIGIT peaked in compensated and acute decompensated cirrhosis stage, but diminished in ACLF stage.118 This may partly be explained by that the compensated cirrhosis may be a disease with low-grade systemic inflammation, but ACLF may reflect the long-term depletion of adaptive immunity, and increased pro-inflammatory cytokines by reduced TIGIT expression contributed to the poor prognosis of ACLF patients,118 suggesting the timing of ICB therapy may affect the clinical benefits.
5.2.2 TIGIT in COVID-19
Studies addressing immune checkpoints in COVID-19 are relatively limited. Herrmann et al. investigated TIGIT expression in patients with COVID-19, finding that CD226 was more often expressed on CD8+ T cells in patients contrasted with healthy individuals, while TIGIT expression does not differ greatly, and even CD4+ T cell subsets showed lower TIGIT expression during acute infection.119 Since TIGIT and CD226 compete for binding to the same ligand CD155, they suggested that the increased CD226 and decreased TIGIT expression may be beneficial in maintaining the cytotoxic effector functions of COVID-19. Similarly, Schultheiß et al. demonstrated that in COVID-19 patients, TIGIT expression in NK cells was downregulated and associated with disease severity.120
Nevertheless, several groups reported opposite findings. Bernal et al.121 found that in COVID-19 patients, the presence of an NK cell subtype resulted in a high upregulation of TIGIT expression and positively correlated with disease severity. Another research found that the expression of TIGIT and PD-L1 was elevated among hospitalized and nonhospitalized samples compared to the healthy counterparts.122 Similarly, Mazzoni et al. identified four subtypes (PD-1+TIGIT+, PD-1+TIGIT−, PD-1−TIGIT+, and PD-1−TIGIT−) of COVID-19-specific CD4+CD154+ T cells and found that the PD1+ TIGIT+ subtype was significantly dominant, thinking that different combinatorial patterns of immune checkpoints indicated various stages of T-cell activation.123 To sum up, the expression of TIGIT in subtypes of lymphocytes at various stages of COVID-19 is still unclear. Considering the complexity of COVID-19 disease and the different activation mechanisms of TIGIT, the conflict between existing studies is reasonable, and further research is needed to explain these differences.
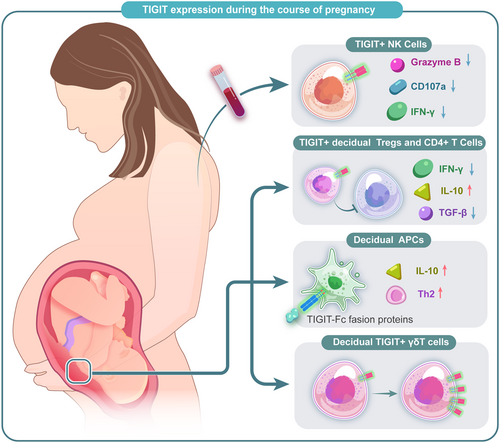
5.3 Human pregnancy and pregnancy-related diseases
Previous studies have clarified that Tregs were vital in embryo implantation, placenta formation, and maternal-fetal tolerance induction.124 Recently, a research reported that TIGIT+ Tregs had a unique ability to establish immune tolerance at the human maternal-fetal interface.125 Another study also showed that the TIGIT gene was upregulated in uterine Tregs (uTregs), especially at the placental bed site.124 Wang et al. found that TIGIT expression on peripheral blood NK (pNK) cells increased gradually during pregnancy, along with a decreased expression of granzyme B, IFN-γ as well as CD107a in pNK cells.125
In fact, several studies have verified that TIGIT low expression is related to adverse pregnancy outcomes and may be a potential target for therapy. A study about unexplained recurrent miscarriage patients and normal pregnancy showed that an increased expression of TIGIT in the decidual γδT cells significantly plays essential roles in maintaining pregnancy,126, 127 which was also confirmed in a study of patients with pre-eclampsia.128 Fu et al. detected the expression of TIGIT in decidual immune cells at the maternal-fetal interface. Then by generating recombinant TIGIT-Fc fusion proteins, they confirmed that treatment with TIGIT-Fc of human decidual immune cells could increase IL-10 production, leading the decidual CD4+T cells to a Th2 phenotype, suggesting that TIGIT-Fc fusion protein has therapeutic potential in pregnancy failure.129 All the existing studies indicated that treatment targeting TIGIT pathway has seen promising clinical benefits in adverse pregnancy outcomes, further studies are needed to better understand its function in maintaining maternal-fetal tolerance and the potential value in the diagnosis and treatments of pregnancy-related diseases (Figure 7).
6 PROSPECT AND FUTURE
Though a growing number of clinical trials evaluating the value of targeting TIGIT pathway, there is still several challenging or interesting issue in preclinical and clinical aspects.
It is changed to explore the specific function of TIGIT in different immunocytes which is not as clear as PD-1 and CTLA-4, though its antitumor clinical trials have emerged worldwide. Meanwhile, it is expected to investigate the synergistic effect of coblockade of CD112R/TIGIT or CD96/TIGIT, or adopted CD226 agonist in clinical trials in the soon further.
In conclusion, a range of ongoing preclinical experiments and clinical trials indicate that targeting TIGIT is an attractive approach for cancer therapy, especially with the coblockade of PD-1/PD-L1 blockade. And we look forward to the progress and expect more exciting outcomes in cancer and noncancerous diseases.
AUTHOR CONTRIBUTIONS
Conceptualization, methodology: Zhihui Li and Han Luo. Collected and prepared the resources: Liying Wang. Writing – review and editing: Jiaye Liu, Xiaofei Wang, Xiuhe Zou, Xun Zheng, Feng Ye, and Heng Xu. Writing – original draft, visualization, investigation: Shu Rui and Xiangyu Kong. All authors revised the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The study was supported by the National Natural Science Foundation of China (82103031, 82272933, 81972498, and 81973408); the International Cooperation Project of Chengdu Municipal Science and Technology Bureau (2020-GH02-00017-HZ); “1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University” (ZYJC18035, ZYJC18025, ZYYC20003, ZYJC18004); and Natural Science Foundation of Sichuan Province (2022NSFSC1314). Clinical Research Incubation Project, West China Hospital, Sichuan University (22HXFH019).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.