Metabolic regulation of intestinal homeostasis: molecular and cellular mechanisms and diseases
Abstract
Metabolism serves not only as the organism's energy source but also yields metabolites crucial for maintaining tissue homeostasis and overall health. Intestinal stem cells (ISCs) maintain intestinal homeostasis through continuous self-renewal and differentiation divisions. The intricate relationship between metabolic pathways and intestinal homeostasis underscores their crucial interplay. Metabolic pathways have been shown to directly regulate ISC self-renewal and influence ISC fate decisions under homeostatic conditions, but the cellular and molecular mechanisms remain incompletely understood. Understanding the intricate involvement of various pathways in maintaining intestinal homeostasis holds promise for devising innovative strategies to address intestinal diseases. Here, we provide a comprehensive review of recent advances in the regulation of intestinal homeostasis. We describe the regulation of intestinal homeostasis from multiple perspectives, including the regulation of intestinal epithelial cells, the regulation of the tissue microenvironment, and the key role of nutrient metabolism. We highlight the regulation of intestinal homeostasis and ISC by nutrient metabolism. This review provides a multifaceted perspective on how intestinal homeostasis is regulated and provides ideas for intestinal diseases and repair of intestinal damage.
1 INTRODUCTION
The small intestine serves as the primary organ for food digestion and nutrient absorption, functioning as a critical immune barrier as well. Intestinal epithelial cells, known for their rapid turnover, undergo a complete renewal every 3–5 days.1, 2 However, this delicate balance can be disrupted by various internal and external stimuli, including mechanical stress, dysbiosis of the gut microbiota, and exposure to radiotherapy, chemotherapy, and inflammatory bowel diseases (IBDs). Such disruptions lead to compromised intestinal homeostasis, resulting in damage to intestinal epithelial cells and a decline in overall intestinal function.3, 4
Intestinal stem cells (ISCs) play a central role in maintaining intestinal homeostasis. The successful culture of small intestinal organoids in vitro and the use of transgenic mouse models contribute significantly to our understanding of ISC self-renewal and differentiation. Digestive diseases are increasingly prevalent in recent years, and China may become the nation with the highest number of IBD cases globally.5, 6 Consequently, research efforts are increasingly focused on strategies to maintain and restore intestinal homeostasis.
ISCs exhibit remarkable plasticity, with the ability to self-renew and differentiate into the various cell types necessary for normal intestinal function. The balance between ISC self-renewal and differentiation is meticulously regulated by multiple signaling pathways, enabling ISCs to proliferate and give rise to specialized cell lineages. In this review, we provide a comprehensive overview of recent development in the regulation of intestinal homeostasis. We explore the status of the intestine and the properties of stem cells under various conditions, including homeostasis, injury, and aging. Additionally, we present the regulation of intestinal homeostasis from multiple perspectives, including the regulation of intestinal epithelium, microenvironment, and metabolic regulation. We also discuss the cellular and molecular mechanisms that underpin intestinal homeostasis, with a particular focus on the impact of metabolic processes within the intestine. Finally, we underscore the significance of nutrient metabolism in intestinal function, suggesting new avenues for metabolic research that may inform the treatment of intestinal diseases and facilitate the repair of intestinal damage.
2 CHARACTERISTICS OF THE INTESTINE
2.1 Characteristics of the intestine under homeostasis
The small intestine is the fastest renewing tissue in the adult body. The small intestine contains a significant number of immune cells and serves as a crucial immunological barrier in the body. In response to internal and external environmental stimuli, the intestinal epithelium is continuously resupplied by self-renewal and migratory differentiation of stem cells to maintain intestinal homeostasis.1, 7, 8 The intestinal epithelium is lined by finger-like structures called villus (Figure 1), which contain absorptive and secretory cells.9 Absorptive cells are responsible for nutrient and water uptake, while secretory cells include goblet cells, which secrete mucus; enteric endocrine cells, which produce hormones; and Paneth cells, which release antimicrobial peptides.10 These cells play a role in regulating intestinal immunity and other physiological functions.1, 2, 9 Lieberkühn crypts reside at the bottom of the villus, there are wedge-shaped crypt base columnar (CBC) cells, which are also referred to as ISCs (Figure 1). Self-renewal and differentiation of leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5+) ISCs are critical for maintaining a stable turnover of intestinal epithelial cells.10-12 Lgr5+ ISCs located at the base of the crypts are widely regarded as the primary agents of intestinal epithelial renewal. There is also a type of reserve stem cells at the +4 position of the crypt, and these cells take over stem cell functions after the loss of Lgr5+ ISCs.13 Recent studies have shown that the main source of Lgr5+ ISC is in the upper crypts. Researchers have found a type of progenitor cell that expresses fibroblast growth factor-binding protein 1 (Fgfbp1) in the upper crypts. These Fgfbp1+ cells, distinct from the previously identified +4 cells, possess the capability to proliferate bidirectionally. Under homeostatic conditions, Fgfbp1+ cells can migrate upward to differentiate into mature cells or migrate downward to form Lgr5+ ISCs.14 Intestinal homeostasis is coregulated by multiple signaling pathways. The wingless-related integration site (WNT)/β-catenin signaling is highly enriched in crypts and drives ISC self-renewal. The bone morphogenetic protein (BMP) signaling pathway promotes differentiation of ISCs into mature cells. When intestinal epithelial cells are damaged, the Yes-associated protein (YAP) signaling pathway induces intestinal cell reprogramming, enabling rapid repair.15-19 Small intestinal organoids have emerged in recent years as powerful tools for studying intestinal development and disease. Intestinal organoids are three-dimensional (3D) mini-intestines with intact crypts and villus structural domains. These organoids can be cultured from a crypt or a single Lgr5+ ISC.20
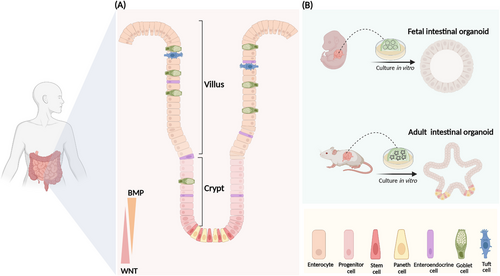
The intestine initially arises from endodermal cells.21 At days 8–9.5 of embryonic development (E8–E9.5), cells of the endodermal lineage form a closed intestinal tube and give rise to the regions of the anterior, middle, and posterior intestine.22 From E9.5 to E14.5, the intestinal tube becomes a pseudostratified layer and the intestinal epithelium and mesenchyme proliferate rapidly.23 Lineage tracing shows that Lgr5+ ISC first appears at E12.5.24 Around E16.5, epithelial cells differentiate into absorptive and secretory intestinal epithelial cells. By 14 days after birth (P14), the intestinal crypts and villus structure resemble those of the adult mouse.23 The fetal intestinal organoids derived from the early fetal intestine, fetal organoids have a greater area for proliferation, evenly dispersed proliferating cells on the surface and present a spherical structure without budding.25 The proliferation of adult intestinal organoids is restricted to the crypt structural domains, and the crypt domains extend outward to form branching structures known as budding (Figure 1). Fetal intestinal organoids and adult intestinal organoids display notable differences in their transcriptomes.25 But when intestinal epithelial cells sustain injury, adult intestinal organoids undergo transient reprogramming to generate fetal-like organoids that proliferate rapidly to promote damage repair.16, 25, 26 We have effectively restored the damaged intestine by transplanting and colonizing intestinal organoids into the injured intestinal mucosa.3
2.2 Characteristics of the intestine during injury
The intestinal epithelium acts as a barrier against the harsh environment. It hosts a diverse and complex community of microorganisms, chemicals, and metabolites, some of which possess pathogenic and toxic properties.27 The intestinal tract is very susceptible to injury from various external factors, including mechanical stress, radiation, chemotherapy agents, pathogenic bacteria, and intestinal diseases. Such insults cause damage to the intestinal epithelium.1 However, the epithelium possesses a remarkable capacity for rapid self-repair, efficiently restoring its structural integrity and thereby mitigating the risk of infection.28 Intestinal surgery, irritable bowel syndrome, intestinal obstruction, and Crohn's disease (CD) result in elevated intraluminal pressure, leading to deformation of the intestinal mucosa. This deformation can cause mucosal atrophy, which negatively impacts both intestinal physiology and the healing processes.29 In mice, mechanical stress resulting from simulated colon tumor expansion activates WNT target genes in adjacent normal tissue. This process accompanied by crypt enlargement during the formation of early tumorous aberrant crypt foci.30 Irradiation and 5-fluorouracil (5-FU) are widely used anticancer treatments in clinical practice.31, 32 However, the intestine is particularly sensitive to these treatments. After irradiation, the apoptotic factors such as p53, poly ADP-ribose polymerase 1 (PARP-1), and caspase-3 being highly expressed in crypt cells.33 High doses of radiation can lead to the loss of Lgr5+ ISCs and the elimination of proliferating progenitor cells, resulting in crypt atrophy.34 After irradiation, endothelial cells generate significant amounts of reactive oxygen species (ROS) and secrete ceramide and acidic sphingomyelinase, leading to increased vascular permeability and tissue hypoxia.35, 36 Immune cells, such as M1 macrophages and mast cells are activated, triggering a long-term inflammatory response and inhibiting crypt regeneration.37, 38 Acute inflammation and IBD can also cause the loss of Lgr5+ ISCs.39 Irradiation can also disrupt the balance of the gut microbiota, leading to an increased abundance of pathogenic bacteria, such as Helicobacter and Clostridium. While concurrently reducing the relative abundance of beneficial phyla like Bacteroidetes and Firmicutes.40 Bacterial, viral, or parasitic infections can cause extensive damage to the intestinal tract, inducing the formation of wound-associated epithelial (WAE) cells. These cells rapidly cover the damaged regions, facilitating the swift repair of the intestinal epithelium.41, 42
However, earlier studies have demonstrated that Lgr5+ ISC are not essential for intestinal regeneration. Even when Lgr5+ ISC are depleted following radiation-induced damage, the intestinal epithelium retains the ability to recover.43 Studies show that differentiated cells can undergo dedifferentiation upon injury, giving rise to stem cells with self-renewal capacity.44-46 Additionally, dormant reserve stem cells located at position +4 are activated after damage to perform stem cell functions.47 Current findings suggest isthmus progenitors within the crypts are now considered the primary contributors to intestinal homeostasis and regeneration.48 Following ISC loss after injury, differentiated epithelial cells can revert to a stem cell state with self-renewal capabilities. Secretory lineage cells such as delta-like canonical Notch ligand 1 (Dll1) and atonal basic helix-loop-helix (bHLH) transcription factor 1 (Atoh1) secretory progenitors, as well as lysozyme 1 (Lyz1+) Paneth cells and goblet cells, have the potential to generate fully functional ISCs.44-46, 49 After the depletion of Lgr5+ ISCs, cells marked by Bmi1, mouse telomerase reverse transcriptase (mTert), homeodomain only protein (Hopx), or leucine-rich repeats and immunoglobulin-like domains 1 (Lrig1) are activated at the +4 position above the crypts. These cells take on stem cell functions and contribute to epithelial repair.13, 47, 50-56 A population of cells highly expressing clusterin (Clu) has also been found in the intestinal epithelium after irradiation. These cells are known as the revival stem cell.26 After irradiation, Lgr5+ ISC ablation mediated by diphtheria toxin (DT) or dextran sulfate sodium salt (DSS) injury, these cells undergo transient expansion, reviving the homeostatic stem cell compartment and replenishing the damaged intestinal epithelium. During the repair process, the damaged epithelial cells undergo transient reprogramming, resulting in the upregulation of fetal and regeneration markers, while the markers of adult stem cell and differentiation are suppressed.16, 26 When cultured in vitro, parasite-infected crypts can develop a spheroid with a fetal-like transcriptome, known as fetal-like organoid.57 The WNT, Notch, and YAP/transcriptional coactivator with PDZ-binding motif (TAZ) signaling pathways are currently recognized as key contributors to intestinal damage repair.3, 16, 58, 59 The WNT pathway is essential for maintaining stem cell homeostasis, and its inhibition impairs crypt repair following irradiation.58 Upon intestinal injury, Notch pathway target genes such as Hes family bHLH transcription factor 1 (Hes1) and the Notch intracellular domain (NICD) are upregulated.46 The YAP/TAZ pathway plays a critical role in damage repair.60 YAP expression increases rapidly after irradiation and DSS injury, and blocking YAP/TAZ activity hinders the repair process.16 Additionally, inhibiting YAP/TAZ can prevent the overactivation of the WNT pathway during injury and restrain stem cells from excessively differentiating into Paneth cells.61 The genes regulated downstream of YAP are markedly enriched in small intestinal organoids derived from embryonic mice. Furthermore, YAP overexpression in adult organoids significantly enhances organoid formation efficiency and induces fetal-like organoids.62, 63 However, the mechanisms by which these pathways interact to coordinate tissue repair remain poorly understood.
2.3 Characteristics of ISC and the intestine during aging
Aging is accompanied by progressive changes in the intestines, which are linked to a higher incidence of intestinal diseases, including malnutrition, chronic constipation, and colorectal cancer (CRC).64-67 These age-related alterations in the intestines encompass loss of stem cells, downregulation of cellular metabolism, dysfunction of the intestinal barrier, and dysbiosis of the gut microbiota.68 The aging process of the intestine is characterized by notable morphological alterations. In aged mice, the villus are significantly elongated and twisted compared the young.69 Additionally, ISCs tend to differentiation of secretory lineage. A decrease in Notch1 expression was observed in the aged intestinal epithelium, which in turn leads to a higher abundance of Paneth cells and goblet cells.70 Mechanistically, the excessive accumulation of proinflammatory cells in the lamina propria of the aging intestine leads to elevated levels of interferon gamma (IFNγ). This increased IFNγ activates signal transducer and activator of transcription 1 (Stat1) in ISCs, resulting in the overexpression of secretory lineage marker genes and causing ISC to preferentially differentiate into secretory cells.71 The number of apoptotic cells increases in senescent crypts, while the number of ISCs decreases.70 This is accompanied by a functional decline. Compared to young mice, 17–24-month-old mice exhibited a notable 62% reduction in Lgr5-GFPHigh ISCs,72 accompanied by a decline in the ISC marker, olfactomedin 4 (OLFM4).69, 70, 73 After two rounds of irradiation (10 Gy + 10 Gy), the rate of crypts regeneration is significantly lower in aged mice (18–22 months).70 In vitro, crypts isolated from aged mice (24 months) demonstrate a markedly reduced ability to form organoids. Crypts from young mice can produce organoids that can be passed down through successive generations. While organoids from senescent crypts form fewer colonies after three to four passages. That indicates a decline in ISC function with aging.74-76 Several signaling pathways are involved in regulating the downregulation of senescent ISC function, such as perturbation of WNT, mammalian target of rapamycin (mTOR) signaling, and reduction of fatty acid oxidation (FAO). In senescent ISCs, Paneth cells, and mesenchymal cells, the key Wnts such as Wnt3 are downregulated.70 Meanwhile, the extracellular WNT inhibitor Notum is significantly upregulated in senescent Paneth cells, leading to reduced WNT signaling and a consequent WNT deficiency in aged ISCs.74 In aged mice (17.5 months), mechanistic target of rapamycin complex 1 (mTORC1) is hyperactivated in ISCs and progenitor cells. Hyperactivated mTORC1 drives villus aging by inhibiting ISC/progenitor cell proliferation through amplifying the MKK6-p38-p53 stress response pathway.77 Aged ISCs have impaired FAO and reduced lipid utilization, while FAO agonists and short-term fasting can activate FAO and restore and improve the activity of ISCs and progenitor cells.73 In addition, the N-terminal domain of protein tyrosine kinase 7 (PTK7) released by senescent fibroblasts is a senescence-associated secretory phenotype (SASP). PTK7 activates WNT/Ca2+ signaling, which in turn triggers nuclear translocation of YAP, reduces stem cell activity and differentiation, and ultimately impairs crypts formation.78 It is evident that ISC function declines with age, and is accompanied by a compromised intestinal barrier and immune function.79-82 These declines may be a key factor contributing to the development of age-related intestinal diseases and possibly even intestinal cancer.
3 REGULATION OF INTESTINAL HOMEOSTASIS
The health of the intestinal tract relies on the maintenance of intestinal homeostasis, which is largely supported by the autoregulation of intestinal epithelial cells and closely linked to the regulation of the tissue microenvironment. Recent studies have shown that nutrient metabolism is crucial for stem cell self-renewal and the preservation of intestinal homeostasis.83 Several transcription factors have been identified as key players in gut development and ISC fate.1, 84-86 The intestinal tissue microenvironment consisting of nonepithelial components such as mesenchymal cells, immune cells, and the diverse gut microbiota.28 This microenvironment interacts with intestinal epithelial cells to preserve ISC function and enhance regenerative responses. These interactions are governed by various signaling pathways, essential for intestinal repair, particularly in response to injury or disease.87-89 Recent studies have identified cellular metabolism as a fundamental regulator of ISC homeostasis,83 positioning nutrient metabolism as a promising avenue for future research.
3.1 Regulation of intestinal homeostasis by epithelium
The maintenance of intestinal homeostasis relies on the self-regulation on ISCs, which is closely tied to stem cell fate determination. ISCs undergo self-renewal to produce new ISCs while also differentiating into progenitor cells with proliferative capacity. These progenitor cells continue to differentiate, eventually giving rise to mature epithelial cells.90, 91 The processes of ISC self-renewal and differentiation are maintained in a dynamic balance, and any disruption to this balance can compromise intestinal homeostasis.92, 93 Numerous transcription factors play a critical role in determining ISC fate. In Beumer and Clevers’ review, the key transcription factors and classical signaling pathways that regulate the differentiation of ISCs are described in detail.1 Recently, more key genes and transcription factors have been identified as crucial regulators of intestinal development and ISC fate determination. Our laboratory has identified hepatocyte nuclear factor 4 (HNF4) as a key transcription factor that regulates intestinal development and maintains intestinal function.83, 94-96 Embryonic ablation of HNF4 induces extensive cytoskeletal reorganization across multiple organs, including the intestine, kidneys, and yolk sacs, culminating in the absence of brush borders.95 Moreover, Hnf4αγDKO at E18.5 results in pronounced intestinal dysplasia, characterized by a translucent and distended intestinal lumen, as well as a loss of villus differentiation.85 In the intestine of adult mice, the HNF4–SMAD4 signaling pathway plays a crucial role in regulating the fate of enterocytes. The deletion of HNF4 promotes ISC differentiation into progenitor and secretory cell lineages.96 Additionally, HNF4 is instrumental in the activation of intestinal FAO and supports ISC self-renewal.83 Similarly, deletion of Krüppel-like factor 5 (KLF5) results in the loss of ISC identity, while the Lgr5ΔKlf5 causes accelerated ISCs proliferation but a loss of self-renewal capacity. This imbalance leads to excessive progenitor cell proliferation within the crypts, eventually exhausting ISCs and triggering premature differentiation of enterocyte.86 Caudal type homeobox 2 (CDX2) and TATA-box-binding protein-associated factor 4 (TAF4) are both critical transcription factors in establishing the gut. CDX2 is essential for the interplay between intestinal epithelium and mesenchyme, and its ectopic expression in the gastric epithelium can induce a transformation into intestinal epithelium.97 When deletion of Cdx2 during embryonic development, the transcriptome of the mutant ileum is highly similar to the esophagus, with intestinal epithelial cells in the distal small intestine being replaced by keratinocytes.98 Similarly, loss of Taf4 in mice at E18.5 results in a villus-deficient intestine with a flat epithelium. In adult mice, the ablation of Taf4 in the epithelium leads to a highly fragile intestine, with the cecum becoming filled with gas.99 Dachsous cadherin-related 1 (Dchs1) and FAT atypical cadherin 4 (Fat4) are essential for the formation of villus and mesenchymal clusters. In E15.5 mice, Dchs1CKO and Fat4CKO led to abnormally flat villus, with mesenchymal cells beneath the villus failing to cluster properly. Instead, these disorganized mesenchymal cells prevent the formation of fully functional villus.100 Special AT-rich sequence-binding protein 2 (SATB2) and metastasis-associated 1 family member 2 (MTA2) are crucial transcription factors involved in regulating the fate of colon stem cells and colon formation. In adult mice, deletion of Satb2 causes colon stem cells to transform into ileum-like stem cells, resulting in the conversion from colon cells to small intestinal cells.84 While the small intestine is responsible for absorbing nutrients such as lipids and carbohydrates, the colon primarily absorbs electrolytes. Loss of Mta2 in the colon of adult mice leads to the activation of Hnf4α, which upregulates the expression of lipid transporter fatty acid-binding protein 6 (FABP6) and microsomal triglyceride transfer protein (MTTP), consequently increasing lipid uptake in the colon.101 Furthermore, the forkhead box A (FOXA) shapes the intestinal microbiota in mice by controlling the glycosylation of intestinal epithelial cells. When Foxa1 and Foxa2 are knocked out in intestinal epithelial cells, the glycosylase network is disrupted, leading to drastic changes in microbial composition and spontaneous IBD.102
3.2 Regulation of intestinal homeostasis by mesenchymal cells
The maintenance of intestinal homeostasis is not only dependent on the regulation of epithelial cells. The tissue microenvironment also plays a crucial role (Table 1). Intestinal mesenchymal cells work synergistically to regulate intestinal homeostasis through interactions with epithelial cells and immune cell.103 Mesenchymal cells are a highly heterogeneous population of cell types (Figure 2), including fibroblasts, myofibroblasts, pericytes, smooth muscle cells, and mesenchymal stem cells.88 Crypts myofibroblasts located at the base of crypts, and they produce WNT ligands that maintain ISC growth.104 Platelet-derived growth factor receptor alpha (Pdgfrα+) mesenchymal cells express high levels of R-spondin3, which amplifies WNT signaling.105 Villus fibroblasts (VF) are a major source of BMP ligands.106, 107 Pdgfrα-high VF progenitors secrete the epidermal growth factor (EGF) family ligand neuregulin 1 (NRG1), which induces ISC differentiation toward to the secretory cell lineage.108 NGR1 was strongly upregulated in macrophages and Pdgfrα+ stromal cells during injury, and activation of the mitogen-activated protein kinase (MAPK) pathway and phosphatidylinositol 3-kinase (PI3K)/AKT, also known as protein kinase B (PKB) signaling supported proliferation of stem and progenitor cells during repair.87 Irradiation and DSS resulted in a large expansion of fibroblasts near the injury site and expression of large amounts of R-spondin3 to promote intestinal repair.49, 109 There is a subset of MAP3K2-regulated intestinal stromal cells (MRISC) in the basal part of colonic crypts that upregulate R-spondin1 expression through the ROS-MAP3K2-Extracellular signal-regulated kinase 5 (ERK5)-KLF2 pathway and enhance WNT signaling. It maintains the function of Lgr5+ ISC, and resists DSS-induced colitis.103 Smooth muscle cells in the mucosal muscularis propria, located near the base of colonic crypts, serve as a source of BMP antagonists while also enhancing the YAP signaling pathway. These cells express the matrix metallopeptidase 17 (MMP17), which remodels the extracellular matrix and plays a crucial role in tissue repair.89 Beneath the crypts lies a rare class of prostaglandin-endoperoxide synthase 2 (Ptgs2+) fibroblasts that secrete prostaglandin E2 (PGE2), which activates YAP and promotes the expansion of the lymphocyte antigen 6 family member S (Ly6a) cells population, resembling a reserve stem cell pool.88
| Classification | Microenvironment component | Effect on intestinal | Refs. |
|---|---|---|---|
| Mesenchymal cells | Pdgfrα+ mesenchymal cells |
Pdgfrα+ mesenchymal cells express high levels of R-spondin3, which amplifies WNT signaling. |
105 |
| Villus fibroblasts | Villus fibroblasts are the major source of BMP ligands that promote intestinal epithelial cell differentiation. | 106, 107 | |
| Pdgfrα-high villus fibroblasts | Pdgfrα-high villus fibroblasts secrete the EGF family ligand NRG1, which induces ISC differentiation toward to the secretory cell lineage. | 108 | |
| MRISC | MRISC can upregulate R-spondin1 expression and enhance WNT signaling. | 103 | |
| Smooth muscle cells | Smooth muscle cells can secrete BMP antagonists, enhance the YAP signaling pathway and MMP17, remodeling the extracellular matrix. | 89 | |
| Ptgs2+ fibroblasts | Ptgs2+ fibroblasts secrete PGE2, which activates YAP. | 88 | |
| Mesenchymal stem cell | Mesenchymal stem cells secrete CCL2 and CXCL12, which promote the M2 polarization of macrophages. | 110 | |
| Immune cells | Macrophages | Macrophages secrete high levels of TGFB1, and TGFB1 activates the regenerative transcription factors YAP. | 3 |
| ILC3 | ILC3 secretes IL-22, which induces STAT3 phosphorylation and promotes ISC proliferation. | 111 | |
| Gut microbiota | Lactobacillus reuteri | Lactobacillus reuteri stimulates the secretion of IL-22, induces the expression of R-spondins, enhances the expression of antimicrobial peptides, and promotes oxytocin secretion. | 112-114 |
| Bacteroides thetaiotaomicron | Bacteroides thetaiotaomicron enhances intestinal transit after colonization of GF mice. | 115 | |
| Bacteroides fragilis | Bacteroides fragilis derivatives 3-phenylpropionic acid and promotes the integrity of the intestinal epithelial barrier by activating AhR signaling. | 116 | |
| Clostridium bifermentans | Clostridium bifermentans increases the expression of the Dgat2, enhances oleic acid uptake and regulates lipid absorption. | 117 |
- Abbreviations: AhR, aryl hydrocarbon receptor; BMP, bone morphogenetic protein; CCL2, C–C motif chemokine ligand 2; CXCL12, C–X–C motif chemokine ligand 12; EGF, epidermal growth factor; ILC3, innate lymphocyte type 3; IL-22, interleukin-22; ISC, intestinal stem cell; MMP17, matrix metallopeptidase 17; MRISC, MAP3K2-regulated intestinal stromal cells; NRG1, neuregulin 1; PGE2, prostaglandin E2; STAT3, signal transducer and activator of transcription 3; TGFB1, transforming growth factor beta 1; WNT, wingless-related integration site.
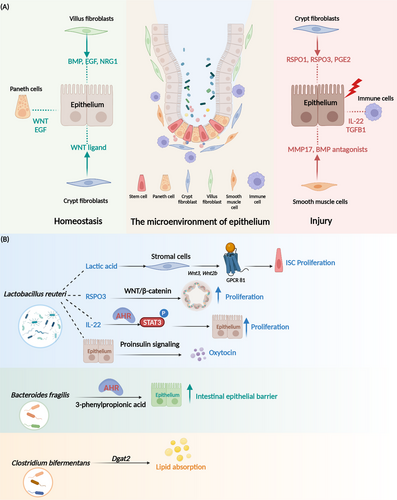
3.3 Regulation of intestinal homeostasis by immune cells
During intestinal injury, the immune system activates (Figure 2) and leads to the recruitment of a substantial number of immune cells to the site of damage.118, 119 Our study found that on day 2 after irradiation, macrophages accumulate at the base of crypts. These cells secrete high levels of transforming growth factor beta 1 (TGFB1) and activates the regenerative transcription factors YAP-SRY-Box transcription factor 9 (SOX9) circuit. It induces the epithelium to undergo reprogramming, and generates fetal-like cells to promote intestinal repair.3 Additionally, bone marrow-derived mesenchymal stem cells secrete C–C motif chemokine ligand 2 (CCL2) and C–X–C motif chemokine ligand 12 (CXCL12), which promote the polarization of M2 macrophages and activate interleukin-10 (IL-10+) T cells and IL-10+ B cells within the intestinal tract, exerting an inhibitory effect on colitis.110 Recent findings highlight the critical role of innate lymphocyte type 3 (ILC3) and interleukin-22 (IL-22) in the process of intestinal regeneration. After injury, ILC3 activates and secretes IL-22. It induces signal transducer and activator of transcription 3 (STAT3) phosphorylation and promotes ISC proliferation.120 Additionally, ILC3 detects lysophosphatidylserine (LysoPS) released from apoptotic neutrophils through its membrane receptor G-protein-coupled receptor 34 (GPR34), which triggers the PI3K-AKT and RAS-ERK pathways, leading to the release of IL-22 and initiation of tissue repair.111 Furthermore, both adrenalines secreted by intestinal nerve cells and viral infections can activate ILC3, drive IL-22 production and enhance the regeneration of intestinal epithelium.121, 122
3.4 Regulation of intestinal homeostasis by gut microbiota
The intestinal lumen harbors a vast and diverse community of gut microorganisms, which play a crucial role in maintaining gut health and function.123, 124 These gut microbiotas are essential for the establishment of a mature immune system during early embryonic development.125, 126 The gut microbiota stimulates the formation of the intestinal capillary network.127 Chorionic capillaries in germ-free (GF) mice are underdeveloped after weaning and remain so into adulthood.127 Additionally, early gut microbiota induces the secretion of antimicrobial peptides by Paneth cells, shaping the innate immune response in the intestinal epithelium.128 Microbes during the early stages of intestinal development also activate the WNT pathway, enhance the expansion of Lgr5+ ISC. They promote the growth of intestinal organoids and facilitate regeneration of crypts after injury.129 The intricate interactions between specific gut microbiota an ISC niches have been increasingly recognized as essential for maintaining intestinal homeostasis.130, 131 Two excellent reviews offer comprehensive insights into the intricate crosstalk between ISCs and the gut microbiota, elucidating the specific molecular mechanisms involved in this dynamic interaction.130, 131 Here, we have added the latest discoveries about the gut microbiota (Figure 2). The intestinal probiotic Lactobacillus reuteri has been shown to stimulate the secretion of IL-22 from lamina propria lymphocytes (LPLs), leading to the activation of STAT3 and subsequent acceleration of intestinal epithelial cell proliferation. Additionally, Lactobacillus reuteri promotes the proliferation of intestinal organoids by inducing the expression of R-spondins under homeostatic conditions, thereby sustaining the activation of the WNT/β-catenin pathway.112 This probiotic also reduces the secretion of proinflammatory cytokines in the intestine, lowers concentrations of serum lipopolysaccharides (LPS), enhances the expression of antimicrobial peptides, and inhibits the colonization of Citrobacter rodentium.112 Furthermore, a recent study revealed that Lactobacillus reuteri can promote oxytocin secretion from human intestinal tissues and organoids via proinsulin signaling.113 Lactic acid produced by Bifidobacterium and Lactobacillus has been shown to stimulate Paneth and stromal cells via G-protein-coupled receptor 81 (GPR81), thereby promoting epithelial regeneration. Furthermore, preadministration of lactic acid has demonstrated protective effects against irradiation-induced damage.114 While Bacteroides thetaiotaomicron produces tryptamine, which enhances intestinal transit after colonization of GF mice.115 Bacteroides fragilis—a species known to reinforce the intestinal epithelial barrier. It can derivative 3-phenylpropionic acid and promotes the integrity of the intestinal epithelial barrier by activating aryl hydrocarbon receptor (AhR) signaling.116 Additionally, various specialized gut microbiota is implicated in host metabolism. The Clostridium bifermentans and its metabolites increase the expression of the diacylglycerol O-acyltransferase 2 (Dgat2), which is associated with lipid absorption in the small intestine. It can enhance oleic acid uptake and regulate lipid absorption.117 In GF mice, the absence of intestinal microbiota impairs glucose uptake and storage capacity in skeletal muscle. It leads to an increase in brown adipogenesis, which prevents obesity. However, this metabolic alteration is accompanied by reduced ATP production, resulting in diminished exercise capacity. The specific microbial strains responsible for regulating this process remain unidentified.132
4 METABOLIC REGULATION OF INTESTINAL HOMEOSTASIS
The process of renewing small intestine epithelial cells is characterized by high dynamism, and this constant turnover is closely linked to intense energy metabolism. The metabolism of carbohydrates, lipids, and proteins serves as a vital source of energy for the body. In addition, the metabolic products participate as precursors in the production of substances for regulating the body's physiological function. We previously conducted a study that discovered that HNF4, a transcription factor responsible for regulating the process of FAO, enhances the renewal of ISCs. This finding suggests that alterations in nutrition and metabolic pathways have the potential to impact the quantity and renewal capacity of ISCs.83 The core of intestinal homeostasis regulation depends on ISCs, which are highly sensitive to the nutritional status of the organism. Therefore, it is critical to understand how the nutritional pathways (Table 2) and their metabolites affect the ISCs, as well as intestinal epithelial cells.
| Metabolic pathway | Protein | Metabolism function | Phenotype of genetic model | Refs. |
|---|---|---|---|---|
| Mitochondria and oxidative phosphorylation |
YY1 |
A transcription factor that regulates mitochondrial structure and OXPHOS | The intestinal epithelium deficiency decreases ISC number and villus length. | |
| FOXO | A transcription factor that regulates mitochondria activity and division | The intestinal organoids deficiency decreases ISC markers and increases secretory cell markers. | 135 | |
| HSP60 | A mitochondrial chaperone | The intestinal epithelium deficiency in mice causes transient loss of ISCs and initiates a transition of ISCs toward a Paneth cell-like phenotype. | 136, 137 | |
| DARS2 |
An aspartyl-tRNA synthetase that involves in mitochondria respiratory chain assembly |
Intestinal epithelium-specific deletion results in accumulation of lipid droplets in the proximal small intestine and severe intestinal lesions with reduced numbers of stem, proliferative, secretory, and absorptive cells. | 138 | |
| UCP4C | Uncoupling protein | Overexpression in the Drosophila intestine extends lifespan and rescues the intestinal aging phenotype. | 139 | |
| Glucose metabolism | HK2 | Generation of glucose 6-phosphate (G6P) | Intestinal epithelium-specific deletion Hk2 leads to a decrease in crypt organoid-forming capacity, ISC conversion to secretory cells. | 140 |
| MPC1 | Mitochondrial pyruvate carrier | Specific deletion of Mpc1 in Lgr5+ ISCs expands the intestinal stem cells compartment and increases proliferation. | 141 | |
| Fatty acid oxidation | PRDM16 | A transcription factor that drives oxidative metabolism in brown fat | Prdm16 deletion in mice triggers progenitor cells apoptosis, leading to diminished epithelial differentiation. | 142 |
| HNF4 | A transcription factor that regulates fatty acid oxidation | Loss of Hnf4 in the intestinal epithelium triggers Lgr5+ stem cell loss, and Hnf4 DKO organoid lacks branched crypt domains. | 83 | |
| CPT1A | The carrier of long-chain fatty acids and a rate-limiting enzyme for fatty acid oxidation | Long-term Cpt1a deletion decreases ISC numbers and function and impair organoid-forming capacity. | 73 | |
| HMGCS2 | A rate-limiting enzyme for ketone bodies synthesis | Loss of Hmgcs2 in the intestinal epithelium compromises ISC stemness and regeneration after radiation. | 143 | |
| Fatty acid synthesis | FASN | An insulin-responsive enzyme essential for de novo lipogenesis | Loss of Fasn in the colonic epithelium blocks MUC2 production and induces intestinal inflammation. | 144 |
| ACC1 | An insulin-responsive enzyme essential for de novo lipogenesis | Loss of Acc1 in the intestinal epithelium results in impaired crypt structures in the distal intestine (colon and ileum), a decrease in OLFM4-positive cells, and an increase in the number of Paneth cells. | 145 | |
| Amino acid metabolism | TSC2 | A negative regulator of mTORC1 | Ablation of Tsc2 enhances proliferative activity of IECs in intestinal crypts but increases ectopic Paneth cells. | 146 |
| TSC1 | A negative regulator of mTORC1 | Ablation of Tsc1 of young mice causes premature senescence of intestine. | 77 | |
| SLC7A5 | Reverse transporter of Gln | Intestinal epithelial deletion of Slc7a5 significantly delays tumor proliferation. | 147 | |
| SLC25A22 | Mitochondrial glutamate transporter | SLC25A22 promotes proliferation and migration of CRC cells with mutations KRAS. | 148 | |
| SLC1A3 | Asp/Glu transporter | Deletion of SLC1A3 inhibits human colon cancer cell growth. | 149 |
- Abbreviations: ACC1, acetyl-CoA-carboxylase 1; CPT1A, carnitine palmitoyl-transferase1 alpha; CRC, colorectal cancer; DARS2, aspartyl-tRNA synthetase2; FASN, fatty acid synthase; FOXO, forkhead box O; HK2, hexokinase 2; HMGCS2, HMG-CoA synthase 2; HNF4, hepatocyte nuclear factor 4; HSP60, heat shock protein; MPC1, mitochondrial pyruvate carrier 1; mTORC1, mechanistic target of rapamycin complex 1; OLFM4, olfactomedin 4; PRDM16, PR/SET domain 16; SLC1A3, solute carrier family 1 member 3; SLC25A22, solute carrier family 25 member 22; SLC7A5, solute carrier family 7 member 5; TSC1, TSC complex subunit 1; TSC2, TSC complex subunit 2; UCP4C, uncoupling protein 4C; YY1, neuronal Yin Yang1.
4.1 Regulation of ISC by nutrient metabolism
4.1.1 Dietary regulation of ISC self-renewal
Diet and metabolism intricately intertwine, and the ISCs constantly adjusting its fate decisions based on diet and nutritional status. Various dietary regimens alter the composition of gut microbiota, which in turn impacts the self-renewal and differentiation of ISCs. This dynamic interaction underscores the profound influence of diet and gut microbiota on ISCs behavior.150 Fasting and caloric restriction (CR) have been shown to enhance ISCs self-renewal and regeneration of small intestinal epithelium.73, 151 Fasting increased the number of Lactobacillus and Bifidobacterium in the gut.152, 153 Oral administration of probiotics (containing Bifidobacterium and Lactobacillus) induced more ISCs and was accompanied by an increase in the number of Paneth cells and goblet cells. Additionally, Lactobacillus protects intestinal epithelium from damage after radiation.114 Dietary restriction, such as CR, intermittent fasting, and ketogenic diets, are the most effective antiaging interventions.154 CR prevents or reduces the accumulation of senescent cells in the mouse colon and delays kidney aging.155, 156 The mechanism by which dietary restriction extends lifespan begins with promotion of lipid utilization. Fasting activates peroxisome proliferator-activated receptors (PPARs), increases FAO in intestinal stem and progenitor cells, and enhances ISC self-renewal.73 In addition, researchers have found that CR leads to an increase in the number of functional Lgr5+ ISC that compete for ecological niches.157
Ketogenic diet and high-fat diets (HFD) also promote self-renewal of ISCs.143, 158 It was found that beta-hydroxybutyrate (β-OHB) produced by the ketogenic diet reinforces Notch signaling, instructing ISC self-renewal and lineage decisions.143 It also inhibits the growth of intestinal Bifidobacteria, which leads to a decrease in the level of intestinal CD4+ T helper cells 17 (Th17) cells and modulates the intestinal immune response.159 HFD increased numbers of ISCs through PPAR-δ-dependent activation, and promoted crypt regeneration after irradiation.158 However, long-term HFD activated the growth-promoting pathways, MAPK/ERK and PI3K/AKT/mTOR, which induced tumor growth in a colon cancer model.160 In addition, PPAR-δ activation induced progenitor cells to take on the characteristics of ISCs, resulting in greater susceptibility to tumorigenesis.158 High-sugar diet increased tumor incidence, and the administration of a high-fructose beverage to Apc-Min mice significantly increased tumor size.161 High-glucose diet also inhibited self-renewal of ISCs. Mice provided with glucose-containing drinking water for 4 weeks exhibited impaired crypt regeneration.143
4.1.2 Mitochondria and oxidative phosphorylation
Mitochondria play a crucial role in intracellular oxidative phosphorylation (OXPHOS) for ATP production. Recent studies have revealed that mitochondria also contribute to the regulation of organismal homeostasis by participating in pathways associated with apoptosis and tissue senescence. A study demonstrated that the pyruvate/lactate ratio was higher in ISCs than their neighbors, Paneth cells. This ratio indicates the relative contribution to cellular bioenergetics of mitochondrial respiration versus glycolysis. It suggests that ISCs are more dependent on mitochondrial metabolism as a source of energy.162 Intestinal epithelial development is directly influenced by mitochondria, and YY1 is a crucial transcription factor that regulates both the structure and function of mitochondria as well as OXPHOS. Specific deletion of Yy1 in the epithelium of embryonic mice resulted in severe hypoplasia of the villus and defective differentiation of enterocytes. Additionally, mitochondrial inhibitors hindered the elongation of the villus. YY1 loss in the intestinal epithelium of adult mice resulted in impaired ISCs renewal and led to an imbalance between crypts and villus.133, 134 The FOXO, a transcription factor, plays a significant role in governing mitochondrial activity and division. Deletion of FOXO inhibits Notch and prompts the transformation of ISC into secretory cells, leading to a reduction in the expression of stem cell markers. This observation demonstrates the intricate regulatory mechanisms by which mitochondria influence ISC fate determination.135 Additionally, mitochondrial dysfunction impairs intestinal homeostasis and contributes to intestinal inflammation. DARS2 is involved in mitochondrial respiratory chain assembly. Specific ablation of Dars2 in the intestinal epithelial cells led to severe mitochondrial impairment.138 DARS2 deficiency obstructs the outward transport of lipids absorbed in the small intestine, causing the accumulation of numerous lipid droplets in the proximal small intestine. The Dars2KO mice manifest severe lesions in the intestine, with a notable decrease in the populations of ISCs, progenitor cells, secretory cells, and absorptive cells.138 Loss of the mitochondrial chaperone HSP60 in the intestinal epithelium leads to a decrease in the number of OLFM4-positive cells, a conversion of Lgr5+ ISCs into abnormal Paneth cells,136 as well as an induction of macrophage aggregation at the base of the crypts, exacerbating intestinal inflammation.136, 137
Aging is associated with significant metabolic alteration, and mitochondria playing a central role in age-related pathologies. Aging is closely linked to mitochondrial dysfunction and mutations in mitochondrial DNA (mtDNA).163, 164 The increased production of ROS during aging further exacerbates mitochondrial damage, and mitochondria-derived ROS are thought to be key drivers of cellular senescence.165, 166 Mitochondrial dysfunction also results in a reduced nicotinamide adenine dinucleotide (NAD+/NADH) ratio, leading to the repression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a crucial gene for glycolysis. This repression causes ATP depletion, activation of AMP-activated protein kinase (AMPK), and subsequent cell cycle arrest.167 Factors associated with mitochondrial dysfunction, such as mutations in mtDNA, deletion of mitochondrial chaperone proteins, and mitochondrial protein deacetylases, contribute to cellular senescence. Mice with high mtDNA mutations lead to NAD+ depletion in the intestine, and the mice show marked senescence features in the intestine at 8 months. Mechanistically the accumulation of mtDNA depletes NAD+, leading to the accumulation of large amounts of unfolded proteins in the mitochondria, thereby exacerbating premature senescence of the small intestine.72 The uncoupling protein UCP4C has been identified as a significant factor in extending the lifespan of Drosophila. UCP4C maintains stable proliferation of ISCs via decreasing ROS levels.139
4.1.3 Glucose metabolism
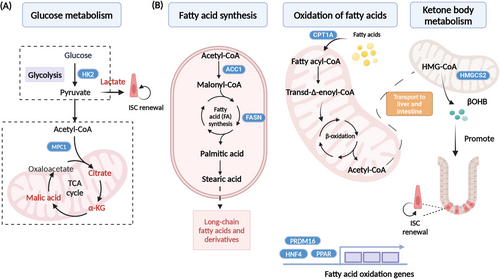
The glycolytic metabolism is the primary source of energy for organismal activity, and its intermediates serve as ample precursors for the synthesis of amino acids and nucleotides. While most cancer cells adhere to the “Warburg” metabolism, ISCs predominantly rely on aerobic mitochondrial metabolism, owing to their higher mitochondrial activity. The Paneth cells utilize anaerobic glycolysis to produce large amounts of lactate to fuel ISCs.162 Organoid formation was impaired with treatment of sodium dichloroacetate (DCA; glycolysis inhibitor) or OXPHOS inhibitor.162 Glycolysis is also closely linked to ISC fate decisions (Figure 3). In the intestinal epithelium, deletion HK2, an enzyme that controls the speed of glycolysis, resulted in reduced ISC self-renewal and increased secretory cells. Mechanistically, HK2 regulates secretory cell differentiation through increased ATOH1 expression and activation of p38 MAPK.140 Interestingly, lactate was able to rescue the phenotype caused by HK2 deletion.140 Under aerobic conditions, pyruvate produced from glycolysis is transported into the mitochondria through the mitochondrial pyruvate carrier 1 (MPC1), where it enters the tricarboxylic acid (TCA) cycle and undergoes aerobic catabolism. MPC1 is lowly expressed in the Lgr5+ ISC, but its expression significantly rises in the villus as ISC differentiation progresses. Deletion of MPC1 in the Lgr5+ ISC leads to an increase in the number of ISCs, but the number of mature differentiated cells (like goblet cells) remains unchanged. MPC1-deficient organoids derived from Lgr5-EGFP mice maintain a larger proportion of GFP-positive stem cells,141 suggesting that inhibition of MPC1-mediated pyruvate metabolism drives ISC self-renewal. But histone acetylation is reduced, and pyruvate metabolism is increased after MPC1 deletion. However, the mechanism by which MPC regulates ISC has not been determined.141
4.1.4 Fatty acid metabolism
FAO (Figure 3) is essential for maintaining stem cell function.168, 169 Our previous study has demonstrated a higher rate of fatty acid uptake in crypts compared to villus. We found deletion of HNF4 in the intestinal epithelium, which plays a critical role in regulating FAO. After loss of HNF4, FAO-related genes downgraded and resulted in the loss of ISCs and a failure of organoid growth.83 The PR/SET domain 16 (PRDM16) along with PPAR proteins regulates small intestinal epithelial renewal via promoting FAO. Intestinal ablation of PRDM16 led to the apoptosis of progenitor cells, significant shortening of the villus, notable lesions in the small intestine, and dysplasia in organoids.142 Fasting and CR induce accelerated FAO and activate ketone body metabolism. Mice under calorie restriction exhibit downregulated mTOR pathway activity, which fosters self-renewal of Lgr5+ ISCs at the expense of differentiation and enhance the number of crypts after injury.73, 151 Twenty-four-hour fastening enhances FAO and accelerates production of passaged organoids, but acute ablation of Cpt1α inhibited fasting-induced organoid formation. In homeostatic, acute Cpt1α loss is largely dispensable for Lgr5+ ISCs maintenance, but chronic deficiency reduces the number and function of ISCs, suggesting the maintenance of ISC function is dependent on long-term FAO.73 HMG-CoA synthase 2 (HMGCS2) is a rate-limiting enzyme in ketone body metabolism, and deletion of Hmgcs2 in the intestinal epithelial cells reduces the number of OLFM4-positive cells in the crypts and leads to premature conversion of ISCs to secretory cells.159 Mechanistically, Hmgcs2 loss depletes β-OHB levels in Lgr5+ ISCs, leading to Notch inhibition, impairing ISC stemness and biasing them toward secretory lineage differentiation (Figure 3). Exogenous fatty acid supplementation, such as acetate, can rescue impaired organoid caused by defects in FAO-related genes.83, 142
Genes involved in fatty acid synthesis have also been shown to regulate ISCs function. Acetyl coenzyme A carboxylase (ACC) is a rate-limiting enzyme for fatty acid synthesis, and specific deletion of Acc1 in the intestinal epithelium results in impaired crypt structures in the distal intestine (colon and ileum), a decrease in OLFM4-positive cells, and an increase in the number of Paneth cells. The organoids deficient in Acc1 resulted in extensive cell death, and supplementation with acetate failed to rescue this defect. Mechanistically, ACC-mediated de novo fatty acid synthesis maintains PPAR-δ/β-catenin levels in the ISCs for supporting organoid formation and differentiation.145 In conclusion, both fatty acid catabolism and synthesis play key roles in maintaining ISC function, and FAO is a key pathway for maintaining ISC function. Deficiency of fatty acid synthase (FASN) in the colonic epithelium blocks mucin 2 (MUC2) production, disrupts the intestinal barrier, and induces enterocolitis.144 However, deletion of the oleic acid rate-limiting enzyme stearoyl-CoA desaturase (SCD1) in intestinal epithelial cells did not affect ISC self-renewal and differentiation, but more and larger tumors were found in Apc-Min mice when loss of SCD1.170
Overall, lipid metabolism is essential for the maintenance of ISC function. PPAR-FAO is currently thought to be closely related to stem cell self-renewal, while pyruvate metabolism and glycolysis promote ISC differentiation into the secretory lineage.73, 135, 141, 158 Both short-term fasting and HFD can increase FAO gene expression and activate PPAR, which in turn increases WNT/β-catenin expression and activates ISC self-renewal.73, 158 HNF4 and PRDM16 can also activate FAO genes and are required for ISC renewal.83, 142 Inhibition of MPC1, a key gene that inhibits pyruvate metabolism, also promotes FAO and ISC self-renewal.141 OXPHOS is required for the differentiation of intestinal epithelial cells during crypt formation. A decrease in mitochondrial function is associated with an increase in the number of secretory cells.135
Recent research has demonstrated that senescence significantly impacts intracellular lipid metabolism. In senescent cells, there is an upregulation of FASN, a key enzyme in fatty acid synthesis, along with related genes involved in β-oxidation, which may contribute to the increased secretion of the SASP.171 Moreover, senescent cells exhibit increased production of polyunsaturated fatty acid (PUFA) derivatives, including oxylipins, dihomo-prostaglandins, and leukotrienes. These PUFAs activate the renin–angiotensin system (RAS) and promote both SASP secretion and cellular senescence.172 It has also been hypothesized that senescent cells utilize PUFA desaturation as a mechanism to convert NADH to NAD+, thereby maintaining NAD+ redox homeostasis.173 Additionally, a recent study revealed an increase in phosphatidyl di(monoacylglycerol) species containing PUFAs across various organs in senescent organisms, while levels of phospholipids containing saturated and monounsaturated fatty acids decrease.174 This alteration in lipid composition further underscores the complex metabolic reprogramming that occurs during cellular senescence.
4.1.5 Amino acid metabolism
Amino acids serve as the basic structural units of proteins, and the breakdown products can enter the TCA cycle to provide energy for the organism. Amino acids are also involved in the mTOR signaling pathway, a key pathway for maintaining cell growth, metabolism, and immunity.175 The mTOR pathway is actively involved in the renewal of ISCs and the maintenance of intestinal homeostasis.146 Glutamine (Gln), arginine (Arg), and leucine(Leu) are known to activate the mTOR pathway.175 Disruption of mTOR signaling in the intestinal epithelium resulted in flattened villus and developmental defects in the ileum.146 Overactivation of the mTOR pathway by depleting Tsc1 in the IECs resulted in the development of senescent features of the intestinal tract in young mice, including reduced crypt height and number, reduced villus length and density, and impaired crypt regeneration after irradiation.77 Loss of Tsc2 in the colonic epithelium suppressed WNT pathway, leading to a decrease in the number of Lgr5+ colonic stem cells and an increased susceptibility to DSS-induced colitis. After small bowel resection, the remaining bowel undergoes compensatory effects that results in deeper crypts in the remaining bowel and more cell regeneration. Tsc1-induced overactivation of mTOR causes larger hyperproliferative crypts and leads to uneven distribution of Paneth cells after small bowel resection.176 Therefore, mTOR may not be essential for stimulating crypt proliferation under homeostatic conditions, but activation of the mTOR pathway is necessary to repair intestinal damage during injury after irradiation, DSS, or small bowel resection.176
Amino acids can produce alpha-ketoglutarate (α-KG), which is involved in OXPHOS as well as sugar and lipid synthesis. It is increasingly recognized that tumor growth heavily relies on the uptake of exogenous amino acids, particularly Gln, aspartate (Asp), and serine (Ser). Studies are now delving into amino acid uptake preferences of various tumors, aiming to devise therapeutic strategies that target amino acid metabolism for combating tumors.175 Gln is the most consumed nonessential amino acid in the human body. Major consumers of Gln include intestinal cells, immune cells, and tumor cells.177 Gln plays an important role in colon cancer growth and invasion. Hypoxia induces high expression of glutaminase 1 (GLS1), the hydrolysis enzyme of Gln, in colon cancer cells, which promotes colon cancer cell migration and tumor growth.177
Exogenous Gln deprivation stimulates p53 activation and promotes the expression of the Asp/Gln transporter protein SLC1A3. The absence of SLC1A3 resulted in the inability of cancer cells to utilize Gln, thereby inhibiting colon cancer cell growth.149 Gln reverse transporter protein, SLC7A5, maintains intracellular amino acid levels. In KRAS-mutant CRC mice, SLC7A5 functions through transcriptional and metabolic reprogramming to support amino acid requirements for cell proliferation. The intestinal epithelial deletion of Slc7a5 significantly reduces the number of tumors.147 These studies suggest that Gln metabolism and its transporter proteins are attractive targets for the treatment of CRC. Mechanistically, Asp inhibits AMPK-mediated p53 activation via serine/threonine kinase 11 (STK11) and promotes tumor cell proliferation.178 SLC25A22 is an amino acid transport protein that promotes Asp synthesis. Knocking down SLC25A22 leads to a reduction in Asp biosynthesis and cell proliferation, and an increase in apoptosis, which in turn reduce tumor formation and metastasis in KRAS-mutant CRC cells in mice.148 Ser is a precursor for the synthesis of nucleic acids, proteins, lipids, and antioxidants. It is essential for the metabolic processes that support cell growth and survival in cancer development. Restricting Ser effectively induced the production of deoxysphingolipids, leading to a reduction in tumor growth.179 In summary, tumor cells favor specific amino acids, suggesting potential treatment avenues by targeting these preferences. Either by knocking down their transport carriers or by restricting their intake could provide a novel approach for cancer therapy. Intriguingly, ISCs have metabolic characteristics similar to those of tumor cells, yet the direct role of these amino acids in regulating ISCs is poorly understood. This raises the pivotal question: Could the convergence of ISCs and tumor cells metabolic pathways hold the key to innovative cancer therapies, reshaping the future of oncology?
4.2 Metabolites regulate ISC function
Metabolites are intricately linked to the renewal of ISCs. As mentioned previously, Paneth cells produce large amounts of lactate, which enhances ISC OXPHOS to support stem cell function.162 Lactate stimulated the growth of intestinal organoids, and prompted a notable upregulation of Wnt3 and Wnt2b in stromal cells.114 Thus, coculture of stromal cells and lactate with intestinal organoids induced larger spherical organoids.114 Fatty acids play a critical role in maintaining ISCs, with exposure to palmitic or oleic acid resulting in increased Lgr5+ ISCs in the organoids. Palmitic and oleic acid also enhanced generation of organoids. Mechanistically, fatty acids activate PPAR-δ, which drives the organoid self-renewal process.158 The β-OHB is key product of ketone body metabolism, supplementation of exogenous β-OHB increased Lgr5+ ISCs and improved the integrity and survival of crypts after injury.143 Exogenous supplementation β-OHB has been shown to upregulate Oct4 and lamin B1 in vascular smooth muscle and endothelial cell, thereby mitigating aortic senescence in mice.180
Amino acids serve not only as building blocks for proteins but also as precursors for the synthesis of a variety of signaling molecules and hormones. Gln is the most abundant nonessential amino acid in the body. In early weaned mice, the self-renewal process of ISCs was impaired, but exogenous Gln activated the WNT signaling pathway and promoted ISC self-renewal.181 Short-term Gln deprivation led to a reduction in the crypt domains of the organoids, while long-term Gln deprivation resulted in organoid atrophy.181 Gln also activated the YAP pathway, significantly attenuated intestinal damage in burned mice, and promoted crypt repair and regeneration.182 Cysteine (Cys), an essential amino acid, and its derivative—taurine are strongly associated with the aging process. The number of ISCs recovered after taurine supplementation in aging mice.183 Methionine (Met) is one of the essential amino acids, and its metabolite, S-adenosylmethionine (SAM), is critical for ISCs. Met suppressed stem cell proliferation and promoted intestinal organoid differentiation. Additionally, deprivation of Met caused a reduced number of ISCs in Drosophila.139, 184 Tryptophan (Trp) is one of the essential amino acids and a precursor of hormones and neurotransmitters. Serotonin (5-HT) is derived from Trp, it serves as a neurotransmitter produced and secreted by enterochromaffin cells in the intestine. 5-HT plays a crucial role in driving self-renewal of ISCs. Valeric acid, a metabolite produced by gut microbes, stimulates the production of 5-HT in the intestine. Inhibition of 5-HT production led to reduced numbers of ISCs, as well as atrophy of crypt and villus. Additionally, it attenuated crypt regeneration after irradiation. Refeeding 5-HT significantly restored the number of ISCs. Mechanistically, 5-HT activated macrophages to produce PGE2, which in turn promoted ISC self-renewal via WNT/β-catenin.185
4.3 Metabolic intermediates against pathological stimulation in the intestine
Metabolic intermediates play an important regulatory role in maintaining intestinal homeostasis against pathological stimulation. For example, lactate and pyruvate contribute to increasing intestinal resistance to Salmonella infection, potentially enhancing intestinal immunity.186 The α-KG and malate, key products of the TCA cycle, have recently been shown to both activate macrophages and promote damage repair in the intestinal mucosa.187 Supplementation with α-KG leads to a reduction in the abundance of pathogenic bacteria, including Ehrlichia and Enterococcus. It also enhances intestinal barrier function and facilitates the restoration of colon damage induced by DSS.188 Gln also plays an important role in maintaining the intestinal barrier during intestinal injury. Gln supplementation increases blood glutathione (GSH) levels, reduces intestinal permeability, and restores small intestinal barrier function after ischemia/reperfusion injury.189 Deprivation of Gln or inhibition of Gln synthetase in Caco-2 cells significantly diminishes the expression of tight junction proteins.190 However, the specific mechanism by which Gln regulates intestinal barrier function is poorly understood. Additionally, the abundance of the Trp metabolites, xanthurenic acid (XANA) and kynurenic acid (KYNA), is negatively correlated with IBD. Supplementation with XANA and KYNA increases mitochondrial respiration, promotes intestinal epithelial cell proliferation, and facilitates repair in a DSS-induced colitis model. XANA and KYNA also facilitate the differentiation of Th17 cells, which strengthens the tight junctions of the intestinal epithelium to maintain the intestinal barrier.191
Metabolites also play roles in colorectal tumors. For example, α-KG promotes DNA and histone H3K4me3 hypomethylation, inhibits WNT signaling, and promotes cellular differentiation. Moreover, it inhibits CRC tumor growth and shows promising anticancer effects.192 Oleic acid reduces tumors in APC-Min mice,170 while arachidonic acid (AA) promotes tumorigenesis. Mechanistically, feeding AA enriches gram-negative bacteria, which promotes the conversion of AA to PGE2 and ultimately promotes CRC development.193 Gamma amino butyric acid (GABA) is an inhibitory neurotransmitter expressed in enteric nerves, immune cells, and endocrine-like cells.194, 195 GABA is involved in the regulation of gastrointestinal motility.196 Activation of GABA receptors inhibits autophagy and enhances the immune response to prevent bacterial infection.197 Additionally, GABA receptors have been found to be upregulated in the intestinal epithelium after treatment with 5-FU, irradiation, and DSS.194, 198 Both endogenous and exogenous GABA exacerbate intestinal injury by activating GABA receptors.194 GABA regulates macrophages and participates in immune regulation. Supplementation with exogenous GABA promotes colon tumor growth. Mechanistically, GABA may facilitate the differentiation of monocytes into anti-inflammatory macrophages that secrete IL-10, consequently inhibiting the cytotoxic function of CD8 T cells.199
4.4 The role of microbial metabolite in gut homeostasis
Metabolites produced by the gut microbiota are key molecular mediators in the crosstalk between the microbiota and the intestine (Table 3). These microbial metabolites can be broadly classified into two categories. The first category includes compounds produced by bacteria that may induce toxic responses in the host, such as LPS and bacterial endotoxins. The second category consists of two subgroups of metabolites associated with the gut microbiota. The first subgroup comprises metabolites generated by intestinal bacteria from dietary components, including short-chain fatty acids (SCFAs), tryptophan, and indole derivatives. The second subgroup involves metabolites initially produced by the host and subsequently modified by intestinal bacteria, such as bile acids.200 LPS enhances apoptosis through the modulation of Toll-like receptor 4 (TLR4), leading to a reduction in the proliferation of stem and progenitor cells.201, 202 Clostridioides difficile exerts its deleterious effects on colonic stem cells via the exotoxin TcdB, impairing the colon's capacity to repair damaged.203 SCFAs, such as acetate, butyrate, and propionate, are metabolites produced through microbial fermentation in the gut.204 SCFAs are capable of activating GPR43 in immune cells, thereby playing a significant role in the resolution of inflammation.205 Butyrate suppresses the proliferation of stem cells exposed to the intestinal lumen by acetylating histones and modulating the activity of FOXO3.206 Tryptophan and its metabolites play a crucial role in regulating intestinal homeostasis, particularly under pathological conditions. Elevated concentrations of tryptophan activate AhR, thereby inhibiting intestinal tumorigenesis through the regulation of E3 ubiquitin ligases ring finger protein 43 (RNF43) and zinc and ring finger 3 (ZNRF3), which suppress WNT/β-catenin signaling to prevent the overproliferation of ISCs.207 Additionally, indole-3-aldehyde stimulates lamina propria LPLs to secrete IL-22 via AhR activation, which subsequently induces STAT3 phosphorylation, accelerating the proliferation of intestinal epithelial cells and promoting the restoration of damaged intestinal mucosa.208 Indoleacrylic acid (IA) and indolepropionic acid (IPA) contribute to the protection of the intestinal epithelial barrier by enhancing macrophage function and modulating the inflammatory response, partially through pregnane X receptor (PXR) signaling.209, 210 Polyamines are small molecules derived from the metabolism of L-arginine, and the addition of spermidine (SPMD) resulted in the transformation of naive T cells from differentiation into Th17.211 The intestine is rich in bile acids, and bile is involved in various physiological processes through various receptors such as farnesoid X receptor (FXR), PXR, and vitamin D receptor (VDR).212 Bile extract or lithocholic acid (LCA) promotes intestinal organoid growth by activating G-protein-coupled bile acid receptor (TGR5) in the ISC, and LCA inhibits nuclear factor kappa B (NF-κB) signaling and activates SIRT1/Nrf2 by activating VDR, thereby increasing tight junction proteins and strengthening the epithelial barrier.213, 214 However, due to the vast variety of metabolites, many remain unknown and unidentified. In the future, advanced approaches in metabolic research are expected to identify more metabolites involved in regulating intestinal homeostasis.
| Metabolites | Regulation of the intestine | Refs. |
|---|---|---|
| Lactate | Lactate promotes organoid growth and enhances intestinal immunity. | 114 |
| β-OHB | β-OHB increases the number of Lgr5+ ISCs and improves the integrity and survival of the crypts after irradiation. | 143 |
| Gln | Gln supplementation activates the WNT signaling pathway and self-renewal of ISCs, and significantly alleviates the intestinal damage caused by burning. | 181, 182 |
| Taurine | Taurine accelerates regeneration of ISCs, promotes intestinal peristalsis, and accelerates glucose metabolism in middle-aged and elderly mice after supplementation with taurine. | 183 |
| SAM | Lack of Met in organoid medium inhibits stem cell proliferation in intestinal organoids. | 184 |
| 5-HT | 5-HT activates PGE2 production in a PGE2 macrophage, promotes WNT/β-catenin signaling to promote self-renewal in ISCs. | 185 |
| α-KG | Modulating macrophage polarization alleviates DSS-induced colitis and suppresses tumor growth in colon cancer. | 187 |
| XANA/KYNA | Supplementation with XANA or KYNA decreases colitis severity through effects on intestinal epithelial cells and T cells. | 215 |
| GABA | B cell-derived GABA promotes monocyte differentiation into anti-inflammatory macrophages that secrete interleukin-10 and inhibit CD8 T cell killer function. | 199 |
| LPS | LPS enhances apoptosis through the modulation of TLR4, leading to a reduction in the proliferation of stem and progenitor cells. | 201, 202 |
| TcdB | Exotoxin TcdB impairs the colon's capacity to repair damaged. | 203 |
| SCFAs | SCFAs activate GPR43 in immune cells and play a significant role in the resolution of inflammation. | 205 |
| Butyrate | Butyrate suppresses the proliferation of stem cells exposed to the intestinal lumen. | 206 |
| Tryptophan | Tryptophan suppresses WNT/β-catenin signaling to prevent the overproliferation of ISCs. | 207 |
| Indole-3-aldehyde | Indole-3-aldehyde accelerates the proliferation of intestinal epithelial cells by IL-22 and STAT3. | 208 |
| Indoleacrylic acid and indolepropionic acid | They enhance macrophage function and modulates the inflammatory response, partially through PXR signaling. | 209, 210 |
| SPMD | SPMD causes the transformation of naive T cells from differentiation into Th17. | 211 |
| LCA | LCA promotes intestinal organoid growth by activating TGR5. | 213, 214 |
- Abbreviations: α-KG, alpha-ketoglutarate; β-OHB, beta-hydroxybutyrate; DSS, dextran sulfate sodium salt; GABA, gamma amino butyric acid; IL-22, interleukin-22; ISC, intestinal stem cell; KYNA, kynurenic acid; LCA, lithocholic acid; LPS, lipopolysaccharides; PGE2, prostaglandin E2; PXR, pregnane X receptor; SAM, S-adenosylmethionine; SCFA, short-chain fatty acid; SPMD, spermidine; STAT3, signal transducer and activator of transcription 3; TGR5, G-protein-coupled bile acid receptor; Th17, T helper cells 17; TLR4, Toll-like receptor 4; WNT, wingless-related integration site; XANA, xanthurenic acid; 5-HT, serotonin.
5 FUTURE PERSPECTIVES: ADVANCED APPROACHES IN METABOLIC RESEARCH
5.1 Untargeted and targeted metabolomics
The maintenance of intestinal homeostasis is inextricably linked to the dynamic metabolic processes of enterocytes and the intestinal microbiota.139 Dietary intake includes approximately 8000 non-nutritive compounds, such as dietary fiber, and polyphenols.216 Most of these compounds cannot be digested by human digestive enzymes and are instead catabolized by gut microbiota, leading to the production of a wide array of metabolites.217 The metabolic processes and pathways involved are highly dynamic. Metabolomics, the comprehensive analysis of metabolites in tissues and cells, allows for the detection of subtle changes in biological pathways. This analysis provides critical insights into the mechanisms underlying various physiological conditions and pathological processes. Moreover, metabolomics can be utilized to track metabolites produced by both the host and gut microbes, thereby enhancing our understanding of the complex metabolic interactions between gut microbiota and their hosts.218 Metabolites are predominantly characterized using both untargeted and targeted mass spectrometry (MS)-based metabolomics approaches. Untargeted metabolomics aims to detect the dynamic changes of metabolites within a sample, followed by biostatistical analysis to identify differential metabolites. Targeted metabolomic focuses on the qualitative and quantitative analysis of a specific class of metabolites of interest. Most analyses of fecal metabolomics can be conducted with targeted metabolomics approaches.219, 220 Liquid chromatography (LC) and gas chromatography (GC) coupled with MS are among the most widely employed platforms in metabolomics research.221-224 LC–MS and GC–MS have different application scenarios. LC–MS is highly sensitive and excels in the separation of thousands of small molecules, including polar metabolites such as organic acids, organic amines, nucleosides, nucleotides, and polyamines. GC–MS is particularly effective for fractionating less polar small molecules, such as alkyl derivatives, AAs, essential oils, esters, fragrances, terpenes, waxes, volatiles, carotenoids, flavonoids, and lipids.221 In addition, GC–MS usually requires the derivatization of certain substances through alkylation, acylation, or silylation reactions to enable analysis.
5.2 Advances in metabolomics technologies
In fact, there are some challenges and advances in the application of metabolomics. First, both untargeted and targeted metabolomics do not provide information on intracellular metabolic rates and the relative activity of metabolic pathways. The most effective method for elucidating the dynamic metabolic status is metabolic flux analysis (MFA), which involves the use of stable isotopes, such as carbon-13 (13C), nitrogen-15 (15N), or deuterium (2H), for tracing purposes.225 Isotopic labeling of specific compounds, including nutrients or substrates, allows for the detailed tracking of their metabolic fate within the body.226, 227 Using 13C16-palmitate and 13C2-acetate, we followed the metabolic flux and found that loss of HNF4 in small intestinal organoids resulted in impaired TCA cycle but increased fatty acid synthesis.83 Second, some metabolites may remain undetectable due to their low abundance or poor ionization efficiency. Derivatization can alter the properties of metabolites and enhance detection sensitivity. It can also facilitate the design of subsequent enrichment steps. For example, ketones and aldehydes are particularly challenging to detect and identify with LC–MS. Conway et al. developed a chemoselective probe immobilized on magnetic beads to improve the metabolites concentration and ionization efficiency. They successfully identified 112 ketone and aldehyde metabolites, and elucidated their exact chemical structures.228 Third, the identification of metabolites typically depends on database searches, which can result in the neglect of many unknown metabolites. The focus of microbiome research is increasingly shifting from the gut microbiota to their small molecules.229 However, a large number of microbiome-derived metabolites are not recorded in databases. To address this, researchers have proposed a new approach to metabolomics research—reverse metabolomics. It first requires newly synthesized compounds, followed by LC–MS/MS to obtain the mass spectra of the compounds. Then researchers utilized the Mass Spectrometry Search Tool (MASST) program to search the obtained MS spectra within the existing MS datasets and explored associations between metabolites and various phenotypes, species, and sample types. Using this approach, researchers have identified that bile acids bound to amino acids, such as glutamate, isoleucine/leucine, phenylalanine, threonine, tryptophan, and tyrosine, are elevated in the feces of patients with CD. Additionally, they discovered that cholestatic amide compounds produce by Bifidobacterium, Clostridium, and Enterococcus may contribute to intestinal inflammation by modulating IFNγ production and activating the PXR in T cells.230
5.3 Future directions in metabolomics research: Single-cell and spatial levels of metabolomics
Currently, using untargeted and targeted metabolomics techniques can obtain information at the bulk tissue level. However, these methods are limited in their ability to track dynamic metabolism at the single-cell and spatial levels. For example, stem and progenitor cells within the intestinal epithelium are relatively scarce, but understanding their metabolic characteristics at the single-cell level is crucial for studying intestinal homeostasis or disease pathogenesis. Metabolomics analysis typically requires millions of cells.231 Consequently, the use of small-scale metabolomics to analyze metabolic changes within cellular subpopulations and achieve spatially resolved metabolic profiling represents a critical direction for future research in metabolomics. Recently, researchers have employed flow cytometry in conjunction with hydrophilic interaction liquid chromatography (HILIC) and high-sensitivity Orbitrap MS. A total of 160 metabolites were identified using this advanced analytical technique in 10,000 cells.232 Additionally, hyperpolarized micromagnetic resonance spectroscopy (HMRS) technology has been developed by integrating nuclear magnetic resonance (NMR) spectroscopy and imaging with hyperpolarization of nuclear spins. This method allows for real-time metabolic analysis in intact cells or organs and requires only approximately 10,000 cells.232 Furthermore, a class of metabolite-specific biosensors has been developed, which can be combined with single-cell techniques to dynamically monitor changes in specific metabolites at the single-cell level. Wu et al. developed a lactate biosensor. When paired with single-cell imaging, it enables the measurement of glycolytic metabolites. The lactate, glucose, pyruvate, and ATP can be detected in individual endothelial cells.233 Similarly, Tao et al. designed a genetically encoded fluorescent indicator for nicotinamide adenine dinucleotide phosphate (NADPH). It is capable of quantifying cytosolic and mitochondrial NADPH pools in a single living cell.234
Matrix-assisted laser desorption/ionization-mass spectrometry imaging (MALDI-MSI) enables spatial localization of target metabolites and proteins within tissues.229, 235 Recently, researchers have developed SpaceM, a technique based on MALDI imaging MS combined with optical microscopy. This method utilizes optical microscopy for image segmentation and calibration, followed by MALDI imaging to analyze metabolites. SpaceM offers nontargeted metabolic profiles, fluorescence intensity measurements, and spatial morphological characteristics at the single-cell level.236 For example, MALDI-MSI has been employed to detect and spatially map 42 lipid species produced by Bacteroides thetaiotaomicron in the mouse colon.237 Furthermore, combining MALDI-MSI with stable isotope tracing allows for the assessment and visualization of region-specific metabolism. 13C-labeled glycerol and glucose were used to directly visualize glycolytic and gluconeogenic activities in distinct regions of the mouse kidney.235 Additionally, to study metabolic differences within organelles, techniques such as organelle precipitation enrichment have been developed. Mitochondrial immunopurification (MITO IP) enables the quantification of metabolites specifically within mitochondria,238 and this approach has now been extended to lysosomes and peroxisomes.239, 240 In summary, single-cell and spatial levels of metabolomics show tantalizing promise, but large-scale applications have yet to be realized.
6 CONCLUSIONS AND PERSPECTIVES
Extensive research has underscored the pivotal role of the tissue microenvironment in sustaining intestinal homeostasis. This microenvironment comprises mesenchymal cells, immune cells, bacteria, enteric neuronal cells, extracellular matrix, and nutritional factors, all of which interact synergistically to create a milieu conducive to regeneration. The homing capacity of intestinal epithelial cells during tissue repair is intricately regulated by this microenvironment. However, the direct link between the microenvironment and cellular plasticity remains largely unexplored in most studies. Therefore, it is possible to coculture nonepithelial components such as immune cells, bacteria, or fibroblasts with intestinal organoids. It provides an approach to elucidate how the regenerative processes of the gut are perceived and initiated. Future research should prioritize the identification of additional microenvironmental components that facilitate the regeneration of intestinal epithelial cells.
Due to the complex and diverse nature of the gut microbiota, there is a significant gap in understanding the detailed interactions between the gut and ISCs. Although current research on the role of gut microbiota in maintaining homeostasis has primarily concentrated on two commensal species, Lactobacillus and Bifidobacterium, many questions remain unanswered. Specifically, which other microbial species are involved in the regulation of intestinal homeostasis, and what are the precise mechanisms underlying these regulatory processes? These questions require further investigation. Fecal microbiota transplantation (FMT) has demonstrated significant potential in treating gastrointestinal disorders. However, variations in FMT approaches, including differences in dosage and routes of administration, have led to heterogeneous clinical outcomes.241 Consequently, there is an increasing emphasis on optimizing FMT through the development of more precise and effective delivery systems, alongside the formulation of personalized therapeutic strategies tailored to the specific needs of individual patients.
Our review enumerates the various metabolic pathways and metabolites that modulate the function of ISCs and maintain intestinal homeostasis through distinct mechanisms in both physiological and pathological states. In the future, targeting specific metabolic pathways and metabolites may emerge as a novel therapeutic strategy for intestinal diseases. Additionally, dietary restriction and the supplementation of specific metabolites hold promise as innovative forms of adjuvant therapy. However, current metabolic analyses predominantly focus on bulk tissue levels. Understanding the dynamic metabolic processes of ISCs and progenitor cells is crucial. Furthermore, recent research on gut microbiota has increasingly shifted from examining the microbiota itself to investigating the metabolites it produces, a transition made possible by the advent of advanced metabolomic techniques. In conclusion, achieving spatially resolved metabolism is a critical direction for future research in metabolomics.
AUTHOR CONTRIBUTIONS
Lei Chen developed the initial idea, conceived, and supervised the study. Ruolan Zhang wrote the initial draft of the manuscript; Ansu Perekatt edited the draft. Lei Chen revised and approved the final draft. All the authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
Lei Chen is supported by grants from the National Key R&D Program of China (2023YFA1801500), the National Natural Science Foundation of China (32270830), the Nature Science Foundation of Jiangsu Province (BK20230026), the Southeast University Interdisciplinary Research Program for Young Scholars (2024FGC1003), and the Start-up Research Fund of Southeast University (RF1028623015). Ansu Perekatt was supported by the National Cancer Institute of the National Institutes of Health under award number K22CA218462. All illustrations are created with Biorender.com.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




