Biomarkers of ageing: Current state-of-art, challenges, and opportunities
Ruiye Chen, Yueye Wang, and Shiran Zhang contributed equally to this study.
Abstract
Given the unprecedented phenomenon of population ageing, studies have increasing captured the heterogeneity within the ageing process. In this context, the concept of “biological age” has been introduced as an integrated measure reflecting the individualized ageing pace. Identifying reliable and robust biomarkers of age is critical for the accurate risk stratification of individuals and exploration into antiageing interventions. Numerous potential biomarkers of ageing have been proposed, spanning from molecular changes and imaging characteristics to clinical phenotypes. In this review, we will start off with a discussion of the development of ageing biomarkers, then we will provide a comprehensive summary of currently identified ageing biomarkers in humans, discuss the rationale behind each biomarker and highlight their accuracy and clinical value with a contemporary perspective. Additionally, we will discuss the challenges, potential applications, and future opportunities in this field. While research on ageing biomarkers has led to significant progress and applications, further investigations are still necessary. We anticipate that future breakthroughs in this field will involve exploring potential mechanisms, developing biomarkers by combining various data sources or employing new technologies, and validating the clinical value of existing and emerging biomarkers through comprehensive collaboration and longitudinal studies.
1 INTRODUCTION
The scientific and medical community has observed enormous advances in prolonging life expectancy in the past few decades. According to a report by World Health Organization (WHO), the global population aged 65 and over is projected to double to 1.5 billion between 2019 and 2050, with one in six people being aged 65 years or over.1 While the rapid global increase in lifespan is striking, the health span, also known as a disease-free lifespan, has not increased at the same pace. Age-related comorbidities now occupy roughly 16%–20% of one's time while they are alive, bringing into question the quality of life for elderly people living longer.2 In addition, the global burden of disease is increasing dramatically due to high rates of age-related functional decline, non-communicable chronic diseases, and mortality.3 In 2010, there were 777 million years lived with disability (YLD) from all causes, up from 583 million in 1990. Age-related disorders, including cancer, cardiovascular diseases (CVD), and neurodegenerative diseases contributed to over 30% of the YLD and are major life threats to adults of advanced age.3 The elderly are also increasingly vulnerable to other nonnegligible social-public issues including elder abuse and poor mental health, and in many developed countries, healthcare expenditure consumes over 10% of gross domestic product (GDP).4-6 This makes the burden of ageing an unignorable problem, and effective, accessible, and affordable strategies to handle the ageing population are urgent priorities for health systems.
Chronological age, measured as the time since the date of birth, is strongly linked with deteriorating health, morbidity, and mortality. However, ageing is a heterogeneous process with great variation in health outcomes among people of the same chronological age.7 At the individual level, different cells, tissues, and organs exhibit different ageing trajectories. For example, different models to predict an organ's age have been proposed for the brain, heart, retina, and other major organs.8-10 At the population level, heterogeneous ageing is evident by elderly individuals of the same age living completely different lives, with some being completely independent and others requiring complex care for daily activities.11 Additionally, centenarians often concentrate in certain geographical regions (e.g., Ikaria in Greece, Sardinia in Italy) and are healthier than their neighbors.12 Taken together, the heterogeneity of ageing is evident at all levels.
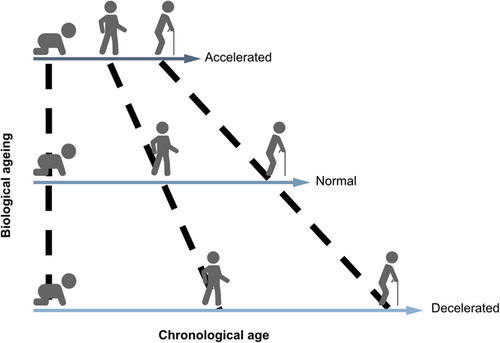
Given ageing is a heterogenous process, chronological age poorly reflects internal biological processes and intra-individual variation.13 As such, the scientific community has shifted their focus to biological markers of ageing, which quantify biophysiological ageing processes.14 The concept of “a biomarker of ageing” was firstly introduced in 1988 by Sprott et al., described as a biological parameter of an organism to predict functional capability.15 Based on this, the concept of biological age has gradually emerged as a measurement which determines individual-specific, age-related risk of adverse outcomes (Figure 1).16 Though biological age lacks a clear definition so far, it can be indicated that this parameter is driven by interactions between cellular and biochemical processes, leading to a thoughtful and individual-specific reflection on physiological function and overall health.17 Countless potential candidate biomarkers of ageing have been proposed, ranging from molecular changes and imaging characteristics to clinical phenotypes. Identifying reliable and robust biomarkers of age is critical for the accurate risk stratification of individuals and exploration into anti-ageing interventions.
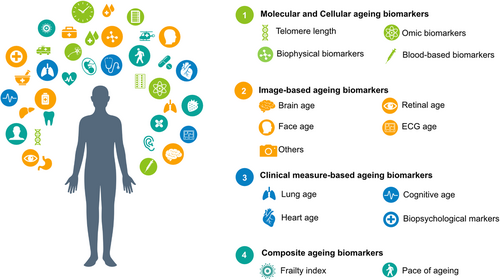
In this review, we focused on ageing biomarkers identified in humans. We will start off with a discussion of the development of ageing biomarkers, followed by an introduction of the main ageing biomarkers, summarized in Figure 2. Our aim was not to present an exhaustive account of all ageing biomarkers but to introduce the rationale underlying each biomarker, highlight their accuracy in age prediction and summarize their clinical value in determining age-specific outcomes all with an up-to-date perspective. In the following sections, challenges, potential applications, and future opportunities in the field are also discussed.
2 DEVELOPMENT OF AGEING BIOMARKERS
2.1 General requirements of ageing biomarkers
The American Federation for Aging Research (AFAR) developed criteria for ageing biomarkers based on Johnson and Butler et al.18 According to their guidelines, a biomarker of age needs (1) better performance for predicting age and age-associated outcomes, (2) monitor the ageing process in systems rather than effects of the disease, (3) have the ability to be repeatedly tested in a harmless way, both in humans and experimental animals.19 As these requirements are too strict that few biomarkers could fulfill, currently there are no gold standard criteria or method established for developing ageing biomarkers.
2.2 General methods for developing ageing biomarkers
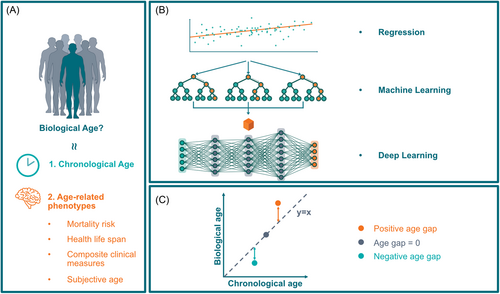
Methods for identifying ageing biomarkers have been developed mainly through the assumption that biological age is aligned with the chronological age in healthy populations.20 In addition, numerous groups have trained prediction models on age-related phenotypes and used them as proxies of biological ageing (i.e., mortality risk, health life span, composite clinical measures of phenotypic age, or subjective age).21-23
The accuracy of age estimators could be assessed through mean absolute error (MAE) determined as the mean absolute value of the difference between the predicted age and the ground truth. In brief, a smaller MAE indicates a higher accuracy of the ageing biomarker to predict chronological age.24 When the model is then applied to general populations, any deviation between predicted age and chronological age suggests the rate of ageing.25, 26 This was coined the “biological age gap,” where a positive age gap infers an accelerated ageing process while a negative one shows a decelerated process. The age gap is a widely accepted and validated concept in the field of ageing biomarkers.27-29 Figure 3 depicts the overview of the classic method of developing ageing biomarkers.
3 MOLECULAR AND CELLULAR AGEING BIOMARKERS
3.1 Telomere length
Telomeres are regions of repetitive nucleotide sequences at the ends of chromosomes which can protect DNA from damage and instability. In most somatic cells, telomeres will shorten 50–150 base pairs after every cell cycle.30 Telomere length is considered as a marker of ageing considering most human somatic cells will undergo a limited number of divisions until the protective function of telomeres is exhausted, leaving cells vulnerable to mutations. Previous studies found telomere length shortened predictably with age, and this process occurred in most cells and tissues including fibroblasts,31 peripheral blood cells,32 and colonic mucosa.33 Growing evidence also demonstrates a strong correlation between age and telomere attrition.32, 34, 35
Telomere length has proved itself to be a promising ageing biomarker and was significantly associated with frailty and functional decline of the lung,36, 37 heart, and kidney.38, 39 Participants with Type 2 diabetes mellitus (T2DM),40 coronary heart disease,41 lung cancer,42 lymphocytic leukemia, and lymphoma43, 44 generally exhibited shorter cellular telomere length compared to that of control groups. A large number of cohort studies have consistently reported an association between telomere length and mortality risk.45-47 Shortening of leukocyte telomere length (LTL) is also associated with the incidence and progression of age-related diseases, including CVD,48 neuropsychiatric disorders,49, 50 diabetes,51 and multiple cancers (e.g., gastrointestinal, head and neck cancers).52 However, due to the high sensitivity of telomere length to a wide range of factors and great variability within different cell types and tissues, controversies still exist over the relationship between telomeres and ageing.53 Ongoing research is focusing on refining its measurement and interpretation, which may ultimately promote the reliability of telomere length as an ageing biomarker.54
3.2 Omics biomarkers
The term “omics” refers to the high-dimensional analysis of whole molecular biology.55 The concept of omics started with genomics (the study of genes), as DNA was the first discovered biological macromolecule. More recently, technical advances in methodologies including proton nuclear magnetic resonance (NMR), mass spectrometry, and array/chip-based methodologies,56, 57 transcriptomics (the study of all RNA molecules) and proteomics (the study of proteins) have brought to light biomarkers of ageing.
Currently, omics can interpret near-entire biologic data from organisms including epigenomics (the study of supporting structure of genome including modifications on DNA), transcriptomics (the study of all RNA types), metabolomics (the study of small molecules), microbiotics (the study of genes and genomes of microbiota), and so on.
3.2.1 Epigenetics
DNA methylation (DNAm), which refers to the covalent attachment of a methyl group to the fifth carbon of a cytosine residue, determines the epigenetic clock that regulates gene transcription.58, 59 DNAm is a sophisticated chemical modification system, where depending on its activation or inhibition, can alter genomes of different cell types and development processes.60-62 In human, DNAm occur most frequently at cytosine–guanine dinucleotides (CpGs), which is associated with ageing.63
DNAm status is important in the field of ageing because as one gets older the profile of the methylation status of CpGs alters at different sites of the genome.59, 64 It was also demonstrated that millions of CpGs are involved in the ageing process, and a growing body of evidence revealed DNAm status to be significantly associated with chronological age and age-related diseases.65-70
Given the strong links between DNAm status and ageing, DNA methylation is the most studied epigenetic trait to estimate biological age in the last decade.19 In 2013, the epigenetic clock was proposed by Hannum et al. based on whole blood from full age range samples including 71 CpGs.71 Soon after, Horvath proposed the multi-tissue-based epigenetic clock and identified 353 CpGs within their database.72 Subsequently, many other epi-clocks have been discovered based on different biological samples, populations, and statistical modelling methods. Existing epi-clocks may vary in the amount and site of CpGs, but most of them exhibited high accuracy in predicting chronological age with a correlation coefficient of approximately 0.9 with chronological age and an MAE < 5 years. Table 1 summarises the accuracy and other characteristics of the epi-clocks.
| Clock (author, year) | Bio-sample | Sample size | Sample age range (years) | Identified CpG sites | Platform | Target | Regression model | Accuracy |
|---|---|---|---|---|---|---|---|---|
| Hannum, 2013 | Whole blood | 656 | 19–101 | 71 | Illumina HiSeq platform | Chronological age | Multivariate linear model (elastic net algorithm) | R: 0.96; MAE: 3.9 years |
| Horvath, 2013 | Multitissue (51) | 72 | 0–100 | 353 | Illumina 27 K or Illumina 450 K array platform | Chronological age | Penalized regression model | R: 0.96; MAE: 3.6 years |
| Horvath, 2013 | Multitissue (51) | 72 | 0–100 | 110 | Illumina 27 K or Illumina 450 K array platform | Chronological age | Penalized regression model | R: 0.95; MAE: 4.0 years |
| Weidner, 2014 | Blood | 575 | 0–78 | 102 | HumanMethylation27 BeadChip platform | Chronological age | Multivariate linear model and leave-one-out validation | R2: 0.98; MAE: 3.34 years |
| Lin, 2016 | Blood | 2756 | - | 99; | Illumina 450 K | Chronological age | Multivariate linear model | R: 0.97 Median error: 3.45 years |
| Lin, 2016 | Blood | 2756 | - | 3 | Illumina 450 K | Chronological age | Multivariate linear model | R: 0.79 Median error: 10.9 years |
| Horvath, 2018 | Multi-tissue | 896 | 0.28–94 | 391 | Infinium 450 K and the EPIC array | Chronological age | Elastic net regression | R: 0.74– 0.99 MAE: 1–18 years |
| McEven 2020, | Buccal cell, saliva, and blood | 1721 | 0-20 | 94 | Infinium 450 K and the EPIC array | Chronological age | Elastic net regression | R: 0.98 Median absolute error: 0.35 years |
| Zhang, 2017 | Blood and saliva | 13,661 | 2–104 | 514 | HumanMethylation450 chips and Illumina EPIC (850 K) arrays | Chronological age | Elastic net and best linear unbiased prediction | R: 0.99 RMSE: 2.04 years |
| Levine, 2018, (PhenoAge) | Blood | 456 | 21–100 | 513 | Illumina Infinium 450 K array and Illumina EPIC methylation array | Phenotypic age | Cox penalized regression model | R: 0.71 |
| Lu, 2019 (GrimAge) | Blood | 2356 | - | 1030 | Illumina Infinium 450 K array and Illumina EPIC methylation array | Lifespan | Elastic net regression | R: 0.82 |
| Liu, 2020 | Blood | 2993 | - | - | Illumina array | All-cause mortality | Elastic net Cox regression | R: 0.63–0.82 |
Data from participants with age-related diseases have established the concept of epigenetic age acceleration (EAA), which is either defined as the residual of regressing DNAm age on chronological age or the difference between DNAm age and chronological age. For instance, patients with ischemic stroke are considered epigenetically older than healthy controls despite having the same chronological age.73 In African Americans, EAA was significantly associated with CVD risk factors, such as systolic blood pressure and fasting glucose.74 In terms of functional markers, it was found that people with greater EAA had poorer performance on lung function, walking speed, grip strength, cognitive ability,75, 76 verbal fluency,77 selective attention task, and frailty.78, 79
Longitudinal studies have further revealed an association between EAA and a higher risk of all-cause mortality,80, 81 and specific leading causes of deaths, such as cancer-related mortality and CVD-related mortality in EAA.65, 72, 82-84 EAA is not only associated with risk of cancer but also the prognosis of cancer.85-90 Independent of chronological age and traditional CVD risk factors, greater EAA was a promising predictor for incident fatal coronary heart disease, peripheral arterial disease, heart failure, and ischemic stroke outcome.74, 91-93 Moreover, EAA was associated with higher risk for incident CVD events, and a predictor for dementia,94 Alzheimer's Disease,95 Parkinson's Disease,96 and posttraumatic stress disorder (PTSD).97 EAA is also documented to be associated with changes in frailty over time.98
3.2.2 Transcriptomics
Transcriptomics study the complete set of RNA transcripts in cells or tissues.99 Initially the transcriptome was restricted to messenger RNA (mRNA) coded by DNA when the central dogma was established, but was later extended to transfer RNA and ribosomal RNA.100 The recent advance of Next-Generation Sequencing (NGS) technology founded noncoding RNAs (ncRNA), which expanded the bank of transcriptomics available for analysis.101
Previous studies show age-associated changes in RNA expression patterns of different tissues including the brain, skin, blood, and kidney.102-104 MicroRNAs (miRNAs), which are newly identified noncoding RNAs, were strongly correlated with ageing and age-related diseases.105-107
Harries and colleagues first developed the concept of transcriptomic age, which used five transcripts derived from blood samples based on linear model analysis to predict chronological age.108 It achieved 95% accuracy when distinguishing young (<65 years) from old individuals (≥75 years).108 Later, Fleischer et al. constructed a transcriptome-derived ageing clock using RNA sequences from fibroblast cell lines which had a median error of 4 years.109 Using similar statistical models, subsequent studies on transcriptomic age achieved correlations of 0.348–0.744 when comparing chronological versus transcriptome-estimated age in different cohorts, achieving an MAE of 7.8 years.110 Recently, Holzscheck leveraged artificial neural networks to predict age using mRNA-sequenced data from 887 subjects aged 30–89 years, and the MAE was reduced to 4.7 years.111 In addition to mRNA, miRNA age constructed by Huan and colleagues used an elastic net regression model which incorporated 80 miRNA expressions and showed moderate correlation with chronological age in the replication sets (r = 0.65).112 Another study used seven machine learning algorithms to predict age from six age-related miRNAs from blood samples, with the AdaBoost model exhibiting the best performance and an MAE of 5.52 years.113 Table 2 summarizes performance-related characteristics of transcriptomic age.
| Clock | Author year | Biosample | Sample size | Sample age range (years) | Identified sites | Sequence technology | Target | Regression Model | Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| Transcriptomic age | Harries, 2011 | Blood | 698 | 30–104 | 5 transcripts | Transcriptomic microarray | To distinguish young (<65 years) from old (≥75 years) | Linear model analysis | 0.95% variation |
| transcriptomic age | Fleischer, 2018 | Dermal fibroblasts | 133 | 1–94 | Transcripts | Genome-wide RNA-seq | Chronological age | Linear discriminant analysis (LDA) classifiers | Median error: 4 years; MAE: 7.7 years |
| Transcriptomic age | Peter, 2015 | Blood | 7074 samples | - | 1497 transcripts | Gene chips | Chronological age | Linear model analysis | MAE: 7.8 years |
| Transcriptomic age | Holzscheck, 2021 | Skin | 887 | 30–89 | Transcripts | RNA sequencing | Chronological age | Artificial neural networks | MAE: 4.7 years |
| MicroRNA age | Huan, 2018 | Blood | 2610 | - | 80 microRNAs | TaqMan chemistry-based miRNA assays | Chronological age | Elastic net regression | R: 0.65 |
| MicroRNA age | Fang, 2020 | Blood | 100 | 21–68 | 6 miRNAs | Massive parallel sequencing | Chronological age | AdaBoost-machine learning | MAE: 5.52 in male; MAE: 7.46 in female |
| GlycanAge | Kristic, 2013 | Blood | 2035 | - | - | Hydrophilic interaction chromatography | Chronological age | Regression | 58% variance |
| IgG N-glycan age | Yu, 2016 | Plasma | 701 | NA | 10 IgG N-glycans | Hydrophilic interaction liquid chromatography | Chronological age | Binominal regression | R: 0.56 29.4% variation |
| Proteomics age no | Tanaka, 2018 | Plasma | 240 | 22–93 | 76 proteins | SOMAscan assay | Chronological age | Elastic net regression | R: 0.94 MAE: 5.7 years |
| Proteomics age | Johnson, 2020 | Plasma | 3301 | 18–76 | 23 proteins | - | Chronological age | Linear models | R: 0.97 MAE: 5.5 years |
| IgG N-glycan age | Lehallier, 2019 | Plasma | 4263 | 18–95 | 373 proteins | The SOMAScan platform | Chronological age | LASSO model | R: 0.93–0.97 |
| metabolic age | Hertel, 2016 | Urine | 1700 women; 1911 men | NA | 59 metabolites | NMR spectroscopy | Chronological age | Linear regression | R: 0.74 in men R: 0.78 in women |
| MetaboAge | Robinson, 2020 | Urine, serum | 2239 | 19.2–65.2 | 1311 features | NMR and liquid chromatography–mass spectrometry (UPLC-MS) | Chronological age | Elastic net models | R: 0.86, MAE: 3.71 years |
| MetaboAge | Akker, 2020 | Blood | 25,000 | - | 56 metabolites | NMR | Chronological age | Liner regression | - |
| Microbiome age | Galkin, 2020 | Stool | 1165 | 18–90 | - | Gut metagenomics | Chronological age | Deep neural network (DNN) | MAE: 5.91 years |
| Microbiome age | Gopu, 2020 | Stool | 90,303 | <1–104 | - | Microbial genes | Chronological age | Elastic Net | MAE: 7.64 years |
| Biophysical age | Denis Wirtz, 2017 | Dermal fibroblast | 32 | 2–96 | 2 biophysical parameters | - | Chronological age | Regression | 6–7 years |
Cross-sectional studies have shown older transcriptome-derived age was associated with several age-related phenotypes including higher blood pressure, body mass index, serum cholesterol, glucose, urea and albumin levels, and lower interleukin-6.110, 114 The transcriptomic clock based on skin fibroblasts also predicted higher ages in progeria patients compared with age-matched controls.109 This miRNA age was also associated with coronary heart disease, hypertension, blood pressure, glucose levels, and an increased risk of all-cause mortality.112 The validity of transcriptomic age in predicting age-specific outcomes requires further validation in larger and more diverse longitudinal cohort studies.
3.2.3 Proteomics
Proteomics study the entire set of proteins produced or modified by an organism or system. The recent advance in mass spectrometry allows for high-throughput characterization of large-scale proteins, making it suitable for disease screening.115
Proteins drive different biological functions and processes, including ageing,116, 117 and numerous studies have demonstrated that proteins may drive ageing, as evidenced by samples from serum, bone marrow, cerebrospinal fluid (CSF), and skin.118-122 For example, Baird et al. detected age-dependent changes of 82 proteins in CSF samples from normal ageing adults.122
A GlycanAge was constructed by Kristic et al. that utilized immunoglobulin G (IgG) glycosylation data from multiple cohorts to demonstrate this protein explains 58% of the variance in chronological age.123 Yu developed a similar IgG glycosylation age using 10 IgG N-glycans and found a moderate correlation between predicted age and chronological age, with a correlation efficient of 0.56.124 Other studies have built proteomic clocks based on plasma protein levels that change with age and reported good accuracy in predicting chronological age.119, 125-127 The proteomic age based on 76 proteins created by Tanaka et al. was highly correlated with chronological age (r = 0.94) with an MAE of 5.7 years.119 Another study built a proteomic age profile using 23 proteins and reported an MAE of 5.5 years and correlation efficiency of 0.97.126 Detailed characteristics of proteomics-derived age are described in Table 2.
Proteomic clocks are reassociated with several ageing phenotypes, including physical function, cognitive function, and ageing markers such as glucose and triglycerides levels.123, 124 From a longitudinal perspective, proteomic age is also an independent predictor for future risk of all-cause mortality, multimorbidity, health-span, and lifespan.119, 125 Despite these promising findings, the high complexity of the proteome and lacking of standardized measurement tools make the proteomic age still in its early stages.
3.2.4 Metabolomics
Metabolomics is the systematic study of metabolites.128 Previously the definition of a metabolite was defined as a molecule less than 1.5 kDa in size, but technical advancements have evolved this definition to amino acids, nucleic acids, carbohydrates, fatty acids (FAs), functional nutrients (e.g., vitamins and cofactors), inflammatory factors, hormones, and any other cell metabolism products.129
Ageing and metabolism are undoubtedly linked, given the evidence that metabolites are actively involved in the ageing processes.130, 131 For example, alterations in metabolites such as lipid profiles,132-134 steroid hormones,131, 135 oxidative products, and inflammatory factors136 are considered to underpin parts of the ageing processes. Thus, these metabolites could be used to develop biomarkers of age.
As detailed in (Table 2), the metabolic age score was first constructed by Hertel in 2016 based on 59 metabolites from human urine samples, where metabolome-derived age was highly correlated with the chronological age (r = 0.74–0.78).137 Using multiple metabolomic data sets, Robinson and his colleagues proposed a prediction model using elastic net models, observing an MAE of 3.71 years and a correlation of 0.86 between predicting and chronological age in the training set.138 Another study used over 25,000 samples to develop a 56 metabolite-based age predictor (metaboAge).139
Metabolic age is associated with obesity, diabetes, and depression.138 In independent cohorts, the value of the metaboAge gap, the difference between the metabolic age and chronological age, was significantly associated with an increased risk for mortality, cardiovascular events, and prospective functional decline.138, 139 Similar to proteomic age, metabolic age also presents several challenges including the complex and interconnected nature of metabolic pathways and the lack of standardized protocols.
3.2.5 Microbiotics
Microbiotics refers to the study of the microbiome to characterize its structure, function, and dynamics. The gut microbiota plays a major role in human healthy ageing through its principle functions of maintaining gut diversity and homeostasis.140 In the past decade, a large number of age-related changes in gut microbiota has been noted,141, 142 and these modifications were associated with the interactions between the gut immune system and microbiota.143
In 2020, Galkin first developed a gut microbiome age clock based on gut metagenomic profiles. This was trained by data from 1165 healthy individuals using a deep neural network (DNN), achieving an MAE of 5.91 years (Table 2).144 In an elastic net model, Gopu et al. built a similar clock with the expression of microbial genes in stool samples from 90,303 individuals and reported an MAE of 7.64 years.145 The microbiome clock is currently limited to one cross-sectional study and was associated with diabetes.144
3.3 Biophysical biomarkers
Apart from biochemical signals, cell mechanics and tissue rigidity have also emerged as crucial aspects of ageing.146 Cell mechanical properties refer to the cells' dynamic resistance to deformation due to applied forces or cell rheology.147 Previous studies have shown age-associated biophysical properties of cells.148 In general, cell stiffness increases while the cell's ability to undergo reversible deformations and rearrangement of the cytoskeleton reduces with ageing.149-152 Alterations in cell mechanics have been linked to functional impairments of tissues and organs, leading to a variety of age-related diseases.148
Denis Wirtz and colleagues have recently reported a new set of biomarkers based on cellular biophysical properties.146 By using a combination of two biophysical parameters and a cohort aged between 2 and 96 years, they achieved an average predictive error ranging from 6 to 7 years. A recent review has discussed the promising potential of combining biophysical and biochemical changes of T cells in age prediction.153 However, further studies are still needed to validate this.
3.4 Blood-based biomarkers
Blood tests are common in clinical practice for the surveillance of certain diseases and conditions. With an abundance of historical data sets, blood tests provide biologically relevant and standardized data for age prediction. Table 3 summarizes blood-based ageing biomarkers.
| Clock | Author, year | Bio-sample | Sample size | Sample age range (years) | Blood-profile | Target | Regression model | Accuracy |
|---|---|---|---|---|---|---|---|---|
| Blood Biochemistry and cell count | Putin, 2016 | Blood | 62,419 | - | 41 blood markers | Chronological age | DNN | R: 0.91 R2: 0.82 MAE: 5.55 |
| blood biochemistry and blood count | Mamoshina, 2018 | Blood | 55,751 | 20–80 years | 19 blood biochemistry and blood count | Chronological age | DNN | MAE: 5.94 years R2: 0.65 |
| Blood Biochemistry and cell count | Mamoshina, 2019 | Blood | 149,000 | 51–60 years | 23 blood tests | Chronological age | Deep neural network | MAE: 5.72 years R2 = 0.56 |
| blood biochemistry and blood count | Gialluisi, 2022 | Blood | 23,858 | ≥35 years | 36 circulating markers | Chronological age | DNN | R: 0.76 R2: 0.57 MAE: 6.00 years |
| Physiological age | PeretZ, 2022 | Blood | 472,189 | 37–82 years | 61 laboratory tests | Chronological age | XGboost model | MSE: 6.67 years |
| IMM-AGE | Alpert, 2019 | Blood | 135 | 20–32, 60–96 years | Cell subsets | Chronological age | Linear regression | - |
| Immune age | Lambert, 2022 | Blood | 28 + 28 + 25 | 2–55 years | 19 immune cell subsets | Chronological age | Linear regression model | R2: 0.92 |
| iAGE | Sayed, 2021 | Blood | 1001 | 8–96 years | 50 circulating immune proteins | Chronological age | Auto-encoder (DNN) | R: 0.78 |
| ipAGE | Yusipov, 2022 | Blood | 159 | 24–89 years | 38 circulating inflammatory/immunological proteins | Chronological age | Elastic net regression model | R: 0.79 MAE: 6.82 years |
3.4.1 Blood routine and biochemistry
Blood draws are part of routine examinations which test the quantitative and morphological changes in blood cells, including red blood cells, white blood cells, and platelets. Analysis of blood biochemistry provides the biological characteristics to estimate the function of organs. These are commonly used and carry low economic burden for tracking overall health in clinical settings.
Since 1991, studies exploring the influence of age on peripheral blood parameters have detected changes in blood cell counts with ageing.154, 155 With advancing age, routine blood tests exhibited decreased levels of hemoglobin levels, white blood cell, and platelet count,155-157 while blood biochemistry revealed impaired physiological function as indicated by decreased glomerular filtration rate and elevated creatinine levels.157-159 Liver enzymes such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transferase (GGT) also showed age-dependent changes both in men and women.160
The first biochemistry-based clock for ageing was developed by Zhavoronkov's lab in 2016 using 41 markers from blood biochemistry. Using DNNs, this clock predicted chronological age with an MAE of 5.55 years (Table 3).161 Similar ageing clocks were developed from the same authors using DNN but with just 19 and 23 parameters, achieving an MAE of 5.94 and 5.72 years, respectively.162, 163 Another blood-based ageing clock, using DNNs for 36 circulating biomarkers from Italian populations found an MAE of 6.00 years.164 Lastly, Peretz et al. trained an XGboost model using 61 blood parameters from 472,189 subjects aged 37–82 years old and achieved a RMSE of 6.67 years.165
Biochemistry-based clocks suggested younger biological age is associated with fewer comorbidities, operations, and prevalence of age-related diseases, alongside a better physical and mental wellbeing than age-matched controls.164, 165 Biochemistry-based age clocks could predict mortality, dementia, and hospitalization risk.163, 164, 166
3.4.2 Immune profiles
The immune system defends and protects the body from foreign pathogens. The important role of the immune system in maintaining the homeostasis within human body has been recognized for hundreds of years. The term immunosenescence has been developed to describe the age-related changes of both innate and acquired immunity at the cellular and serological level.167 This is usually characterized by the senescence of specific immune cell populations and impaired immune function to mount appropriate responses to immunogenic stimuli,168 leading to an increased vulnerability to infectious and chronic diseases.169 Additionally, age-related changes in the immune system's composition can contribute to low-grade systemic inflammation, which is one of the key factors for the pathogenesis of many morbidities including cardiovascular diseases and neurodegenerative diseases.170
Alpert and colleagues have recently developed an aggregated score IMM-AGE which remodeled the immune cell composition derived from flow cytometry (Table 3).171 Another study generated immune clocks using 19 immunity-related parameters trained using healthy participants and demonstrated a high correlation with chronological age (r2 = 0.92).172 People with Down's syndrome have shown accelerated immune age from childhood.172 Immune age can also predict all-cause mortality, but a paucity of data exists.171
3.4.3 Inflammatory profiles
Inflammation is a defense mechanism mediated by inflammatory proteins in response to perceived noxious stimuli. Concomitant with the broad deregulation of functioning immune cells with ageing, accumulating evidence indicates that inflammatory molecules moderately rise, touted as “inflammageing.”173 Most older individuals have numerous elevated proinflammatory markers (termed the senescence-associated secretory phenotype or SASP) like C-reactive protein (CRP),174 interleukin 6 (IL-6),175 interferon α and interferon β,176 transforming growth factor-β (TGF-β),177 and serum chemokines MCP-1.178 These SASP would modify the tissue microenvironment and alter the function of nearby normal cells.179 A prolonged and persisting inflammation will result in the accumulation of tissue damage, ultimately leading to age-related pathologies.170
Sayed et al. proposed an inflammatory ageing clock (iAge) from 50 circulating markers of inflammation, including cytokines and growth factors, all of which highly correlated with chronological age (r = 0.78).178 A similar inflammatory clock derived from 38 inflammatory markers was subsequentially developed using machine learning and achieved an MAE of 6.8 years.180 Inflammation age was associated with ESRD status and could predict multimorbidity and frailty.180, 181
4 IMAGE-BASED AGEING BIOMARKERS
4.1 Brain age
The concept of brain age was proposed with the knowledge that structures and functions of the brain change with the ageing process. Neural ageing is a significant part of human ageing, and neuroimaging, such as magnetic resonance imaging (MRI) and Positron Emission Tomography (PET), provides a unique window to identify neural ageing.182 For instance, structural MRI showed various age-related brain alterations including gray matter (GM) volume, GM concentration, connectivity of white matter, cortical thickness, cerebrospinal fluid increment, and so forth.183-185
Brain age was first built upon structural MRI scans where features such as the gray matter volume, cortical thickness were extracted to predict chronological age.183, 186 In 2010, Franke et al. used a correlation vector machine to predict age based on gray matter density with an MAE of 4.98 years and correlation coefficient of 0.92.186 Later, other processing methods, such as Gaussian processes regression model187 and support vector regression188 were applied to predict age using neuroimaging. Though overall these models achieved high accuracy in age prediction, the image-processing before model constructions was time-consuming and labor-intensive.
Recent emergence of artificial intelligence (AI) techniques, especially deep learning (DL), could enable age predictions from raw and unprocessed neuroimaging data, thus obviating the reliance on time-consuming preprocessing and improving the efficiency of age prediction. Cole et al. leveraged convolutional neural networks (CNN) to predict brain age based on raw T1-weighted MRI data, achieving an MAE of 4.16 years and correlation of 0.96.26 Various models trained on neuroimaging (T1/2 weighted MRI,magnetoencephalography, etc.) by DL techniques (Table 4) have been developed to predict age with reasonable accuracy and demonstrate strong correlation between predicted brain age and chronological age.189-192 Recent studies demonstrated the utility of multimodality neuroimaging data in terms of age prediction.193, 194
| Clock (author, year) | Sample size | Sample age range (years) | Image modality | Target | Regression model | Accuracy |
|---|---|---|---|---|---|---|
| Franke, 2010 | 655 | 19–86 | T1W MRI | Chronological age | Kernel method | R: 0.92 MAE: 5 years |
| Lin, 2016 | 112 | 50–79 | T1W MRI | Chronological age | Principal component analysis | R: 0.8 MAE: 4.29 years |
| Cole, 2017 | 2001 | 18–90 | T1W MRI | Chronological age | CNN | R: 0.96 MAE: 4.16 years |
| Chung, 2018 | 953 | 3–21 | T1W MRI | Chronological age | Supervised machine learning | R2: 0.84 MAE: 1.69 years |
| Kuhn, 2018 | 765 | 20–78 | Diffusion tensor imaging | Chronological age | Support vector regression | R: 0.84 MAE: 7.39 years |
| Beheshti, 2019 | 675 | 45–95 | T1W MRI | Chronological age | Support vector regression | R2: 0.88 MAE: 2.36 years |
| Wang, 2019 | 3688 | - | T1W MRI | Chronological age | CNN | R: 0.85 MAE: 4.45 years |
| He, 2021 | 16,705 | 0–97 | T1W MRI | Chronological age | CNN | R: 0.98 MAE: 3 years |
| Hwang, 2021 | 1530 | 20–90 | T2W MRI | Chronological age | CNN | R: 0.98 MAE: 4.22 years |
| Rockicki, 2021 | 750 | 18–86 | Multimodal MRI (T1W, T2W ASL) |
Chronological age | Random forest | R2: 0.77 MAE: 6.4 years |
| Porxas, 2021 | 613 | 18–88 | MRI + MEG | Chronological age | Principal Component Analysis (PCA) and Canonical Correlation Analysis with Gaussian process regression model | R: 0.98 MAE: 4.88 years |
The difference between brain age and chronological age, also known as brain age gap, has been found to be associated with brain conditions in an array of cross-sectional studies. Accelerated brain ageing, indexed by a positive value of brain age gap, was reported to be associated with poorer cognitive performance,27, 195, 196 increased odds of dementia (Alzheimer's disease),187, 197, 198 major depressive disorders (depression, anxiety),199-201 schizophrenia,188, 202 and epilepsy.203 In addition, larger brain age gap was linked with diabetes.204
In longitudinal studies, brain age gap was found to be an independent predictor for incident dementia.204 Subjects with older brain age in childhood had lower cognitive ability in adulthood.205 In addition to brain diseases, accelerated brain ageing was also associated with increased future risk of mortality.24
4.2 Retinal age
An increasing amount of evidence points to the retina as a window to the body. The retina shares similar embryological origins, anatomical structures, and physiological features with vital organs, such as the heart, brain, and kidney.206 Originating from the diencephalon, the retina is composed of several neuronal cell types and is connected to the central nervous system (CNS) through the optic nerve.207 The microvasculature of the retina is closely connected with that of the brain, heart, and kidney.208 A growing number of studies suggest retinal microvascular alterations in the neurons and microvasculature could reliably reflect cerebrovascular diseases, neurodegenerative diseases, and systemic circulation health outcomes.206, 209, 210
Given the strong association between eyes and vital organs of the human body, age estimation from retinal imaging has become a hot topic. Liu et al. proposed a CNN model for age estimation from fundus images and achieved an MAE of 3.73 years in a Chinese population.211 Similarly, Zhu et al. developed an age prediction model based on fundus images from a healthy population in the UK biobank data set, which achieved a correlation of 0.81 between predicted retinal age and chronological age, and an MAE of 3.55 years (Table 5).212 A research team from Singapore developed RetiAGE, which determined the probability of age being ≥ 65 years from a fundus photo, achieving an AUROC of 0.968 and correlation of 0.62 with chronological age.208
| Clock | Clock (author, year) | Sample size | Sample age range (years) | Image modality | Target | Regression model | Accuracy |
|---|---|---|---|---|---|---|---|
| Retinal age | Liu, 2019 | 8736 | >50 | Fundus image | Chronological age | CNN | MAE: 3.73 |
| Retinal age | Zhu, 2022 | 19200 | 40–70 | Fundus image | Chronological age | Xception architecture | R: 0.81 MAE: 3.55 |
| RetiAGE | Nusinovici, 2022 | 40,480 | - | Fundus image | Probability of age being ≥65 years | Visual Geometry Group (VGG)-DNN | R: 0.62 |
| OCT age | Shigueoka, 2021 | 7217 | 20.8–85.8 | Optical coherence tomography | Chronological age | CNN | R: 0.86 MAE: 5.82 |
| Facial age | Geng, 2007 | 1002 | 0–69 | 2D human face images | Chronological age | Primary component analysis | MAE: 6.22-14.83 |
| Facial age | Guo, 2008 | 8000 | 0–93 | 2D human face images | Chronological age | Manifold learning and locally adjusted robust regression | MAE: 5.25, 5.30 |
| PhotoAgeClock | Bobrov, 2018 | 8414 | 20–80 | Eye corners images | Chronological age | Xception model | R: 0.95 MAD: 2.3 |
| Facial age | Chen, 2015 | 322 | 17–77 | 3D human face images | Chronological age | Partial least squares regression model; support vector regression model | R: 0.85 MAD: 6.10–6.21 |
| Facial age | Xia, 2020 | 4719 | 20–80 | 3D human face images | Chronological age | CNN | R: 0.95 MAE: 2.9 |
| ECG-age | Starc, 2012 | 377 | 4–75 | 12-lead ECG | Chronological age | Multiple linear regression | R2: 0.76 |
| ECG-age | Ball, 2014 | 1438 | 20+ | 5-min 12-lead ECG test | Chronological age | Bayesian approach | - |
| ECG-age | Attia, 2019 | 774,783 | 18+ | 12-lead ECGs | Chronological age | CNN | MAE: 6.9 years R2: 0.7 |
| ECG-age | Lima, 2021 | 1,558,415 | - | 12-lead ECG | Chronological age | DNN | MAE: 8.38–10.04 years |
| abdominal age | Goallec, 2022 | 82,336 | 37–82 | Liver and pancreas MRIs | Chronological age | CNN | R2: 73.3 MAE: 2.94 years |
| CXR-Age | Raghu, 2021 | 116,035 | 40–100 | Chest X-ray | Chronological age | CNN | R: 0.37 |
- Abbreviations: CNN, convolutional neural networks; ECG, electrocardiogram; MRI, magnetic resonance imaging.
A breakthrough using optical coherence tomography (OCT) for age estimates has been noted once, with Leonardo et al. developing a DL-based age prediction model using B-scans, which obtained an MAE of 5.82 years and strong correlation between the predicted age and chronological age (r = 0.860).213 Compared with two-dimensional (2D) fundus images, OCT could capture three-dimensional (3D) and micrometer level structures of the retina, providing optical biopsy of neuroanatomical and vascular changes with high resolution (~5 μm).214 More research is needed to test the value of OCT for biological markers of ageing.
Longitudinal studies showed the retinal age gap independently predicted the risk for all-cause and cause-specific mortality208, 212 and age-related morbidities, including neurodegenerative disease,215 cardiovascular diseases,208, 216 and kidney failure.217
4.3 Face age
Ageing of facial features is a phenotype of human ageing,218-221 where alterations in facial skin texture, soft tissue, and bone volume lead to the sunken appearance of eye sockets, reduced angle of brow, and changes to the angles of the lower jaw.218, 222
In 2007, 2D facial images first predicted chronological age using regression statistical method and achieved an MAE of 6.22 years (Table 5).223 Using a manifold learning method and a locally adjusted robust regression model, Guo et al. devised an automatic 2D-image estimation system for age prediction with an MAE of 5.25 years for female and 5.30 years for male.224 In 2018, Eugene et al. developed the PhotoAgeClock, using deep-learning network that focused on photographs of eye corners from facial images to predict chronological age. It could predict chronological age with an MAE of 2.3 years.225 As 3D imaging techniques improved, features of 3D facial images were integrated into biomarkers of ageing. Using a partial least squares regression model and support vector regression model, the 3D-based face age revealed a correlation coefficient of 0.95 and an MAE of 6.11 and 6.10 years in females and males, respectively.218 Later, the application of CNNs on 3D images achieved an MAE of 2.79 years.22
The difference between face age and chronological age (face age gap) was associated with health and lifestyle parameters (obesity, blood pressure, transglutaminase, alkaline phosphatase, cholesterol, etc.), inflammation, and innate immunity at the transcriptome level.22 Notably, face age could better reflect general health status than chronological age.218 Previous studies reveal face age reflects the heterogeneity of the human ageing rate and the face age gap could predict mortality risk among older people.226 However, the use of antiageing skin medications (i.e., tretinoin), natural fat mass in the face, and cosmetic procedures could obscure and confound the accuracy of face age.
4.4 Electrocardiogram (ECG) age
Recently the ECG was explored for its reflection of biological cardiac age, considering older individuals have age-related changes to wave amplitude, duration, inter-interval variability, and electrical axis of ECG leads.227, 228 Additionally, older individuals are more likely to have ECG abnormalities such as atrial fibrillation or flutter, complete right bundle branch block, and ischemic changes.229, 230
The first ECG-derived age prediction model used multiple linear regression analysis from five ECG parameters with the resulting predicted age being highly correlated with chronological age (r2 = 0.76, Table 5).231 Another ECG-based age was developed using a Bayesian approach derived from normal 12-lead ECG of 1438 healthy populations.232 Attia et al. later trained a CNN model to predict a person's age using the 12-lead ECG signals from 499,727 patients and the ECG-age achieved an MAE of 6.9 years in testing data sets and an r2 = 0.7).233 Later, deep neural networks were trained and achieved an MAE of 8.38–10.04 years in different testing cohorts.234
ECG-derived age was associated with lower ejection fraction, and an risk of hypertension, coronary diseases, and mortality.232-234
4.5 Others
The usefulness of abdominal MRI scans as an ageing biomarker was explored using CNN analysis and achieved an MAE of 2.94 years (Table 5).235 Chest X-ray age (CXR-Age), also derived through CNNs detecting characteristics of chest radiographs, obtained a correlation of 0.37 with chronological age (Table 5) and was associated with cardiovascular risk factors.236
Skeletal parameters derived from X-ray images were also used for estimations of both dental age and skeletal age.237-241 However, these are used to estimate chronological age for forensic practices rather than to reflect biological ageing.242-244
5 OTHER CLINICAL MEASURE-BASED AGEING BIOMARKERS
5.1 Lung age
The concept of lung age was developed in 1985 to show the premature ageing of the lung in smokers.245 By recording the volume and speed of air inhaled and exhaled, spirometry provides a subjective indicator of pulmonary function.246
Spirometric lung-age (SLA) was first proposed in 1985 using spirometry values from healthy adults.245 Linear equations were developed using the forced expiratory volume at 1 s (FEV1), and the estimated SLA achieved a standard error of 15.8 years.245 Newbury et al. updated this equation for Australians and found lung age to be equivalent to chronological age in the never-smoker group.247 Subsequently, several research teams developed different SLA-calculation equations based on different populations and spirometry variables.248-250
Lung age was significantly older in patients with morbid obesity, severe airflow limitation, and COPD.248-251
5.2 Cognitive age
Normal ageing is associated with a decline in cognitive function, specifically in memory, language, visuospatial, and executive function abilities.252 Besides, older individuals exhibit higher risk for cognition-related illnesses, such as dementia or mild cognitive impairment (MCI).253, 254 Therefore, cognitive clocks have been developed as a novel indicator of brain health.
Machine learning was first used to generate a model for neurocognitive age in healthy adults based on nine standardized cognitive tests, achieving an MAE of 2.2 years in healthy populations.255 Another study characterized nonlinear cognitive trajectory patterns indexed as mini-mental state examination scores across years until death. The correlation between this cognitive age and chronological age was 0.56 at baseline, and 0.43 at death.256
Cognitive age demonstrated strong associations with neuropathology and brain volume, and this method outperformed chronological age for predicting AD, dementia, MCI, and mortality.255, 256
5.3 Heart age
The concept of heart age was initially introduced into CVD management as an alternative way to express CVD risk scores.257 Age, sex, hypertension, dyslipidemia, diabetes, and unhealthy lifestyles are widely established risk factors for developing CVD.258 Developed from these factors, vascular age now is commonly considered a way for tracking vascular health.257
In 2008, the Framingham Heart Study introduced the concept of heart/vascular age based on the CVD risk factor profile.257 The heart age was calculated as the chronological age of a person with the same predicted CVD risk but with other risk factors in normal ranges.257 Another study extended the use of heart age with the SCORE project scales most used in Europe.259 Following studies incorporated atherosclerosis indexed from imaging into the current vascular age calculation, such as intima-media thickness (CIMT)260, 261 and coronary arterial calcification (CAC).262, 263 Emerging studies confirmed the value of heart/vascular age in the prediction of all-cause mortality and cardiovascular diseases.264-266
5.4 Biopsychological markers
The ageing process is more psychological than just physical. Psychological age refers to an individual's perception or experience of ageing as well as ageing-related physical frailty and mortality.267 A higher psychological age is related to higher psychological stress and detrimental psychological–physical interplay,268 which may significantly influence age-related biological changes, including systemic inflammation,269, 270 obesity, pulmonary, and muscular dysfunction.269, 271, 272 Subjective age, evaluated by personal feelings of “how old oneself is,” is the most studied form of psychological age and is determined by personal experiences, social relationships, cultural values, and health status.272-274
Recently, a DL-based model enabled quantitative assessment of an individual's psychological age based on multiple features such as health condition, headache frequency, relationship status, and expectations from sex life. This method achieved a mean absolute error of 6.7 years (R2 = 0.56).275 As a biopsychological marker of ageing, older subjective age than chronological age, namely feeling older, has been shown to associate with a higher risk of mortality,276, 277 frailty,278 depressive symptoms, activities of daily living,279 and well-being.267, 280 DL-based psychological age was also predictive of all-cause mortality risk.275
6 COMPOSITE AGEING BIOMARKERS
6.1 Pace of ageing
The pace of ageing acknowledges ageing by tracking and quantifying biomarkers that index cardiovascular, metabolic, renal, immune, and biochemical functions simultaneously to holistically address accelerations in ageing.281, 282 By combining changes in these system across time, a single measurement (e.g., biomarker z-score) can determine the pace of ageing. In a Dunedin cohort, a faster pace of ageing was associated with cognitive deficits, signs of advanced brain ageing (i.e., volume, hippocampal atrophy), diminished sensory-motor functions, and older appearance.281, 283
6.2 Frailty index
Frailty is a functional state of physiological vulnerability and the degree of frailty increases with age.284-286 Derived from frailty, the frailty index is a composite biomarker of biological age and comprises multiple health items (various signs, symptoms, laboratory measurements, disabilities, and diseases).287, 288 It is widely acknowledged that the frailty index is a measurement of malnutrition, wasting, weakness, slowness, and inactivity, and a sign of advanced ageing.288, 289 Frailty index is linked to mental health,290 risks of falls and fractures,291 all-cause mortality, and cause-specific mortality.287, 292-295
7 APPLICATION OF AGEING BIOMARKERS
7.1 Anti-ageing research
Interventions aimed at slowing the ageing process and extending a healthy life span are equivalent to preventive interventions for a positive biological age gap (see the concept of biological age gap in Part 2). Future antiageing research will benefit from an understanding of the emerging predictors of ageing and the exploration of potential biological changes in accelerated ageing.
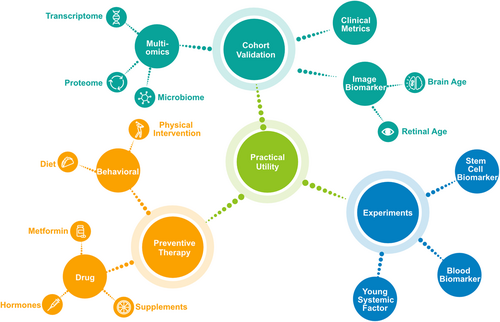
An increasingly important use of biological age in recent years has been as a metric to validate existing behavioural and pharmacological interventions. For example, several clinical trials have used the DNA methylation clock to measure the effects of physical exercise, diet, and antioxidant supplementation.296, 297 In addition, emerging ageing biomarkers, such as proteomic biomarkers, provide new perspectives for targeted drug discovery, although most of these studies have remained experimental with limited clinical application. Furthermore, the field of anti-ageing regenerative medicine is advancing, with investigations exploring the effects and mechanisms of young stem cell components and regulatory factors. These efforts offer inspiring opportunities for the development of novel therapeutic strategies to combat ageing (Figure 4).
7.1.1 Behavioural strategies
An inestimable part of biological ageing is the result of people's physical and social behaviours, including physical activity, diet, smoking and drinking habits, and socioeconomic status, and so forth. Physical activity, especially at moderate to vigorous levels, can provide robust improvements in physiological capacity and multiple organ function, as well as reduce the risk of age-related adverse events.298 Physical activity has also been shown to be effective in ameliorating age-related immune decline, another major contributor to multimorbidity and mortality.299, 300 Another important behavioural approach to ageing is diet. Dietary interventions in patients with diabetes and hypertension have been recommended to reduce the risk of age-related morbidities, with benefits equivalent to or even better than medication.301 Dietary intervention is particularly applicable to the growing ageing population because of its advantages in terms of reducing care, low cost, wide universality, and positive feedback on the mental and social status of the elderly.302
7.1.2 Pharmacological intervention
However, in most situations, behavioural interventions alone are insufficient to treat age-related diseases, possibly due to inconsistent patient compliance and complex interactions with other confounding factors. Targeted pharmacological modulation is a practical approach to further extending lifespan and health against the functional decline of biological ageing. Based on identified biomarkers and pathways of biological ageing, reducing inflammation, suppressing aberrant immune responses, and maintaining cellular homeostasis are current targets of mainstream pharmacological strategies to counteract this process.303 Preventive drug use for common diseases such as diabetes and cardiovascular disease in high-risk populations is already widely used as an anti-ageing tool, like Metformin, doxazosin, and so forth.304-306 The biological age gap can be used to better monitor ageing preclinically, allowing more precise identification of the time point for pharmacological intervention.
7.1.3 Drug discovery
In addition, the discovery and development of novel anti-ageing drugs have emerged as a promising avenue that could significantly alter the future prospects of individuals.307 The identification of emerging biomarkers of ageing, particularly those at the molecular and cellular levels, has provided researchers with a wealth of targets for anti-ageing therapies. With the aid of advanced multi-omics technology and large-scale molecular databases, researchers have accurately pinpointed the dominant plasma proteomic dataset associated with immunological ageing and have identified targeted immunological drugs relevant to age-related diseases.308 Nevertheless, the majority of current research has concentrated on the investigation of existing pharmaceuticals. While experimental studies have explored the utility of emerging biomarkers for the identification of new drug targets, their clinical application remains limited.
7.1.4 Regenerative strategies
During biological ageing, the ability of our stem cells to differentiate into tissue cells gradually decreases. In general, most adult stem cells are in a quiescent state rather than an active state as in an embryo. Emerging regenerative medicine focuses on restoring and rebuilding this regenerative potential by inducing quiescent stem cells to proliferate.309 Transplantation of neural stem cells has been used in the treatment of brain-ageing diseases such as Alzheimer's disease, reducing amyloid deposition and improving cognition in animal models.310, 311 In addition, injecting the blood of younger animals into older animals resulted in the rejuvenation of their aged stem cells, with restored youthful functional and molecular states.310-313 However, it remains unclear whether the effective components are young stem cells or young regulatory factors. Advances in regenerative medicine provide insights for future anti-ageing interventions.
7.2 Promotion in geroscience
7.2.1 Surrogate in clinical outcomes
Ageing biomarkers are popular surrogate endpoints in geroscience trials.314 The use of ageing biomarkers provides a practical solution to the challenges of using traditional primary clinical endpoints, which can occur so infrequently that the statistical power of the trial is compromised. A potential application of biomarkers as surrogate endpoints is measuring the biological age gap in large-scale screening of diseases for preventive purposes. Most screening participants may be asymptomatic, free of age-related morbidities, but still have increased risks of ageing, indexed by high biological age gaps. A positive biological age gap can help us to identify those at high risk of future disease and allow early gero-protective interventions.
7.2.2 Incorporate social policy
Multidisciplinary cooperation with health professionals and society as a whole is needed to tackle the problem of ageing.315 At present, most social programmes and policies for older people are developed based on chronological age as a criterion for eligibility. However, due to the heterogeneity of ageing, there is still a large number of people who do not meet the criteria yet suffer from accelerated biological ageing and impaired health status. With the emerging application of ageing biomarkers in geroscience, the target population of ageing social policies can be identified with greater precision than by reference to chronological age alone. In line with the public health response of the UN Decade of Healthy Ageing (2021–2030), the use of ageing biomarkers can update our perceptions of ageing, facilitate research into the interactions between social determinants and biological changes, and provide individualised health services based on personal characteristics of biological ageing. The subsequent changes in such biomarkers can provide traceable evidence for long-term care.
8 CHALLENGES
Advances in ageing biomarkers may uncover the physiological changes driving the ageing process and provide insight on “healthy” ageing. However, many challenges must be addressed before widespread adoption of ageing biomarkers. First, the methods of defining the ground truth have been varying and undetermined; second, the sources of data that best represent biological ageing remain unknown; third, there is unavoidable bias in improving the model accuracy and a paradox may exist between improving the accuracy of age prediction and validity of reflecting ageing rates; fourth, clinical value of different ageing biomarkers remains to be investigated, especially in longitudinal studies.
8.1 Ground truth of biological age
Of note, there has been no standard way of defining biological age (see details in Part 2).315 Generally, various objective and subjective assessments have served as ground truth of biological age, including objective metrics covering chronological age in the healthy population and age-related phenotypic data (e.g., mortality risk), as well as subjective metrics such as perception of ageing. The prediction of the ageing process may have certain characteristics.Chronological age in a healthy population is considered as the most common way of defining biological age. However, consensus around the definition of a “healthy” ageing population is lacking, and noise or dirty data potentially exist despite the strict definition of the healthy population.316
Given these limitations of defining biological age from the chronological age, age-related phenotypic data, such as mortality risk, healthy life span, and composite clinical measures of phenotypic age are also used to train the model.16, 21-23 Although this avoids using healthy populations and makes the ageing rate determined directly by biologically-related process, it remains unclear which age-related phenotypes best represent the ageing of the body. Some raised that the best way to define biological age may be mortality risk since this metric is an absolute objective estimate of the overall ageing outcome.65 However, very few models are developed based on mortality risk due to the lack of large-scale longitudinal data.23, 80 Regarding the subjective assessment of perceived health, the performance of the ageing biomarker may be subject to recall bias introduced by raters.317, 318
8.2 Data sources for age prediction
This review has classified the data sources of ageing biomarkers into three types: molecular and cellular tests, medical imaging, and other clinical measures. Molecular and cellular biomarkers are derived from human blood or other tissues analyzed either by clinical laboratory tests or multi-omics technology.119, 161 Imaging-based markers are based on medical imaging for clinical practice such as brain MRI, ECG, and fundus images.189, 212, 232 Other clinical measures such as physical assessments and functional markers are also commonly evaluated sources for biological age prediction. Composite biomarkers integrating different types of data sources to predict ageing have also been proposed.281 We also searched patents claiming ageing biomarkers and summarized the relevant patents in Supporting Information: Table S1.
Given molecular and cellular biomarkers based on molecular levels, the mechanism underlying the biomarkers is easier to interpret than imaging and clinical profiles. However, heterogeneity, access, and reproducibility of methodologies and analytical protocols may lead to batch-to-batch variations. In fact, there is little overlap of the molecules used in the “same” ageing clocks from different studies.319 Additionally, the biological ageing rate in one tissue can be quite different from that of another and it is not realistic to obtain a combination of age estimates in several tissues.
Imaging-based markers have the advantage of not requiring invasive sample collection and can be readily acquired. Previous age models need image-processing, which was time-consuming and labour-intensive. Recent emergence of artificial intelligence techniques, especially deep learning, could enable age predictions from raw and unprocessed data while achieving high accuracy. However, the reliability of AI algorithms is still limited due to their “black box” nature.320
Other clinical measures such as physical assessment or functional tests are readily assessable and lend themselves as decent initial screening tools capable of identifying individuals at risk of unhealthy ageing in a clinical setting. However, physical assessments only reflect the functional status of one organ system, such as spirometry used in the estimation of lung age.249, 259
An integration of different types of data in composite biomarkers can reflect the multiple traceable footprints across many different levels and body systems, which improves the quantification of the overall impact of ageing. However, this requires strong computing power and advanced algorithm.
8.3 Model performance accuracy
As discussed in Part 2, previous studies have tried to identify a better-performing marker by comparing the accuracy indicators (e.g., MAE) of ageing biomarkers before incorporating measures of biological age into clinical applications. Although various statistical approaches have been proposed to improve the model's accuracy, some concerns need to be noted. One concern is the dilute effect; that is the age of younger subjects is overestimated while the age of older subjects is underestimated because the models are often influenced by the mean.321 This may cause a negative correlation between age gap and chronological age, leading to spurious associations between age gap and clinical outcomes.321
Another concern is that improved accuracy in predicting chronological age may not be beneficial for age gap reflecting rates of biological ageing. There is still a pitfall that the age gap may be just a measurement error after subtraction or regression. Zhang's group reported that as the accuracy of chronological age prediction increases using an epigenetic clock, the association between biological age gap and mortality or other age-related outcomes attenuates.65, 322 This paradox may remind us that the performance of the model should not be evaluated mainly by accuracy in predicting age but by the value in revealing ageing phenotypes.
8.4 Clinical value validation
Currently, the clinical evaluation for ageing rate estimator is based on health or ageing-related diseases associated parameters. While most estimators mentioned in this review showed associations with health parameters, few studies have compared the validity of different ageing biomarkers predicting health outcomes. Such comparative head-to-head studies incorporating different markers in one study are still lacking,- due to the lack of large cohorts with different data types and difficult access to different algorithms for age estimators.
It is important to note that the vast majority of current ageing biomarkers have been validated on cross-sectional data, thus causal insights into ageing biomarkers have not been studied until recently. Emerging studies of longitudinal cohorts have also provided supporting evidence for the predictive value of established ageing biomarkers in predicting poor health outcomes, (e.g., brain age in predicting age-related strength loss, and retinal age in predicting incident neurodegenerative diseases).323-325 More longitudinal human studies are still warranted with a larger sample size to address questions on the utility of ageing biomarkers in the prediction of pathological ageing and age-related outcomes.
As estimates of ageing were only captured at a single point in current studies, few studies have investigated the dynamic change in these ageing biomarkers in response to environmental factors or antiageing interventions. Protective and risk factors could be identified by comparing the dynamic changes of the ageing biomarkers. Therefore, tracking and reporting the long-term effects of anti-ageing interventions on the biological ageing rate indexed by biomarkers remain as major focuses of future research.
To effectively tackle the challenges posed by ageing research, it is imperative to concentrate efforts, promote multidisciplinary communication, and encourage collaboration. In recognition of this need, the Biomarkers of Aging Consortium (https://www.agingconsortium.org) was recently established by experts in fields such as ageing and longevity, cellular rejuvenation, and multi-omic ageotyping. Given the emerging trends in ageing research, the consortium has emphasized the necessity of systematic validation efforts to identify and establish reliable ageing biomarkers. This requires addressing the challenges posed by the compatibility and generalizability of current biomarkers, as well as the need to evaluate the efficacy of longevity interventions.
9 OUTLOOK
Research on ageing biomarkers has led to significant advances and applications, but much work is still needed to further explore their potential in the future. Breakthroughs are expected in several areas including exploring potential mechanisms, constructing potential biomarkers by combining different data sources or applying new technologies, and validating the clinical value of existing and emerging biomarkers through extensive collaboration and longitudinal study investigation.
Although clinical and population studies have suggested several biomarkers of ageing, we still need laboratory research to elucidate the biological processes and molecular mechanisms involved. Laboratory work is underway to reveal the remarkable malleability of ageing. For example, to investigate how the alterations in CpG sites affect downstream physiological age-related changes, in vitro work is warranted to identify key relevant molecules (e.g. hypoxia-related factors and key methylation enzymes) during epigenetic reprogramming.326, 327 Using experimental vertebrate animals, DNAm age estimators across species can advance our understanding of epigenetic ageing and contribute to the future development of anti-ageing interventions.328-330 Current emerging genetic methodologies, such as Mendelian randomization, are effective in revealing the deep causality of ageing clock theories, providing support for further interpretation of mechanisms.331 The Aging Biomarker Consortium conducted a thorough review that comprehensively evaluated age-related pathophysiological, functional, and morphological changes at the cellular and organ levels, which could serve as a significant foundation for future research.332
Standardized data collection and combination of multimodality data are needed in the future investigation of constructing potential biomarkers. Since each “level” or type of biomarker conveys different information about biological ageing, to obtain optimal performance and biological relevance, multiple data involving a plethora of modalities and functions may have to be used to track multimodality-specific ageing patterns. Michael Snyder and his colleagues have adopted deep multi-omics data to profile “Ageotype” as a pattern of personal ageing.333 Based on such a combination of multimodality data, existing ageing research centers should be engaged to develop standardized measures and methods across research teams, allowing the pooling of study populations and results.
Applying new technologies might also help constructing potential biomarkers. Advances in AI, such as machine learning and DL, may provide advocated solutions to untangle the complexity of ageing.334 AI-based models have unique advantages in handling a large amount of data and show better performance than traditional regression models. With the application of new algorithms such as generative adversarial network (GAN),335 we could generate medical images of a person at an older age that present age-related changes in a more intuitive way.
To further validate the clinical value of existing and emerging biomarkers, constructing different ageing biomarkers in one cohort should be explored to characterize how these biomarkers interact with each and compare their validity in predicting ageing-related health outcomes. Moreover, changes in the different ageing biomarkers over time should also be recorded for tracking protective or risk factors and identifying effective antiageing interventions. Future research should focus on longitudinal studies with a larger sample size to address challenges on the utility of ageing biomarkers in the prediction of pathological ageing and age-related outcomes. Measures of resilience can also be included since resilience is a key feature of intrinsic ageing while age-related outcomes only document damage accumulation over time.
AUTHOR CONTRIBUTIONS
Outline of the review: Zhu ZT, He MG, Gao YX, Cheng CY, Ge ZY, Clark M. Performed the search: Chen RY, Wang YY, Zhang SR. Drafting of the manuscript: Chen RY, Wang YY, Zhang SR, Bulloch G, Zhang JY. Critical revision of the manuscript: Chen RY, Wang YY, Zhang SR, Bulloch G, Zhang JY, Liao H, Shang XW, Peng QS. Obtained funding: He MG, Zhu ZT. Administrative, technical, or material support: Zhu ZT, He MG. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (82101173), High-level Talent Flexible Introduction Fund of Guangdong Provincial People's Hospital (KJ012019530), Fundamental Research Funds of the State Key Laboratory of Ophthalmology (303060202400362), NHMRC Investigator Grants (2010072), and Research Accelerator Program of University of Melbourne.
CONFLICTS OF INTEREST STATEMENT
Yuanxu Gao is an Editorial Staff of MedComm-Future Medicine. Author Yuanxu Gao was not involved in the journal's review or decisions related to this manuscript. The other authors declared no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.




