Cardiomyopathy: pathogenesis and therapeutic interventions
Abstract
Cardiomyopathy is a group of disease characterized by structural and functional damage to the myocardium. The etiologies of cardiomyopathies are diverse, spanning from genetic mutations impacting fundamental myocardial functions to systemic disorders that result in widespread cardiac damage. Many specific gene mutations cause primary cardiomyopathy. Environmental factors and metabolic disorders may also lead to the occurrence of cardiomyopathy. This review provides an in-depth analysis of the current understanding of the pathogenesis of various cardiomyopathies, highlighting the molecular and cellular mechanisms that contribute to their development and progression. The current therapeutic interventions for cardiomyopathies range from pharmacological interventions to mechanical support and heart transplantation. Gene therapy and cell therapy, propelled by ongoing advancements in overarching strategies and methodologies, has also emerged as a pivotal clinical intervention for a variety of diseases. The increasing number of causal gene of cardiomyopathies have been identified in recent studies. Therefore, gene therapy targeting causal genes holds promise in offering therapeutic advantages to individuals diagnosed with cardiomyopathies. Acting as a more precise approach to gene therapy, they are gradually emerging as a substitute for traditional gene therapy. This article reviews pathogenesis and therapeutic interventions for different cardiomyopathies.
1 INTRODUCTION
Cardiomyopathies are a group of heterogeneous diseases characterized by morphological and functional abnormalities of the heart, leading to a wide spectrum of clinical manifestations, from asymptomatic left ventricular (LV) dysfunction to severe heart failure and sudden cardiac death (SCD). Cardiomyopathy is the predominant indication for pediatric heart transplantation, especially in children over the age of one.1 With increasing recognition of their impact on public health, cardiomyopathies have become a major focus in the field of cardiology.
According to the American Heart Association (AHA) 2006 classification system, primary cardiomyopathies are diseases characterized by having direct, targeted impacts on the heart muscles.2, 3 The most effective categorization of cardiomyopathies is through their classification as primary or secondary. Primary cardiomyopathies can be classified as genetic, mixed (genetic and nongenetic), or acquired (Figure 1).3 Breakthroughs in the fields of genomics and proteomics have illuminated the underlying molecular mechanisms behind hereditary, mixed, and acquired cardiomyopathies.
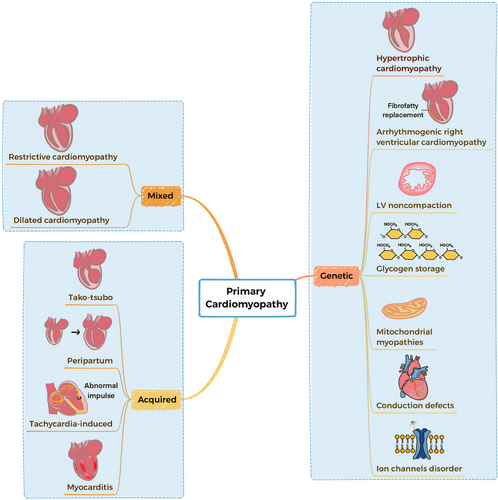
In recent years, with the growing comprehension of the molecular and cellular mechanisms underlying cardiomyopathy, the molecular mechanism of disease-causing mutations is increasingly being discovered. Genetic factors have been identified as a significant component in the etiology of many cardiomyopathies, with hundreds of genes linked to disease development, we can facilitate early diagnosis and therapy by periodic screening. The advent of gene therapy, cell therapy, and precision medicine offers promising avenues for disease modification and personalized care. Ongoing investigations involve the exploration of genetic manipulation using specific viral vectors and genome editing strategies including antisense oligonucleotides (AONs), transcription activator-like effector nucleases (TALENs), AAV, iPSCs, and CRISPR systems. In the future, they have the potential to emerge as a promising therapeutic approach for primary cardiomyopathies, offering in vivo genome editing capabilities.4
Despite these advances, challenges remain in the early diagnosis, risk stratification, and management of cardiomyopathies. There is a pressing need for continued research to elucidate the complex interplay between genetic, environmental, and lifestyle factors that contribute to the development and progression of these diseases. This review will delve into the intricate details of the pathogenesis of different types of cardiomyopathies, explore the current landscape of therapeutic interventions, and highlight the emerging strategies that hold the potential to transform the future of cardiomyopathy.
2 GENETICS AND CARDIOMYOPATHY
2.1 Genetics in the pathogenesis of cardiomyopathy
Cardiomyopathies are a diverse array of conditions, each with a unique set of genetic underpinnings, that manifest as structural and functional anomalies within the myocardium.5 In recent decades, the advent of next-generation sequencing (NGS) has revolutionized genetic analysis, rendering it more accessible than ever before. Utilizing NGS, high-throughput genetic research has yielded significant insights into the genetic architecture of cardiomyopathies, leading to the identification of numerous associated gene mutations.6 These genetic variations can impair the heart's ability to systole and dilate, thereby influencing the initiation and progression of the disease. Furthermore, as the correlation between clinical phenotypes and their genetic determinants becomes more defined, there is a growing understanding of the underlying mechanisms and the potential to develop innovative therapeutic strategies. Recent findings suggest that the development of cardiomyopathy is influenced not only by rare genetic variants but also by common genetic variations.
Genes are pivotal in the pathogenesis of cardiomyopathies, with myocardial structural and functional abnormalities often stemming from genetic influences or environmental triggers. The goal of gene therapy is to ameliorate disease symptoms by introducing new genetic material or by genetically modifying existing genes and their regulatory sequences. This is achieved through strategies such as gene replacement and gene editing. Consequently, uncovering the genetic mechanisms underlying cardiomyopathies and their progression is essential for establishing a foundation for effective gene therapy.7
The molecular mechanisms through which specific mutations precipitate cardiomyopathy can be categorized into three types: (1) loss of function, typically arising from nonsense or frameshift mutations that result in the production of partially or entirely nonfunctional proteins, or even in the complete absence of the protein due to nonsense-mediated decay; (2) gain of function, often due to missense mutations that lead to the creation of a protein with enhanced or altered activity compared with its wild-type counterpart; and (3) dominant negative effects, usually caused by missense mutations that impede the normal biological function of the wild-type protein, a phenomenon most frequently observed in proteins that form homomeric complexes, where the mutant subunits can disrupt the complex assembly.8
Hypertrophic cardiomyopathy (HCM) is one of the most common hereditary cardiomyopathies, usually caused by mutations in genes encoding sarcomere proteins. These genes include beta-myosin heavy chain (MYH7), myosin-binding protein C (MYBPC3), and troponin T2 (TNNT2). These mutations can lead to abnormal contraction of cardiac muscle cells, causing myocardial hypertrophy and heart dysfunction.9-12
The hereditary form of dilated cardiomyopathy (DCM) is usually associated with mutations in various genes encoding structural proteins of cardiac muscle cells, such as titin (TTN), actin (ACTC1), and MYBPC3.13 Mutations in these genes can lead to structural and functional abnormalities of cardiac muscle cells, ultimately resulting in ventricular dilation and reduced heart pumping function.
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a cardiomyopathy characterized by the gradual replacement of myocardium with fat and fibrous tissue. Its hereditary form is associated with mutations in genes encoding cardiac cell junction proteins, such as desmoplakin (DSP), plakophilin-2 (PKP2), and desmoglein-2 (DSG2).14-16 These mutations affect the intercellular connections of cardiac muscle cells, leading to ventricular dysfunction and arrhythmias.
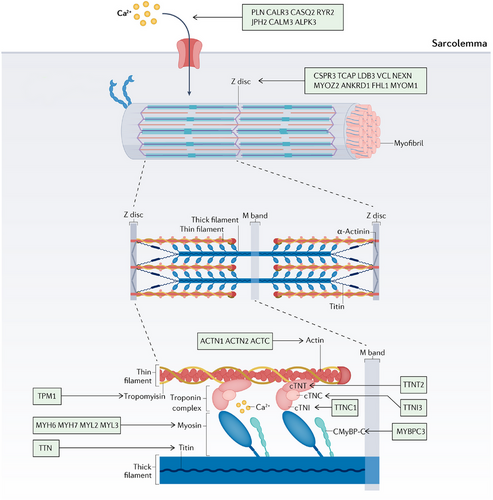
The hereditary form of restrictive cardiomyopathy (RCM) is rare, but mutations in certain genes, such as the gene for TTN, are known to be associated with it.17 Left ventricular noncompaction (LVNC) is a cardiomyopathy characterized by an excessive and abnormal trabeculation in the LV cavity. This condition may be associated with mutations in various genes, including those encoding sarcomere proteins.18 These cardiomyopathies involve mutations in genes of metabolic pathways, such as glycogen metabolism disorders, fatty acid oxidation disorders, lysosomal storage diseases, and mitochondrial diseases. These diseases affect the energy metabolism of cardiac muscle cells, leading to heart dysfunction.
2.2 Genetics in the diagnosis of cardiomyopathy
Genetic testing is crucial for the early diagnosis and differential diagnosis of patients with cardiomyopathies and their family members. For example, for patients with DCM, LMNA, and sodium voltage-gated channel alpha subunit 5 (SCN5A) genetic testing is recommended if they have associated cardiac conduction diseases or a family history of sudden death at an early age.19 In patients with HCM, if a pathogenic gene mutation has been identified in the family, family members should undergo genetic testing to help reclassify the mutation level and intervene early in high-risk members.20 In addition, genetic counseling is an essential component of the genetic testing and family screening process, helping patients and family members understand genetic risks and providing advice on prevention, management, and family planning.
It is important to note that genetic test results are usually probabilistic rather than deterministic, so they need to be interpreted in conjunction with the patient's medical and family history. For example, family history information and the distribution of presumed disease-related mutations in the family may be important for guiding clinical interpretation, especially when identifying new genetic variants. In addition, family studies have noted that up to 10% of ARVC families have multiple pathogenic variants.21
When conducting genetic testing, it is important to consider using large gene panels, as these panels can increase the likelihood of identifying the molecular cause, especially when patients exhibit mixed phenotypes or lack typical features. However, as the number of genes tested increases, the possibility of identifying variants of uncertain significance (VUS) also increases, adding complexity to interpretation and genetic counseling.22
In summary, genetic testing and family screening for cardiomyopathies are key components in managing these diseases, helping with early diagnosis, risk assessment, and the formulation of personalized treatment strategies.23 As our understanding of the genetic basis of cardiomyopathies deepens, our understanding and treatment of cardiomyopathies will become more precise and effective.
3 GENERAL THERAPEUTIC INTERVENTIONS OF CARDIOMYOPATHIES
The management of cardiomyopathies is multifaceted, requiring a tailored approach that considers the specific type of cardiomyopathy, the severity of symptoms, and the presence of complications. This section outlines the general therapeutic interventions applicable to various forms of cardiomyopathies.
3.1 Pharmacological therapy
Pharmacological interventions are the cornerstone of cardiomyopathy treatment, aimed at alleviating symptoms, improving cardiac function, and reducing the risk of complications. Common pharmacological agents include beta-blockers, calcium channel blockers, diuretics, inotropic agents, and antiarrhythmic medications.24
3.2 Surgical interventions
The surgical treatment of cardiomyopathy includes a variety of different surgical procedures, which specifically depend on the type of cardiomyopathy and the patient's condition.
Cardiac resynchronization therapy (CRT) is a therapeutic option for patients with DCM who have intraventricular conduction delays and for patients with HCM who have reduced left ventricular ejection fraction (LVEF), improving cardiac synchronization and function.25 Sidhu et al.26 observed that systolic function is enhanced in patients with LMNA-related cardiomyopathy following CRT. It involves the use of a specialized pacemaker that stimulates both ventricles of the heart to contract in a coordinated manner.
Current guidelines advocate the use of implantable cardioverter-defibrillators (ICDs) for primary prevention of SCD in patients with symptomatic nonischemic cardiomyopathy who have LVEFs below 35%.27 These devices monitor heart rhythm and deliver a shock to restore normal rhythm if a life-threatening arrhythmia is detected.
Surgical treatment for cardiomyopathy encompasses a variety of procedures, contingent upon the type of cardiomyopathy and the specifics of the patient's condition. For instance, in the case of HCM, particularly for patients with left ventricular outflow tract obstruction (LVOTO), septal myectomy (Morrow surgery) is a commonly employed surgical approach.28
For end-stage cardiomyopathy, heart transplantation remains the ultimate therapeutic option, providing a potential cure and significant improvement in quality of life. Candidates for transplantation are carefully selected based on medical criteria and the severity of their condition.29
3.3 Gene therapy
The concept of gene therapy emerged during the 1970s. Despite the initially high expectations, early development in this field encountered setbacks. Genetic analysis has advanced significantly with the development of NGS. Recent studies showed that the variation of the disease-causing genes plays a critical role in the morbidity and development of cardiomyopathy. An increasing number of cardiomyopathies have been recognized as monogenic diseases with a genetic component.7, 30, 31 Gacita et al.32 proved that promoters and enhancers modify the expression of cardiomyopathy genes, so genetic variations in promoters and enhancers within the human genome could potentially contribute to the development of cardiomyopathy. The mutations in the noncoding parts of the genome are also involved in the pathogenesis of cardiomyopathy, namely, microRNA, promoter elements, enhancer/silencer elements, and long noncoding RNAs (lncRNAs), so that therapeutic genes are not limited only to protein-coding complementary DNAs, thus broadening the spectrum of possible applications.33, 34
Therefore, defining the associations between gene variation, gene expression, and disease as well as novel potential therapeutic targets will accelerate progress in the study of cardiomyopathies.7, 35 Cardiac gene-targeted therapies include identifying pathogenic genes, selecting appropriate vectors, constructing the gene of interest, and transporting organ-targeted vectors. There are some standard techniques that introduce exogenous genes in cardiac gene therapies (Figure 2).
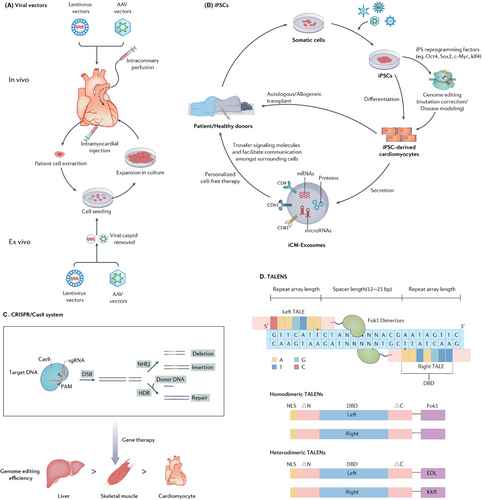
3.3.1 Viral vectors
Lentivirus and adeno-associated viruses (AAVs) are the most commonly used viral vectors. With strong cardiomyocyte transduction ability and low immunogenic response, the improved lentivirus has been tested to treat various human diseases.36-39
Lentiviral vectors are frequently employed for gene transfer within the nervous system. They have a large capacity of 10 kb. Thus, adequate genes could be transferred in vivo via lentiviral vectors, and they could stabilize long-term transgene expression by integrating into the chromosomes of transduced cells.40 Although lentiviral vector gene therapy has many advantages, it also has some disadvantages: lentiviral vectors may cause immune responses in the host, which could not only reduce the therapeutic effect but also cause adverse immune-related side effects. In addition, there are still potential biosafety issues with the viruses. Future research needs to further improve the lentiviral vector system to enhance its safety and application efficiency.41
AAV, a nonenveloped virus, can be employed as a vehicle to transport DNA into specific target cells.42 The AAVs vector is genetically modified to avoid integration into the host cell genome, thereby ensuring its stable expression within the host cells over an extended period, lasting long time.43, 44 Even though AAVs, with their single administration, can provide stable long-term expression and high efficiency in cardiomyocytes, and despite their lower inflammatory profile compared with other viral vectors, the host's immune responses to the vector components and the products of the transgene still pose challenges to the efficacy and safety of gene therapy.45 AAVs vector has already been widely used for phenotypic assessment and heart failure prevention in animal models of cardiomyopathy.46, 47 AAV vector technology is mature and rapidly progressing in various practical and therapeutic applications. In mice models, specific targeting of the heart can be achieved after systemic administration by utilizing a combination of cardiac AAV serotype and a promoter that is specific to cardiomyocytes, as demonstrated by various studies.33, 48 Moreover, isolating novel AAV variants with increased tropism for cardiomyocytes can refine AAV delivery methods, thereby increasing cardiomyocyte transduction.49 The integration of AAV vector-derived DNA into the host genome may have potential carcinogenicity and may also interfere with the function of normal genes, causing genomic instability.50 Therefore, it is necessary to conduct long-term monitoring for patients who have received AAV gene therapy.51
The most widely used gene delivery techniques via viral vectors are intramyocardial (IM) injection and intracoronary (IC) perfusion. IM injection is a catheter-based minimally invasive procedure that directly injects a virus carrying a gene or a cytokine into the tissue to increase the local concentration of the vectors. It has the highest local retention.52 However, uniform diffusion of the carrier tissue is difficult to achieve with direct IM injection, and the proportion of cells transfected is insufficient for ideal therapeutic effects. Furthermore, the injection efficiency of IC injection may be reduced due to the rapid coronary circulation.53-56 Based on these limitations, coronary venous retro-infusion has garnered significant attention for therapeutic strategies aimed at precisely delivering drugs, genes, or cells to the ischemic myocardium. It can not only integrate homogenous intravascular delivery, but also increase the retention of angiogenic substrates.57, 58
In addition to IM injection and IC perfusion, intravenous (IV) administration and local delivery techniques are also pivotal in the arsenal of gene delivery methods utilizing viral vectors. Salami et al.59 discovered that a single IV injection of 10^11 genome copies of AAVrh.10hFXN, an AAV serotype rh10 vector engineered to deliver the human FXN gene, effectively corrected the stress-induced ejection fraction and fractional shortening phenotypes. Kevany et al.60 identified a novel AAV capsid that enhances tissue specificity and expression within the target tissues, namely, the heart, muscle, and central nervous system, following IV administration. Vassalli et al.61 successfully instilled AAV vectors into the pericardial space, achieving transduction of epicardial myocytes in mice, achieving transduction of epicardial myocytes in mice.
3.3.2 RNA editing
Recent studies have suggested that the mutant pre-mRNA could be repaired by trans-splicing to treat autosomal-dominant diseases. Mearini et al.62 demonstrated that Mybpc3 mRNA could be repaired by 5′-trans-splicing in cardiac myocytes of homozygous Mybpc3-KI mice.
Another prevalent RNA editing technique is spliceosome-mediated RNA trans-splicing, which entails the fusion of two distinct RNA molecules to generate a complete, repaired mRNA through splicing.63
The third strategy of RNA editing is mRNA silencing. Jiang et al.64 demonstrated that AAV-mediated RNAi delivery allele-specific silenced the mutant Myh6 mRNA and the manifestation of the disease phenotype was postponed in heterozygous Myh6-KI mice. In another study, Bongianino et al.65 demonstrated the efficacy of allele-specific silencing by RNA interference (RNAi) to prevent catecholaminergic polymorphic ventricular tachycardia phenotypic manifestations in a mouse model.
3.3.3 Antisense oligonucleotides
Antisense oligonucleotides (AONs/TASOs) is an approach that internally deletes a small part of the targeted protein but maintains its function through in-frame skipping of mutated exons. Thus, AONs are expected for the therapeutic correction of many genetic diseases. Gedicke-Hornung et al.66 successfully transduced AONs into cardiomyocytes of neonatal mice to prevent the MYBPC3 gene mutation. Hahn et al.67 offered the pioneering program represents the initial systematic effort to design and evaluate AONs specifically targeting mutated TTN target exons, improving the future therapeutic potential in titin-based DCM. Gramlich et al.68 demonstrated that AONs could restore disruption of the titin reading through exon skipping, as observed in both patient cardiomyocytes and mouse hearts.
3.3.4 CRISPR/Cas9 systems
The CRISPR/Cas9 system used an approximately 100-nucleotide RNA molecule derived from Streptococcus pyogenes and other bacterial species to facilitate the precise targeting of the Cas9 protein and a specific genomic site for cleavage. Consequently, the host cell activates endogenous DNA repair pathways to repair the damage by either nonhomologous end-joining (NHEJ) or homology-directed repair (HDR).69 Therefore, gene editing therapy often favors the use of HDR. However, the efficiency of HDR compared with NHEJ is typically low, primarily due to the relative rarity of HDR events in cardiomyocytes. Furthermore, even in mitotic cells, HDR is constrained to the S and G2 periods in the cell cycle.70 CRISPR HDR genome editing corrected MYBPC3 mutations and restored MYBPC3 protein expression, offering a promising therapeutic approach for treating HCM associated with these mutations. This underscores the immense potential of CRISPR technology in addressing hereditary heart diseases.71
In addition, CRISPR/Cas9 system can not only correct disease-related mutations but also introduce protective mutations and targeted viral genome.72, 73 Thus, CRISPR/Cas9 gene editing has the potential hope for correct genetic diseases in the future of personalized medicine because of its ability to make precise gene-specific corrections.
Despite the remarkable advancements in genome editing technology brought about by the CRISPR/Cas9 system, challenges persist. The full therapeutic potential of genome editing for cardiovascular diseases is still hindered by a range of biological and technical obstacles. It is crucial to acknowledge the variations in the efficiency of CRISPR–Cas9 genome editing in different organs within an organism. Specific attention should be given to the notably low efficiency observed in skeletal muscle and, particularly, in cardiomyocytes when compared with the liver. This distinction could be attributed to the postmitotic nature of cardiomyocytes, resulting in differences in CRISPR–Cas9 accessibility for delivery, or it may be due to inherent variations in the activity of CRISPR–Cas9 among assorted cell types.74
One other significant concern regarding the application of the CRISPR/Cas9 system to cardiomyocyte mutagenesis is the high frequency of off-target effects. Although off-target events may be infrequent, their potential impact should not be downplayed, as mutations in other genes could lead to detrimental effects. This is particularly crucial for clinical applications of genome-edited cells or tissues, where it is imperative to entirely prevent the occurrence of off-target effects.75 Another issue is immunogenicity of Cas9 protein. CRISPR–Cas9 evokes a host cellular and humoral immune response with distinct cellular and molecular signatures, indicating that Cas9 can serve as an antigen in mammals.76 The recent finding that routine prior exposure to Staphylococcus aureus and Streptococcus pyogenes can lead to preexisting immunity against Cas9 presents a significant challenge for its clinical advancement. Nonetheless, pioneering studies employing immune-privileged sites, immunosuppressive strategies, or novel Cas9 sources not previously encountered offer promising avenues to navigate around these initial hurdles in gene-editing-based therapeutics.
3.3.5 Transcription activator-like effector nucleases
Transcription activator-like effector nucleases (TALENs) consist of a specific DNA-binding domain comprising tandem repeats from transcription activator-like effectors (TALEs) found in Xanthomonas bacteria, combined with a nonspecific DNA-cleaving nuclease domain.77, 78 TALENs, similar to zinc finger nucleases (ZFNs), offer a versatile tool for precise genome editing purposes.79 The utilization of TALENs enables precise and targeted genetic modifications within the human genome, offering the capability for specific site-specific alterations of the desired gene.80 Recent studies have started to apply TALENs to clinical trials, which made DNA editing possible for the first time.81, 82 Furthermore, TALENs have the advantages of low cytotoxicity and stability compared with other gene-editing technologies.
Although TALENs are a powerful genome-editing tool, they also have some limitations and disadvantages. First, the design of TALENs requires precise DNA-binding modules to recognize the target DNA sequences, which may involve complex molecular cloning and sequencing operations. Second, despite the high specificity of TALENs, they can still potentially cause DNA cutting at off-target sites, leading to unintended effects. Finally, the expression of TALENs may have toxicity to certain cell types, especially when expressed at high concentrations or over long periods.83, 84
3.4 Cell therapy
Cell therapy is an evolving field of research in the application for cardiomyopathy, and the most widely used cell therapy in cardiomyopathy at present is induced pluripotent stem cell (iPSC) therapy. iPSCs are reprogrammed cells analogous to embryonic stem cells. They have the capacity for self-renewal and multidirectional differentiation.85 Genome editing would take place in ex vivo iPSCs, which after editing or differentiating into the desired tissue type, and then be transplanted back into the patient.86 Ong et al.87 suggested the transplantation of human iPSC-derived cardiomyocytes (hiPSC-CMs) could improve LV function and attenuate cardiac remodeling in an acute mouse myocardial infarction (MI) model. However, nonfatal ventricular tachycardia may occur in the process of transplantation. Thus, the prevention of arrhythmogenesis is one of the next areas of study for hiPSC-CMs.88 Moreover, the exosomes secreted from the hiPSC-CMs exert protective effects to salvage the injured neighboring cells by transferring the endogenous molecules.89 Tachibana et al.90 demonstrated that induced cardiomyocytes (iCMs) exhibited superior efficacy in salvaging injured myocardium compared with undifferentiated stem cells. This enhanced outcome was attributed to the paracrine effects exerted by and iCMs.90 The integration of iPSCs with genome editing technology is beneficial to expand the understanding of the gene's biological function and the pathological implications of genetic variants in cardiomyopathies. The CRISPR/Cas9 technology has been proven to be particularly useful for editing iPSC.75, 91
4 HYPERTROPHIC CARDIOMYOPATHY
HCM stands as the prevalent primary cardiomyopathy and can cause exertional dyspnea, presyncope, atypical chest pain, heart failure, and SCD in adults under 50 years of age, especially among young athletes.92-94 Asymmetric septal hypertrophy represents a frequently observed characteristic of HCM, which is subsequently accompanied by contractile dysfunction, myocardial fibrosis, and arrhythmias. HCM is a genetically diverse disorder that exhibits heterogeneity, often attributed to mutations in sarcomeric genes. These genetic mutations give rise to LV hypertrophy, fibrosis, hypercontractility, and decreased compliance. HCM follows an autosomal dominant inheritance pattern, meaning it is passed on to offspring with a Mendelian frequency of 50%.95-97 Many studies have shown that family screening in HCM contributed to the prediction of morbidity.98, 99
4.1 Pathogenesis and disease-causing genes
The pathogenesis of HCM involves the participation of several genes responsible for encoding sarcomeric proteins, Z-Disc proteins, and calcium-handling proteins. The genes associated with HCM are summarized here (Table 1 and Figure 3).9-12 The primary genetic cause of HCM is the presence of dominant pathogenic mutations in genes that encode sarcomere proteins, specifically those involved in thick and thin filament formation.100 Numerous clinical genomic studies suggest that mutations in the sarcomere protein gene MYH7 and MYBPC3 may cause HCM. Mutations in MYH7 and MYBPC3 can lead to altered sarcomere function, which may increase myocardial load due to impaired contractility. Mutation of the MYBPC3 gene promotes the expression of cMyBP-C protein and results in excessive contraction of myosin,101, 102 which induces aberrant cross-bridge kinetics, resulting in severe myocardial contractile dysfunction and eventually leading to HCM. In a study consisting of 26 HCM patients (11 with MYBPC3 mutations, nine with MYH7 mutations, and six with no sarcomere mutations, referred to as HCMsmn), it was observed that sarcomere mutations disrupt the energetic expenditure of cardiac contraction.103 Furthermore, several studies have proposed that disturbed metabolic signaling and impaired mitochondrial function represent prevalent pathogenic mechanisms in individuals diagnosed with HCM.104
| Gene | Locus | Protein | Frequency |
|---|---|---|---|
| HCM-associated genes | |||
| Sarcomere HCM | |||
| Giant filament | |||
| TTN9 | 2q31 | Titin | Rare |
| Thick filament | |||
| MYH79 | 14q11.2-q12 | β-Myosin heavy chain | 25–40% |
| MYH69 | 14q11.2-q12 | α-Myosin heavy chain | Rare |
| MYL29 | 12q23-q24.3 | Regulatory myosin light chain | 0.5–1% |
| MYL39 | 3p21.2-p21.3 | Essential myosin light chain | 0.5–1% |
| Intermediate filament | |||
| MYBPC39 | 11p11.2 | Cardiac myosin-binding protein C | 25–40% |
| Thin filament | |||
| TNNT29 | 1q32 | Cardiac troponin T | 3–5% |
| TNNI39 | 19p13.4 | Cardiac troponin I | 1–5% |
| TPM19 | 15q22.1 | α-Tropomyosin | 1–5% |
| ACTC9 | 15q14 | α-Cardiac action | Rare |
| TNNC19 | 3p21.1 | Cardiac troponin C | Rare |
| MYOM19 | 18p11.31 | Myomesin 1 | Rare |
| MYOZ29 | 4q26-q27 | Myozenin 2 | Rare |
| Z-Disc HCM | |||
| CSRP312 | 11p15.1 | Muscle LIM protein | Rare |
| TCAP12 | 17q12-q21.1 | Telethonin | Rare |
| LDB312 | 10q22.2-q23.3 | LIM binding domain 3 | Rare |
| ACTN112 | 14q24.1 | α-Actinin 1 | Rare |
| ACTN212 | 1q42-q43 | α-Actinin 2 | Rare |
| VCL12 | 10q22.1-q23 | Vinculin/metavinculin | Rare |
| ANKRD112 | 10q23.31 | Cardiac ankyrin repeat protein | Rare |
| FHL112 | Xq26.3 | Four-and-a-half LIM domains 1 | Rare |
| NEXN12 | 1p31.1 | Nexilin F-actin binding protein | Rare |
| BAG312 | 10q26.11 | BAG cochaperone 3 | Rare |
| Calcium-handling HCM | |||
| PLN12 | 6q22.1 | Phospholamban | Rare |
| CALR312 | 19p13.11 | Calreticulin 3 | Rare |
| CASQ210 | 1p13.3-p11 | Calsequestrin | Rare |
| RYR210 | 1q42.1-q43 | Ryanodine receptor 2 | Rare |
| JPH210 | 20q13.12 | Junctophilin 2 | Rare |
| CALM310 | 19q13.2–q13.3 | Calmodulin 3 | Rare |
| ALPK311 | 15q25.2 | Alpha-protein kinase 3 | Rare |
| ARVC/D-associated genes | |||
| Desmosomal | |||
| PKP2105 | 12p11 | Plakophilin-2 | 20–46% |
| DSP106 | 6p24 | Desmoplakin | 3–15% |
| DSG2106 | 18q12.1 | Desmoglein-2 | 3–20% |
| DSC2106 | 18q12.1 | Desmocollin-2 | 1–15% |
| JUP107 | 17q21 | Plakoglobin | Rare |
| Nondesmosomal | |||
| CTNNA3107 | 10q21.3 | αT catenin | Rare |
| CDH2108 | 18q12.1 | Cadherin-2 | Rare |
| PLN109 | 6q22.31 | Phospholamban | 0–4% |
| TMEM43109 | 3p25.1 | Transmenbrance protein 43 | 0–2% |
| TGFB3109 | 14q24.3 | Transforming growth factor beta 3 | Rare |
| SCN5A109 | 3p22.2 | Nav1.5 | 2% |
| LMNA109 | 1q22 | Lamin A/C | 0–4% |
| FLNC109 | 7q32.1 | Filamin C | 0–3% |
| DES109 | 2q35 | Desmin | Rare |
| RYR2109 | 1q43 | Ryanodine receptor-2 | Rare |
| TJP1109 | 15q13.1 | Zona occludens-1 | Rare |
| TTN109 | 2q31.2 | Titin | 0–10% |
In a multicenter multinational study of 358 patients with consecutive genotyped HCM, MYH7 (n = 53) and MYBPC3 (n = 75) were identified as the most common genes, respectively, at 33.1 and 47% of gene positive patients.110 Out of more than 1400 reported pathogenic variants, MYH7 and MYBPC3 genes account for approximately 70–80% of the causal genes in HCM.111 Homozygous or compound heterozygous frameshift mutations in MYBPC3 can be the underlying cause of neonatal HCM, which promptly progresses to systolic heart failure and usually results in mortality within the first year of life.112 According to a comprehensive study conducted in Iceland, it has been found that a founder mutation of MYBPC3, which originated more than 550 years ago, is the primary cause of HCM in the country. Additionally, the genotype of this mutation can influence the prognosis of individuals with HCM. Recent research has indicated that the MYBPC3 c.927-2A:G mutation is linked to lower rates of adverse events (AEs), but it is associated with earlier cardiovascular mortality.113 Montag et al.114 applied TALEN-mediated genome editing to create a porcine model that demonstrated characteristics of HCM by introducing the HCM-point mutation R723G into the MYH7 gene.
Furthermore, changes in the promoter region of MYH7 could be linked to the risk of developing HCM, and the patients harboring likely pathogenic or pathogenic variations in the MYH7 gene exhibited a higher incidence of developing new-onset atrial fibrillation.115-117 Apart from the mutations with MYBPC3 and MYH7, the variants in other genes encoding sarcomeric proteins also cause HCM. Compound DSG2/DSC2/MYH6 mutations were found in an athlete, and these variants determined a mild hypertrophic phenotype associated with both ventricular tachyarrhythmias and atrioventricular block.118 Mutations in troponin T, I, and Tm represent less than 10% of diagnosed cases of HCM. Pua et al.119 demonstrated that Chinese HCM patients often have low penetrance risk alleles in TNNT2 or TNNI3 compared with white patients.120
In general, patients with sarcomere mutations receive an earlier diagnosis and exhibit more severe hypertrophy compared with those without mutations.121, 122 Differences in disease onset may be due to the gene-specific severity of cardiac abnormalities.103
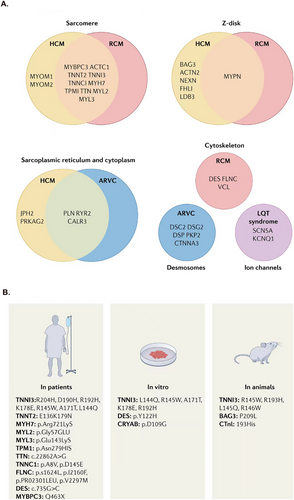
Recent research indicates that mutations in genes encoding the proteins forming the cardiac Z-disc, located adjacent to each other, are prevalent in both HCM and DCM. The proteins at the Z-disc facilitate the anchoring of thin filaments in neighboring cardiac sarcomeres, serving as a mechanical integration site for transducing sarcomere force generation through the myofilaments.123 Some genes encoding Z-disc proteins have been demonstrated to result in the onset of HCM (Table 2). Wang et al.124 demonstrated that mutations in NEXNs, a recently identified member of the Z-disc gene family, may be associated with HCM. Gallego-Delgado et al.125 suggested that HCM caused by FHL1 mutations has a very aggressive course and poor prognosis.
| Intervention/treatment | Targets | Mechanisms | ClinicalTrials.gov ID | Study population | Primary endpoint | Phase | References |
|---|---|---|---|---|---|---|---|
| Mavacamten | β-Myosin heavy chain | Stabilizes the super relaxed state | NCT03470545 | HCM | 1.5 mL/kg per min or greater increase in pVO2 and at least one NYHA class reduction or a 3·0 mL/kg per min or greater pVO2 increase without NYHA class worsening | Phase III | 126 |
| NCT04349072 | oHCM | The proportion of patients proceeding with SRT or remaining guideline-eligible at 32 weeks in both treatment groups | Phase III | 127 | |||
| NCT05174416 | Chinese oHCM | Change in Valsalva LVOT peak gradient | Phase III | 128 | |||
| Aficamten | Cardiac myosin | Decreases the number of active actin-myosin cross-bridges | NCT0518681 | HCM | Adverse cardiac events | Phase III | 129 |
| AAV1/SERCA2a | SERCA2a | Corrects abnormal Sarcoplasmic reticulum Ca2+–ATPase activity | NCT01643330 | Heart failure | Cardiovascular hospitalizations and time to terminal events | Phase II | 56 |
| Belantamab mafodotin | B-cell maturation antigen | Induces apoptosis | NCT04484623 | Relapsed or refractory myeloma | Progression-free survival | Phase III | 130 |
| NCT03525678 | Relapsed or refractory myeloma | The proportion of randomly assigned patients in the intention-to-treat population who achieved an overall response | Phase II | 131 | |||
| NCT04162210 | Multiple myeloma | Progression-free survival in all patients who were randomly allocated | Phase III | 132 | |||
| rAAVrh74.MHCK7.micro-dystrophin | DMD | Transfers microdystrophin gene | NCT03375164 | DMD | Safety | Phase I/IIa | 133 |
| Givinostat | Histone deacetylase | Inhibits histone deacetylase | NCT02851797 | DMD | Results of the four-stair climb assessment | Phase III | 134 |
| Eteplirsen | Exon 51 | Exon skipping to restore the dystrophin open reading frame | NCT0229655552 | DMD | Change in 6-min walk test | Phase III | 135 |
| Bromocriptine | Prolactin | Inhibits the production of antiangiogenic cleaved prolactin fragment | NCT00998556 | PPCM | LVEF change (delta) | Multicenter randomized study | 136 |
| Inotersen | TTR | Inhibits hepatic production of transthyretin | NCT01737398 | Cardiac amyloidosis | Modified neuropathy impairment score+7 | Phase III | 137 |
| VEGF165 | VEGF receptors | Induces angiogenesis in ischemic tissues | NCT00744315 | Refractory angina | Worsening of rest ischemia scores | Phase I/II | 138 |
| AdVEGF121 | VEGF receptors | Mediates the generation of new blood vessels and reverse coronary ischemia | NCT01174095 | Late-stage, diffuse coronary artery disease | Death | Phase I | 139 |
- Abbreviations: pVO2, peak oxygen consumption; oHCM, obstructive hypertrophic cardiomyopathy; SRT, septal reduction therapies; NYHA, New York Heart Association; LVOT, left ventricular out flow tract; nHCM, nonobstructive hypertrophic cardiomyopathy; KCCQ-CSS, Kansas City Cardiomyopathy Questionnaire—Clinical Summary Score; rAAVrh74, recombinant adeno-associated virus serotype rh74; DMD, Duchenne muscular dystrophy; PPCM, peripartum cardiomyopathy; LVEF, left ventricular ejection fraction; TTR, transthyretin; VEGF, vascular endothelial growth factor.
An increasing number of studies have indicated that the alteration of Ca2+ homeostasis may be associated with prohypertrophic remodeling.140 Therefore, apart from sarcomeric HCM and Z-disc HCM, genes encoding Ca2+-handling or Ca2+-regulatory proteins (PLN, CALR3, CALM3,141 Junctophilin 2 [JPH2],142, 143 CASQ2, RYR2) have been proposed as potential causal genes for HCM,144 further indicating the heterogeneity in the etiology and pathogenesis of cardiomyopathy. The JPH2 mutant gene has been considered one of the causal genes in familial HCM.145 Matsushita et al.143 demonstrated that the JPH2 gene mutation could lead to the diagnosis of HCM. Vanninen et al.146 proposed that the heterozygous JPH2 p.(Thr161Lys) variant could lead to heart failure in atypical HCM.
4.2 Therapeutic interventions
The treatment strategies for HCM include pharmacological therapy, surgical treatment, and emerging gene therapy approaches. The goals of HCM treatment are to reduce mortality, improve cardiac function, alleviate clinical symptoms, and slow down the progression of the disease.
4.2.1 Pharmacological treatment
Traditional pharmacological treatment
Traditional medications mainly include β-blockers, nondihydropyridine calcium channel blockers, late sodium current inhibitors, angiotensin receptor blockers (ARBs), and the antiarrhythmic drug disopyramide. The traditional pharmacological treatment options for HCM are limited to nonspecific drugs that can alleviate symptoms to varying degrees but do not truly slow the progression of the disease. In recent years, new compounds that directly address myocardial hypercontraction and energy changes have been developed.147
Mavacamten
Mavacamten (MYK-461) is a small molecule allosteric inhibitor of cardiac myosin that inhibits the excessive formation of myosin-actin cross-bridges at the sarcomere level.148 Mavacamten has successfully advanced to Phase III clinical trials. The EXPLORER-HCM study is a 30-week, double-blind, placebo-controlled RCT, showing that its primary composite endpoint [increase in peak oxygen consumption (pVO2) by ≥1.5 mL kg−1 min−1, along with an improvement of ≥1 in New York Heart Association (NYHA) functional classification; or an improvement in pVO2 by ≥3.0 mL kg−1 min−1 without a decline in NYHA classification] achieved significant statistical difference (p = 0.0005). It also confirmed that Mavacamten can significantly improve patients’ exercise capacity (p = 0.0006), LVOTO (p < 0.0001), NYHA functional classification (p < 0.0001), and health status (p < 0.0001). The proportion of patients achieving complete resolution in both groups was 27% and less than 1%, respectively.126 The study demonstrated that Mavacamten, as a specific treatment for HCM, can bring about significant improvements in hemodynamics, cardiac function, and quality of life scores with clinical significance. Moreover, in terms of safety and tolerability, the results for the treatment group were similar to those of the placebo patients, with adverse reactions during treatment usually being mild. Another Phase III study, the VALOR-HCM study, aimed to assess whether the treatment with Mavacamten could reduce the need for septal reduction therapies (SRTs) in patients with obstructive HCM.127 The study demonstrated that the addition of Mavacamten treatment for 16 weeks on top of maximally tolerated background medical therapy significantly reduced the proportion of patients who still met the guidelines for SRT indications (p < 0.0001), which may also be beneficial for improving the quality of life in patients with severe symptomatic disease.
Aficamten
Aficamten (CK-274/CK-3773274) is a novel selective small molecule inhibitor of cardiac myosin. The drug has demonstrated safety and tolerability in healthy populations, with no serious AEs observed during treatment, and no clinically meaningful changes in vital signs, electrocardiograms, or laboratory test results.149 Based on this, the drug has initiated Phase III (SEQUOIA-HCM) clinical trials.129 Preliminary results from the Phase II study indicate that Aficamten can significantly reduce LV outflow tract gradient and NT-proBNP levels.
Furthermore, potential pharmaceutical interventions might exist for mitigating HCM resulting from MYBPC3 mutations. In their study, Singh et al.116 observed that administering rapamycin at a dose of 2.24 mg/kgxd or implementing a 40% caloric restriction for a duration of 9 weeks enhanced Akt–mTORC1 signaling, partially reinstated autophagic flux, and successfully rescued cardiomyopathy in Mybpc3-targeted KI mice. These findings serve as evidence that autophagy modulators can effectively impede the progression of cardiomyopathy in KI mice.150
4.2.2 Surgical treatment
The surgical treatments for HCM mainly include ventricular septal myectomy (VSM) and alcohol septal ablation (ASA). VSM involves the surgical removal of a portion of the hypertrophied myocardium from the interventricular septum to reduce or eliminate LVOTO, thereby improving cardiac function and symptoms. This is an effective treatment for obstructive HCM, especially for patients with ineffective medical treatment or severe symptoms. ASA, on the other hand, involves the percutaneous injection of alcohol into the septal branches of the coronary artery, causing local MI, reducing the thickness of the interventricular septum, and thus alleviating LVOTO.151 This method is suitable for patients with high surgical risk or contraindications to surgery.
4.2.3 Gene therapy
Myosin-binding protein C3
Gene therapy approaches aimed at addressing HCM primarily concentrate on targeting MYBPC3 mutations, which offer promising potential as a successful translation from laboratory research to practical clinical interventions. This focus is justified by the fact that MYBPC3 mutations are the most frequently observed genetic abnormalities in patients with HCM.152 Merkulov et al.153 carried out gene transfer by using a specific lentiviral vector, which increased the expression level of the MYBPC3 gene in HCM mice, thereby restoring the abnormal dynamics of myocardial cross-bridges, improving myocardial contractile function, delaying or reversing the pathogenesis of cardiac hypertrophy and myocardial fibrosis. On account of most MYBPC3 mutations leading to cMyBP-C haploinsufficiency, the potential strategy to treat MYBPC3-caused HCM is the introduction of wild-type MYBPC3 cDNA into abnormal cardiomyocytes.154 Recently, Prondzynski et al.155 introduced the full-length MYBPC3 cDNA into abnormal cardiomyocytes induced by pluripotent stem cells from HCM patients caused by mutation of the MYBPC3 gene, hence increasing the cMyBP-C expression level and successfully improving cardiac hypertrophy. Gedicke-Hornung et al.66 transduced the AON, which mediates exon skipping, into cardiomyocytes of neonatal mice by exon skipping therapy and inhibiting MYBPC3 gene mutation. The expression of abnormal transcriptional mRNA increased the expression of the deleted exon and successfully inhibited the progression of cardiac hypertrophy. Recent studies have found that in the HCM model of MYBPC3 gene mutation, the entire MYBPC3 mRNA mutation can be repaired by PTMs (pretrans-splicing molecule); theoretically, 40–60% of HCM patients can be cured.156-158
In addition, the full-length and functional repair of the cMyBP-C protein can be achieved using trans-splicing technology, enabling a faster and more accessible approach. Mearini et al.159 showed the feasibility of employing the 5′-trans-splicing strategy to repair Mybpc3 mRNA in a mouse model of HCM with a Mybpc3 mutation. The repaired Mybpc3 mRNA constituted approximately 66% of the overall Mybpc3 transcripts present in cardiac myocytes. Moreover, they correctly repaired the cMyBP-C protein incorporated into the sarcomere in cardiac myocytes. Mearini et al.159 also found that a single systemic administration of AAV9–Mybpc3 in mice could ameliorate cardiomyopathy by increasing Mybpc3 mRNA and cMyBP-C protein levels in a dose-dependent manner. Moreover, Li et al.160 also indicated that AAV9 gene transfer of cMyBP-C N-terminal domains that contained domains C0C2 prevented the development of cardiac hypertrophy and dysfunction in cMyBP-C-deficient mice, which genetically mimic this human cardiomyopathy.
Beta-myosin heavy chain
Mutations in the MYH7 R403Q in HCM patients result in a particularly severe cardiomyopathy characterized by progressive myocardial dysfunction. In a recent study, Anderson et al.161 identified that the MYH7 sequence could be targeted, referenced, or alternated selectively by three SNPs in MYH7 or ASOs libraries and suggested that SNP-targeting ASOs are a promising therapeutic strategy for treating cardiomyopathy. Yue et al.162 discovered that CASAAV (CRISPR/Cas9–AAV9-based somatic mutagenesis) technique successfully silenced Myh6 and Myh7 in cardiomyocytes. Bu et al.163 recently discovered ventricle myosin heavy chain like (vmhcl) as the zebrafish equivalent of human MYH7 and subsequently showcased the therapeutic advantages of inhibiting mTOR and mitogen-activated protein kinase (MAPK) pathways in vmhcl homozygous mutants.
Myosin heavy chain 6
Heterozygous MHC403/+ mice express the R403Q mutation in Myosin heavy chain 6 (Myh6), which causes changes in sarcomere function, including increased actomyosin sliding rate and hydrolysis of ATP.148, 164 Jiang et al.64 delayed the development of HCM by introducing a specific RNA inhibitor into the HCM animal model induced by the new mutation of the MYH6 gene via the viral vector AAV9 (AAV-9–cTnT–EGFP–RNAi).165, 166 In their research, Ma et al.167 developed a novel adenine base editor platform known as ABEmax-NG system, which exhibited the capability to effectively rectify a pathogenic Myh6 mutation through embryonic gene correction in mouse embryos. This correction mechanism proved instrumental in averting the progression of HCM.167
Chromatin remodeling protein
Han et al.168 discovered that chromatin remodeling protein (BRG1) plays a pivotal role in governing early-age myocardial development, differentiation, and gene expression. The MYH7 in healthy adult hearts can be cleaved by cardiac-specific antisense transcription to generate a batch of lncRNA molecules called Mhrt (Myosin Heavy Chain Associated RNA Transcripts); Mhrt can antagonize the effect of Brg1 on chromatin. A pressure load stimulus activates Brg1, resulting in cardiac hypertrophy. In hiPSC-CMs, MYH7 could be decreased, but MYH6 could be increased by inhibition of BRG1, which suggests BRG1 assumes a regulatory role in the pathological imbalance of the two myosin heavy chain isoforms in individuals with HCM.169 The team found a lncRNA that blocks pathological cardiac hypertrophy by antagonizing the effects of Brg1. Moreover, when subjected to adverse stimulus in the myocardium, the activated Brg1 will form a BRG1–Hdac–Parp complex, binding to the Mhrt promoter and inhibiting Mhrt transcription, forming a complete cardioprotective feedback loop.168 The discovery of the HCM regulatory protein BRG1 and the myocardial protection sequence Mhrt gene promotes the development of biochemical markers, enabling early identification and targeted treatment of HCM through designing novel, targeted drugs.
Sarcoplasmic reticulum Ca2+-ATPase 2a
A decreased ratio of sarcoplasmic reticulum Ca2+-ATPase 2a (Serca2a) to phosphoprotein (PLB) can affect the activity of the sarcomere and reticulum calcium pump, resulting in the myocardial inability to maintain normal diastolic function.170 Pena et al.171 indicated that the Serca2a/PLB ratio can be improved by upregulating Serca2a gene expression using viral vectors, which may enhance myocardial cell diastolic function and delay cardiac hypertrophy and myocardial fibrosis. Phospholamban (PLN) is an inhibitor of cardiac muscle Serca2a in the unphosphorylated state. Gaffin et al.172 demonstrated that the enduring mitigation of familial HCM, resulting from mutations in genes encoding thin filament protein and tropomyosin, could potentially be achieved through the modulation of a calcium cycling protein, specifically the deletion of the PLN gene. The CUPID study is the first clinical study to use gene transfer technology for individual therapy. In this study, the patients with severe heart failure received a single IC infusion of AAV1/SERCA2a. A CUPID Phase II study included 39 patients with heart failure compared with the placebo group. The high-dose group significantly decreased cardiovascular event rate (HR = 0.12, p = 0.003) and hospitalization (0.4 d vs. 4.5 d, p = 0.05) at 1 year.173 After 3 years, the high-dose group had an 82% reduction in cardiovascular events compared with the placebo group (p = 0.048). Cardiac biopsy was performed on three high-dose patients to confirm the presence of therapeutic genes.55 Despite the same treatment, in the CUPID IIb phase study with more subjects, the 1-year follow-up showed no improvement in clinical outcomes or ejection fraction in patients with heart failure.56 Nevertheless, the study provides a perspective for the future use of adenoviruses in the gene therapy for cardiomyopathy.
Protein kinase AMP-activated noncatalytic subunit gamma 2
Mutations occurring in the protein kinase AMP-activated noncatalytic subunit gamma 2 (PRKAG2) gene, responsible for encoding the γ2-subunit of AMPK, give rise toHCM and familial Wolff–Parkinson–White syndrome.174, 175 Ben Jehuda et al.176 generated iPSC-HCMs with PRKAG2 mutations, which demonstrated both functional and structural abnormalities in cardiac myocytes, indicative of HCM. The researchers successfully employed CRISPR technology to rectify the mutation in the patient's iPSCs, thereby eliminating the HCM-associated characteristics.176 Zhan et al.177 utilized CRISPR–Cas9-mediated genome editing to rectify the R302Q mutation in hiPS-CMs. These cardiomyocytes harbored a heterozygous missense mutation (c.905G>A, R302Q) in the PRKAG2 gene.177
Long noncoding RNAs
Apart from the therapy targeting causal genes, Mosqueira et al.178 proposed potential diagnostic biomarkers and therapeutic targets. They used CRISPR/Cas9 to produce isogenic sets of C9123T–MYH7 (R453C–bMHC) mutants in hiPSC-CM. The discovery of previously unidentified lncRNAs and potential gene modifiers opens up opportunities for gaining fresh insights into molecular mechanisms and functional aspects through techniques such as knockout, overexpression, and pathway analysis.178
5 ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY
ARVC is an uncommon hereditary cardiac disorder that ranks among the leading causes of SCD in young individuals.107, 179 Therefore, professional societies in Europe and North America advise individuals with ARVC to refrain from engaging in high-intensity exercise.180-182
5.1 Pathogenesis and disease-causing genes
Alterations in genes encoding desmosomal proteins or proteins that interact with desmosomal proteins have been identified as a disease-causing factor in ARVC, which contribute to the occurrence of the disease in more than 50% of individuals diagnosed with classical ARVC.183, 184 The most common defective ARVC genes have been discovered in genes encoding desmosomal proteins, including JUP, PKP2, DSP, DSG2, and DSC2,14-16 in which 87% of the genetic variants were found within the five genes.105, 106 Nondesmosomal pathogenic variants have been described in DES, LMNA, SCN5A, CDH2, CTNNA3, FLNC, PLN, TGFβ3, TMEM43, RYR2, TJP1, and TTN106, 107, 109, 179, 180 (Table 1). Brun et al.185 identified two unique FLNCtv variants in two families causing ARVC. Currently available therapeutic tools include antiarrhythmic drugs, catheter ablation, and implantable cardioverter defibrillators.186 In accordance with the desmosomal model, recent studies have shown that exercise often triggers SCD in ARVC. Gene therapy facilitated by recombinant AAV (rAAV) offers a compelling approach for precise, targeted interventions, holding the potential to revolutionize treatment strategies for patients with ARVC.187
The propensity of ARVC to cause arrhythmias is intricate, involving multiple mechanisms. The abnormal signal transduction and the establishment of macro-reentry circuits, triggered by the deposition of fibrofatty scar tissue, can result in the formation of malignant ventricular arrhythmias. Moreover, the intricate interplay among desmosomes, voltage-gated sodium channels, and gap junction proteins within the intercalated disc is associated with the disruption of normal cell signaling, further promoting arrhythmogenesis.24
5.2 Therapeutic interventions
Although ARVC significantly contributes to SCD among young individuals, there is currently no effective method to reverse its progression. Consequently, recent therapeutic approaches have shifted their focus toward inhibiting or delaying the advancement of ARVC. Numerous preclinical studies have been conducted in an effort to uncover additional evidence that could aid in the management of this condition.
5.2.1 Pharmacological treatment
The data related to drug treatment for ARVC are relatively scarce. In clinical practice, some antiarrhythmic drugs are commonly recommended to slow down the progression of the disease.
5.2.2 Surgical treatment
When using ICD as primary prevention, it is necessary to weigh the absolute risk of SCD and device-related complications, including inappropriate shocks and infections.188 For patients with ARVC, a combination of endocardial and epicardial ablation is usually required. Some researchers suggest starting with endocardial ablation followed by epicardial ablation. In experienced centers, catheter ablation is an important adjunctive treatment for ARVC patients with ventricular arrhythmias.189 Patients who have undergone catheter ablation and meet the indications can still receive an ICD implant. The main indication for heart transplantation is severe right ventricular dysfunction, and patients with refractory ventricular arrhythmias can also be considered for heart transplantation.190
5.2.3 Gene therapy
Plakophilin-2
PKP2, a key desmosome component, plays a crucial role in cell–cell adhesion. Mutations in the human PKP2 gene are linked to the severe, life-threatening ARVC.191 Preliminary research into gene therapy for PKP2 has utilized rAAV as a vector. Wu et al.192 demonstrated that a single administration of AAV9:PKP2 gene delivery effectively prevents the onset of ARVC by restoring the integrity of desmosomal and gap junctional cellular structures. This intervention not only maintains or enhances LVEF but also arrests or reverses right ventricular dilation. Furthermore, it mitigates the frequency and severity of ventricular arrhythmias and averts detrimental fibrotic remodeling, showcasing the therapeutic potential of this targeted gene therapy approach.192 van Opbergen et al.193 determined that the delivery of PKP2a via AAVrh.74–PKP2a significantly enhanced survival rates in the PKP2–cKO murine model, which features cardiac-specific, tamoxifen-inducible PKP2 deletion. The therapeutic advantage was pronounced in mice that received AAVrh.74–PKP2a postonset of the disease. Echocardiographic evaluation disclosed that AAVrh.74–PKP2a efficaciously averted dilation of the right ventricle, halted the progressive decline in LV function, and alleviated the severity of arrhythmias. The study presented robust preclinical evidence supporting the candidacy of AAVrh.74–PKP2a (RP-A601) as a promising therapeutic intervention for PKP2-associated ARVC, efficacious in both the incipient and advanced stages of the disease.193
Phospholamban
PLN is a critical regulator of calcium cycling and contractility in the heart. The loss of arginine at position 14 in PLN (R14del) is associated with DCM with a high prevalence of ventricular arrhythmias.194 Karakikes et al.195 derived iPSCs from a patient with the PLN R14del mutation and successfully differentiated these into cardiomyocytes (iPSC-CMs). Their research revealed that gene correction employing TALENs effectively mitigates the disease phenotypes associated with the R14del mutation in the iPSC-CMs.195 Dave et al.196 employed the CRISPR/Cas9 system in conjunction with a cardiotropic AAV9 to perform in vivo genome editing. Their study successfully demonstrated a reduction in end-diastolic and stroke volumes, as well as a decreased susceptibility to ventricular tachycardia in young adult mice that express the human PLN-R14del mutation.196 This preclinical research presents encouraging, potentially translatable methods for the detection and therapeutic modulation of the arrhythmogenic phenotype in individuals with PLN-R14del disease and may also be applicable to other inherited cardiomyopathies.
Desmoglein-2
DSG2, a protein encoded by the DSG2 gene, is integral to the desmosomal complex that upholds tissue integrity, particularly within the cardiac muscle. Genetic alterations in the DSG2 gene are known to precipitate arrhythmogenic cardiomyopathy, a condition predominantly associated with the Japanese variant of ARVC.197, 198 Shiba et al.199 generated iPSC from a patient carrying the DSG2 (c.C355T, p.R119X) mutation (R119X-iPSC). They successfully heterozygously corrected the mutated DSG2 gene locus to a normal allele using HDR, resulting in HDR-iPSCs. In the cardiomyocytes derived from these HDR-iPSC, the previously observed phenotypes, including abnormal desmosome protein deposition and disrupted intercalated disk structures, were notably restored.199
6 DILATED CARDIOMYOPATHY
DCM can be an end-stage form and a common feature of several known causes (e.g., hypertension, ischemia, diabetes, etc.). Nevertheless, DCM is usually associated with a genetic predisposition caused by specific gene mutations, often inherited in an autosomal dominant pattern. Patients with DCM may have multiple affected members within their family, and risks can be identified through genetic counseling and testing. In addition, certain individuals exhibit clinical symptoms such as enlarged ventricular cavities and reduced contractility even without a definitive diagnosis of an underlying primary disease.
DCM is characterized by ventricular enlargement and systolic dysfunction.200 Although the left ventricle (LV) quality in DCM is usually significantly increased, the LV wall thickness is reasonable compared with HCM.3 It is estimated that the incidence of primary DCM has exceeded one out of 250, and it has a trend of increasing year by year.201
6.1 Pathogenesis and disease-causing genes
In about 35% of patients with DCM, genetic mutations can be identified, which usually involve genes encoding ion channels, cytoskeletal, sarcomere, and nuclear envelope proteins.202 Disease-causing mutations have been identified in more than 50 genes,13 including TTN, DSP, MYH7, BAG3, TNNT2, TNNC1, PLN, ACTC1, NEXN, TPM1, VCL, LMNA, MYBPC3, ABCC9, ACTN2, ANKRD1, CAV3, CHRM2, CRYAB, DES, DMD, DOLK, DSC2, DSCG2, DTNA, EMD, FHL2, GATAD1, GATA4, GLA, LK, JPH2, JUP, LAMA4, LAMP2,LDB3, MURC, MYH6, MYL2, MYL3, MYLK2, MYOM1, MYOZ2, MYPN, NEBL, PDLIM3, PKP2, PRDM16, PRKAG2, PTNP11, RAF1, RBM2, RIT1, RYR2, TNNI3, RBM20, SCM5A, and so on.200, 203 Furthermore, more than 40 genes are known to be associated with DCM, and mutations in these genes could also lead to phenotypes of other types of cardiomyopathies. Although each type of cardiomyopathy has its unique characteristics, there may be a certain degree of overlap in clinical presentation and genetic background. DCM often has shared genes and overlapping phenotypes with other cardiomyopathies (Figure 4A). Significant evidence exists for variants within the top 12 genes, which could potentially account for 17% of the cases observed in the outpatient clinic cohort with DCM.204 The high-evidence DCM genes are recommended for use in clinical practice.205
6.1.1 Phospholamban
DCM can be caused by mutations in the gene encoding the cardiac protein PLN. Disease-causing mutations for familial DCM have been identified in the PLN gene. The p.(Arg14del) pathogenic variant (R14del) of the PLN gene has been regarded as a common cause of DCM with heart failure. Eijgenraam and colleagues206 discovered changes in proteostasis and aggregation of PLN protein as the initial indicators of PLN-R14del-related DCM, suggesting a novel therapeutic target. Yost et al.207 described a missense G>A mutation in exon 1 of the PLN gene that changed an amino acid arginine to histidine in a spontaneous canine model of familial DCM. hiPSC-CMs harboring the R9C PLN mutation showed activation of a hypertrophic phenotype and also perturbed the expression of several miRNAs involved in fibrosis, hypertrophy, and cardiac metabolism.208
6.1.2 Titin
Recent studies have shown that mutations in the gene encoding giant-muscle filament titin cause autosomal dominant DCM linked to chromosome 2q31 (CMD1G; MIM 604145).209 Truncating variants in the TTN gene (TTNtv) have been identified as the most common cause of heritable DCM. Yoskovitz et al.210 determined the sequences of the gene in an Israeli Arab family. The linkage studies and direct sequencing excluded LMNA, MYH7, TNNT2, TNNI3, SCN5A, DES, SGCD, ACTC, PLN, and MYH6 but found a linkage between the TTN locus at chromosome 2q31 and DCM. Sequence analysis identified an insertion (c.58880insA), which finally caused protein truncation after 19,628 amino acids (p.S19628IfsX1). Hinson et al.211 indicated that titin mutations disrupted critical linkages between sarcomerogenesis and adaptive remodeling and caused DCM in iPSCs. TTNtv results in reduced phosphorylation levels of Troponin I (TnI) and MYBP-C in the LV, contributing to the manifestation of frequent arrhythmias.212, 213
6.1.3 GATA binding protein 4
In cardiac development, the cardiac transcription factor GATA binding protein 4 (GATA4) is essential, and mutations in GATA4 have been associated with a wide variety of congenital heart diseases and DCM.214, 215 In recent studies, various new heterozygous GATA4 mutations—namely, p.V291L, p.V39L, p.P226Q, and p.T279S—have been detected in three unrelated patients with sporadic DCM and in a family exhibiting DCM inheritance via an autosomal dominant pattern.216, 217
6.1.4 Beta-myosin heavy chain
The majority of pathogenic mutations found in DCM affect genes responsible for both sarcomeric and cytoskeletal proteins. Among these, mutations in the MYH7 gene are the most prevalent. Patients with MYH7 variants exhibit a specific correlation with LV noncompaction characteristics.218 Rani et al.219 found a novel mutation in the β-MYH7 gene in Indian patients with DCM.
6.1.5 Lamins
Lamins (LMNA) PP-associated cardiomyopathy is a form of DCM with poor prognosis and high mortality. LMNA DCM has gender difference, which is more severe in males in both human patients and a knock-in mouse model carrying a homozygous p.H222P mutation (LmnaH222P/H222P).220 Cai et al.221 found that the LMNA-R225X nonsense mutation induces cardiac conduction defects through AV node fibrosis, resulting in DCM.
6.1.6 Junctophilin 2
Recent studies have identified two novel variants by ultra-sequencing in patients with DCM: the p.Asn1474Lys variant in the SCN5A gene and the p.Glu85Lys variant in the JPH2 gene.222 However, pathogenic JPH2 variants are rare among patients with DCM.223
6.1.7 Sodium voltage-gated channel alpha subunit 5
Mutations in the Sodium voltage-gated channel alpha subunit 5 (SCN5A) gene have been associated with the development of DCM, a total of 12 family members (10 males, 83.3%), 2 of them carriers of the p.Asn1474Lys variant in the SCN5A gene.222 Mann et al.224 indicated that the R222Q SCN5A variant activates sodium channel function and is associated with reversible ventricular ectopy and DCM.
6.1.8 BAG Cochaperone 3
BAG Cochaperone 3 (BAG3) has been identified as one of the most common DCM causative genes in recent human genetic studies, with its variants contributing to 2.3–6.7% of DCMs.225 BAG3-related DCM is characterized by a high penetrance in patients >40 years of age and a high risk of progressive heart failure.226 The mechanism of BAG3 mutations caused DCM possibly to interfere with Z-disc assembly and enhance sensitivity to apoptosis in cardiomyocytes.227 Hakui et al.228 identified that loss-of-function mutations in BAG5 caused inherited DCM in five patients among four unrelated families with complete penetrance.
6.2 Therapeutic interventions
To date, there is no evidence-based treatment for DCM, and DCM is treated the same way as heart failure.229 In many cases, heart transplantation remains the only option when it is impossible to inhibit the progression of heart failure through drug therapy.230 On this issue, gene therapy may be a promising treatment option or supplement for patients with DCM caused by genetic mutations.
6.2.1 Gene therapy
More than 50 causal genes associated with DCM have been identified. The use of contemporary genetic testing has also demonstrated the underlying pathogenesis in between 50 and 75% of patients with multiple causes found in 25%.231 Recent studies have suggested that therapeutic strategies to attenuate disease-causing gene activity may rescue depressed cardiac contractility in patients with DCM.102
Troponin T2
The DCM caused by TNNT2 mutations tends to have severe clinical phenotypes and develop markedly enlarged hearts with LV systolic dysfunction and frequent SCD.232 Li et al.233 found that the expression of cardiac XIN protein was decreased in TNNT2-1 K210 hESCs-derived cardiomyocytes and the heart-specific delivered overexpression of XINB via AAV9 could ameliorate DCM remodeling in Tnnt2-1 K210 mice. Migliore et al.234 identified that allele-specific silencing by RNAi (ASP-RNAi) could specifically knock down mutant alleles coding for R92Q and R173W mutant TNNT2 proteins in HCM and DCM.
The point mutation in the LMNA gene can lead to Hutchinson-Gilford progeria syndrome (HGPS), which might cause heart complications. Lee et al.235 found that the antisense oligonucleotide therapy (siRNAs) targeting exon 11 could rescue LMNA-relate progeria and reduce prelamin A/progerin in favor of the alternative splicing of lamin C. Genome editing was used to correct LMNA-relate progeria in two similar recent preclinical study,236, 237 suggesting that in other malignant LMNA missense variants, the HGPS gene editing model could be recapitulated. PTC124 induces translational read-through across the premature stop codon and restores the production of the full-length proteins encoded from the mutated genes. Lee et al.238 found that the production of full-length LMNA proteins was increased by PTC124 treatment and improved the excitation–contraction coupling of the affected cardiomyocytes in the R225X mutant.
Titin
TTN is the largest protein in humans and is required for sarcomere assembly.239, 240 It provides most of the motility and regulates the active contractile force in the striated muscle.241 TTN mutations and consequent truncated protein abnormalities are among the most common genetic causes of DCM in approximately 25% of idiopathic DCM family cases and 18% of sporadic cases.242 Gramlich et al.68 found that AON treatment in Ttn knock-in mice improved sarcomere formation and contractile properties in homozygous embryos and prevented the development of DCM phenotypes in heterozygous animals. The intervention of calcium sensitivity may promote myofilament function in DCM patients with sarcomere gene mutations, thereby preventing cardiomyocyte dysfunction.243 Romano et al.244 indicated that using CRISPR to ablate A-band variant-specific truncation peptides by introducing a proximal I-band TTNtv restored functional deficits and could be adapted as a potential genome editing strategy to target above 30% of DCM-associated TTNtvs.
Phospholamban
Therapeutic genome editing could be effectively applied to the PLN, encoding the protein functions to regulate the kinetics of calcium flux in cardiomyocytes.195, 245, 246 Recent studies found that irregular Ca2+ handling, abnormal cytoplasmic PLN protein distribution, and increased cardiac hypertrophy marker expression could be exhibited in the iCMs carrying a deleterious PLN R14del mutation.195 A TALEN vector pair introducing a double-strand break adjacent to the mutation and the gene correction matrix incorporating the wild-type copy of the gene into the DNA via recombination could be used to correct the R14del mutation. TALEN-mediated genetic correction restored contractile function in this model that has been impaired by PLN R14del mutation.246 Feyen et al.194 modeled the PLN R14del cardiomyopathy with isogenic pairs of hiPSC-CMs, and single-cell RNA sequencing revealed that the unfolded protein response pathway (UPR) had been induced in PLN R14del. Therefore, modulation of the UPR might be exploited therapeutically.194 Hoshijima et al.247 trans-coronary delivered S16EPLN gene via rAAV vector, myocardial SR Ca2+ uptake and LV systolic function could be improved. Beverborg et al.248 used ASOs to target Pln mRNA and interfered with the PLN/SERCA2a interaction in the heart of murine HF models. The progression of LV dilatation had been suppressed by this therapeutic modality.248
Immunoglobulin Mu DNA binding protein 2
The therapeutic strategy of a tissue-specific requirement for immunoglobulin Mu DNA binding protein 2 (IGHMBP2) has been verified effectively in cardiomyocyte maintenance and survival, and a genetic modifier has been found that it can alter the course of DCM through cardiac functional adaptation and physical remodeling.249 Maddatu et al.250 indicated that transgenic expression of the Ighmbp2 cDNA prevented the process of impairing the function of skeletal and cardiac myocytes in mouse.
Apoptosis signal-regulating kinase 1
Hikoso et al.251 indicated that the rAAV expressing an N-terminal truncated form of the dominant-negative mutant of apoptosis signal-regulating kinase 1 (ASK1) inhibited ASK1 protein activation in the hamster hearts and suppressed the progression of ventricular remodeling such as chamber dilation, impairment of contractile and relaxation functions, and fibrosis.
Vascular endothelial growth factor B
Vascular endothelial growth factor B (VEGF-B) gene transfer has shown beneficial effects in experimental models of cardiac injury.252, 253 VEGF-B is one of the five members of the mammalian VEGFs family and is a major presurvival factor.254 Its remarkable cytoprotective/antiapoptotic and minimal angiogenic effects make it particularly suitable for gene therapy for nonischemic DCM.255-257 Woitek et al.258 used a dog DCM model to inject the adeno-associated-9 virus carrying the VEGF-B167 gene into the coronary arteries of dogs with compensated heart failure. Compared with the control group, the VEGF-B167 group revealed significant retention of diastolic and systolic function and reduced ventricular remodeling that prevented progression from compensated to decompensated heart failure.
MicroRNAs
MicroRNA can be highly expressed in cardiomyocytes and plays a vital role in muscle growth, regeneration, and fibrosis processes. Misexpression of miRNA could have severe effects on cardiomyocytes. Studies have shown that down-regulation of miR-448-3p can trigger reactive oxygen species (ROS) production and lead to cardiac hypertrophy, atrial fibrillation, myocardial fibrosis, and inflammation, resulting in DCM.259
Studies have shown that approximately 8% of sporadic and 25% of familial DCM are associated with truncated mutations in the gene encoding casin. Moreover, the frameshift mutation of the annexin-encoding gene mediated by antisense oligonucleotides is one of the critical genetic forms of DCM. Gramlich et al.68 exhibited that the beneficial potential of AON-mediated exon skipping could be used to reframe titin transcripts in humans. The reframed TTN could produce three major isoforms (N2A, N2B, and N2BA) by alternative splicing, which predominately differ in the length of the extensible I-band domains.260 Quattrocelli et al.261 demonstrated that intraventricular delivery of AAV vectors induces long-term (18 months) miR-669a overexpression significantly improved myocardial structure and cardiac function in Sgcb gene knockout mice, and it reduced adverse LV remodeling, thereby attenuating malnutrition and improving survival in mice with severe cardiomyopathy rate. In addition, drug therapy can also regulate the expression of mRNA in cardiomyocytes. Sukumaran et al.261 found that olmesartan treatment upregulated myocardial protein and mRNA levels of ACE-2, ANG 1−7 receptor but effectively suppressed the myocardial protein and mRNA expressions of inflammatory markers compared with the vehicle-treated DCM rats.
6.2.2 Therapeutic dilemma
The degree of genetic mutation varies from gene to gene. For example, the LAMIN A/C mutation is highly porous and therefore requires correction by silencing the mutated gene.262 siRNA-mediated deleterious allelic silencing can be used to treat more malignant genetic mutations. Conversely, some benign gene mutations (such as TTN truncation mutations) with cardiac function that are likely to improve after treatment do not benefit from gene silencing therapy.263 The phenotypic manifestation of a genetic variation will also determine the treatment. Antiarrhythmic therapy is more often considered in DCM patients with LMNA or RBM20 gene mutations, whereas standard heart failure therapy may be sufficient in other benign DCM patients.264-267
7 RESTRICTIVE CARDIOMYOPATHY
RCM is a rare form of cardiomyopathy characterized by restrictive ventricular physiology in the presence of normal diastolic volume and normal ventricular wall thickness. Genes associated with RCM include those encoding myosin heavy chain, myosin binding protein C, troponin I and T, tropomyosin, and desmin (DES). RCM is rarer than HCM and DCM, but the prognosis is worse, and the risk of pulmonary hypertension, thromboembolic events, and sudden death is higher.268, 269 Although the primary molecular mechanism in RCM is not fully understood, recent studies have advanced hypotheses based on experimental models.
7.1 Pathogenesis and disease-causing genes
Primary RCM is usually related to genetic factors, and its pathogenesis mainly involves the increased sensitivity of cardiac muscle filaments to calcium, as well as the accumulation of DES and type III collagen within the cardiac muscle cells, which are usually caused by mutations in the related encoding genes. Alterations in genes encoding for sarcomeric proteins, Z-disc proteins, or transthyretin (TTR) have been identified as the potential disease-causing genes of RCM.17 Causes of RCM with associated genetic perturbations included mutations in genes encoding sarcomeric proteins (MYH7−4, MYBPC3−3, TNNI3−2, and TNNT2−1), genes encoding structural and genes encoding cytoskeletal proteins (BAG3, JUP, ACTN2, DES). Among 24 variants of unknown significance, 19 were identified in structural and cytoskeletal genes (TTN, SYNE, MYOM1, CACNB2, FKTN, LDB3, EMD, MYOZ, DSP, TMPO), two in ion channel genes, and two in genes encoding mitochondrial proteins.270, 271 Gallego-Delgado et al.272 analyzed the variants in genes associated with RCM in 32 unrelated patients and found that mutated genes included MYH7 (four patients), DES (three), FLNC (three), MYBPC3 (two), LMNA (two), TCAP (one), TNNI3 (one), TNNT2 (one), TPM1 (one), and LAMP2 (one). Eleven patients (34%) exhibited only VUS and two patients(6%) did not bear any mutation. DNA samples from 12 children have been analyzed in a recent study, which identified sarcomere protein gene mutations in four patients (33%): two in TNNI3 and one each in TNNT2 and ACTC genes.273 Caleshu et al.17 first reported that mutations in TPM1, MYL3, and MYL2 were associated with primary, nonhypertrophied RCM in two DCM patients.
Recent studies have found different mutations associated with RCM in patients, in vitro, and in animals (Figure 4B).17, 273-284, 444
7.2 Therapeutic interventions
RCM is relatively rare compared with HCM and DCM, with diverse inherited and acquired causes and manifestations. Elimination of the pathogenic protein and organ recovery is the goal of effective treatment.271 There is no approved therapy that targets the underlying genetic cause. Zaleta-Rivera et al.285 found that a one-time injection of AAV9–M7.8L R into 3-day-old humanized regulatory light chain mutant transgenic mice silenced the mutated allele (RLC-47K). The expression of hypertrophic biomarkers was suppressed and the pathological increase was attenuated in the LV mass, which showed RNAi therapeutics may be a feasible and safe treatment strategy directed toward human RCM. This is a promising step toward targeted gene therapy for RCM.286
8 LEFT VENTRICULAR NONCOMPACTION
LVNC, characterized by abnormal trabeculations in the LV, is the third most common cardiomyopathy in children. The intrauterine arrest of compaction of the loosely woven meshwork that constitutes the fetal myocardial primordium leads to an altered myocardial wall. In at least 30−50% of patients, the development of the condition can be attributed to genetic inheritance, and researchers have identified several genes responsible for LV noncompaction.286
8.1 Pathogenesis and disease-causing genes
LVNC, characterized by abnormal trabeculations in the LV, is the third most common cardiomyopathy in children. The intrauterine arrest of compaction of the loosely woven meshwork that constitutes the fetal myocardial primordium leads to an altered myocardial wall. In at least 30−50% of patients, the development of the condition can be attributed to genetic inheritance, and researchers have identified several genes responsible for LV noncompaction.286 The pathogenesis of LVNC involves mutations in multiple genes. These include genes encoding for sarcomeric (MYBPC3, TPM1, MYH7, ACTC1, TNNT2,18 TNNI3, MYL2, and MYL3287), Z-disc (LDB3, Cypher, ZASP, DSC2287, 288), nuclear envelope (e.g., LMNA), mitochondrial (SCO2, SDHA, TAZ), ion channel proteins (ABCC9, ANK2, CACNA1C, KCNE3, KCNH2, KCNQ1, RYR1, RYR2, SCN5A, HCN4289, 290), α-dystrobrevin (DTNA), and NOTCH pathway regulators (e.g., MIB1), muscle-specific intermediate filament protein desmin (e.g., DES), AMP-activated protein kinase (PRKAG2). The different genotype may correlate with different phenotype.291-293
Kolokotronis et al.294 revealed that severe cardiomyopathy accompanied by LVNC could be caused by a missense variant in either MYH7 or MYBPC3. Kodo et al.295 discovered that by inhibiting TGFβ signaling and correcting the TBX20 mutation in iPSC-CMs from LVNC patients, the disease phenotype associated with the cardiac transcription factor TBX20 mutation could be reversed.
8.2 Therapeutic interventions
8.2.1 Pharmacological treatment and surgical treatment
The drug treatment for LVNC primarily targets the symptoms of heart failure, arrhythmias, and thromboembolism that may arise from the condition. In treating heart failure, standard antiheart failure medications are typically used, and for end-stage heart failure patients, heart transplantation should be considered. For ICDs, the indications for secondary or primary prevention are the same as for other cardiomyopathies, and appropriate ICD implantation can effectively prevent sudden death and reduce mortality rates. Additionally, since patients with LVNC may be at high risk for thromboembolism, long-term anticoagulation therapy is recommended for patients with atrial fibrillation, a history of systemic embolism, or a LVEF less than 40%. In the case of drug treatment for patients with LVNC, there are currently no specific drug guidelines and treatment mainly involves symptomatic therapy and management of complications.296
8.2.2 Gene therapy
Although numerous mutations have been discovered in the genes associated with LVNC, the exact mechanism behind ventricular compaction and the pathogenesis of LVNC remains to be fully elucidated. With a large number of genes leading to LVNC, genetic testing plays a minor role in clinical practice at this time.297 There is no targeted gene therapy at present, but we believe that the molecular underpinnings and genetic mechanisms behind LVNC will soon be discovered, and then LVNC patients will be able to receive the proper treatment targeted to their specific pathogenic gene mutation.
9 GLYCOGEN STORAGE CARDIOMYOPATHY
Glycogen storage cardiomyopathy is a group of genetic disorders characterized by the abnormal accumulation of glycogen in cardiac muscle tissue, leading to impaired heart function. This disease can result in glycogen accumulation in various tissues, especially in skeletal and cardiac muscle tissue, causing a genetics storage cardiomyopathy with enlarged glycogen vacuoles.298, 299 Glycogen storage cardiomyopathy usually contains Pompe disease (PD), PRKAG2 syndrome, and LAMP2 syndrome.
9.1 PRKAG2 cardiomyopathy: pathogenesis and therapeutic interventions
HCM is characterized by mutations in the contractile elements of the sarcomere or Z-disc. Moreover, certain cardiomyocytes exhibiting unexplained hypertrophy may harbor mutations in additional genes. One such gene is PRKAG2, responsible for encoding the gamma2 subunit of AMPK, causing an accumulation of cardiac glycogen and LV hypertrophy.298, 300-302 Laforêt et al.303 reported 38-year-old male presenting with HCM, which was attributed to a recently discovered heterozygous PRKAG2 mutation (Ser548Pro). Recent research has provided evidence of an elevated susceptibility to arrhythmic and myocardial complications in individuals affected by cardiac glycogenosis caused by PRKAG2 mutations.304, 305 CRISPR technology could correct the mutation and ameliorate disease in the patient's iPSCs.176 Xie et al.306 injected AAV9–Cas9/sgRNA either on postnatal day 4 or day 42, resulting in significant restoration of the cardiac morphology and function in H530R Prkag2 transgenic and KI mice.
9.2 LAMP2 cardiomyopathy: pathogenesis and therapeutic interventions
Human mutations in the X-linked LAMP2 gene can cause Danon disease (DD), a lysosomal glycogen storage disease with fatal cardiomyopathy.307, 308 In LAMP-2 deficient myocytes, cardiac contractile function is significantly attenuated.309 Dvornikov et al.310 discovered that partial mTOR deficiency had the ability to restore certain features of lamp2 KO in zebrafish, such as ejection fraction and actomyosin activation kinetics. These findings hold promise for the development of targeted gene therapies aimed at addressing these specific characteristics.310 In an open-label Phase 1 clinical trial, a single IV administration of RP-A501 (AAV9.LAMP2B) was shown to effectively deliver and transduce cardiomyocytes in patients afflicted with DD—an X-linked monogenic cardiomyopathy attributed to mutations in the LAMP2 gene. This targeted therapy not only arrested but also demonstrated the potential to reverse the disease's rapid progression of cardiomyopathy.311
9.3 Pompe disease: pathogenesis and therapeutic interventions
PD, also known as glycogen storage disease type II, is an autosomal recessive metabolic disorder that ultimately causes damage to muscle and nerve cells throughout the body. The condition arises due to a deficiency in the lysosomal enzyme acid α-glucosidase (GAA), leading to the accumulation of glycogen within the lysosome.312 Recently, a large number of studies have demonstrated the feasibility of AAV vectors for in vivo GAA gene therapy are available. Pauly et al.313 constructed an E1-deleted recombinant adenovirus encoding human GAA. They demonstrated that recombinant acid GAA could express at a high level in treated target tissues, which supported the availability of gene replacement strategies for PD.313 Mah et al.314 showed that the deficiency of cardiac and diaphragmatic Gaa enzymatic activity in mice with PD could be successfully restored through the use of a rAAV serotype 1 (rAAV2/1) vector administered in vivo. Keeler et al.315 found that both AAVB1 and AAV9 vectors expressing GAA transduced the heart efficiently, leading to glycogen clearance. Stok et al.316 reported the normalization of glycogen in heart tissue by ex vivo hematopoietic stem cell gene therapy. They have used a codon-optimized GAA (GAAco) to normalize the glycogen in the heart, muscles, and brain in enzyme levels.316 Liang et al.317 utilized a fusion approach by combining insulin-like growth factor 2 (IGF2) with a codon-optimized variant of GAA (LV-IGF2.GAAco) to enhance cellular uptake. This fusion construct demonstrated the ability to fully restore glycogen levels, alleviate pathology, and improve impaired autophagy in both heart and skeletal muscles. Importantly, these effects were achieved at a clinically relevant vector copy number of 3.317
10 BARTH SYNDROME
10.1 Pathogenesis and disease-causing genes
Barth syndrome (BTHS) arises from mutations in the gene responsible for encoding tafazzin (TAZ), leading to a mitochondrial disorder. The mutations resulted in muscle dysfunction and DCM, characterized by abnormal sarcomere assembly, compromised contractility, and an excessive presence of ROS.318 In a cellular experiment, Wang et al.319 utilized iPSCs derived from a healthy individual and introduced TAZ mutations. They successfully demonstrated that TAZ mutation alone is both necessary and sufficient to induce the cardiac myocyte dysfunction associated with BTHS. These findings suggest potential new treatment approaches, such as in vivo gene therapy targeting the TAZ gene, to rescue cardiomyopathy in patients with BTHS.319
10.2 Potential therapeutic interventions
In recent studies, AAV has been used for gene therapy to deliver Taz since AAV is long-lasting and provides stable gene transfer.320 Wang et al.47 showed that the replacement of Taz through AAV gene therapy effectively rescued neonatal death, cardiac dysfunction, and fibrosis in Taz-KO mice. Suzuki-Hatano et al.321 indicated that AAV-mediated TAZ gene replacement restored mitochondrial and cardioskeletal function by improving the Des promoter. Moreover, the therapeutic targeting of YAP signaling has emerged as a promising approach for the treatment of pressure overload-induced heart disease, suggesting potential efforts to treat BTHS by targeting YAP.322
11 CONDUCTION AND ION CHANNEL DISORDERS
11.1 Lamin A/C gene (LMNA) cardiomyopathy
11.1.1 Pathogenesis and disease-causing genes
LMNA cardiomyopathy is caused by mutations in the LMNA, encoding the nuclear proteins lamin A/C. The main clinical manifestations are cardiomyopathy and conduction disorders. In an iPSCs model of the K219T mutation on LMNA, Salvarani et al.323 revealed that the K219T-LMNA mutation synergistically interacted with PRC2, leading to the downregulation of SCN5A, resulting in a reduction in sodium current density and a slowdown in conduction velocity. This mechanism potentially contributes to the conduction abnormalities observed in LMNA cardiomyopathy.323 Gerbino et al.324 investigated a newly discovered frameshift variant on the LMNA gene (p.D243Gfs*4), which was found in three individuals from an Italian family and was found to be cosegregating with a severe manifestation of cardiac conduction defects. The results indicated that the aberrant CX43 expression participated in the pathogenic mechanism for this LMNA truncating alteration.324 Moreover, Cai et al.221 indicated that the LMNA-R225X nonsense mutation induced cardiac conduction defects and DCM.
11.1.2 Potential therapeutic interventions
In terms of treatment, there is currently no specific therapeutic method for LMNA-related cardiomyopathy. However, research is exploring the possibility of interventional treatment by targeting epigenetic modifiers or specific signaling pathways. Sun et al.325 identified that whole-body supplementation with LMNA using rAAVs partially ameliorated the cardiac abnormalities in mouse models harboring Lmna truncating variants. This finding implicates AAV-mediated gene supplementation as an emerging and promising therapeutic approach for the treatment of LMNA-related cardiomyopathy.325 Lee and his team326 established an in vitro model for LMNA-DCM by utilizing patient-specific iPSC-CMs. Through this model, they discovered that the activation of the PDGF pathway plays a significant role in the pathogenesis of LMNA-DCM. They also proposed that PDGF receptor beta (PDGFRB) might represent a promising therapeutic target for this condition.326 Chen et al. identified the E2F/DNA damage response/TP53 axis as a mechanism underlying the development of DCM in laminopathies and suggested it as a potential target for interventions.327 Upregulated MAP kinase signaling pathway in the heart was detected in the lamin A/C gene mutated mouse. Loss of functional lamin protein leads to activation of the p38 MAPK pathway, secondary to cardiomyocyte apoptosis and interstitial fibrosis.328 Therefore, the MAP kinase signaling pathway may potentially serve as a therapeutic target for LMNA-related cardiomyopathy. Choi et al.329 found that in vivo administration of the rapamycin analog temsirolimus prevented the hyperactivation of the AKT–mTOR pathway in the hearts of mice with cardiomyopathy caused by the LMNA mutation and suppressed the deterioration of cardiac function. Chatzifrangkeskou et al.330 showed that TGF-β/Smad signaling participated in activated ERK1/2 signaling in LMNA cardiomyopathy, leading to altered activation of CTGF/CCN2 to mediate fibrosis and altered left cardiac function, which indicated a novel therapeutic target in the treatment of LMNA cardiomyopathy. Wu et al.331 also demonstrated that inhibitors of ERK and JNK signaling could potentially be used to treat humans with DCM caused by LMNA insufficiency. Tan et al.332 found that upregulation of Yy1 suppressed Lmna DCM and cardiac fibrosis by inducing Bmp7 expression and preventing upregulation of Ctgf, which offered novel therapeutic strategies for the treatment of LMNA-related DCM.
11.2 Brugada syndrome
11.2.1 Pathogenesis and disease-causing genes
Brugada syndrome (BrS) is an inherited disease associated with loss-of-function mutations in the cardiac sodium channel Nav1.5, which is encoded by the SCN5A gene.333 Genetic tests for diagnosis of BrS should include screening for the pathogenic variant in one of 23 genes: ABCC9, CACNA1C, CACNA2D1, CACNB2, FGF12, GPD1L, HCN4, KCND2, KCND3, KCNE5, KCNE3, KCNH2, KCNJ8, PKP2, RANGRF, SCN1B, SCN2B, SCN3B, SCN5A, SCN10A, SEMA3A, SLMAP, and TRPM4.334, 335 Currently, SCN5A and SCN10A have attracted the most attention as significant susceptibility genes for BrS.336, 337 Furthermore, the mutations in different genes are related to phenotype severity. Individuals with mutations in the SCN5A gene show heightened epicardial electrical abnormalities and a more severe clinical presentation.338
11.2.2 Potential therapeutic interventions
In recent years, ICDs have been proven effective for the treatment of BrS, while epicardial ablation of the right ventricular outflow tract has shown success in reducing the incidence of arrhythmias and normalizing the ECG patterns in BrS patients.339
Disease models and experimental therapeutic techniques have developed rapidly in recent years. Cellular reprogramming and gene editing through the technology of IPSCs has been described for different primary cardiomyopathies with cardiac conduction dysfunction.340 Liang et al.341 used iPSC-CMs to explore the pathomechanism of BrS. They then corrected the proposed causative SCN5A variant and rescued the phenotypes, including abnormal Ca2+ transients, via CRISPR/Cas9 genome editing.341 Teng et al.342 suppressed these SCN5A nonsense mutations by utilizing two readthrough-enhancing methods (either aminoglycosides or a siRNA-targeting eukaryotic release factor eRF3a (a GTPase that binds eRF1)), which suggested nonsense mutations in SCN5A could be suppressed. The expression of full-length channels could be restored effectively via readthrough-enhancing methods. Yu et al.343 employed AAV9 vector-based delivery of MOG1 to enhance MOG1 expression. This increase in MOG1 led to elevated cell surface expression of NaV1.5, resulting in augmented ventricular INa levels. As a result, they were able to successfully mitigate the symptoms of cardiac arrhythmias and contractile dysfunction in heterozygous humanized knock-in mice carrying the SCN5A mutation p.D1275N. Chakrabarti et al.344 discovered that MOG1 effectively restored diminished PM expression. Consequently, utilizing MOG1 to enhance Nav1.5 trafficking holds promise as a targeted therapeutic approach for certain patients with BrS in future applications.
11.3 Long QT syndrome
11.3.1 Pathogenesis and disease-causing genes
The most common forms of long QT syndrome (LQTS) mutations with LQTS are uncovered in KCNQ, KCNH2, and SCN5A, accounting for approximately 75% of genotype-positive LQTS cases.345 LQTS, BrS, and cardiomyopathy may all be caused by SCN5A mutations,336 encoding the alpha-subunit of the Nav1.5 ion channel protein responsible for the sodium inward current (INa).334 Investigating SCN5A variations in various SCN5A-related cardiac conditions and exploring newly developed therapeutic strategies could prove valuable in the clinical setting for the prevention and treatment of these disorders.346
11.3.2 Potential therapeutic interventions
Given that LQTS arises from a gain-of-function in the SCN5A channel, sodium channel blockers, SGLT2 inhibitor and SGK1 inhibitors are potential candidate drugs for its treatment.347 Recently, research has shown that SGK1 inhibitors can selectively diminish the late sodium current (INa-L) and abbreviate the action potential duration (APD) in various contexts. Their efficacy has been demonstrated in iPSC-derived cardiomyocytes (iPSC-CMs) harboring the SCN5A–N406K mutation, as well as in iPSC-CMs treated with dofetilide.348, 349 Furthermore, an SGLT2 inhibitor has been demonstrated to decrease the INa-L in cardiomyocytes from mice with heart failure, as well as in cells expressing the SCN5A–R1623Q or SCN5A–ΔKPQ mutations.350
Dotzler and colleagues developed a dual-component gene therapy called KCNQ1 Suppression-and-Replacement (SupRep) by introducing a KCNQ1 short hairpin RNA and a short hairpin RNA-resistant KCNQ1 cDNA into LQT1 iPSC-CMs. The KCNQ1 SupRep gene therapy effectively reduced APD, thereby eliminating the characteristic feature of LQT1, which is a significant step in the treatment of this condition.351 They provided a promising therapeutic approach for LQTS.
11.4 Short QT syndrome
11.4.1 Pathogenesis and disease-causing genes
Short QT syndrome (SQTS) is a rare, life-threatening, inherited heart disease presenting a family history of SCD in 15%. Therefore, genetic testing is important in the diagnosis of SQTS, but the causative mutation has been found in <25% of cases up to now.352 The mutations associated with SQTS usually happened in genes including KCNH2, KCNQ1, KCNJ1, CACNA1C, CACNB2, and CACNA2D1, responsible for SQT-1–8 subtypes, respectively.353 In children carrying a KCNH2–V141M mutation, a nonthreatening form of the disease has been noted. Additionally, recent studies suggest that SQTS may arise from a mutation in the cardiac Cl/HCO3 exchanger AE3.352
11.4.2 Potential therapeutic interventions
At present, the primary treatment approach for individuals with SQTS involves the use of an ICD, while quinidine remains the sole medication to have undergone clinical evaluation for this condition.353 Targeted gene therapy is not currently available. Some studies have found that KCNH2 mutation may be the potential target for therapy in SQTS patients.354 To uncover fresh potential targets, an exome or genome sequencing methodology is essential.355
11.5 Sudden unexpected nocturnal death syndrome
Sudden Unexpected Nocturnal Death Syndrome (SUNDS) is a phenomenon that predominantly affects young, seemingly healthy Southeast Asian individuals, with a higher prevalence among men. The exact pathophysiological mechanisms of SUNDS remain elusive; however, several factors and genetic predispositions have been proposed to contribute to its development.356 Roughly 50% of the ion channel anomalies linked to SUNDS are attributed to variations in the SCN5A gene or abnormalities in other constituents of the sodium channel macromolecular complex.357 Variants in this gene can lead to sodium channel dysfunction, potentially resulting in disturbed cardiac conduction and an increased risk for life-threatening arrhythmias, such as ventricular fibrillation, especially during sleep.
While the precise mechanisms of SUNDS are not fully understood, it is believed to be a multifactorial condition involving genetic predispositions, possibly interacting with environmental and lifestyle factors to increase the risk of SCD during sleep. Further research is needed to elucidate the pathophysiology of SUNDS and to develop effective preventive strategies.
12 CARDIAC AMYLOIDOSIS
Cardiac amyloidosis (CA) is a progressive cardiomyopathy caused by an accumulation of endogenous proteins that fold and degrade in the heart (mainly in the kidneys, liver, gastrointestinal tract, and soft tissues with amyloid fibrils). Clinically, systemic amyloidosis is divided into five types, primary amyloidosis (AL) (or light chain amyloidosis), secondary (AA) amyloidosis (or reactive amyloidosis), familial amyloidosis (ATTR or hereditary amyloidosis), dialysis-related amyloidosis and senile systemic amyloidosis (SSA). Of these, AL, ATTR, and SSA are commonly involved with the myocardium.358 ATTR has become the most frequent type of CA in clinical practice.359 However, the prognosis of amyloidosis with cardiac involvement is poor. The average survival time in AL-CA patients is 6 months, and in ATTR-CA patients is 26−43 months.360 Cardiac involvement in ATTR typically manifests in the sixth and seventh decades of life, presenting as heart failure with preserved ejection fraction (HFpEF), with “wild-type” or “senile systemic amyloidosis” being the predominant cause in the United States.361, 362
12.1 Primary amyloidosis
12.1.1 Pathogenesis
Plasma cells in the bone marrow (a type of B lymphocyte) abnormally proliferate to form clonal plasma cells. These abnormal plasma cells produce abnormal monoclonal immunoglobulin light chains, which under normal conditions should bind with other parts of the immunoglobulin, but may exist in a free form in pathological states. Due to their structural characteristics, these monoclonal light chains are prone to misfolding and form beta-sheet structures, which then spontaneously aggregate to form oligomers and fibrils. They further aggregate to form insoluble amyloid protein deposits, which accumulate in cardiac tissue, particularly in the subendocardial and myocardial interstitial regions. The deposition of amyloid proteins leads to an increase in the extracellular matrix of cardiomyocytes, affecting the normal contraction and relaxation functions of the heart, causing an increase in cardiac stiffness, and ultimately may progress to heart failure.363
12.1.2 Potential therapeutic interventions
Previous treatments for AL amyloidosis have often been based on bortezomib-based regimens for multiple myeloma, but there has still been a significant unmet clinical need, and researchers have never stopped exploring new protocols. In recent years, with the emergence of immunotherapies such as monoclonal antibodies, the survival rate of patients with AL amyloidosis has improved compared with the past. Dara (daratumumab) is a monoclonal antibody targeting the CD38 antigen on the surface of plasma cells and has shown good efficacy in the treatment of AL-CA, especially when used in combination with the CyBorD regimen.364 As research progresses, some new drugs, such as Belantamab mafodotin, are being evaluated in clinical trials for their efficacy and safety in patients with AL-CA.130-132
12.2 Familial amyloidosis
12.2.1 Pathogenesis and disease-causing genes
The pathogenesis of ATTR mainly involves the deposition of misfolded TTR in cardiac muscle tissue. Under normal conditions, TTR is a soluble tetramer responsible for transporting thyroxine and retinol, but when TTR dissociates into monomers and misfolds, it forms amyloid substances that deposit in the myocardial interstitium, ultimately leading to myocardial disease and progressing to progressive heart failure.
Researchers believe that approximately 10% of amyloidosis gene variants are present in suspected patients with systemic AL amyloidosis.365 The TTR gene, located on chromosome, 18 has more than 130 known pathogenic variants that result in different phenotypic manifestations. Genetic variation is largely inherited by autosomal dominant inheritance, and its penetrance often changes.
The Val30Met mutation is the most common in the TTR gene worldwide.366 This mutation showed a variety of phenotypic expressions, and almost all forms have neurological symptoms, among which lower extremity neuropathy (familial amyloid polyneuropathy) is more common than others. Usually, the disease has a bimodal manifestation, early-onset (30–40 years of life) rarely with cardiomyopathy, and late-onset (50–60 years of life) with heart involvement.367 The Val122Ile mutation is the most prevalent genetic variant in the United States, which mainly occurs in patients of African descendant and causes almost only heart disease.362 The Cardiovascular Health Study reported that after 65 years of age, Val122Ile patients had a higher incidence of heart failure symptoms (38 vs. 15%) and mortality (76 vs. 53%) compared with the general population. However, there was no difference between the two groups was found in patients <65 years of age.368 In Europe, Val122Ile is also considered to be a common cause of heart failure.369 The most common variant leading to cardiomyopathy in the UK is Thr60Ala.370 In the United States, this mutation is found in 20% of patients with THAOS.362 In an epidemiological analysis of British patients with the Thr60Ala mutation, cardiac involvement was found in 93% of patients by echocardiography. Most patients also show autonomic or peripheral neuropathy. In Japan, the first case of sporadic hereditary V122I ATTR amyloidosis was reported in male patients.371
Apart from these common mutations associated with ATTR amyloidosis, there are some rare mutations in the TTR gene have been found. Nakase et al.372 reported that a novel mutation (Y114S, p.Y134S) in the TTR gene was found in a 65-year-old Japanese man who suffered from hereditary amyloidosis, which was characterized by progressive cardiomyopathy with a poor vital prognosis. Bauer et al.373 found a predominantly cardiac phenotype with high penetrance and late onset of symptoms in ATTR amyloidosis caused by the TTR Val20Ile mutation and progressed to end-stage heart failure within a few years. Moreover, p.Ser43Asn is a sporadic TTR mutation leading to familial ATTR amyloidosis, associated almost invariably with an isolated cardiac phenotype.374
12.2.2 Potential therapeutic interventions
Genetic variation in amyloid heart disease provides an opportunity to explore potential treatments for the disease, especially for ATTR. Based on the elucidation of the mechanisms of amyloid formation, targeted gene therapies are now approved for treating ATTR-CA.375 Genetic treatment for ATTR involves inhibiting the production of abnormal TTR proteins by the liver, stabilizing the TTR tetramer, and breaking down amyloid fibrils by interfering with the protein. Gene-based therapies work by preventing the liver from producing TTR. These newly developed therapies can be achieved by using small interfering ribonucleic acid (siRNA), ASOs, or CRISPR–Cas9 system.376
RNA interference
The purpose of siRNA is to knock down the production of hepatic mutants and wild-type ATTR, thereby reducing unstable cyclic TTR tetramers and preventing organ deposition of TTR monomers and amyloid fibrils, eventually resolving the disease. The feasibility of this method was confirmed using siRNA in a mouse model of hereditary ATTR. Moreover, the extent of TTR tissue sediment degradation appears to be linearly related to RNAi-mediated knockdown and serum TTR protein exposure.377
Antisense oligonucleotides
ASO can hybridize with native mRNA to form double-stranded RNA, which is recognized and cleaved by RNase. Inotersen is a 2′-O-methoxyethyl-modified ASO that selectively binds to mRNA encoding TTR and causes mutant and wild-type TTR mRNA degradation, preventing synthesis of TTR proteins (mainly in the liver). TTR ASOs suppressed hepatic TTR mRNA levels and serum TTR levels by as much as 80%, leading to a significant decrease in circulating and wild-type TTR protein levels, thereby reducing amyloid deposition.378, 379 The Phase III NEURO-TTR trial showed that inotersen improved neurological disease and quality of life in patients with ATTR.137 Recent studies have shown that ASO treatment of patients with moderate to advanced ATTR cardiomyopathy could prevent the disease progression, and therefore the life expectancy may be enhanced.380 Currently, regulatory reviews of inotersen are conducted in the US and Canada.
CRISPR–Cas9 system
Gillmore and colleagues proposed NTLA-2001 as an in vivo gene-editing therapeutic utilizing the CRISPR–Cas9 system. This agent comprises a lipid nanoparticle encapsulating messenger RNA for the Cas9 protein and a single guide RNA targeting TTR. The administration of NTLA-2001 resulted in a reduction of serum TTR protein concentrations achieved by specifically knocking out the TTR gene.381
13 MUSCULAR DYSTROPHIC
13.1 Pathogenesis and disease-causing genes
Muscular dystrophies encompass a range of genetic conditions characterized by gradual muscle weakening and wasting. Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD) are closely associated disorders primarily impacting skeletal and cardiac muscles, leading to progressive muscle weakness, cardiomyopathy, and a reduced lifespan.
In DMD, the absence or dysfunction of dystrophin leads to muscle fiber damage, necrosis, and a reduced capacity for regeneration, which manifests clinically as progressive muscle weakness and eventual replacement of muscle tissue with connective and fatty tissue, leading to pseudohypertrophy, particularly in the calves. The disease typically presents in early childhood, with symptoms such as difficulty in running, jumping, climbing stairs, and rising from the floor, as well as the development of a characteristic waddling gait and Gower's sign. BMD is a milder form of the disease, also caused by mutations in the dystrophin gene, but with later onset and slower progression.382
Cardiomyopathy, which emerges in adulthood, affects nearly all patients with DMD and stands as the primary cause of death in this condition. Both DMD and BMD are caused by mutations in the X-linked dystrophin (DMD) gene. Wong et al.383 showed that deletion mutation of exons 52−54 led to the absence of dystrophin, presenting with early-onset HCM in mice models. In recent years, there have been significant advances in the understanding of the pathogenesis of DMD, leading to the development of targeted therapies such as exon-skipping drugs, gene replacement using AAVs, and CRISPR gene editing technologies, which are currently at the forefront of research and emerging as promising treatment options for DMD.
13.2 Potential therapeutic interventions
13.2.1 Duchenne muscular dystrophy
AAV-mediated micro-dystrophin gene therapy has significantly prevented the progress of the disease in rodent models associated with DMD. Validation of these encouraging results in large animal models will outline the path forward to human trials.384, 385 Mice are engineered to replicate a frequently observed set of mutations found in patients with DMD, and this dystrophin-deficient mouse DMD model is named mdx mice. Recent studies have shown that intravascular delivery of AAV micro-dystrophin could significantly ameliorate muscle pathology, attenuate dystrophic cardiomyopathy, and normalize the heart rate, PR interval, and QT interval in animals.386, 387 These discoveries hold significant implications for utilizing AAV gene therapy in managing DCM and heart failure.388 Moreover, AAV-mediated cardiac transduction with other proteins also could attenuate dystrophic cardiomyopathy. Bauer et al.389 indicated that AAV9–βARKct—cDNA with a cardiac-specific promoter injection into mdx mice over a long time could obviously improve LV systolic function and ameliorate myocardial hypertrophy. Xu and colleagues discovered that administering rAAVrh74.MCK.GALGT2 to mdx hearts prevented initial LV remodeling and the expression of fibrotic gene markers.390 Several clinical trials are now underway to advance therapy to DMD patients.133-135
CRISPR-mediated genome editing has proven effective in enhancing dystrophin expression and improving cardiac function in mdx mouse and deltaE50-MD dog models following a sole systemic administration of recombinant AAVs.391, 392 Xu et al.393 suggested that in vivo CRISPR genome editing could be developed as a safe treatment for DMD and did not lead to other deleterious defects, and dystrophin restoration could be increased. Fibrosis could be reduced via systemic AAV CRISPR therapy with an increased dose of the guide RNA vector in all striated muscles at 18 months in a mouse mdx model of DMD.394 In mdx mice, recent studies found that a premature termination codon located in exon 23 of the DMD gene has been pinpointed. Three research teams employed AAV vectors to administer guided RNAs (gRNAs) and CRISPR/Cas9 into mdx muscles, targeting the mutated exon 23 of the DMD gene. This approach effectively removed the premature termination codon, restored the expression of truncated yet partially functional dystrophin through exon skipping, and notably extended the survival of mdx mice.395-398 Immunofluorescence data suggested that the expression of dystrophin protein was restored to a level approaching 40% via CRISPR/Cas9 in dystrophic cardiac muscles.391 Zhang et al.399 deployed a unique class 2 CRISPR effector Cpf1, the DMD mutations could be successfully corrected through Cpf1, and pathophysiological hallmarks of muscular dystrophy in patient-derived iPSCs and mdx mice also could be improved.
Goyenvalle et al.400 presented a new class of AONs consisting of tricyclo-DNA (tcDNA) that could be fully absorbed by many tissues after systemic administration in mice characterized by DMD. The rescue of dystrophin expression could be promoted by systemic delivery of tcDNA-AONs in skeletal muscles, heart, and brain, thereby improving physiological cardio-respiratory functions and correcting behavioral features.
13.2.2 MicroRNA
Previous studies have shown that miRNAs have been implicated as fine regulators in the progression of cardiomyopathy, so miRNAs have become genetic targets for therapy.401 Quattrocelli et al.261 demonstrated that miR-669a downregulation linked to the severe DCM progression in Sgcb-null dystrophic mice, the intraventricular delivery of AAV vectors induced the overexpression of miR-669a and reduced the mortality of Sgcb-null mice. miR-669a treatment could reduce adverse remodeling, enhance systolic fractional shortening of the LV, and ameliorate the gene/miRNA profile of DCM markers in treated dystrophic mice. Apart from the therapeutic target, miRNAs also have the potential to play a role as a biomarker in early disease detection.
13.2.3 Transient receptor potential vaniloid 2
Transient receptor potential vaniloid 2 (TRPV2), a stretch-sensitive Ca2+-permeable channel, can accumulate and become activated in the sarcolemma of cardiomyocytes/myocytes in cardiomyopathy and MD. The mitigation of muscle dysgenesis may be achieved through TRPV2 inactivation, leading to enhanced cardiac function and improved survival prognosis. While TRPV2 presents as a promising therapeutic target for cardiomyopathy and MD, research on specific inhibitors is currently underway.402
14 ISCHEMIC CARDIOMYOPATHY
In developed countries, coronary heart disease continues to be a major contributor to illness and death. Although revascularization strategies, such as coronary artery bypass grafting (CABG), percutaneous coronary intervention, and enhanced medication significantly improve outcomes, approximately 30% of patients still develop chronic heart failure.403, 404 Ischemic heart disease is characterized by shoddy cardiac remodeling, including cardiac hypertrophy, increased fibrosis, and sparse capillaries. Therefore, gene therapy for ischemic heart disease, such as improving systolic function and promoting new blood vessel formation, appears promising.405
14.1 Pathogenesis and disease-causing genes
Ischemic cardiomyopathy (ICM) is primarily characterized by the pathogenesis involving long-term cardiac ischemia that leads to localized or diffuse myocardial fibrosis, which impairs the heart's contractile and/or diastolic functions, resulting in clinical manifestations such as cardiac dilation or stiffness, congestive heart failure, and arrhythmias. The etiology of ICM is typically associated with atherosclerotic narrowing or occlusion of the coronary arteries; these pathological changes cause an imbalance between the oxygen supply and demand of the myocardium, leading to degeneration, necrosis, myocardial fibrosis, and scar formation of the cardiac muscle cells, and ultimately may lead to heart failure, arrhythmias, and cardiac chamber enlargement.406
14.2 Potential therapeutic interventions
In acute MI, a single gene therapy application provides continuous therapeutic protein at the site of infarction and may lead to a pathophysiological reversal. Utilizing innovative gene constructs through genetic modification enables the regulation of gene expression based on the intracellular milieu, thereby reducing unrestricted protein synthesis. Stem cell therapy, combined with gene therapy, can promote cardiac regeneration with a high success rate.407-409 Therefore, gene therapy can improve heart function and relieve symptoms, potentially delaying or reducing the need for heart transplantation.
14.2.1 Vascular endothelial growth factor
VEGF binds to distinct receptors on endothelial cells, playing a vital role in the process of angiogenesis.410 The mammalian genome contains five isoforms within the VEGF family: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and the placental growth factor. Among these, VEGF-A and VEGF-B activate VEGF receptor one and VEGF receptor two, thereby governing vascular physiology.254, 411, 412
Transcripts for VEGF-121 and VEGF-165 isoforms were found in the majority of cells and tissues expressing the VEGF gene. VEGF-165 gene therapy has been found to be very effective in promoting angiogenesis.413 Gene therapy with VEGF-165 is successful in the Kuopio Angiogenesis Assay (KAT trial). In the KAT trial, adenovirus-mediated VEGF-A165 gene therapy showed an improvement in coronary perfusion when IC injections in percutaneous transluminal coronary angioplasty (PTCA) patients with grade 2−3 angina were performed.414 The research monitored patients over an 8-year period, showcasing both safety and effectiveness in individuals with coronary heart disease.415 Giusti et al.138 performed a Phase I/II prospective and time-controlled series of clinical trials. Thirteen patients were maintained under optimized clinical management for at least 6 months and then received an IM injection of 2000 µg of plasmid VEGF-165. After treatment, the third month of single-photon emission computed tomography (SPECT) under stress and the sixth month under rest had a transient increase in myocardial perfusion. One year later, the treadmill test and oxygen consumption of the experimental group improved. For patients with advanced ICM, high-dose gene therapy has also proven feasible and safe.
In the REVASC trial, patients with severe refractory angina who were not eligible for standard drug therapy were treated with adenovirus for VEGF-121 gene therapy by IM injection after mini-thoracic incision. At 26 weeks of follow-up, the exercise time before the ischemic change of ECG was prolonged, the total exercise time was increased, and the symptoms of angina were improved.416 A clinical study of 31 patients with advanced diffuse coronary artery disease who underwent direct IM injection of AdVEGF121 showed that patients with gene therapy had a low incidence of malignant diseases and retinopathy at a mean follow-up of 11.8 years. Given the expected prevalence of the age-matched general population, it was concluded that adenovirus-mediated VEGF could be safely used in the myocardium of patients with severe coronary heart disease. However, conducting trials in a larger population is necessary to assess efficacy.139
14.2.2 Granulocyte-macrophage colony-stimulating factor
The versatile granulocyte-macrophage colony-stimulating factor (GM-CSF) oversees the production, differentiation, proliferation, and survival of leukocytes.417 Although GM-CSF is not required for hematopoietic function under normal physiological conditions, its yield is significant after myocardial injury. GM-CSF is the primary coordinator of the white blood cell supply chain during inflammation. It plays a pivotal role in the development of MI and represents a promising therapeutic target.418
Seiler et al.419 randomized 21 patients who did not meet the CABG criteria but had coronary heart disease who were treated with an IC injection of 40 mg GM-CSF or placebo and subcutaneous GM-CSF (10 mg/kg) or placebo respectively for 2 weeks. The invasive collateral flow index of the GM-CSF group was significantly increased. However, there are still a few contradictory findings. For instance, increased serum levels of GM-CSF in MI patients correlate with acute decompensated heart failure and subsequent extensive cardiac remodeling,420 and administration with exogenous GM-CSF aggravates heart failure.421 These findings suggest that the role of GM-CSF in MI needs further investigation, and that GM-CSF may become a potential therapeutic target for ICM.
14.2.3 Synthetic-modified mRNA
Synthetic-modified mRNA (modRNA) represents a novel gene delivery vector known for its stability, low immunogenicity, and high expression levels, rendering it a promising candidate for treating ICM, particularly post-MI. After luciferase modRNA was injected IM into the LV, high luciferase activity could be identified within 3 h after injection. The activity reached a peak at 18 h after injection and gradually declined for 6 days. modRNA has been identified as an attractive vector to quickly and efficiently express gene or gene combinations due to its expression kinetics, which could lead to minimizing heart injury and induce regeneration.422
15 ACQUIRED CARDIOMYOPATH
15.1 Tako-tsubo cardiomyopathy
15.1.1 Pathogenesis
Tako-tsubo cardiomyopathy (TTC), also known as stress-induced cardiomyopathy, has a pathogenesis that is not yet fully understood, but it is widely believed to be related to intense emotional or physical stress, involving the massive release of catecholamines.423 This release may lead to direct toxic effects on the myocardium, microcirculatory disturbances, and multivessel coronary artery spasm, thereby causing temporary changes in cardiac structure and function. Specifically, catecholamine toxicity is an important factor in the pathogenesis of TTC; elevated levels of catecholamines in the blood of patients may cause microcirculatory disturbances and myocardial stunning.424 In addition, metabolic abnormalities in the myocardium and multivessel coronary spasm may also be one of the mechanisms of the disease.
15.1.2 Therapeutic interventions
Because TTC is a self-limiting condition, treatment mainly targets symptoms, such as using antianxiety medications to alleviate emotional stress and using diuretics, ACE inhibitors, and so on, to treat heart failure.
15.2 Peripartum cardiomyopathy
15.2.1 Pathogenesis
Peripartum cardiomyopathy (PPCM) is a form of myocardial disease that emerges during the final month of pregnancy or within the first 5 months postpartum, hallmarked by the onset of LV dysfunction that can range from acute to gradually progressive. While the exact etiology of PPCM remains elusive, it is thought to result from a confluence of contributing factors. The influence of genetic factors in the development of PPCM is underscored by numerous studies, revealing a genetic etiology in as many as 20% of the patients examined.425 Truncating mutations in the TTN, DSP, FLNC, and BAG3 genes have been identified in women with PPCM, with relative prevalence rates (TTN: 10%; DSP and FLNC: 1%; BAG3: 0.2%) that are nearly identical to the rates found in the DCM cohort, further supporting a high degree of genetic similarity between PPCM and DCM.426 However, it is still unclear how these genetic mutations predispose individuals to PPCM.
15.2.2 Potential therapeutic interventions
Treatment for PPCM typically focuses on symptom management and may include diuretics, angiotensin-converting enzyme inhibitors, ARBs, beta-blockers, and anticoagulant drugs. As a dopamine D2 receptor agonist, bromocriptine, which blocks the production of prolactin, has emerged as a potential disease-specific treatment for PPCM. The 2018 ESC guidelines recommend considering the use of bromocriptine in women newly diagnosed with PPCM.427 A recent multicenter randomized study evaluated the effects of two distinct bromocriptine dosing regimens-2.5 mg daily for 1 week versus 5 mg daily for 2 weeks, followed by a continuation of 2.5 mg daily for 6 weeks—on patients with severe PPCM. The results demonstrated a high rate of LV recovery after 6 months, with zero mortality, no need for assistive devices or heart transplantation. These findings suggest a significant positive correlation between bromocriptine treatment in the acute phase of PPCM and improved clinical outcomes.136
15.3 Tachycardia-induced cardiomyopathy
15.3.1 Pathogenesis
Prolonged episodes of tachycardia are a recognized cause of LV systolic dysfunction. Tachycardia-induced cardiomyopathy is characterized as a heart failure syndrome resulting from the sustained elevation of atrial or ventricular heart rates.428 The exact mechanisms of how tachycardia leads to cardiomyopathy are not completely understood.
15.3.2 Potential therapeutic interventions
Treatment for TIC often involves controlling the heart rate and rhythm to prevent further damage and may include medications, catheter ablation, or other procedures to manage the underlying cause of the tachycardia. If the tachycardia is effectively treated, the heart may recover its normal function over time, although this is not guaranteed and depends on the extent of the damage and the individual's response to treatment.
15.4 Myocarditis
15.4.1 Pathogenesis
Myocarditis is a prevalent precursor to DCM and SCD, often arising from cardiotropic viral infections that lead to subsequent active inflammatory myocardial damage.429 In addition, some noninfectious factors (including toxins, immunological syndromes, and hypersensitivity) may also lead to the occurrence of myocarditis.
15.4.2 Potential therapeutic interventions
IV immunoglobulin is a highly effective immunomodulatory treatment, commonly utilized for patients afflicted with systemic autoimmune diseases characterized by antibody-mediated pathogenesis, including the particularly aggressive giant-cell myocarditis.430 At present, there are no specific pathogen-targeted or antiviral treatments approved for patients with viral myocarditis. While the use of aciclovir, ganciclovir, or valaciclovir for herpesvirus infections could be contemplated, their effectiveness has not been explicitly assessed in myocarditis patients.431 A pioneering in vitro model replicating human viral myocarditis has been established by exposing hiPSC-CMs to coxsackievirus. These infected cells exhibit detrimental alterations in cardiomyocyte structure and function, and they demonstrate varied responses to an array of antiviral agents. This hiPSC-CM model represents a promising tool for investigating the underlying disease mechanisms and serves as a platform for high-throughput screening of potential new therapies.432
16 CONCLUSION AND PROSPECTIVE
Cardiomyopathies are a group of heterogeneous diseases characterized by structural and functional impairment of the heart. The pathogenesis of cardiomyopathy is complex and diverse, involving genetic mutations, immune responses, apoptosis, and energy metabolism imbalances, among other aspects. We emphasize the importance of a deep understanding of these mechanisms, which not only helps to identify new therapeutic targets but is also crucial for the development of personalized medical strategies. This review has summarized the pathogenesis of cardiomyopathies, current treatment strategies, and emerging gene and cell therapies. We have gained insights into the molecular mechanisms of cardiomyopathies, including genetic mutations, cell death, fibrosis, and immune responses, providing new perspectives for early diagnosis and treatment. Recent preclinical animal experiments and clinical trials have demonstrated that gene therapy and cell therapy have shown great potential in the treatment of cardiomyopathies, especially for hereditary forms, by targeting pathogenic genes and offering more precise therapeutic directions (Tables 2 and 3).
| Cardiomyopathy | Targeted genes and molecules | Therapeutic intervention | Mechanism of action | References |
|---|---|---|---|---|
| Hypertrophic cardiomyopathy | MYBPC3 | Rapamycin | Enhances Akt–mTORC1 signaling | 150 |
| AAV-U7–AON-5+6 | Increases Var-4 mRNA/protein levels and reduces aberrant mRNAs | 66 | ||
| AAV9–Mybpc3 | Increasing Mybpc3 mRNA and cMyBP-C protein levels | 159 | ||
| CRISPR/Cas9 | Corrects the variant using HDR | 71 | ||
| cMyBPC | AAV9–C0C2 | cMyBPC gene transfer | 160 | |
| Lentiviral vector | cMyBPC gene transfer | 153 | ||
| MYH7 | CASAAV | Silences Myh6 and Myh7 and early depletion of Myh7 | 162 | |
| CRISPR/Cas9 | Inhibits mTOR or MAPK | 163 | ||
| MYH6 | RNAi cassette | Silences Myh6 R403Q mutation | 64 | |
| PRKAG2 | CRISPR/Cas9 | Rectifies heterozygous missense mutation (c.905G>A, R302Q) in the PRKAG2 gene | 177 | |
| Arrhythmogenic right ventricular cardiomyopathy | PKP2 | AAVrh.74–PKP2a | Recovers the expression of PKP2a | 193 |
| PLN | TALEN | Corrects PLN R14del mutation | 195 | |
| AAV9–CRISPR/Cas9 | Disruption of hPLN-R14del allele | 196 | ||
| DSG2 | HDR-iPSC | Heterozygously corrects mutated DSG2 gene locus to a normal allele | 199 | |
| Dilated cardiomyopathy | TNNT2 | AAV9 | Heart-specific delivery overexpression of XINB | 233 |
| ASP-RNAi | Specifically knocks down mutant alleles coding for R92Q and R173W mutant | 234 | ||
| siRNAs | Rescues LMNA-relate progeria and reduces prelamin A/progerin in favor of the alternative splicing of lamin C. | 235 | ||
| PTC124 | Full-length LMNA proteins were increased by PTC124 treatment and improves the excitation–contraction coupling of the affected cardiomyocytes in the R225X mutant | 238 | ||
| TTN | AON | Reframing titin transcripts by AON-mediated exon skipping | 68 | |
| CRISPR/Cas9 | Ablates A-band variant-specific truncation peptide | 244 | ||
| ASK1 | rAAV | Inhibits ASK1 protein activation | 251 | |
| Restrictive cardiomyopathy | MYL2 | AAV9–M7.8L R | Silences the mutated allele (RLC-47K) | 285 |
| PRKAG2 cardiomyopathy | PRKAG2 | AAV9–Cas9/sgRNA | Disrupt the mutant PRKAG2 allele encoding H530R while leaving the wild-type allele intact | 306 |
| Pompe disease | GAA | AAVB1 and AAV9 | Combines IGF2 with a codon-optimized variant of GAA (LV-IGF2.GAAco) to enhance cellular uptake | 317 |
| Lamin A/C gene (LMNA) cardiomyopathy | LMNA | rAAVs | Whole-body supplementation with LMNA using rAAVs | 325 |
| Temsirolimus | Prevents the hyperactivation of the AKT–mTOR | 329 | ||
| Brugada syndrome | SCN5A | CRISPR/Cas9 | Utilizes two readthrough-enhancing methods (either aminoglycosides or a siRNA-targeting eukaryotic release factor eRF3) | 342 |
| MOG1 | AAV9 | Enhances MOG1 expression, resulting in augmented ventricular INa levels | 343 | |
| Long QT syndrome | KCNQ | SupRep | Introduces a KCNQ1 short hairpin RNA and a short hairpin RNA-resistant KCNQ1 cDNA into LQT1 iPSC-CMs. | 351 |
| Cardiac amyloidosis | TTR | RNAi | Reduces unstable cyclic TTR tetramers and Prevents organ deposition of TTR monomers and amyloid fibrils | 377 |
| CRISPR/Cas9 | specifically knocks out the TTR gene | 381 | ||
| Muscular dystrophies | DMD | AAV9–βARKct–cDNA | Increases cardiac GRK2 activity with βARKct expression | 389 |
| CRISPR/Cas9 | Removes the premature termination codon and restores the expression of truncated dystrophin through exon skipping | 395-398 | ||
| Systemic AAV CRISPR therapy | Increases dose of the guide RNA vector in all striated muscles | 394 | ||
| tcDNA-AONs | Improves physiological cardio-respiratory functions | 400 |
- Abbreviations: cMyBPC, cardiac myosin binding protein C; HDR, homology-directed repair; CASAAV, CRISPR/Cas9-AAV9-based somatic mutagenesis; MAPK, mitogen-activated protein kinase; RNAi, RNA interference; AAVrh.74, adeno-associated virus vector of serotype rh.74; PKP2a, PKP2 variant A; ATTR-CA, hereditary amyloidosis; TTR, transthyretin; GRK2, G-protein-coupled-receptor-kinase-2; tcDNA, tricyclo-DNA; ASP-RNAi, allele-specific silencing by RNA interference; AON, antisense oligonucleotide; IGF2, insulin-like growth factor 2; SupRep, suppression-and-replacement.
With an enhanced understanding of the molecular mechanisms of cardiomyopathies, future treatments will become more personalized, tailored to the patient's genetic background and disease characteristics. Gene editing technologies such as CRISPR/Cas9, TALENs, and applications of cell therapy like iPSCs offer new strategies for the treatment of cardiomyopathies. Although gene therapies open a new era for genetic diseases, the results have various limitations. For example, most studies have shown that gene therapy can inhibit or delay the progression of cardiomyopathy only for animals born 1 day, and some of the inhibitory effects will gradually disappear over time and cannot reverse the already thickened myocardium.433 The challenges in the future would be how to strictly control the effect of the target gene with gene transfer techniques and reduce side effects or excessive expression of exogenous proteins; how to avoid potential immunity to the transgene and viral capsid; how to find a more specific cardiac-targeted virus, and reduce the uptake of “off-target” viral vectors by other tissues because incorrect intake may impair the function of different tissues and the carrier of viral vectors. Besides, most animal experiments have been performed in mice or dogs, so the dose of virus injected, the dose-effect relationship, along with the injection interval in higher mammals need to be further explored.
Cardiac gene therapy is evolving into a replacement for traditional treatments, significant advances have been reported in preclinical models of cardiomyopathy, but technical challenges remain. Therefore, the development of new, more efficient, and smaller gene vectors is particularly important, such as micro circles and mini-intronic plasmids that exhibit superior transfection efficiency and greater biological effects.434 At the same time, exosomes are a new delivery platform for gene therapy. Besides, cell-containing RNA-containing exosomes can serve as functional genetic biomarkers for disease.435-437 While animal models often replicate the clinical features of cardiomyopathy, its crucial to acknowledge the physiological and physical distinctions between these models and humans. Recently, a large number of reports on stem cell therapy have provided us with a better choice for assessing cardiomyopathy in a real clinical setting.438-440 Stem cell therapy has been successfully used in small studies of ischemic, dilated, and RCM. In the near future, stem-cell-based gene therapy and cell therapy might be an effective way for cardiomyopathy treatment.
In summary, the research and treatment of cardiomyopathy are in a rapidly advancing phase. Through interdisciplinary collaboration and the application of innovative technologies, we have reason to believe that in the future, we will be able to more effectively prevent, diagnose, and treat cardiomyopathy, thereby improving the quality of life for patients.
AUTHOR CONTRIBUTIONS
Abdelouahab Bellou, Jian Zhuang, and Liming Lei conceived the manuscript. Shitong Huang, Qiuying Li, and Jiaxin Li wrote the initial draft of the manuscript. Shitong Huang drew figures. Xianwu Zhou, Xuanhui Chen, and Jimei Chen participated in the revision of the manuscript. All authors have read and agreed on the submission a publication of this manuscript.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Funds of China (NO. 82270308), Science and Technology Planning Project of Guangdong Province (NO. 2020B1111170011), and Science and Technology Program of Guangzhou, China (NO. 202102080379 and No. 202206010049).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This systematic review adhered to the ethical guidelines for research and publication as outlined by the Guangdong Provincial People's Hospital. Since this review did not involve the collection or analysis of primary data, no formal ethics approval was required. However, the authors ensured that all data sources used in this review were obtained and analyzed in accordance with the principles of ethical research.
Open Research
DATA AVAILABILITY STATEMENTS
Data availability is not applicable to this article as no new data were created or analyzed in this study.




