Modifying the electrical, optical, and magnetic properties of cancer cells: A comprehensive approach for cancer management
Abstract
Despite the recent successes of cancer therapy, numerous obstacles remain. The medical community of the 16th century believed that magnets might be used to cure or prevent illness. However, this concept has only recently been put into practice in the treatment of cancer. Cancers vary greatly not only at the patient, tissue, and cellular levels but also at the molecular level. Because of this multiscale heterogeneity, effective treatments that not only differentiate between cancerous and healthy tissues but also target a wide variety of tumor subclones are difficult to develop. Most treatments either take advantage of a specific biological characteristic shared by cancer cells (e.g., their propensity for rapid division) or indiscriminately eradicate every cell in an area of interest. In this article, we review the physical, chemical, electrical, optical, and magnetic properties of cancer cells, before discussing how these properties may be modulated by current and future cancer therapies.
Abbreviations
-
- ACEK
-
- alternating current electrokinetic
-
- ACET
-
- alternating current electro-thermal
-
- AMF
-
- alternating magnetic field
-
- CAFs
-
- cancer-associated fibroblasts
-
- CAR
-
- chimeric antigen receptor
-
- CHO
-
- Chinese hamster ovary
-
- CTCs
-
- circulating tumor cells
-
- CtDNA
-
- circulating tumor DNA
-
- DEP
-
- dielectrophoretic
-
- ECM
-
- extracellular matrix
-
- ECT
-
- electroconvulsive treatment
-
- EIS
-
- electrical impedance spectroscopy
-
- EMF
-
- electromagnetic field
-
- EPR
-
- enhanced permeability and retention
-
- ES
-
- electrical stimulation
-
- fMRI
-
- functional MRI
-
- GCO
-
- Global Cancer Observatory
-
- HIF-1
-
- Hypoxia-inducible factor 1
-
- IDEAs
-
- intelligent double-locked deoxyribonucleic acid automata (IDEAs)
-
- IRE
-
- irreversible electroporation
-
- LOC
-
- lab-on-a-chip
-
- MES
-
- micro-electro-mechanical system
-
- MNPs
-
- magnetic nanoparticles
-
- MRI
-
- Magnetic resonance imaging
-
- NK
-
- natural killer
-
- PDT
-
- Photodynamic therapy
-
- PEE
-
- pulsed electric field
-
- PEMFs
-
- pulsed electromagnetic fields
-
- PMFs
-
- permanent magnetic fields
-
- PTT
-
- Photothermal therapy
-
- SMF
-
- static magnetic field
-
- TME
-
- tumor microenvironment
-
- VEGF
-
- vascular endothelial growth factor
1 INTRODUCTION
Cancer affects individuals of all ages and backgrounds. The use of dielectric characteristics in diagnostic medicine is based on the extensive literature that establishes a connection between physiological changes and adjustments in the dielectric properties of tissues or cells. The electrical properties of cells are expected to be influenced by the interaction between the cell membrane and internal cellular components, such as water and ions [1]. These properties are altered in transformed or dead cells. Thus, it is reasonable to assume that cancer cells exhibit electrical behavior that differs from that of normal cells. Among these alterations is the lower transmembrane potential of cancer cells, which strongly coincides with their increased mitotic activity [2]. The optical detection of structural alterations in biopsy samples has led to significant advancements in cancer staging [3]. The examination of morphological alterations in stained biopsy samples is the gold standard for the pathological identification of cancer stages. However, the structural changes that occur in healthy tissues prior to or in the early phases of tumor formation remain a mystery. This is largely due to the limited resolution of conventional microscopes, which are constrained by diffraction. Healthy cells undergo structural changes owing to the rearrangement of macromolecules (100–200 nm in size) such as DNA, RNA, and lipids. The neoplasm center is not the only place where genetic and epigenetic modifications occur; field cancerization is also a well-recognized concept. Field cancerization begins at the nanoscale level prior to tumor development and migration, whereby anomalies arise in the tissue around the cancerous area or in the altered cells of the primary tumor [4, 5]. For instance, cancerous cells are characterized by higher concentrations of salt and chlorine [6, 7] but lower concentrations of potassium, calcium, zinc, and magnesium than healthy cells. The ionic composition within cancerous cells is altered because their cell membrane permeability has been compromised. A study comparing normal and cancer cells of the large intestine reported that cancer cells differed from normal cells in their membrane makeup [8]. Current cancer treatment methods, including tumor removal, chemotherapy, immunotherapy, and hormone therapy, all have drawbacks. Surgery on a tumor has little impact on its metastases, while radiation treatment is both time-consuming and costly. Chemotherapy drugs exhibit high levels of systemic bioavailability, meaning that they will not kill metastatic cancer cells even upon reaching them. The severe toxicity of chemotherapeutic agents has also been linked to worse patient survival rates. Moreover, dermatological, gastrointestinal, endocrine, hepatic, pulmonary, and renal toxicities and side effects have been reported (in 1%–95% of patients) with the use of cancer immunotherapy [9]. Genetic modifications are regarded as a major contributor to cancer development [3]. Researchers predict that 18.1 million people will be diagnosed with cancer this year (2023) alone [10]. The Global Cancer Observatory (GCO) projects that by 2030, cancer will claim the lives of 30 million individuals each year [11]. This colossal loss of life is just one aspect of cancer's enormous toll on society. Thus, research into cancer prevention, detection, and treatment is essential. Cancer cells survive and proliferate due to flaws in the normal mechanisms that regulate their division and death. It is common for signaling pathways to be altered throughout the progression of cancer. For instance, cancer development and its resistance to chemotherapy and radiotherapy are both influenced by the inhibition of physiological apoptosis [12]. Pulmonary, colon, breast, head and neck, kidneys, bladder, prostate, and ovarian cancers are only a few examples of cancers that are categorized by the organs from which they arise [13]. Meanwhile, imaging, laboratory testing, and morphological analysis of tissues and cells are examples of methods used for cancer detection, which are generally considered reliable [14].
2 THE PHYSICAL AND CHEMICAL ALTERATIONS OCCURRING IN CANCER
Cancer formation is a dynamic process involving a wide variety of changes, which occur at the cellular and tissue levels, including aberrant cell proliferation and differentiation as well as extracellular matrix (ECM) and blood vessel remodeling. In the case of solid tumors, rapid cellular growth depletes the localized oxygen supply much more quickly than in normal tissues [15]. The resulting hypoxic microenvironment changes cellular behavior because the cells have to adapt to survive in their new environment. Hypoxia-inducible factor 1 (HIF-1), which is more stable at low than normal oxygen levels, controls the activation of hypoxia-inducible genes. Downstream signaling following HIF-1 activation controls a variety of cellular processes, including apoptosis, cell-cycle arrest, angiogenesis, glycolysis, and low pH adaptation [16]. However, fast and disorganized vascular growth produces a network that does not transmit nutrients efficiently. Thus, the activation of HIF-1 signaling drives the development of new vascular networks inside tumors [17] in a process called angiogenesis. During this time, the tumor microenvironment (TME) undergoes profound physicochemical changes. Variable interstitial pressure, hypoxic zones, and gradients of nutrients and growth stimulants all contribute to the complex TME [18], the establishment of which is facilitated by the leaky and uneven tumor vasculature and inadequate lymphatic drainage. The acidity of the TME arises due to the altered metabolism of hypoxic tumor cells (which depend on glycolysis for energy) and the poor perfusion associated with neoplastic growth and angiogenesis. Evidence suggests that cancer-associated fibroblasts (CAFs), a prominent cellular component of the TME, display elevated proliferation rates, increased ECM synthesis, and altered cytokine secretion [19]. CAFs are the primary scaffolds of tumor tissues. In liver [20], breast [21], and prostate [22, 23] cancers, collagen is deposited in the ECM before undergoing cross-linking to increase tissue stiffness. The tumor tissue stiffening increases despite individual cancer cells being more pliable than normal cells, which is also partly a result of cytoskeleton remodeling [24, 25]. Cancer can be diagnosed by monitoring these micro-environmental changes. For instance, changes in tissue stiffness are detected by mammography to diagnose breast cancer. In diagnostic approaches such as electrical impedance spectroscopy (EIS) [26, 27], micro-environmental electrical differences between healthy and cancerous tissues are compared. Clinical impedance testing was used to detect the reduced electrical reactance of lung cancer patients compared with that of healthy individuals [28]. Moreover, PET-CT scans, which are often used to diagnose cancer, may detect the increased glucose absorption of the acidic TME. Although the physicochemical characteristics of cancer cells and tissues are important for determining the diagnosis and prognosis of cancer patients, they have not been successfully targeted during cancer treatment to date.
The physical characteristics of cancer cells, such as their size, shape, and mechanical qualities, allow for the creation of advanced therapeutic approaches. Nanotechnology makes use of the physical differences between normal and cancer cells (e.g., cell surface abnormalities) to facilitate targeted drug delivery to cancer cells [29]. Furthermore, because cancer cells are more rigid than normal cells, treatment strategies that aim to regulate the cell cytoskeleton to limit cancer cell migration/invasion can be developed. Cancer cells have different metabolic profiles and surface indicators from those of normal cells, offering a variety of opportunities for treatment intervention [30]. Targeted immunotherapies use monoclonal antibodies, which identify and attach to specific surface markers overexpressed on cancer cells. These antibodies can be engineered in a number of ways to destroy cancer cells directly and/or activate the anti-tumor immune response. Drug development may also take advantage of the metabolic abnormalities of cancer cells, such as glycolysis dependence. By blocking the activity of certain enzymes in these pathways, selective cell death can be induced in cancer cells. Therapeutic agents are delivered to cancer cells with precision using targeted drug delivery technologies, such as liposomes and nanoparticles, which minimize collateral harm to healthy tissues. Chimeric antigen receptor (CAR) T cells and immune checkpoint inhibitors are examples of immunotherapies that use the immune system to identify and destroy cancer cells. The goal of metabolic targeting techniques is to hinder the proliferation of cancer cells by depriving them of energy sources or by disrupting important metabolic processes [31]. Photodynamic therapy (PDT) uses light-activated photosensitizing drugs to specifically target and kill cancer cells by taking advantage of their optical characteristics. During PDT, light triggers the production of reactive O2 species from the introduced drug, which in turn causes localized cell death [32].
3 MAGNETIC-FIELD-ASSISTED CANCER TREATMENT
There are three primary mechanisms by which magnetic fields influence cancer cells and tumors: the thermal effect, the cavitation effect, and the non-thermal/cavitation effect [33]. The exposure of magnetic nanoparticles (MNPs) accumulated in cells to permanent magnetic fields (PMFs) has been used to induce localized hyperthermia [34]. Patients with advanced cancer stages (Stages 3 and 4) have also been advised for PMF treatment [35] due to their reduced tolerance to chemotherapy as a consequence of impaired organ function. Furthermore, studies in mouse models have shown that very low-frequency PMF may halt neoangiogenesis, which is required for tumor development [36]. In addition, tumor blood vessel growth is stimulated by magnetic fields due to a phenomenon called Joule's heating. The resulting expansion of arteries increases blood flow to the tumor, and therefore oxygen delivery, which is toxic to cancer cells. In addition, increased blood flow to the tumor increases the infiltration of natural killer (NK) cells, which limit the growth of and actively kill cancer cells [37]. However, cancer cells can also use the newly formed blood vessels to metastasize and spread throughout the body. Because vascular endothelial growth factor (VEGF) is central to angiogenesis, applying a magnetic field to significantly reduce VEGF levels has been shown to inhibit tumor development and metastasis [38]. Recent magnetic-field-aided cancer treatment strategies make use of an electromagnetic field (EMF) to promote hyperthermia; the use of MNPs increases this effect significantly. Here, a tumor is essentially microwaved using an EMF at frequencies that will damage cancer cells but leave normal cells unharmed. However, there are a number of drawbacks associated with hyperthermia-based cancer therapy, including a lack of radiation selectivity, lengthy treatment periods, and the risk of inducing necrosis in surrounding healthy tissues. The way that heat affects tumor tissues at the molecular level has generated interest in the therapeutic use of magnetic fields for the treatment of cancer [39]. These interactions have a substantial influence on angiogenesis and the vascular system. For instance, the induction of hyperthermia in cancer cells may damage their DNA because it activates the immune system and causes the membranes of normal cells to become more permeable and fluid [40]. These changes also alter cancer cell homeostasis via several signaling cascades, which eventually kill the cells [41]. Tumors loaded with iron oxide nanoparticles have been eradicated by subjecting them to an alternating magnetic field (AMF) in addition to heat and thermo-ablation [42]. Magnetic fields play an important role in tumor detection and therefore in the development of improved ways to track cancer cells. Magnetic resonance imaging (MRI) is a well-known application of magnetic-field-assisted cancer imaging. MRI employs a powerful magnetic field to align the protons inside tissues [43]. Then, radiofrequency waves are used to briefly perturb this alignment. When the protons reconnect with the magnetic field, they send out signals, which are picked up and turned into high-resolution detailed images. Cancer imaging with a magnetic field has several benefits. First, it increases the contrast within soft tissues, which makes it possible to see internal structures with great clarity even in complex anatomical areas. These properties of MRI are very useful for tumor identification and characterization. Second, MRI is a non-invasive technique that relies on multiplanar imaging to determine the size of the tumor, its location, and the relationships with the normal tissues that are surrounding it. Third, functional MRI (fMRI) uses magnetic fields to measure blood flow and oxygenation to reveal details related to tumor metabolism [44]. This functional information assists in determining the aggressiveness of the tumor and predicting its response to therapy. Finally, MRS is another magnetic-field-assisted approach, which can be used to monitor metabolic indicators linked to cancer, helping tumor characterization and differential cancer diagnosis.
Magnetic hyperthermia offers a focused approach to treating cancer by exploiting the magnetic characteristics of cancer cells. This method involves the introduction of MNPs into the body, which are often composed of materials such as iron oxide. These nanoparticles are then preferentially transported to malignant tissues. When the nanoparticles reach the tumor, they are subjected to an external alternating magnetic field, causing them to undergo hysteresis and fast changes in magnetic orientation. This movement heats up the tumor, killing cancer cells selectively [45]. Damage to cells occurs as a result of hyperthermia induction, which preferentially induces the necrosis and apoptosis of malignant cells while sparing normal ones. The use of magnetic hyperthermia complements radiation therapy and certain chemotherapy drugs by increasing their effectiveness. This approach also stimulates the anti-tumor immune response. Moreover, the MNPs may contain therapeutic payloads, which are released following hyperthermia initiation, enhancing the localized therapeutic effect. This complex interaction between the magnetic characteristics and nanoparticle-mediated heating properties represents a potential option for precise and regulated cancer treatment; research that aims to optimize the therapeutic efficacy of this strategy is ongoing [46].
4 ALTERNATING MAGNETIC AND ELECTROMAGNETIC FIELDS FOR CANCER TREATMENT
Hyperthermia-based cancer treatments use EMFs with or without high-frequency AMFs/PMFs. A static magnetic field (SMF) is generated using permanent magnets, whereas an EMF is generated using electromagnets. As described earlier, hyperthermia can be induced in cancer cells by exposing MNPs (e.g., iron oxide) to an electromagnetic field [47, 48]. Hysteresis loss, relaxation loss, and frictional loss are three of the most important modes of heat generation. Heat is generated due to a combination of Brownian relaxation (coming from whole particle oscillation or rotation) and Néel relaxation (resulting from internal magnetic domain rotation) (Figure 1). Hyperthermia caused by Brownian relaxation seems to be more successful in halting tumor development because it may generate more heat than Néel relaxation. It is also important to consider the magnetic field design and mode of manipulation in increasing the efficacy of various treatments and protecting healthy cells from the harmful effects of chemotherapy drugs. Triangular magnetic fields applied to the axillary artery of breast tissues have been shown to achieve up to 90% target effectiveness [49].
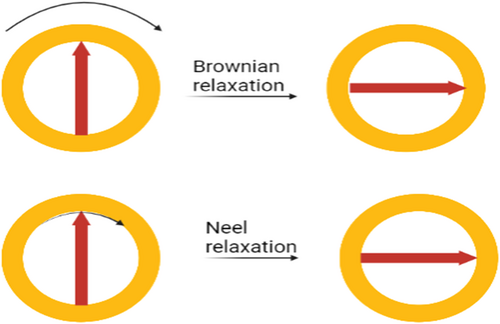
Schematic representation of the effects of AMF and PMF on the Brownian and Néel relaxation times of MNPs.
Treating MNP-loaded tumor cells with an AMF/PMF has been shown to induce their apoptosis in a wide variety of cancers, including osteosarcoma, breast cancer, gastric cancer, colon cancer, and melanoma [50-52]. Extensive in vitro studies of these therapies have been conducted using a wide variety of human cancer cell lines [53, 54], including those derived from pheochromocytomas (PC12), breast cancer (MCF7, MDA-MB-231, and T47D), colon cancer (SW-480 and HCT-116), and liver cancer (MCF7, MDA-MB-231, and T47D). In an innovative in vitro study, human breast cancer cells (MCF7) were selectively targeted using ultraweak low-frequency-pulsed electromagnetic fields (PEMFs) [55]. Specifically, a PMF of 20 Hz and 3 mT intensity was applied to MCF7 cells and normal breast epithelial cells (MCF10) for 30, 60, and 90 min daily over 3 days. After analyzing the effects of a 3-mT flux density on the apoptosis and electrical characteristics of the cells in vitro, the researchers found that MCF7 cells responded dramatically to the treatment, while the normal cells (MCF10) were unaffected. This research shows that cancer cells can be killed with precision by tailoring the frequency, amplitude, and duration of the electromagnetic therapy.
5 ANALYZING THE ELECTRICAL PROPERTIES OF DIFFERENT CANCER CELLS
Cancer is the primary cause of death in many nations, with lung, cervical, and breast adenocarcinomas being the most common malignancies in women. Unfortunately, many patients with cancer already present with distant metastases at the time of diagnosis; moreover, many patients who have had their primary tumor surgically removed go on to develop metastatic disease [56]. In medical care, measures are taken to either prevent a disease from occurring (primary prophylaxis) or stop its progression (secondary prophylaxis). The efficacy of secondary prophylaxis relies on the early diagnosis of the disease. The implementation of cutting-edge approaches is necessary to identify the presence of prospective cellular abnormalities in the earliest stages (incubation period) of a disease. Surgery, imaging, and the morphological and immunohistochemical investigation of tissue samples obtained after surgery are often used together to diagnose cancer. However, using such methods to obtain a diagnosis is intrusive, time-consuming, and challenging; in addition, it requires a carefully controlled laboratory setting. Therefore, new techniques of cancer detection that are accurate, non-lethal, affordable, and user-friendly should be created. Conventional cancer detection methods use a population of cells to model various cellular parameters. Because these parameters are artificially generated, they cannot be used to distinguish between individual cells. Therefore, new techniques for analyzing single cells have emerged. Detailed information about a given cell's electrical characteristics and pathological status may be gained by measuring its impedance, making this a useful tool for single-cell research [57-59]. This method of diagnosis is quick and accurate because it reveals the workings of a single cell. Additionally, the influence of environmental factors, drugs, and even pathogens (e.g., viruses and bacteria) may be investigated using single-cell impedance [60-64]. Single-cell analysis has been possible because of recent advancements in micro-electro-mechanical system (MEMS) technology [65-68]. In the life and pharmaceutical sciences, lab-on-a-chip (LOC) devices and micro total analysis systems (TAS) provide high-resolution, fast, and cost-effective analysis and synthesis solutions. These days, microchipped cells may be influenced in a variety of ways. In most cases, the driving component used to classify the cells can be easily determined. One such technique uses an electric force to temporarily attach cells to a surface for the sake of observation and research without inhibiting their capacity to divide and multiply. Unlike other methods, this strategy does not involve touching the cells, alleviating any risk of accidentally distorting or damaging them. Particles are moved by a gradient force originating from an electric field induced by alternating current electrokinetics (ACEK), which may then be used to manipulate the cells. An alternating current electro-thermal (ACET) effect or AC electro-osmosis may be used by ACEK to transport polarizable particles through the dielectrophoretic (DEP) force or to drift fluids. In the presence of a non-uniform electric field, a polarized particle in the medium will feel an asymmetric electric force, giving rise to the DEP force [69]. The ACET and DEP forces may be used to direct the cell to its proper location [70, 71]. The present work suggests that quadrupole electrodes can be used for cell control, while microwell electrodes with a pair of electrodes can be used for cell capture and impedance evaluation. In the frequency domain, the electrophysiological properties of distinct cell types are used to define the cell type and distinguish between normal and abnormal cells using EIS [72]. The mechanical, physical, and metabolic activities of live cells are influenced by their electrical impedance [73]. Cells might potentially be described by their electrical response across a very small frequency range as indicated by their impedance values. Thus, EIS may be used to classify cancer cells [74].
5.1 Mechanism
Electrochemotherapy is a complex form of cancer treatment, which uses the electrical capabilities of cancer cells to improve their response to chemotherapy. The procedure involves the systemic or localized administration of chemotherapeutic drugs and the subsequent application of electrical impulses under careful supervision [75]. Electroporation occurs when electric pulses temporarily change the cancer cell membrane structure creating temporary holes in the membrane, which increase its permeability. The increased permeability of their outer membrane enables cancer cells to absorb chemotherapy drugs at a much higher rate than before. Ultimately, the cytotoxic effects of the drug are amplified inside the electroporated malignant tissues, which interrupts essential cellular processes and triggers apoptosis [76]. The administration of electric pulses in the vicinity of the tumor minimizes collateral harm to adjacent healthy tissues. This selective targeting, which is a fundamental benefit of electrochemotherapy, increases the therapeutic index of chemotherapy and alleviates resistance to traditional therapies. Localized electric pulses decrease systemic adverse effects, making this therapy more tolerable and patient-friendly. Several types of cancers, including skin cancer and certain solid cancers, have responded well to electrochemotherapy in clinical trials. This approach is useful in cancer therapy because of its versatility and compatibility with other treatment methods [77]. Current investigations are being conducted to improve the pulse parameters, find the best drug combinations, and broaden the use of electrochemotherapy to increase its efficacy against a variety of cancer types.
5.2 Liquid biopsy for cancer diagnosis
Liquid biopsy is an innovative technique in the field of cancer diagnostics, which has surfaced as a non-intrusive approach for cancer surveillance and early detection [78]. This novel method analyzes many circulating biomarkers such as extracellular vesicles, circulating tumor cells (CTCs), and circulating tumor DNA (ctDNA) in physiological fluids (e.g., blood or urine). Because the biomarkers contain tumor-associated genetic and molecular clues, liquid biopsies provide a non-invasive way of characterizing cancer [79]. Accessibility and simplicity of implementation are two of the main advantages of liquid biopsy over conventional tissue biopsies. As an alternative to invasive tissue biopsies, liquid biopsies offer a less stressful and more convenient means of obtaining essential tumor-specific information from patients. The implementation of liquid biopsies might help overcome the constraints of tissue biopsies, which can be difficult to perform frequently and in certain clinical situations. In addition, liquid biopsies enable the dynamic monitoring of tumors in real time. This is useful for measuring therapy responses, diagnosing resistance, and directing therapeutic strategy changes [80]. In addition, liquid biopsies play a crucial role in detecting minimum residual disease by providing information on the survival of cancer cells after therapy and the possibility of disease recurrence. The introduction of liquid biopsies has dramatically transformed the field of early cancer detection. The capacity to detect and analyze circulating tumor biomarkers enables malignancies to be detected even before symptoms appear. Early cancer detection in turn greatly improves patient outcomes. In the age of precision medicine, liquid biopsies are likely to have a profound impact on cancer treatment and eradication. For instance, their use enables physicians to customize therapies according to the unique genetic composition of each patient's tumor [81]. Ultimately, the non-invasive nature, ease, and accessibility of liquid biopsies will improve the accuracy, effectiveness, and accessibility of cancer detection and treatment.
6 PROPOSED MODELS OF CELL POLARIZATION
The molecules that comprise living cells and tissues range from the simplest ions and polar water molecules to more complex macromolecules such as carbohydrates, proteins, DNA, and lipids [82]. When an external electric field is applied to a cell or tissue, it may change the molecular distribution of charges and other substances contained inside. As a consequence, certain molecules spin in space and become polarized, whereas others migrate across long distances and act as conductors. Because of these changes, a cell becomes a polarizable dielectric [83]. The electric permittivity of a material, which measures its capacity to store energy, is governed by the strength of its internal electric field. This internal electric field is produced when the material is polarized; it can then resist and weaken the external electric field. The latter is commonly specified by a material's conductivity or permittivity, whereas the former is defined by a dielectric constant or relative permittivity [84]. Living cells are highly diverse and complex. The membrane-bound organelles inside cells provide even more internal interfaces for the various ions and biomolecules to interact with. Interestingly, the dielectric properties of cells also depend on the frequency of the electric pulse applied [85].
One possible way to make sense of these patterns is to imagine cells as highly conductive spheres of cytoplasm filled with free ions and surrounded by insulating non-conductive cell membranes. In cells, the membranes separate the conducting exterior medium from the insulating interior. An electric double layer is created when a cloud of counterions is absorbed into the cell membrane, canceling out the negative charge of the membrane [86]. Two different mechanisms have been proposed to account for the polarization of living cells (Figure 2a): Different modes of cell polarization. The existence of a cell membrane means that live cells can be polarized in two ways such as (a) the colloidal partial mechanism [87], which indicates that ionic mobility within the double layer mediates the entire process, and (b) the electric double layer mechanism [88], which implies that polarization occurs outside the double layer. Typically, ions are free to move inside the cell; however, their movement is restricted by the insulating cell membrane, resulting in a concentration of ions at the cell interface. In this way, a cell might be thought of as a giant dipole. To facilitate the mass movement of ions across a membrane, an electric field should be applied to the membrane, resulting in the formation of an electric double layer surrounding the pores [89, 90]. When the electrical frequency is increased, this phenomenon ceases and negative dispersion occurs (Figure 2b). The negative dispersion may be explained by the interfacial polarization, which exists between the two phases [91]. It is hypothesized that cancer cells have a more irregular capacity for charge storage than normal cells because of the changes in the membrane permeability, transmembrane potential, surface charge, and ionic concentration that accompany malignant transformation. Most research has either compared the electrical and dielectric characteristics of cancer cells to those of healthy cells or compared the dielectric properties of tumor tissue in vitro or in vivo. In this study, we were interested in examining how cancer cells in suspension might affect the dielectric properties of healthy cells. We produced a cell suspension into which we injected increasing numbers of cancer cells while maintaining a stable population of healthy cells, thus gradually increasing the cancer cell to healthy cell ratio. Cancer arises when the tumor tissue is mostly composed of cancer cells and surrounded by healthy cells. Cancer cells that have metastasized to a substantial area of normal tissue might resemble tumor cells at an initial glance.
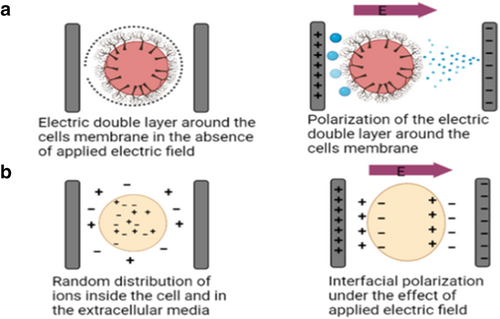
Different modes of cell polarization. The existence of a cell membrane means that live cells can be polarized in two ways: (a) polarization of the electric double layer or (b) interfacial polarization, which both cause net negative dispersions. Net negative dispersions are due to the buildup of counter ions in the extracellular space and the trapping of ions by the ion-impermeable cell membrane.
7 THE IMPORTANCE OF LIGHT-BASED CANCER THERAPY AND IMAGING METHODS
Light therapy is an important mode of cancer therapy because it provides a novel way of harnessing the power of light for therapeutic purposes. For instance, PDT uses a photosensitizing chemical that preferentially accumulates inside cancer cells. Specific wavelengths of light activate the photosensitizer, triggering a chain of reactions, which ultimately create reactive oxygen species and destroy cancer cells [92]. Compared to more conventional cancer therapies, PDT has fewer adverse effects because of its precision in targeting cancer cells while sparing healthy cells. Photothermal therapy (PTT) is another light therapy technique, which uses light-absorbing materials (such as gold nanoparticles) to transform light energy into heat. This method offers a specific and non-invasive way to treat cancer. Light-based optical imaging methods are also used in cancer diagnosis. Fluorescence imaging helps surgeons to see and locate tumors in real time using fluorescent markers that attach exclusively to cancer cells. This improves the accuracy of the surgical procedure and decreases the probability of leaving behind any malignant tissue [93]. The incorporation of light-based therapeutics and diagnostics into multidisciplinary cancer care signals a paradigm change. These methods provide new treatment options and improved imaging tools for tumor identification and characterization. As studies on light-based cancer therapeutics advance, these approaches will be refined and expanded to provide patients with more personalized and effective cancer therapies.
Significant progress in cancer research has been made in recent years [94], thanks to the convergence of the expanding disciplines of molecular biology and cell biology. Recent progress in the molecular research of cancer genes and proteins has allowed us to get a deeper insight into the basic features of this complex disease. Despite these breakthroughs, we still do not have a full picture of cancer pathogenesis, especially the mechanisms of invasion and metastasis. Cancer stem cells have recently come under scrutiny because of their roles in cancer development and treatment resistance. It is now recognized that the blood vessels and interstitial components within the TME form a complex system, which provides essential support to cancer cells. Optical imaging techniques include fluorescence imaging and bioluminescence imaging (both of which rely on the luciferase enzyme), which use fluorescent proteins and dyes. The ability to gather time- and space-specific data on individual cells and biomolecules in vivo (including their functional dynamics) will further enhance our understanding of cancer stem cells and the TME. Thus, optical imaging is one of the most promising imaging modalities for evaluating cancer cell activity and the TME in vivo [95].
A novel tumor-specific fluorescence imaging method has recently been developed, which promises to revolutionize the optical imaging of cancer. The researchers who are leading the study created intelligent double-locked deoxyribonucleic acid automata (IDEAs) and a DNA walking system using multifunctional nanocomposite materials (ZrMOF@MnO2) [96]. This innovative approach proved to be very effective at differentiating between tumor cells and healthy cells. A high level of specificity in molecular identification is guaranteed by the integration of IDEAs, which are built to incorporate a double-locking mechanism. The combination of this smart DNA automata system with the enhanced properties of ZrMOF@MnO2 multifunctional nanocomposites creates a high-tech platform for fluorescence imaging, which specifically targets tumors. This study is expected to transform cancer diagnostics. Further investigation into DNA-based automata and multifunctional nanocomposite materials is essential to unlock their full potential in cancer detection and develop personalized diagnostic approaches [97].
8 OPTIMIZING THE PHYSICAL AND CHEMICAL FEATURES OF CANCER CELLS TO IMPROVE CANCER TREATMENT
Cancers are notoriously diverse, both across and within individuals. The effectiveness of many standard cancer treatments is severely compromised by this multiscale variability. Conventional chemotherapies aim to stop the proliferation of cancer cells, a biological characteristic of the disease. When cancer treatments kill normal cells, they also leave behind groups of resistant cells that might replenish the tumor. However, even treatments such as surgical resection and radiation therapy, which are designed to kill all cells inside a certain area, may leave behind cells that cause the tumor to return. Thus, recent studies in cancer therapy have focused on targeting specific receptors, which are overexpressed by cancer cells. However, as practically all targeted receptors are also expressed by normal cells, off-target effects and associated toxicity remain worry [98-101].
8.1 Interfering with the tumor's chemical microenvironment
8.1.1 Reducing acidity
Low pH has been linked to an increased risk of malignancy and has been reported in a variety of tumor types. Consequently, recent studies have focused on targeting this chemical feature of tumors to treat cancer. For instance, anti-cancer drugs may be delivered selectively to acidic tumors using low pH insertion peptides [102]. As the pH drops, these peptides undergo a process of membrane-associated folding, which transports them from the cell surface (under neutral pH conditions) to within the cell (at low pH) [103]. Therefore, selectively targeting cancer cells based on their acidity might be an innovative method of delivering therapeutic drugs to tumors, especially those resistant to traditional treatments and with the greatest metastatic potential.
8.1.2 Targeting hypoxia
The chemical state of hypoxia has been identified as a potential therapeutic target because of the link between hypoxia-induced changes in the TME and drug resistance. Bio-reductive prodrugs [104] undergo a transformation from a non-toxic substance to a dangerous molecule in the presence of low oxygen levels. Certain chemical moieties are degraded by enzymes in the absence of oxygen. Using this approach, a prodrug radical is created by a one-electron reduction; further reactions might render it cytotoxic to cells, inducing hypoxia-selective death [105, 106]. Researchers have employed various chemical groups, such as nitro groups, aromatic N-oxides, aliphatic N-oxides, and transition metals to create novel drugs that enhance the production of cytotoxic radicals in low-oxygen (hypoxic) environments. This innovative approach aims to develop drugs that are activated specifically in hypoxic conditions, potentially improving their effectiveness in targeting specific tissues or conditions. Numerous investigations [107-109] have shown that many bio-reductive drugs are selectively cytotoxic to hypoxic cells and are thus effective against tumors.
8.2 Interfering with the tumor's physical environment
8.2.1 Using the EPR effect
Macromolecules may pass through leaky and highly permeable arterial walls, and therefore tumor blood vessels facilitate tumor tissue invasion. Improved permeability and retention have been dubbed as the “enhanced permeability and retention” (EPR) effect. Macromolecules bigger than 40 kDa tend to accumulate in tumor tissues due to the selective leaking of tumor arteries [110]. Given that the EPR effect is absent in normal tissues, novel therapeutic alternatives, such as macromolecule therapies, have been developed to preferentially target tumor tissues [111, 112]. Micelles, liposomes, nanoparticles, DNA polyplexes, and lipid particles are all transported selectively via the EPR effect [113-117]. Angiotensin II, nitric oxide (NO), and VEGF are all factors that modify arterial permeability and thus contribute to the EPR effect. Adjusting these parameters has the potential to maximize the EPR effect and improve tumor eradication.
8.2.2 Using a pulsed electric field
PEFs applied uniformly throughout a tumor volume induce a series of biophysical processes that ultimately lead to cell death. Several types of PEF therapy exist, such as nanosecond PEFs, tumor therapy fields, and irreversible electroporation (IRE). They are classified based on the properties of the pulses used. Delivering PEFs with a duration of 10–100 s and a magnitude of hundreds of V/cm to cell membrane results in electroporation [118]. Applying IRE at a threshold electric field causes cell death because cells are unable to close the generated holes. This method of non-thermal tumor ablation has seen extensive use [119].
9 IMMUNE SYSTEM MODULATION THROUGH ELECTRICAL STIMULATION AS A POTENTIAL CANCER TREATMENT
9.1 Stimulation via a direct electrical current
Multiple animal models and clinical studies in humans have shown the efficacy of a low-level direct current (current flowing in one direction) for the treatment of cancer. If the current flow is strong enough and the electrodes are far enough apart, most tumors may shrink or even be eliminated. When the electrodes are too far from the tumor, however, the tumor grows at an accelerated rate [120-123]. In addition to electrotherapy, both sets of mice received intra- or peritumor injections of Chinese hamster ovary (CHO) cells genetically modified to secrete large amounts of interleukin-2 (IL-2). Electrotherapy (at 0.6 and 1.0 mA) significantly slowed the tumor growth in mice with a functional immune system, compared with that in immunodeficient mice. These findings suggest that the immune system greatly impacts the efficacy of electrotherapy. A much higher percentage of mice injected with CHO cells were completely cured or experienced tumor regression after receiving a combination of IL-2 and electrotherapy [121]. These results demonstrate that the combination of direct current stimulation and immunotherapy is superior to the use of either therapy alone.
9.2 Nano-pulsed electrical stimulation
ES with electrical pulses lasting just a few nanoseconds is called nano-pulse ES. When immunotherapy is used to treat cancer, nano-pulse ES is employed more often than any other type of ES. The nano-pulsed ES is highly efficient against cancer because it combines the therapeutic benefits of electroporation with those of immune system stimulation. High-frequency electrical stimulation targets cancer cells, attacking them and causing membrane holes. Fragments of the cell's nucleus (including its DNA) and other organelles subsequently leak out of the cell through these holes. The leakage of intracellular contents activates immune cells, leading to improved tumor eradication [124-126] (Figure 3).

Mechanism through which NPSS modulates the immune system. Cancer cells are electroporated (pores are created in the cell and nuclear membranes) when exposed to nanosecond-long electric fields. This results in the production of cytokines and chemokines, which in turn stimulate the immune system to target cancer cells.
9.3 Immunotherapy for cancer
The field of tumor immunotherapy has undergone substantial progress. Recently, Yang et al. developed a novel ultra-high-efficacy radiotherapy approach by loading metformin into extracellular vesicles encased in hollow MnO2 structures. This innovative nanoplatform not only demonstrated significant efficacy against tumors but also activated the innate immune system by activating NK cells via the cGAS-STING pathway [127]. This pathway is well known for its role in detecting cytoplasmic DNA and initiating immunological responses [128]. Thus, improving the therapeutic effect of radiation may be achieved by the strategic incorporation of metformin, an immunomodulatory medication that is often used to treat diabetes, into a MnO2-based nanoplatform. Controlled drug release and targeted distribution are made possible by the additional layer of complexity provided by the hollow MnO2 encapsulation inside extracellular vesicles. The capacity to activate NK cells and induce a strong innate immune response is what distinguishes this method from others. This pioneering strategy shows that activating the cGAS-STING pathway in conjunction with radiation and metformin treatment may increase the innate immune response against cancer cells. The findings of Yang et al. have far-reaching implications, potentially leading to a transition towards more integrated and efficient strategies in the field of clinical tumor therapy. Many cancer therapies are associated with tumor resistance and recurrence. Using combination therapies that activate the immune system, especially NK cells, could help solve these problems and improve clinical outcomes [129, 130].
9.4 Electrochemotherapy
According to recent research, combining electroconvulsive treatment (ECT) with immunotherapy may be a promising strategy for treating cancer. Electroporation is the process of making a cell membrane more permeable by subjecting it to brief but strong electric pulses. Owing to its effectiveness and safety, electroporation is now being used to deliver materials, such as nucleic acids, cytotoxic drugs, and ions, into malignant cells and tissues [131, 132]. Antitumor ECT uses electroporation to boost the absorption of anticancer drugs (especially those which do not readily enter cancer cells) by cancer cells. ECT is a non-invasive, non-thermal method for treating solid tumors locally [133, 134]. ECT can be modified by varying the electric pulse type, frequency, amplitude, and number (Figure 4). Electroporation may be used to kill cancer cells or administer chemotherapeutic drugs and DNA vaccines. Antitumor ECT was developed to increase anticancer drug absorption via electroporation. When electric pulses are used in conjunction with the administration of non- or poorly penetrant anticancer agents, ECT provides a non-ablative and non-thermal local therapy for solid tumors. The local administration of electric pulses to the whole tumor reversibly increases the permeabilization of the cell without inducing cell death [133, 134]. Thus, when the cancer treatment is subsequently injected directly into the tumor mass, it has a greater chance of reaching every cancer cell in the tumor. Advantages of ECT include its local application, meaning that only the pulsed cells will receive a high dose of anticancer drug, which limits off-target toxicity. ECT has the potential to target and regulate immune cells. To be effective, however, ECT requires two things: (1) that the tumors receive a high enough drug dose; and (2) that the whole tumor is immersed in an electric field, which can permeate the tumor's cell membranes. When these prerequisites are met, ECT eradicates cancer cells without harming surrounding healthy tissue or histological structures [135].
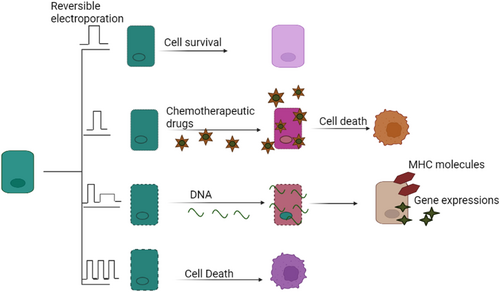
Variations in electroporation results with respect to electric pulse type, frequency, amplitude, and number. Electroporation may be used to kill cancer cells and administer chemotherapeutic drugs or DNA vaccines.
10 CONCLUSION
In this paper, we discuss cancer therapy strategies that exploit the chemical, physical, electrical, optical, and magnetic characteristics of cancer cells. Strategies aimed at altering/targeting these parameters hold great promise for enhancing the efficacy and safety of cancer diagnosis and treatment. The integration of electrical control, visual diagnostics, and magnetic navigation creates new opportunities for individualized and successful therapies. Although further study is required, the work presented establishes a basis for innovative technologies that have the potential to revolutionize cancer treatment and improve treatment outcomes while reducing adverse effects in patients. Future strategies may focus on modulating the immune response through electrical stimulation or combining immunotherapy with electrochemical therapies as potential avenues for treating cancer.
AUTHOR CONTRIBUTIONS
All authors contributed to the study's conception and design. Data collection and analysis were performed by Rishav Sharma and Rishabha Malviya. The first draft of the manuscript was written by Rishav Sharma and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. Data availability is available at the request of different authors for data.




