Salamanders aid research into mechanisms of non-canonical organ regeneration for clinical applications
Binxu Yin and Xiao Cui equally contributed.
Abstract
Salamanders possess remarkable regenerative capacities for organ regeneration among tetrapod vertebrates. Previous research has primarily focused on studying the regeneration of canonical tissues or organs such as limbs, tail, brain, spinal cord, heart, and lens. The advancements made in these areas have broader implications for understanding regeneration and developing therapeutic approaches for these organs, not only in salamanders but also in other vertebrates. In recent years, there has been an increasing interest in studying the regeneration of non-canonical organs in salamanders, such as the liver, lungs, kidneys, and pancreas. This diversification of research has opened up new avenues and provided potential solutions to challenging clinical problems. This review aims to summarize the progress made in the field of non-canonical organ regeneration in salamanders and provides an outlook on future research directions.
Abbreviations
-
- CoMBI
-
- correlative microscopy and block-face imaging
-
- dpa
-
- days post amputation
-
- EdU
-
- 5-ethynyl-2′-deoxyuridine
-
- EMT
-
- epithelial-to-mesenchymal
-
- PHx
-
- partial hepatectomy
-
- PN
-
- nephrectomy
-
- PNX
-
- pneumonectomy
-
- STZ
-
- streptozotocin
-
- TUNEL
-
- terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling
1 INTRODUCTION
In 1768, Lazaro Spallanzani was the first to discover that salamanders have the ability to regenerate organs [1]. To date, salamanders have been bred in laboratories for more than 160 years [2], and many scientists are trying to find the hidden secrets behind this fascinating phenomenon. Salamanders have long been an important resource for understanding the development and regeneration of organs [3]. Their regenerative capacity extends to various organs throughout the body, providing insights that can be applied to other species.
To date, the limb regeneration phenotype is the most intensively studied [4]. All relevant discoveries in this field provide important insights into other organ regeneration. Limb regeneration is regarded as epimorphic regeneration, a canonical process described as wound healing, blastema formation, dedifferentiation and re-patterning [4-6], which has been explored in all its aspects and is fundamental to our understanding of regeneration in other organs. The brain is similar to the limb after an amputation injury in that a proliferating mass of cells accumulates at the wound site; they then cover the wound and finally dedifferentiate to form a large number of functional cells that activate neurogenesis and complete the reconnection of old and new nerves [6, 7]. The heart can regenerate damaged myocardial tissue without scarring after amputation [8]. The process of limb, brain and heart regeneration involves both the repair of organ morphology and the restoration of function. Other organs, such as the tail, heart, and lens, also largely follow this process. Non-canonical organs such as the liver, lungs, kidneys and pancreas, which are equally important, have not been extensively studied. Next, this review will summarize some of the advances in the study of these less-focused, non-canonical organ regeneration.
2 LIVER REGENERATION
The liver is one of the few organs or tissues in the mammalian body that can regenerate [9, 10]. The liver is able to restore the liver-to-body weight ratio up to 100% and liver function even after a 70% partial hepatectomy (PHx) [11]. Seventy percent PHx is a commonly used model to study liver regeneration, where massive damage to liver mass through PHx leads to a cascade of events: remodeling of the extracellular matrix, re-entry of hepatocytes into the cell cycle, restoration of liver mass, and return of the liver to a normal state through tissue remodeling [10, 11].
Recently, Ohashi et al. reported a compensatory congestion regeneration in axolotl livers, which has similarities with compensatory growth in mammalian livers and unique aspects of salamander regeneration [12]. Three-dimensional imaging by CoMBI (correlative microscopy and block-face imaging) significantly revealed that the axolotl liver consists of four lobe-like clefts. Histological results show that the liver in axolotl is similar to that of mammals, which is structured with connective tissue septa, portal area, portal vein, sinusoids, blood vessels, bile ducts and nerves [12-15]. Unlike mammals, axolotl livers consisted mainly of mononuclear cells. Multinucleated cells, a common feature in mammalian livers, were also present in axolotl livers that increased in an age-dependent manner and were also induced after PHx. After 30% PHx in axolotl, hepatocytes are able to respond to the injury and re-enter the cell cycle [12]. Extensive proliferation was evident throughout the liver, rather than the epimorphic regeneration typically seen during limb regeneration, which is dominated by blastema proliferation. A dramatic increase in cell density and a well-maintained liver weight to body weight ratio in about a month strongly suggest liver regeneration. Meanwhile, the expression of genes highly related to liver development is analyzed [12]. The results showed that they were not activated in regeneration, suggesting that salamander liver regeneration is hypomorphic. Similar to mammals, the ERK pathway promotes the proliferation of Alb + hepatocytes [16].
The above studies show that the maintenance of the liver/body weight ratio and the signaling pathways involved in regeneration after hepatectomy are highly conserved in salamanders and mammals [9]. However, in salamander, cell proliferation occurs mainly away from the amputated area and is accompanied by an increase in hepatocyte density. In conclusion, compensatory congestive regeneration of the salamander liver has both similarities and peculiarities compared to mammals, as well as being distinctly different from conventional limb regeneration.
The liver is the only organ that has been observed to have a conserved regenerative capacity across different species. Such unique ability can be possibly attributed to several scenarios: (1) The liver possesses both innate and adaptive response mechanisms [9]. It is well known that immunity is thought to be a key factor in the great regenerative capacity of salamanders [17, 18]; (2) The liver has a robust synthetic capacity and is responsible for synthesizing 90% of all soluble proteins [19]. Recent studies have shown that rapid protein synthesis is essential for limb regeneration in salamanders [20]; (3) The liver stores the energy substance hepatic glycogen. It is well known that organ regeneration is a process that is highly dependent on energy expenditure and requires rapid metabolism of energy in the early stages of regeneration [21-23].
In response to injury, in the acquisition of substances, and in the utilization of energy, the liver perhaps has an innate advantage that is far superior to that of any other organ [9]. The above-mentioned advantages in the liver could potentially endow it with a strong ability to maintain a stronger generative capacity during evolution.
3 LUNG REGENERATION
Diseases of the lungs or respiratory system are the third most prevalent disease worldwide and have a high risk of causing morbidity and mortality [24]. Mammalian lungs generally undergo compensatory growth after injury. Adult alveolar epithelial cells have a very low rate of cell turnover [25] after severe lung injury, which results in limited proliferation. This also leads to the development of adverse epithelial remodeling, which is evidenced by the severe acute respiratory syndrome caused by the COVID-19 virus [26]. It is confirmed that the surface area and tissue mass of human lungs could be restored over a period of more than a decade after pneumonectomy (PNX) [27]. However, there are no effective clinical means to promote lung regeneration. Hence, studying salamanders with potential remarkable lung regeneration capabilities could deepen our understanding of lung regeneration and provide more potential clinical therapeutic options.
The lung of the salamander is a sac-like structure consisting of parallel aligned alveoli similar to those in mammals, pneumocytes, goblet cells, ciliated cells, and neuroepithelial endocrine cells [14, 28-30]. Jensen et al. found that PNX could effectively induce organ-wide proliferation to restore lung mass, and half of the EdU + cells were SFC + pneumocytes, which accounted for about 20% of all pneumocytes [29]. The size and morphology of the lungs would be restored in about 2 months. In addition, the authors showed that Nrg1/ErbB signaling is involved in the development and regeneration of axolotl lungs, and Nrg1 also induces cell proliferation in undamaged lungs.
A study from Yin showed another interesting phenomenon in newt lung regeneration [30]. Organ-wide proliferation in axolotl could also be observed after PNX in larvae newts, with the difference that the proliferating cells appeared to aggregate near the injury and gave rise to blastema-like structures, resulting in whole lung intact regeneration after 1 month. In contrast, after PNX in adult newts, a unique type of rapidly proliferating organ compensatory growth was induced. It primarily relies on alveolar epithelial cells that respond rapidly to injury, undergo a burst of proliferation within a week, return to normal oxygen-consuming function after 30 dpa (days post amputation), and subsequently restore the lost mass of the lungs by thickening the epithelial layer through hypertrophic growth until 90 dpa. More than 90% of the cells activated by this repair process are alveolar epithelial cells, which do not activate cell proliferation in the contralateral uninjured lung. The authors found that the rapid activation of alveolar epithelial cells was associated with intranuclear expression of Yap, with almost all proliferating epithelial cells being Yap-positive at 3 dpa. At the same time, the specific proliferation of epithelial cells disappeared in Yap mutant animals, resulting in the failure of the lung to fully recover its functionality. Through EdU pulse-chase experiments, the authors discovered that epithelial cells that were specifically activated always remained in their original location and did not undergo epithelial-to-mesenchymal (EMT) transfer elsewhere. Furthermore, rapid activation of alveolar epithelial cells can effectively restore ventilatory function in the lungs, and Yap plays a key role in this process, suggesting that lung function might be improved and repaired clinically by stimulating Yap signaling in alveolar epithelial cells.
Previous studies revealed that both larval newt and axolotl, which are considered to be in a lifelong larval state, exhibited organ-wide proliferation after PNX. More interestingly, after larvae newt PNX, there was a blastema-like proliferation similar to that typical in limb regeneration, while after adult newt PNX, there was a shift to single-cell proliferation and hypertrophic growth of tissues. Evolutionarily, salamanders are in an intermediate state of aquatic-terrestrial evolution, and lung function varies considerably before and after salamander metamorphosis, so the mechanisms of lung regeneration in adult salamanders may be more applicable to mammals. In addition, some classical pro-proliferative factors (e.g., Nrg1 and Yap) are indicated to be involved in pneumocyte proliferation after PNX.
4 KIDNEY REGENERATION
Examination of the creatinine and urea nitrogen levels is normally applied in clinics for monitoring kidney function. However, these indicators are often not routinely tested, which may result in a delayed diagnosis and increased chance of occurrence of kidney disease [31]. Severe acute kidney disease may lead to irreversible chronic kidney disease. Currently, there is no effective means to recover the function of the kidney from patients suffering from chronic kidney disease, except for transplantation [32]. It has been confirmed that the kidneys of salamanders contain nephrons, including renal corpuscles and tubules, which are similar to those of mammals in terms of canonical labeling, filtration, and reabsorption functions [33-37]. It is crucial that the kidneys of salamanders and mammals demonstrate extensive similarities in terms of structures and functions. These findings indicate that the regenerative mechanism induced by the injury model can also be applied to mammals and has potential clinical applications.
According to Scadding's study, after nephrectomy (PN), the kidney of newts exhibits hypertrophy and hyperplasia responses similar to those in mammals and does not show regenerative potential [38]. Detected by ³H-thymidine incorporation, Scadding found that the peak of cell proliferation after PN in newt occurred at 12–15 dpa, which is consistent with the peak time point of cell proliferation detected by 5-ethynyl-2′-deoxyuridine (EdU) incorporation in Chen's gentamicin drug injury model in axolotl [37]. In addition, Scadding found that the hypertrophy occurred predominantly at 10–15 dpa after PN, as confirmed by the 3H-leucine incorporation assay. Chen found that cell death peaked at 5 days after drug injury and returned to normal at 14 days using a terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL) assay.
Chen showed that axolotl renal tubule and glomerular could regenerate upon drug injury induced by gentamicin and doxorubicin, respectively [37]. Gentamicin induced large-scale renal tubular disorganization after gentamicin administration for 1 week, and basophilic cell populations aggregated 2 weeks later. Nephrogenesis-related genes in the process of tubular regeneration play crucial roles at both the RNA and protein levels. Consistent with the gentamicin model, doxorubicin treatment significantly induces severe glomerular disruption. The morphology and the reabsorption function were completely restored after 2 months.
Interestingly, the amphibian salamander has a distinguished anatomical structure of nephrons to drain the peritoneal cavity, which is distinct from mammals. This intrinsic characteristic significantly offers an excellent model for studying protein load-induced interstitial fibrosis [31, 39-41], ruling out the effects of glomerular injury and offering more opportunities for detailed mechanistic studies [33]. After intraperitoneal injection of fetal bovine serum, only the proximal tubular epithelial cells of nephrons that drain the coelomic cavity contained protein droplets. Due to the accumulation of protein, luminal dilatation and protein sludge occur along with rapid fibrosis of the interstitial cells and tubulointerstitial activation in response to protein exposure.
Similar to mammals, salamander kidneys did not show any morphological regeneration after PN. Both PN and drug models suggest that the kidney of the salamander undergoes dynamic changes in cell death, proliferation, and hypertrophy. Meanwhile, the drug injury model also revealed dynamic changes in the morphology of the renal tubules and glomeruli from destruction to reconstruction and from loss of function to recovery.
5 PANCREAS REGENERATION
In the last 30 years, the prevalence rates of diabetes in the population have risen dramatically (more than doubled). It is also one of the major diseases contributing to global mortality [42]. Loss or dysfunction of beta (β) cells canonically leads to diabetes.
Sørensen et al. observed that the pancreatic anatomy of the axolotl is comparable to that of mammals, and confirmed the presence of islets using histopathological and immunological methods. They then made a diabetes-like model by treating axolotls with streptozotocin (STZ) [43] and found that β cells could regenerate to recover the functions of the pancreas.
As suggested by these results, the salamander pancreas is highly conserved structurally and functionally from mammals. Establishing a proper model for studying the mechanisms of pancreatic and β cell regeneration in axolotls will open up a whole new vista for human diabetes treatment in the future.
6 CONCLUSIONS AND PERSPECTIVES
We can find that different organs or tissues in salamanders exhibit different regenerative potentials and modes, while at the same time there are some regenerative mechanisms that are conserved with those in mammals. Specifically, canonical organs such as limbs, brain (spinal cord), heart, tail and lens can achieve morphological recovery after amputation injury and show morphological regeneration (Figure 1c). Non-canonical organs such as the liver and lungs show compensatory regeneration after amputation injury, similar to mammals (Figure 1b). Non-canonical organs such as the kidneys, normally exhibiting mild regeneration ability after amputation injury, show structural regeneration to reproduce lost cell types after drug-induced injury (Figure 1a).
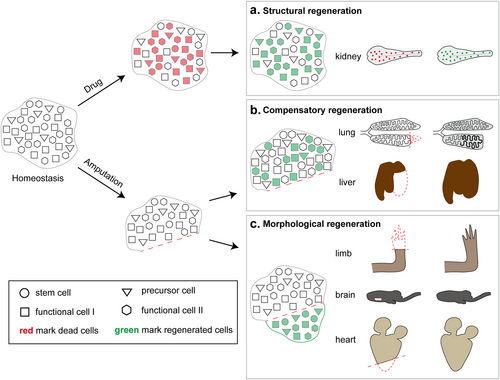
Scheme of different injury and regeneration behaviors of salamander organs. (a) Structural regeneration: In drug-damaged kidneys, there is no obvious change in the morphology of the organ as a whole, but the loss of sub-structures or particular cell types, such as renal tubules and renal corpuscles due to the death of a large number of functional cells. It leads to a temporary imbalance in renal function, and then the relevant cells will re-enter the cell cycle and rebuild the renal sub-structures until the function is restored. (b) Compensatory regeneration: After amputation in the lungs and liver, functional cells such as alveolar epithelial cells and hepatocytes rapidly proliferate in large numbers and compensate for the restoration of the overall function of the organ by spatial occupancy. (c) Morphological regeneration: After amputation with loss of morphology, the regenerative cell population consists mainly of dedifferentiated cells, which proliferate and differentiate into different terminal cells to rebuild tissue sub-structures and achieve restoration of morphology and function.
Why are the regeneration mechanisms of canonical and non-canonical organs so different? The internal microstructures of non-canonical organs such as the liver, lungs, kidneys, and pancreas of salamanders are highly similar and conserved, even though their morphology is quite different from that of mammalian organs. Through comparison, it is found that non-canonical organs have alveoli in the lungs, lobules in the liver, and tubules and glomeruli in the kidneys, and there are a large number of repetitive structural copies of functional units in these organs. Whereas no such repetitive structures can be found in any of the canonical organs, they tend to function as an intact and complex system. Although there are many brain regions in the brain, each has a unique function. The remaining repetitive structures in all non-canonical organs after amputation can be regenerated in a compensatory manner to compensate for the maintenance of total organ function, whereas canonical organs can de novo regenerate to restore organ function. Using drugs such as gentamicin or doxorubicin do not destroy the overall morphology of the kidney, only the repetitive structural units, so regeneration of the original structural units occurs in situ. Whereas drug damage to salamander dopamine neurons remodels the proliferative dynamics and induces robust neurogenesis, histological and functional recovery is accomplished within a month [44-47].
Stem cell activation and differentiation can affect the regenerative capacity of organs. Stem cell-mediated organ regeneration is representative and conserved in organs such as the liver [48], lungs [49, 50], kidneys [51], and intestines [52]. In skeletal muscle regeneration, humans [53], like other mammals [54], share with salamanders the existence of a similar mechanism of muscle stem cell-driven muscle regeneration. Notably, muscle stem cells show different potential for skeletal muscle repair in different species, even in different salamander varieties [55-59]. The activation and differentiation of Pax7+ satellite cells are essential for blastema formation and limb regeneration in newts and axolotls [57-59]. Larval newts, and adult, neotenic axolotls, as well as mammals, have a conserved mechanism for skeletal muscle regeneration, that is, generation of myofibers and muscle through massive proliferation and differentiation of Pax7+ satellite cells [57-60]; whereas adult newt, is restored to a mononuclear myofiber state by multinucleated myofiber de-differentiation, and then regenerates skeletal muscle by mononuclear myofiber proliferation [61, 62]. De-differentiation is unique to adult newt skeletal muscle regeneration. In other organs, like in humans, hepatocyte dedifferentiation is critical for liver regeneration [63]. In the future, if methods can be developed to induce dedifferentiation in mammalian skeletal muscle, it will provide a novel set of solutions for human skeletal muscle regeneration therapy.
The current difficulties in the study of non-canonical organ regeneration in salamanders are mainly due to: (1) The small number of researchers; (2) The lack of technical means for the study; and (3) The lack of background and resources for the study. Recent progress in embryo, tissue and cell transplantation [58, 64-69], virus-mediated cell labeling [70, 71], transgenic [72-79], tissue clearing [80-83] and live imaging [77, 83-86], gene editing [87-90], de novo sequencing [88, 91-97], single-cell [7, 98-105] and spatiotemporal [7, 103-107] multi-omics technologies (Figure 2) have dramatically expanded knowledge about embryonic and tissue development, and the evolution of animal behavior and have advanced human understanding of the spatiotemporal dynamics of cells and genes at the genetic, transcriptional, and protein levels during the regeneration of canonical organs in salamanders, and their regulation of regeneration. Currently, models of pneumonectomy, hepatectomy, nephrectomy, and renal drug injury have been successfully constructed in salamanders, based on which we have discovered the regenerative potential of salamander alveolar epithelial cells, hepatocytes, and other cells and structures such as the alveoli, glomeruli, and renal tubules. In the future, we can try to construct models of other human diseases such as lung drug injury, pulmonary airway damage and diabetes in salamanders to advance human understanding of regenerative therapies for related diseases. Furthermore, we need to develop more transgenic animals (such as those for cell tracking, knockout or knockdown, and cell killing) and combine them with viral-mediated labeling and spatiotemporal multi-omics labeling techniques as a way to improve our research tools, allowing us to study non-canonical and canonical organ regeneration in salamanders in greater depth at broader scales, longer time spans, finer cellular localization, and a fuller array of tools for omics analysis.
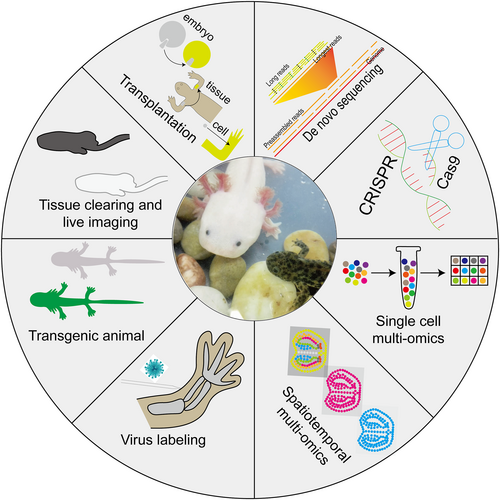
Technological development in the field of salamander regeneration research. Illustration of main available technologies developed in the past years in axolotl, mainly including tissue/cell transplantation, virus-mediated cell labeling, transgenic methods, tissue clearing and live imaging, gene editing, de novo sequencing, single-cell and spatiotemporal multi-omics technologies.
The study of salamander regeneration offers a promising future prospect. It not only helps us to further understand the mechanisms of organ development and regeneration but also promotes potential clinical applications. Further studies on the mechanism of non-canonical organ regeneration in salamanders, especially the exploration of cell behavior and the cell fate of functional cells during non-canonical organ regeneration, will help us to improve the function of human organs. Meanwhile, with the development of biotechnology and synthetic biology, organ regeneration research will also provide new ideas and methods for the treatment of human diseases and the development of new drugs. Therefore, organ regeneration research will have an important impact on human health and disease treatment in the future.
AUTHOR CONTRIBUTIONS
Binxu Yin, Xiao Cui, Yanmei Liu and Ji-Feng Fei wrote the manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
All authors declare that there are no competing interests. Ji-Feng Fei is an Editorial Board Member for Medicine Advances and was not involved in the editorial review or the decision to publish this article.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




