High sucralose intake suppresses autoimmunity and promotes tumor growth by limiting T cell-mediated immune responses
In a recent study, Zani et al. published an article entitled “The dietary sweetener sucralose is a negative modulator of T cell-mediated responses” in Nature.1 They reported that the intake of high amounts of sucralose, a calorie-free sugar substitute, can suppress autoimmunity and promote tumor growth by suppressing the proliferation and function of effector T cells in mice.
With the deepening of research, more and more studies have proved that excessive intake of sugar could cause a variety of diseases, including inflammatory disorders and tumors.2, 3 On the other hand, there are some studies suggesting that calorie-free sugar substitutes may also have some adverse health effects, such as glucose intolerance, by affecting the gut microbiome.4 However, the mainstream view is still that non-caloric sugar substitutes are harmless to humans. As a calorie-free sugar substitute, sucralose was also considered to be safe for people. Therefore, the consumption of sucralose has increased significantly in the past decades. These findings reported by Zani et al. almost upend the way people think about sucralose.1
They first treated the wild-type mice with 0.17 or 0.72 mg/mL sucralose to determine whether sucralose can affect the immune system. They did not identify any detectable effect on the homeostatic levels of immune cells, showing that sucralose had no significant effect on the immune system under immune homeostasis conditions. Then, they investigated whether a high dose of sucralose could affect immune responses in different kinds of immune challenge conditions; they found that high sucralose exposure decreases cell proliferation in Rag2−/− mice transferred with naïve T cells. However, the immune challenges did not affect B cells or macrophages. After that, they found high sucralose exposure suppressed cell proliferation and differentiation of CD4+ and CD8+ T cells in the in vitro cultures, and the suppression is dose-dependent. To find the target of sucralose-mediated limitation of T cell proliferation and T cell differentiation, the authors used Jurkat T cells in the absence of T cell receptor (TCR) stimulation to first determine that the sucralose effect is not mediated by the sweet taste receptor (STR). Given this, the authors conducted an RNA-sequencing analysis to explore the alternative mechanism. Principal component analysis (PCA) identified that T cell subsets exposed to sucralose displayed a unique expression profile compared with control cells, and enrichment analysis of the RNA-seq data identified a number of pathways affected by sucralose, including those associated with proliferation. After further evaluation, the authors found that sucralose specifically impedes TCR-dependent T cell proliferation.
Subsequently, the authors conducted experiments to test at which level(s) sucralose influences the TCR-dependent proliferation pathways and found that the limitation of PLCγ1 activation ultimately blocks TCR signaling (Figure 1). To explain why sucralose affects the activation of PLCγ1, the authors utilized spatial measurement analysis and deep-learning-enabled mass spectrometry imaging platform cryo-OrbiSIMS to show that sucralose primarily acts on the T cell membrane, further decreasing the PLCγ1 clustering and colocalization with TCRβ in response to TCR stimulation (Figure 1).
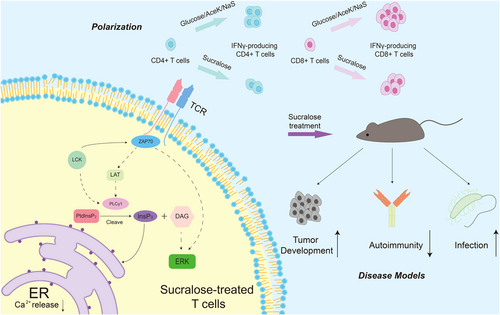
Next, the authors further explored the secondary effects of sucralose on the downstream of PLCγ1. Flow cytometry revealed that sucralose reduced TCR-dependent calcium flux in T cells, while these changes were not detected in dendritic cells and B cells. This led the authors to investigate which calcium source was affected by sucralose. When the authors treated the naïve T cells with EDTA, a chelating agent of calcium, to inhibit entry of extracellular calcium, they determined that sucralose-treated cells retained reduced TCR-dependent calcium flux compared with control cells in the presence of EDTA. These data proved that sucralose impaired the TCR-mediated intracellular calcium release. To confirm this finding, the authors used ionomycin in the absence of exogenous calcium to induce the release of calcium from intracellular stores. They found that sucralose did not affect intracellular calcium release in the presence of ionomycin. Consistent with this, T cell proliferation and cytokine production were partially rescued in the presence of ionomycin. Together, these findings indicate that a high dose of sucralose limits T cell proliferation and differentiation by reducing TCR- and PLCγ1-dependent intracellular calcium release, although the underlying mechanisms of how sucralose affects TCR signaling remain to be revealed.
To examine whether a high dose of sucralose intake has any effect on T cell-related disorders in vivo, they next investigated the effects of sucralose on tumor-specific T cell responses. Zani et al. established a mouse tumor model and found that high sucralose intake through drinking water resulted in increased growth of subcutaneous EL4 cancer cells expressing OVA (EL4-OVA cells). By investigating immune responses against EL4-OVA cells, the investigators found that the tumor-specific T cell response was suppressed in mice treated with 0.72 mg/mL sucralose, mainly by inhibiting interferon-γ (IFN-γ) production of CD8+ effector T cells (Figure 1). To confirm this, they adoptively transferred CD8+ T cells that recognize OVA (OT-I cells) into recipient mice challenged with EL4-OVA cells and treated the mice with sucralose or water. They found that the tumor growth was increased and the antitumor immune response of OT-I cells was reduced in mice treated with sucralose. Moreover, they also activated OT-1 cells in vitro in the presence or absence of sucralose, and OT-1 cells treated with sucralose showed decreased cytotoxic activity against EL4-OVA cells.
Besides the suppression of antitumor immunity, Zani et al. also reported that sucralose could impair anti-infective immunity in an infection model of Listeria monocytogenes expressing OVA (LmOVA). In this infection model, it was determined that treatment with sucralose caused a significant reduction in the frequency of splenic OVA-specific CD8+ T cells, too. Taken together, the data proves that a high dose of sucralose intake promotes tumor growth and limits anti-infective immunity by suppressing T cell-related immune responses (Figure 1).
The findings that sucralose suppresses T cell differentiation and proliferation also encouraged the authors to identify whether sucralose could treat T cell-mediated autoimmunity. Indeed, they determined that sucralose could also suppress T cell-mediated inflammation in disease models of type 1 diabetes and colitis (Figure 1). This study proposes for the first time that sucralose supplementation may be a treatment strategy for diseases characterized by overactivation of T cell-mediated autoimmune responses.
Given that this study shows that sucralose can promote tumor growth, it is necessary to limit the addition of sucralose to foods and beverages, at least not exceeding the dose applied in this study. Although sucralose might be effective in the treatment of T cell-mediated autoimmunity, its tumor-boosting effect and infection-promotion effect limit its clinical application greatly and may reduce patient acceptance significantly. On the other hand, the human need for sweet taste should be respected, so the safety assessment of calorie-free sugar substitutes is particularly important.
Overall, these findings are important not only for tumor patients and infected patients but also for all people; it shows us one possibility that artificial sweeteners may not be always safe. Although there is no epidemiological study to prove these findings now, one study has reported that artificial sweetener consumption is a potential risk factor for well-differentiated thyroid cancer.5 Therefore, reducing the consumption of sucralose-containing beverages may be necessary. In future studies, the effects of all kinds of artificial sweeteners on various diseases, including malignant tumors and infection, need to be systematically and deeply revealed. More importantly, to reveal the effects of sucralose on humans, large-scale epidemiological studies on the relationship between sucralose intake and various diseases are necessary.
AUTHOR CONTRIBUTIONS
Yubin Lin: Writing—original draft (lead). Qipeng Zhan: writing—review and editing (lead). Dunfang Zhang: Conceptualization (lead); funding acquisition (lead); writing—review and editing (supporting). All authors have read and approved the final manuscript for publication.
ACKNOWLEDGMENTS
This study is supported by the National Natural Science Foundation of China (No. 82171829), the Key Project of the Science and Technology Department of Sichuan Province (No. 2022YFH0100), and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYYC21012). The figure in this highlight was created with Adobe Illustrator. Dunfang Zhang sincerely wants to commemorate Dr Sang-A Park, who passed away suddenly in an auto accident in Bethesda, MD, USA.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




