Head and neck cancer: pathogenesis and targeted therapy
Abstract
Head and neck cancer (HNC) is a highly aggressive type of tumor characterized by delayed diagnosis, recurrence, metastasis, relapse, and drug resistance. The occurrence of HNC were associated with smoking, alcohol abuse (or both), human papillomavirus infection, and complex genetic and epigenetic predisposition. Currently, surgery and radiotherapy are the standard treatments for most patients with early-stage HNC. For recurrent or metastatic (R/M) HNC, the first-line treatment is platinum-based chemotherapy combined with the antiepidermal growth factor receptor drug cetuximab, when resurgery and radiation therapy are not an option. However, curing HNC remains challenging, especially in cases with metastasis. In this review, we summarize the pathogenesis of HNC, including genetic and epigenetic changes, abnormal signaling pathways, and immune regulation mechanisms, along with all potential therapeutic strategies such as molecular targeted therapy, immunotherapy, gene therapy, epigenetic modifications, and combination therapies. Recent preclinical and clinical studies that may offer therapeutic strategies for future research on HNC are also discussed. Additionally, new targets and treatment methods, including antibody–drug conjugates, photodynamic therapy, radionuclide therapy, and mRNA vaccines, have shown promising results in clinical trials, offering new prospects for the treatment of HNC.
1 INTRODUCTION
Head and neck cancer (HNC) is a frequently diagnosed malignancy with 946,456 new cases and 482,001 deaths reported in 2022 according to GLOBOCAN.1 About 90% of these cases are head and neck squamous cell carcinoma (HNSCC).2, 3 The incidence of HNSCC is projected to increase by 40% by 2040 reaching nearly 600,000 new cases annually.4 Diagnoses are occurring at younger ages, largely due to the rising infection rates of human papillomavirus (HPV), especially HPV-16 and HPV-18. Besides HPV infection, studies have shown that tobacco use, chronic heavy alcohol consumption, oral sex, mechanical irritation, radiation exposure, and various occupational exposures can also contribute to the increased incidence of HNC.5, 6 Additionally, Epstein–Barr virus (EBV) and hepatitis B virus are other pathogenic factors for HNC and are considered biomarkers for nasopharyngeal carcinoma.7, 8 However, with the implementation of HPV vaccination, decreased smoking rates, enhanced promotion of safe sex practices, and early precision diagnosis, it is anticipated that the incidence of HNC will decline by 2060.3, 6, 9
Although the incidence of HNC might decrease in the future, the 5-year survival rates of HNC remain unsatisfactory. In the United States from 2012 to 2018, the 5-year survival rates of tumors in oral cavity and pharynx were 68 and 61% for tumors in the larynx.10 The prognosis of hypopharyngeal tumor is even poorer, with a 5-year survival rate of only 25%.11, 12 The poor outcomes of HNC are often due to late diagnosis, recurrence, metastasis, and drug resistance.13, 14 Generally, 60−65% HNC patients can be cured by surgery and/or radiotherapy (RT) if detected early. However, patients with locoregional recurrence, metastatic diseases, or second primaries may require chemoradiation.15 Patients undergoing chemoradiotherapy may experience significant side effects and a reduced quality of life.16 Targeted and immune-based therapies, either alone or in combination with conventional treatments, offer promising new strategies for HNC. For example, combining afatinib with cisplatin enhances anticancer activity in HNSCC, inhibiting tumor growth and spread.17 The combination of pembrolizumab with chemotherapeutics is considered a first-line therapy for R/M HNSCC.18
Despite significant improvements in the combined therapeutic efficacy of HNC over the past few decades, further research is required to identify the most appropriate therapeutic strategies, given the genetic heterogeneity among HNC patients and the complexity of the disease. This paper reviews recent advances in understanding the molecular pathogenesis of HNC, including changes in signal pathways, immune microenvironment suppression, and the impact of genetic and epigenetic alterations on disease progression. It also discusses various treatment methods such as targeted therapy, immunotherapy, gene therapy, or combination therapy, and summarizes the drugs currently being researched or available on the market. The goal is to identify the most effective treatment plans from existing therapeutic strategies and provide a theoretical foundation for the clinical management of HNC.
2 PATHOGENESIS OF HNC
As a tumor with high heterogeneity, late diagnosis and high recurrence rate, the pathogenesis of HNC is extremely complex, involving many aspects, including signaling pathway abnormalities, tumor microenvironment (TME) inhibition, genomic changes, epigenetic changes,19 and so on. Additionally, risk factors like HPV infection, smoking, alcohol abuse and poor oral health play significant roles in the onset and progression of HNC.20 We will discuss the factors related to the occurrence and progression of HNC, providing a foundation for its clinical treatment.
2.1 HPV infection in HNC
HPV infection is a major factor contributing to the rising incidence of HNC patients.21 Among all the chronic HPV infections in HNC patients, nearly 85% are caused by HPV16 or HPV18. HPV can cause changes in oncogenes, including amplification, rearrangement, deletion, and translocation, and it induces the expansion and expression of E5/E6/E7 proteins,22-24 leading to the initiation and progression of HNC.25
E6 and E7 proteins are particularly crucial in HPV-positive HNC.25 E6 interacts with the retinoblastoma protein (RB), reducing inhibition of E2F transcription factors, and degrades the tumor suppressor protein p53 through a ubiquitin-dependent pathway. The disrupts normal cell cycle regulation and promotes HNC proliferation and growth.26 Additionally, E6 upregulates proapoptotic proteins (BAK and BAX) by suppressing P300 and cyclic adenosine monophosphate response element binding protein, or by disrupting apoptotic signaling pathways, thus affecting apoptosis.26 E6 also enhances tumor immune evasion by inhibiting interferon regulatory factor 3, Toll-like receptors 9 (TLR9)/CD289, and by activating cyclin-dependent kinase 2.27, 28 E7 also binds to RB, promoting its proteasome degradation, preventing apoptosis, senescence, and cell cycle arrest in host cells, and thereby encouraging viral production and tumor development.29, 30 Expression of p16 inhibits pRb phosphorylation, which is correlated with HPV infection status and survival outcomes in HNSCC.31 In addition, E6 participate in cell division and immortalization by upregulating TERT.32 E5 proteins also play significant role in the development of HNSCC through activation of the epidermal growth factor receptor (EGFR) signaling pathway.33
Beyond pathogenesis, HPV infection status can influence the therapeutic efficacy for HNC. The genetic, epigenetic, and TME differences between HPV-positive and HPV-negative HNC, as well as treatment variations, will be discussed in detail in the following sections.34
HPV infection alone is not enough for tumor development, other risk factors like EBV infection and smoking and alcohol consumption are necessary.21, 35 EBV is another significant factor in the occurrence and development of HNSCC.36, 37 It promotes HNSCC by manipulating various cell signaling pathways to protect infected cells from the immune system.38 Rahman et al.39 found that almost 12% of patients with HNSCC have coinfection with HPV and EBV, and Deng et al.40 has revealed that these two viruses synergistically promote tumor occurrence and development. HPV can facilitate EBV entry into epithelial cells by upregulating integrin and CD21, help establish EBV's incubation period and the activation of its cleavage cycle, and promote local immune escape, leading to secondary EBV infection. Together, HPV and EBV promote the development of HNSCC.41
HPV vaccines targeting E6/E7 have showed the ability to generate virus-specific cytotoxic T lymphocytes (CTLs), but these vaccines have not demonstrated significant antitumor effects.42 Interestingly, recent RNA-Seq and whole genome sequencing (WGS) studies on HPV-positive HNC revealed that most of these cancers express E2 instead of E6. This could explain the limited efficacy of current therapeutic vaccines for HPV-positive cancers and indicate E1/E2 may be better targets for future therapies.43
2.2 The pathogenesis signaling pathways in HNC
HNC is characterized by a complex signaling network involving numerous genes and proteins. In HNC, there is an overexpression of four key cell surface receptors: EGFR, VEGFR, HER2, and MET. These receptors drive downstream pathways that are crucial for cell proliferation, apoptosis, immune escape and metastasis (Figure 1). Here, we will focus on the PI3K/AKT/mTOR, JAK/STAT, NF-κB, HGF/MET, and TP53/RB pathways.
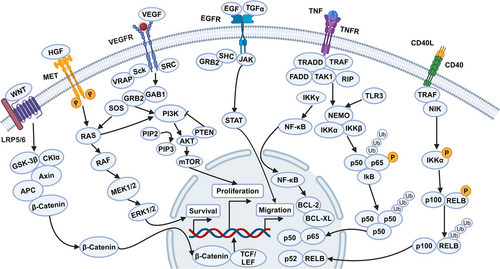
2.2.1 PI3K–AKT–mTOR signaling
Activated PI3K/AKT/mTOR signaling is associated with radiation therapy and cellular drug resistance.44-46 In HNSCC, this pathway is often overactivated due to the amplification or mutation of PIK3CA and the amplification or overexpression of AKT. PIK3CA amplification and PTEN inactivation are common in HPV-positive tumors. The inactivation or deletion of PTEN weaken its lipid phosphatase activity, reducing its inhibition of PI3K pathway. This leads to upregulation of AKT and an increased rate of cell proliferation, which in turn promotes the expansion and metastasis of HNSCC.2 Mutation or amplification of PIK3CA abnormally activate this pathway, accelerating the onset and progression of HNSCC. Several drugs targeting this pathway are currently in clinical trials.46-48 Dual targeting of mTOR and EGFR is recommended to overcome drug resistance.
2.2.2 JAK/STAT signaling
The JAK/STAT signaling pathway is abnormally activated in HNC due to mutations in various oncogenes affecting receptors, downstream mediators, and related transcription factors.49-51 Research has shown that the abnormal activation of STAT3 and STAT5 is related to cell proliferation, angiogenesis, immune escape, metastasis, and resistance to treatment.51, 52 In several types of HNSCC cells, STAT3 signaling has been reported to be activated by the RTK Family, alpha-7 nicotine receptors, the interleukin family (IL-6, IL-10, and IL22 receptors), erythropoietin receptors, TLRs, GPCRs, TGF-α activation, and circular RNA FAT1 (circFAT1).2, 51, 53, 54 Phosphorylated STAT3 upregulates the expression of cyclins and cytokines, thereby promoting the development of HNSCC. Jia et al.54 discovered that circFAT1 can promote phosphorylation of STAT3, and knocking down circFAT1 demonstrated antitumor effects both in vitro and in vivo. Phosphorylated STAT3 can also lead to the inactivation of Caspase-3 and Caspase-9, inhibiting the apoptosis of tumor cells. Additionally, the abnormal activation of the JAK/STAT pathway can promote the differentiation of Th1 and Th2 cells, affecting the type and intensity of the immune response. In HNSCC, the abnormal activation of JAK/STAT pathway can also promote the expression of PD-1 and matrix metalloproteinase 9 (MMP-9), creating an immunosuppressive state and inducing the degradation of the extracellular matrix (ECM), which promotes the progression and metastasis of HNSCC. Currently, Numerous studies have showed that targeting this pathway can inhibit the growth of HNSCC, including compounds or small molecule inhibitors like FLLL12, AG490, AZD1480,55 and so on. JAK/STAT is emerging as a promising pathway for drug development in HNSCC.52
2.2.3 NF-κB signaling
Several studies have shown that the upregulation and abnormal activation of NF-κB family plays significant role in HNSCC.56-58 Among the pathogenic factors of HNSCC, cigarette smoke can lead to phosphorylation and degradation of IκBα, activating the NF-κB pathway. In addition, the NF-κB pathway can interact with the PI3K/AKT, EGFR, and JAK/STAT pathways. Overexpression of EGFR, as upstream signal, can also activate NF-κB signaling, contributing to the production of IL-8 and VEGF, which promote the proliferation and metastasis of HNSCC. Researches indicates that NF-κB can enhance the production and spread of reactive oxygen species (ROS) by inducing enzymes such as nitric oxide synthase (iNOS), causing DNA damage and carcinogenic damage to surrounding cells.59 Carcinogens and ROS-related genetic and epigenetic alterations collectively affect upstream signal transduction, leading to the abnormal activation of IKK and NF-κB, which in turn contributes to the development of HNSCC.
2.2.4 HGF/MET signaling
Research data have showed that HGF and MET proteins were overexpressed in HNSCC.60 These proteins influence the onset and progression of HNSCC through multiple pathways. Notably, MET and EGFR share a downstream signaling pathway, indicating that these receptors collaborate to enhance tumor cell proliferation, movement, invasion, malformation, angiogenesis, metastasis, and resistance to chemotherapy (CT). In addition, the c-Met pathway can upregulate WNT/β-catenin and ERK/c-Fos pathways, further driving tumor proliferation and migration.61
2.2.5 TP53/RB signaling
TP53 mutations are the most frequent among the TSGs in HNSCC.62, 63 The primary causes of p53 protein dysfunction in HNSCC include gene mutation and HPV infection. In HPV-positive HNSCC patients, p53 is inactivated following degradation induced by E6 viral protein binding. While in HPV-negative HNSCC patients, TP53 gene mutations are the primary cause of p53 protein dysfunction.64, 65 In addition, TP53 inactivation can lead to reduced phosphorylation of the RB protein, increasing the activity of the E2F transcription factor. Pathways like WNT/β-catenin pathway also interact with the TP53/RB pathway, contributing to HNSCC mechanisms. Recent studies have proposed p53-based therapeutic strategies, such as introducing WT-TP53 into HNSCC cells to restore p53 activity or using compounds to reactivate WT p53 function in cells with mutant p53. Strategies also include using compounds to target carcinogenic mutant p53 for degradation or hitting downstream pathways of mutant p53, leading to synthesis lethality.62, 65, 66 Although few p53-targeted drugs have advanced to late-stage clinical trials and none have been marketed. TP53 mutation status holds potential for prognosis evaluation and predicting chemoradiotherapy efficacy in HNSCC patients.
2.3 Immune regulation mechanism
The TME in HNC includes various immune cells, which participate in immune escape, suppression and response. This section discusses these immune cells and the mechanisms of immune escape.
2.3.1 Immune microenvironment in HNC
The TME in HNC consists of both nontumor cells and ECM proteins. The cellular components include genetically altered stromal cells, blood and lymph vascular cells, and infiltrating immune cells. Noncellular components include ECM proteins and physicochemical parameters. HNSCC is heterogeneous with two different TMEs based on HPV infection status. HPV-negative HNSCC patients have lower CD8+ T cells infiltration and higher levels of monocytes, macrophages, NK T cells and neutrophils.67, 68 HPV-positive HNSCC patients exhibit higher expression of B cells, plasma cells, Th1 and Th2 CD4+ T cells, CD8+ T cells, Treg cells, dendritic cells (DC), and CD56dim NK cells.69 Tumor-ablating M1 macrophages and protumoral M2 macrophages coexist in the TME. A high M1/M2 macrophages ratio is associated with better prognosis in HPV-positive tumors.70 In addition, cancer-associated fibroblasts (CAFs) produce HGF, VEGF, TGF-β, IL6, CXCL1, CXCL12, and PD-L2, promoting tumor growth and antagonizing antitumor immune responses by recruiting suppressive immune cells.71, 72 Recent single-cell analyses have identified premetastatic cell subpopulations related to AXL and AURK pathways73 (Figure 2).
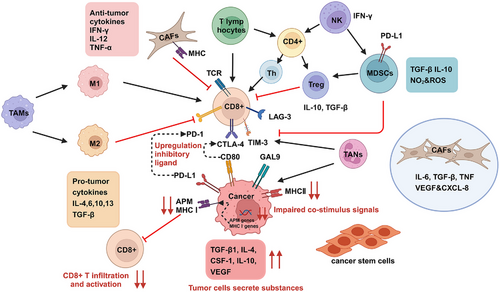
HPV infection interacts complexly with the TME of HNSCC.74 It can affect TAMs, myeloid cells, DC, and NK cells, facilitating immune escape. HPV infection also downregulates the expression of HLA, impairing T cells recognition of HNSCC and promoting immune escape. What is more, HPV increases the expression of IL-6, IL-8, and TGF-β, enhancing tumor cell proliferation, migration, and invasion. It also influences VEGF expression, promoting tumor blood vessel formation and expansion.
On the other hands, HGF/c-Met plays a role in TME alterations, where HGF is paracrinally produced by CAFs in the surrounding TME.61, 75 The activation of HGF and Met promotes HNSCC cell proliferation, growth, spread, and metastasis. HGF influences radio and chemoresistance through glycolytic pathways, leading to drug resistance in HNSCC patients.76 Regarding tumor immunity, HGF/MET signaling converts M1 macrophages to M2-like phenotypes, with M2 macrophages promoting HNSCC cell growth.77 HGF/Met signaling also increases lactic acid secretion via glycolysis, inhibiting human CTL proliferation and activity, and triggering tumor recurrence and metastasis. HGF can upregulate PD-L1 expression, aiding immune evasion.
2.3.2 Immune escape in HNC
The resistant and recurrent nature of HNC suggests that an immune escape mechanism is at play. Recent studies indicate that HNC cancer cells evade immune detection primarily through four mechanisms: upregulation of inhibitory ligands, engagement of immune checkpoints, impaired costimulatory signals, and secretion of substances from tumor cells (Figure 2).
2.3.2.1 Upregulation of inhibitory ligand
The immune system contains inhibitory checkpoints such as CTLA-4, PD-1, TIM-3, LAG-3, TIGIT, B7-H3, and B7-H4, which act as “brakes” to regulate T cell activity. TIM-3 interacts with several ligands, including GAL-9, CEACAM1, phosphatidylserine (PS), and HMGB1 protein. The ligand for LAG-3 is FGL1, while TIGIT interacts with CD96 and CD226.78 Tumor cells expressing PD-L1 and PD-L2 can activate PD-1 on T cells, inhibiting T cell immune responses, promoting apoptosis of lymph node antigen-specific T cells, and reducing apoptosis of Tregs and macrophages. Additionally, PD-L1 from activated T cells can bind to PD-1 on macrophages, promoting M2 polarization. Similarly, CTLA-4 on cancer-activated T cells can bind to the B7 ligand on HNC cells, leading to T cell inactivation.79 In HNSCC, the TME is immunosuppressive, and the upregulation of inhibitory ligands hinders the immune system's ability to eliminate tumor cells, resulting in tumor progression.
2.3.2.2 Engagement of immune checkpoints
Studies show that PD-L1 is expressed in about half of HNC cases.80 Other immune checkpoints, such as CD276 and CD44, are also overexpressed in HNSCC. These proteins decrease the activity of CD8+ T cells by binding to corresponding proteins on T cells, thereby inhibiting the anticancer abilities of immune cells.81 Wang et al.81 established mouse models of HNSCC with in vivo lineage tracing of tumor stem cells, confirming that CD276 is elevated in tumor stem cells and protects them from T cell attacks. Prince et al.82 identified CD44 as a cancer stem cell marker for HNSCC, finding significant increases in CD44 levels and associations with tumorigenesis, radiation resistance, CT resistance, and an increased immunosuppressive phenotype of HNSCC.83-85
2.3.2.3 Impaired costimulus signals
Cancer cells can evade recognition by CD8+ T cells by downregulating the MHC-I/II, making antigens unrecognizable. It is revealed that the expression of MHC-I class molecules is reduced due to the downregulation of human leukocyte antigen genes in HNSCC.86 A latest research showed that MHC-I associated antigen presentation machinery (APM) plays a crucial role in activating tumor-killing effector T cells, contributing to the low response rate of HNSCC to immune checkpoint inhibitors (ICIs).87
2.3.2.4 Secreted substances from tumor cells
HNSCC and its surrounding matrix continuously secrete substances like VEGF, CSF-1, IL-4, IL-10, and TGF-β, which facilitate tumor immune escape and drug resistance. These substances not only directly inhibit T cell function but also promote the aggregation of inhibitory immune cells.88
2.4 Pathogenic genetic factors
In HNC, genetic and epigenetic factors combine to influence gene expression, leading to changes in cell signaling pathways that regulate tumor growth, DNA repair, antiapoptosis, angiogenesis, resistance to external factors, and epithelial mesenchymal transformation.89
Researches on molecular biomarkers of HNSCC indicates frequent chromosomal losses and genetic instability. Losses of 9p21, 3p21, 17p13, 11q13, 13q21, 14q32, 6p, 8, 4q27, and 10q23 are marked at different stages of progression.90 Tumor suppressor genes (TSGs) such as CDKN2A and TP53 are involved in early stages of HNSCC,91 while PTEN is implicated in later stages, rather than oncogene mutation. Key TSGs also include FAT1, NOTCH1, KMT2D, NSD1, and TGFBR2.92 The only oncogene frequently mutated in HNSCC is PIK3CA, with a mutation rate of 14%.93, 94 Mutations in CDKN2A, TP53, NFE2L2, and KEAP1 are restricted to HPV-negative tumors. Conversely, frequent losses of TRAF3, NSD1, FAT1, NOTCH1, and SMAD4, amplifications of E2F1 and genes encoding EGFR, HER2, and FGFR1, and alterations in PIK3CA, PTEN, FBXW7, and KRAS occur in HPV-positive tumors91 (Figure 3). A new study in 2023 identified seven differential genes (CCR4, WDFY4, VCAM1, LYZ, VSIG4, XIRP1, and CMKLR1) as crucial in the TME.95 Recent research also showed that genes including ACTN1 and TNFAIP2 play significant roles in tumor progression and CT resistance, suggesting ACTN1 as a novel target in HNSCC.96, 97 Another study revealed that the metabolic biomarker PYGL promotes progression, metastasis, and CT resistance of HNSCC through the GSH/ROS/p53 pathway.98
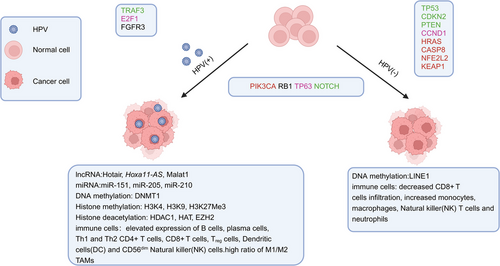
In addition, data from The Cancer Genome Atlas22 showed that smoking-associated HNSCC exhibits widespread gene mutations and continual copy number changes. Functional studies of these genes indicate their critical roles in the development and progression of HNSCC, and targeting these potential genes may be significant for HNSCC treatment.99
2.5 Epigenetic mechanism in HNC
Epigenetic modifications, unlike gene mutations, alter the phenotype without changing the DNA sequence. These modifications are heritable and reversible.100 Epigenetic alterations include DNA methylation,101-103 histones modifications, chromatin remodeling, and noncoding RNA (ncRNA) activity (Figure 3).104, 105
2.5.1 DNA methylation
DNA methylation is a common epigenetic modification involving the covalent addition of methyl groups to the five position of cytosine (5-MC).100 Laytragoon-Lewin et al.106 found DNA methylation in multiple TSGs in HNSCC and linked abnormal methylation to shorter survival after standard therapy. Zhou et al.103 identified and confirmed the diagnostic value of two hypermethylated and two hypomethylated genes as prognostic biomarkers in HNSCC. Pan et al.107 subsequently identified six more methylated genes serving as prognostic markers. The E6 protein inactivates TP53 by promoting the DNMT1 promoter, affecting the methylation of several genes, such as CDKN2A, RASSF1, CCNA1, Cadherin family genes, ITGA4, TIMP3, ELMO1, MEI1, and LINE1. CDKN2A is hypomethylated in HPV-positive HNC cases, while RASSF1 is hypomethylated in normal head and neck cells. Genes like CCNA1, Cadherin family genes, ITGA4, and ELMO1 are hypermethylated in HNC, and LINE1 is hypomethylated in HPV-negative tumors. Further research is required to establish these epigenetic changes as HNC biomarkers.108 In addition, one study has shown that the risk of HPV-HNC can be predicted by detecting DNA methylation levels in HPV late genes.109
2.5.2 Histone modification
Aside from DNA methylation, histone modification is another significant predictive epigenetic mechanism in HNSCC.110 Histones, composed of N-terminal and globular C-terminal domains, undergo posttranslational modifications primarily at the N-terminal. Acetylation, deacetylation, and methylation of histones are closely linked to transcriptional activation and gene expression. Chen et al.111 demonstrated that acetylation of lysine 27 on histone H3 (H3K27ac) at the promoter of long ncRNAs (lncRNA) plac2 leads to upregulation of plac2 and activation of the WNT/β-catenin pathway, impacting HNSCC progression. John et al.89 found that paracrine-induced histone modification increased expression of Bmi-1, a transcriptional suppressor related to HNSCC invasiveness. What is more, Ma et al.112 discovered that a new substance LncMX1-215 directly binds H3K27 acetylase GCN5, blocking H3K27 acetylation and inhibiting the proliferation and transfer ability of HNSCC both in vitro and in vivo. In addition, E7 also regulates HADC1 and EZH2.113
2.5.3 Noncoding RNAs
ncRNAs play a crucial role in various biological processes and are altered in HNSCC tumor tissues. NcRNAs, which include small ncRNAs (miRNAs, siRNAs, and piRNAs) and lncRNAs, are vital for cell homeostasis, development, and differentiation.
lncRNAs can bind to RNAs and proteins, regulating gene expression and infecting cell proliferation, survival, and metastasis. They interact with pathways like JAK/STAT3, TGF-/Smad, and WNT/β-catenin. Key HNSCC-related lncRNAs include HOTAIR, HOXA11-AS, MALAT1, ANRIL, and H19. HOTAIR, for example, recruits EZH2 to catalyze H3K27 trimethylation, inhibiting TSGs, and also acts on miR-206 to activate the PI3K/AKT pathway, promoting HNSCC development114
miRNAs prevent translation or downregulate mRNA of target genes, participating in HNC proliferation, differentiation, development, and apoptosis. They also regulate pathways like WNT/β-catenin, PTEN/AKT/mTOR, JAK/STAT, and TGF-β. Overexpression of carcinogenic miRNAs promotes the occurrence and development of HNSCC. While downregulation of tumor suppressor miRNAs like miR-99a is associated with increased invasion, cell cycle progression, cell proliferation, and clonal formation of HNSCC.104 Overexpression of miR-107, miR-151, miR-182, miR-361, miR-324-5p or low expression of miR-492, miR-20b is correlated with disease-free survival and overall survival (OS) in OC/OPSCC patients. In HPV-positive HNSCC, miR-151 is upregulated while miR-210 and miR-205 are downregulated.104
Other dysregulated miRNAs in HNSCC include miR-99a, HSA-miR-29c-3p, miR-128, miR-375, miR-32-5p, miR-26a/b, miR-376c, miR-876-5p, miR-200a, miR-93, miR-205-5p, miR-124-3p, miR-29s, miR-92a-3p, miR-150, miR-203, miR-545, miR-532-3p, miR-204-5p, miR-200, miR-26a, and miR-145 have been summarized.115 miR-375 is correlated with metastasis and shorter survival, which has promised to be a biomarker of poor prognosis in HNSCC.116 In addition, downregulation of hsa-let-7d and hsa-miR-205 in HNSCC tumors, showing potential as a poor prognosis biomarker of HNSCC.117 Microarray analysis revealed 20 miRNAs118 were highly expressed in HNSCC samples, including miR-372, miR-320, miR-21, miR-34a, miR-200c, miR-223, hsa-miR-32-5p, miR-654-5p, miR-187, miR-510, miR-626, miR-107, miR-103, miR-24, miR-450a, miR-122-5p, and hsa-miR-375,115, 119 and most of these can be considered as diagnostic and prognostic biomarkers in HNC.
In addition, miRNAs in HNC regulate drug resistance through pathways involving the cell cycle, apoptosis, DNA repair, EMT, CSCs, and drug efflux pumps.120, 121
3 TARGETED THERAPY
Traditional treatments for HNC primarily include surgery, RT, and CT. Currently, surgery remains the cornerstone of HNC treatment except for nasopharynx cancer. However, as our understanding of the molecular characteristics and pathogenesis of HNC has deepened over the past decade, treatment strategies have evolved significantly. This evolution has introduced targeted therapy, immunotherapy,122-128 and gene therapy, either alone or in combination with traditional methods like surgery, RT, or CT129 (Figure 4).
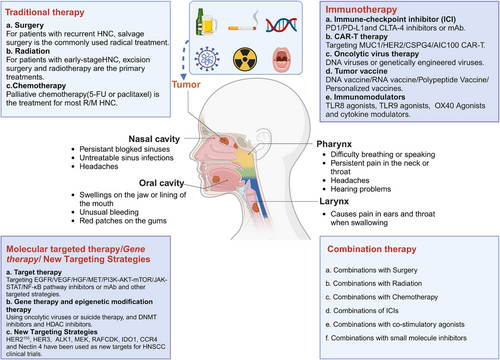
By searching for the keywords “head and neck,” “HNC,” “head and neck squamous cell carcinoma,” “HNSCC,” “nasopharyngeal carcinoma,” “SCC of the nasopharynx,” and “NPC” on PubMed, Web of Science, and the National Cancer Institute (NCI) databases: we summarized the therapeutic drugs currently in use or under clinical investigation worldwide. To date, 20 drugs have been brought to market, including ICIs, antibodies targeting EGFR, reactive oxygen activators, and CT agents. Three single-molecule therapies, cetuximab, pembrolizumab, and nivolumab, have been approved for treatment of R/M HNSCC following platinum resistance. Cetuximab in combination with RT has been approved for locally advanced HNSCC (Table 1). Furthermore, we searched ClinicalTrials.gov for drugs related to HNC and summarized the latest research progress of various drugs in clinical trials. These include molecular targeted therapy drugs, immunotherapy drugs, gene therapy drugs, epigenetic modification therapy drugs, and emerging therapy drugs (Table 2).
| Drug(s) | Target(s) | Indication | First approved country/region |
|---|---|---|---|
| Pembrolizumab | PD-1 | HNSCC, R/M HNSCC, head and neck neoplasms | America |
| Nivolumab | PD-1 | Head and neck neoplasms, HNSCC | America |
| Toripalimab | PD-1 | NPC | China |
| Tislelizumab | PD-1 | NPC | China |
| Camrelizumab | PD-1 | NPC, recurrent NPC | China |
| Nimotuzumab | EGFR | Head and neck neoplasms, NPC | India |
| Cetuximab | EGFR | Head and neck neoplasms, HNSCC | America |
| ASP-1929 | EGFR | Unresectable locally advanced or recurrent HNC | Japan |
| Docetaxel | Tubulin | Head and neck neoplasms | Japan |
| Fluorouracil | TYMS | Head and neck neoplasms | Japan |
| Nitrocaphane | DNA | Nasopharyngeal carcinoma | China |
| Carboplatin | DNA | Head and neck neoplasms, HNSCC | Japan |
| Cisplatin | DNA | Head and neck neoplasms | Japan |
| Cyclophosphamide | DNA | Head and neck neoplasms | China |
| Technetium Tc-99 M Tilmanocept | CD206 | OSCC | America |
| Hydroxycarbamide | RNR | HNSCC, HNC | America |
| Methotrexate sodium | DHFR | HNSCC | America |
| Recombinant human adenovirus type 5 | p53 | NPC | China |
| Bleomycin A5 hydrochloride | – | NPC | China |
| Temoporfin | ROS | HNSCC | European |
- Abbreviations: HNSCC, head and neck squamous cell carcinoma; HNC, head and neck cancer; NPC, nasopharyngeal carcinoma; OSCC, oral squamous cell carcinoma.
| Classifications | Interventions | Target (s) | Clinical trials | Phase | Enrollment | Indication |
|---|---|---|---|---|---|---|
| Molecular targeted therapy | ||||||
| EGFR pathway | Nimotuzumab | EGFR | NCT00957086 | III | 710 (Estimated) | HNSCC |
| Zalutumumab | EGFR | NCT00496652 | III | 619 (Actual) | HNC | |
| Afatinib | EGFR | NCT01427478 | III | 134 (Actual) | HNSCC | |
| VEGF pathway | Bevacizumab | VEGF | NCT05063552 | II/III | 430 (Estimated) | R/M HNSCC, R/M LSCC, R/M Lip and oral cavity carcinoma, R/M PSCC, R/M SSCC, R/M NSCC, metastatic HSCC |
| Sorafenib | VEGFR | NCT00494182 | II | 48 (Actual) | R/M HNSCC, R/M HSCC, R/M lip and oral cavity carcinoma | |
| Sunitinib | VEGFR | NCT00387335 | II | 22 (Actual) | Metastatic SNC with occult primary, recurrent LSCC, recurrent NSCC, recurrent OSCC | |
| Cabozantinib | VEGFR, MET, AMT, AXL | NCT03468218 | II | 36 (Actual) | R/M HNSCC, PSSCC, recurrent HSCC, recurrent LSCC, recurrent OSCC | |
| HGF/MET pathway | Ficlatuzumab | HGF | NCT06064877 | III | 410 (Estimated) | R/M HNSCC |
| PI3K/AKT/mTOR pathway | Buparlisib | PI3K | NCT04338399 | III | 483 (Estimated) | HNC |
| Everolimus | mTOR | NCT00942734 | II | 49 (Actual) | HNC | |
| Ipatasertib | AKT | NCT05172258 | II | 52 (Estimated) | R/M HNSCC, R/M LSCC, R/M OSCC, R/M HSCC | |
| JAK/STAT pathway | Danvatirsen | STAT3 | NCT02499328 | I/II | 340 (Actual) | Advanced solid tumors, metastatic HNSCC |
| Ruxolitinib | JAK1/2 | NCT03153982 | II | 16 (Actual) | HNSCC | |
| New targeting pathways | Trastuzumab | HER2 | NCT06130007 | II | 48 (Estimated) | OSCC |
| Patritumab | HER3 | NCT02633800 | II | 87 (Actual) | Head and neck neoplasms | |
| Immunotherapy | ||||||
| ICIs | Finotonlimab | PD-1 | NCT04146402 | III | 330 (Estimated) | HNSCC |
| Durvalumab | PD-L1 | NCT02551159 | III | 823 (Actual) | HNSCC | |
| Atezolizumab | PD-L1 | NCT01810913 | II/III | 613 (Estimated) | p16INK4a-negative OSCC, LSCC, HSCC, OCSCC | |
| Avelumab | PD-L1 | NCT02999087 | III | 707 (Actual) | HNSCC | |
| Ipilimumab | CLTA-4 | NCT03700905 | III | 276 (Estimated) | HNC | |
| Tremelimumab | CLTA-4 | NCT03624231 | II | 18 (Actual) | HNSCC | |
| CAR-T/NK | CAR T cells | MCU1 | NCT05239143 | I | 100 (Estimated) | Nasopharyngeal cancer, HNSCC |
| HER-2 | NCT03740256 | I | 45 (Estimated) | HNSCC, cancer of the salivary gland | ||
| CSPG4 | NCT06096038 | I/II | 33 (Estimated) | HNC | ||
| LMP-1 | NCT02980315 | I/II | 20 (Estimated) | Nasopharyngeal neoplasms | ||
| CAR-NK cells | PD-L1 | NCT04847466 | II | 55 (Estimated) | Advanced HNSCC | |
| Oncolytic virus | RP3/Herpesvirus | – | NCT05743270 | II | 130 (Estimated) |
HNSCC, locally advanced HNSCC, recurrent HNSCC |
| Tumor vaccines | Anti-MUC1 vaccine | MUC1 | NCT02544880 | I/II | 16 (Actual) | HNC, HNSCC |
| Peptide-based | p16(INK4a) | p16 | NCT01462838 | I/II | 26 (Actual) | HPV-induced cancers |
| UCPVax vaccine | Telomerase | NCT03946358 | I/II | 47 (Estimated) | HNSCC | |
| IO102/IO103 | IDO | NCT04445064 | II | 17 (Estimated) | HSCC, LSCC, OSCC, OCSCC | |
| Nucleic acid-based | ISA101 vaccine | HPV16 E6/E7 | NCT02426892 | II | 33 (Actual) | Solid tumors |
| MEDI0457 | HPV16/18E6/E7 | NCT03162224 | II | 35 (Actual) | HNC, HPV Associated HNSCC | |
| Personalized cancer vaccines | VB10.NEO | – | NCT03548467 | I/II | 41 (Actual) | Locally advanced or metastatic solid tumors |
| AlloVax | – | NCT01998542 | II | 12 (Actual) | HNC, HNSCC | |
| Cell-based vaccine | DC vaccine | p53 | NCT00404339 | I | 17 (Actual) | HNC |
| Immunomodulators | Motolimod | TLR8 | NCT02124850 | I | 18 (Actual) | HNSCC |
| EMD 1201081 | TLR9 | NCT01040832 | II | 107 (Actual) | HNSCC | |
| IRX-2 | IL-2 | NCT00210470 | II | 27 (Actual) | HNSCC | |
| Gene therapy | ||||||
| Adenovirus-carrying endostatin gene | E10A | VEGFR | NCT02630264 | III | 540 (Estimated) | Head and neck neoplasms |
| NCT00634595 | II | 116 (Estimated) | HNSCC, NC | |||
| Recombinant human endostatin adenovirus | EDS01 | VEGFR | NCT02283489 | II | 180 (Estimated) | Head and neck neoplasms |
| Epigenetic modification therapy | ||||||
| DNMT1 inhibitors | Azacytidine | DNMT1 | NCT05317000 | I | 50 (Estimated) | HNSCC |
| Decitabine | DNMT1 | NCT05265962 | II | 85 (Estimated) | ESCC | |
| HDAC inhibitors | Romidepsin | HDAC | NCT00084682 | II | 14 (Actual) | Stage IV HSCC, Stage IV OCSCC, Stage IV OSCC |
| Panobinostat | HDAC | NCT00670553 | I | 7 (Actual) | HNC | |
| CUDC-101 | HDAC | NCT01171924 | I | 47 (Actual) | HNC | |
| Emerging therapies | ||||||
| APP inhibitor | EGCG | APP + DYRK1A + α-synuclein | NCT01116336 | I | 25 (Actual) | Head and neck neoplasms |
| CCR4 antagonist | FLX475 | CCR4 | NCT03674567 | I/II | 323 (Actual) | Advanced cancer |
| Cell INDUCTION | E7 TCR-T cells, aldesleukin | HPV E7 | NCT05639972 | I/II | 15 (Estimated) | HPV+ OSCC, OC |
| mRNA vaccine | BNT-113 | E6, HPV E7 | NCT04534205 | II | 285 (Estimated) | Unresectable HNSCC, R/M HNC |
| Therapeutic vaccine | HB-201 | E6, HPV E7 | NCT04630353 | I | 10 (Actual) | HPV 16+ confirmed OC |
| ADC | OBT076 | CD205 | NCT05930951 | I | 32 (Estimated) | Adenoid cystic carcinoma of HNC |
- Abbreviations: HNC, head and neck cancer; HNSCC, head and neck squamous cell carcinoma; HSCC, hypopharyngeal squamous cell carcinoma; LSCC, laryngeal squamous cell cancer; NC, nasopharyngeal carcinoma; NSCC, nasopharyngeal squamous cell carcinoma; OC, oropharynx cancer; OCSCC, oral cavity squamous cell carcinoma; OSCC, oral squamous cell carcinomas; PSSCC, paranasal sinus squamous cell carcinoma; PSCC, pharyngeal squamous cell carcinoma; SSCC, sinonasal squamous cell carcinoma; SNC, squamous neck cancer.
- Sources: Data were obtained from ClinicalTrials.gov.
3.1 Molecular targeted therapy
Compared with the toxicity and drug resistance caused by traditional treatment methods, molecular targeted therapy offers higher selectivity and fewer adverse reactions.130 By targeting cell surface receptors such as EGFR, VEGFR, HER2, and MET, and their downstream signaling pathways, it is possible to inhibit HNSCC cell proliferation, invasion, and migration. Additionally, targeting epigenetic modification sites like histone acetylation/deacetylation or DNA methylation can inhibit HNSCC migration and proliferation.131
3.1.1 Targeting cell surface receptors
- (1) EGFR inhibitor
Monoclonal antibodies (McAbs) targeting EGFR include cetuximab, nimotuzumab, panitumumab, zalutumumab, duligotuzumab, and imgatuzumab. Tyrosine kinase inhibitors (TKIs) targeting EGFR include selective targeted TKIs (gefitinib, erotinib, sapitinib) and dual-targeted TKIs (afatinib, lapatinib, dacomitinib).
Cetuximab was the first EGFR inhibitor approved for HNSCC in 2006, with monotherapy response rates for recurrent/metastatic (R/M) HNSCC around 10−15%.132 Cetuximab has shown increased efficacy when combined with cisplatin/carboplatin, fluorouracil, and paclitaxel in R/M HNSCC.133 Recent clinical trials combining cetuximab with various inhibitors have demonstrated durable activity and safety.134 However, cetuximab treatments can cause severe skin, mucosal, kidney, and gastrointestinal toxicity.135 New inhibitors like nimotuzumab, panitumumab, and zalutumumab have been developed with fewer side effects and higher EGFR affinity. Nimotuzumab combined with cisplatin/RT demonstrated good effects in a phase II study. Panitumab is being evaluated in several clinical trials for locally advanced HNSCC and R/M HNSCC (NCT00547157, NCT00500760, NCT00820248, and NCT00798655) or R/M HNSCC (NCT00454779). Another clinical trial showed that zalutumumab increased survival to 9.9 weeks from 8.4 weeks in platinum-resistant R/M HNSCC patients.2 Duligotuzumab is a McAb double targeted to EGFR and HER3. Additional antibodies like milatuzumab, a humanized anti-EGFR McAb designed to enhance antibody-dependent cellular cytotoxicity, have shown promising efficacy for metastatic colorectal cancer with KRAS mutations.136
- (2) VEGF/VEGFR inhibitors
- (3) HGF/MET inhibitors
MET activation is associated with resistance to cetuximab in HNSCC. Current inhibitors targeting the HGF/MET pathway include c-Met inhibitors (cabozantinib, stantinib, forvitinib) and HGF inhibitor (ficlatuzumab). Cabozantinib is also a TKI of VEGFR-1/2/3/AXL/c-Met/TAM receptor.145 Cabozantinib also has immunomodulatory effects, including inhibition of AXL19 and MER signaling. A recent clinical trial (NCT03468281) showed that cabozantinib and pembrolizumab had improved PFS and OS in R/M HNSCC, and an overall clinical benefit rate of 91%.146 The clinical trial results of carbozantinib combined with cetuximab (NCT03667482) showed a certain efficacy of carbozantinib in CTX-resistant R/M HNSCC patients.145 However, stanitinib and forvitinib have not demonstrated promising clinical efficacy in R/M HNSCC.
Ficlatuzumab is a humanized anti-HGF IgG1 mAb. Multiple clinical trials (NCT06064877, NCT02277197, NCT03422536) compared the combined efficacy of ficlatuzumab and cetuximab in OS in patients with R/M HNSCC. The results show that this well-tolerated combination regimen has good antitumor activity in cetuximab-resistant advanced HNSCC and better efficacy in HPV-negative HNSCC patients.147, 148
3.1.2 Targeting PI3K–AKT–mTOR
Abnormal activation of the PI3K/AKT/mTOR pathway is observed in 30.5% of HNSCC patients, and clinical trial results show that targeting PI3K/AKT/mTOR pathway-related components can inhibit the metastasis, expansion, and deterioration of HNSCC.
Inhibitors targeting PI3K include generic Class I inhibitors (buparlisib, copanlisib) and only targeting PI3K P110α-specific inhibitor (alpelisib). Buparlisib is a potent pan-class I PI3K inhibitor and showed good efficacy in combination with paclitaxel for advanced HNSCC.149 A KURRENT-HN study showed that more than 45% of patients with PI3KCA mutation and HRAS overexpression in R/M HNSCC benefit from the combination of alpelisib and tipfarnib.150 Copanlisib, a selective PI3K inhibitor, showed adverse toxicity and limited efficacy in a large number of patients with R/M HNSCC when combined with cetuximab.151
mTOR inhibitors include everolimus, temsirolimus, buparlisib, rapamycin, and CC-115. Studies have shown that everolimus is ineffective alone or in combination with erotinib in unselected patients with R/M HNSCC.152, 153 Recently, a clinical study found that everolimus combined with CT for HNSCC was well tolerated, with an overall response rate of 75.6% and tumor size reduction of ≥50% in 20 patients.154 Unlike everolimus, temsirolimus was poorly tolerated when combined with erlotinib.155 However, temsirolimus monotherapy significantly reduced pS6 and p4E-BP1 in tumors, as well as pS6 and pAKT in PBMC with minimal and reversible side effects.156 CC-115 is a novel dual DNA-PK and TOR kinase inhibitor, and the results of clinical trials (NCT01353625) indicate that CC-115 is well tolerated and is considered as a promising new anticancer agent.157
AKT inhibitors such as MK-2206 (NCT01349933), ipatasertib (NCT05172245), BYL719 (NCT02145312), and perifosine (NCT00062387) are under clinical trials. Currently, more clinical research are needed to demonstrate the efficacy of AKT inhibitors in HNSCC.2
3.1.3 Targeting JAK/STAT
JAK/STAT pathway inhibitors like JAK1/2 inhibitor (ruxolitinib and AZD1480) and STAT3 inhibitor (danvatirsen and TTI-101). Ruxolitinib and AZD1480 have shown good antitumor activity in PDX models associated with HNSCC. A clinical trial (NCT03153982) of ruxolitinib in patients with HNSCC is currently underway. AZD1480 showed antitumor efficacy in PDX models, which may provide a new prospect for HNSCC treatment.55 One phase Ib/II clinical trial (NCT02499328) showed that the treatment of danvatirsen combined with durvalumab was more effective than PD-L1 treatment in R/M HNSCC patients.158 TTI-101 has exhibited antitumor activity in many preclinical cancer models, which could inhibit tumor growth and reverse liver damage and fibrosis, and a clinical trial (NCT05668949) is underway to examine its effects.
3.1.4 Targeting NF-κB
Inhibition of the IKKβ/NF-κB signaling pathway can improve treatment efficacy in cisplatin-resistant HNSCC.159, 160 Here, we mainly reviewed two NF-κB inhibitors (bortezomib and triptolide).
Bortezomil is a proteasome inhibitor targeting 26S proteasome and inhibit the activation of NF-κB. The literature suggests that bortezomib may inhibit tumor growth in combination with RT in HNSCC.159 Du et al.161 showed that bortezomib combined with dasatinib may be effective in cisplatin-resistant HNSCC patients. Several phase I studies combining Bortezomib with radiation therapy (NCT01445405, NCT00329589, NCT00629226, and NCT00011778) are currently conducting. Preclinical and clinical data suggest that the combination treatment may be promising; however, further mechanistic studies are still needed. Another NF-κB inhibitor triptolide has immunosuppressive, anti-inflammatory, and antiproliferative effects. Preclinical studies have shown that triptolide and minnelide can induce apoptosis by activating wild-type p53. A clinical trial (NCT05791136) is evaluating its efficacy when combination with radiation therapy for ESCC.162
3.1.5 New targeting strategies
Emerging studies have shown that signaling pathways such as HER2,163 HER3, ALK1, MEK, RAF, CDK, IDO1, CCR4, and Nectin 4 can also be used as targets for HNSCC clinical trials. Trastuzumab selectively binds to HER2 with high affinity.164 Numerous studies have investigated the use of CDX-3379, ISU104, and duligotuzumab targeting HER3 in the treatment of HNSCC. Notably, KTN3379 has been shown to significantly inhibit the proliferation of HPV-positive HNSCC.165 Patritumab is a fully human McAb target HER3, a phase Ib study (NCT02633800) showed that the combination strategy of patritumab, cetuximab, and platinum did not improve PFS and OS, and all patients had more than one adverse event, resulting in early termination of the trial.166
3.2 Immunotherapy
HNC features one of the most inflammatory TMEs among solid tumors,167 making it highly receptive to immunotherapy.168 Current strategies include ICIs, adoptive cell therapies, oncolytic virus (OV) therapies, cancer vaccines, and immunomodulators133, 168 (Figure 5).
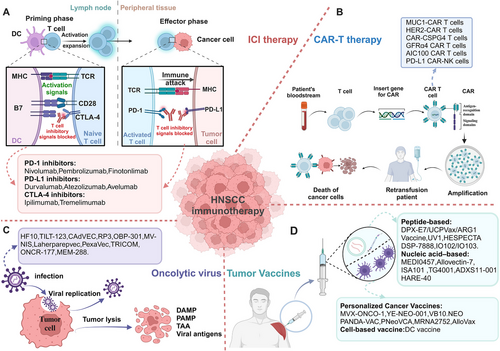
3.2.1 Targeting PD-1/PD-L1
Nivolumab, the first US FDA-approved anti-PD-1 McAb for R/M HNSCC, has shown a significant therapeutic effect in platinum-sensitive R/M HNSCC patients.169-172 Pembrolizumab, another highly selective McAb with high affinity for PD-1132, has also demonstrated good safety and efficacy in various clinical trials.173 Pembrolizumab combined with platinum and 5-fluorouracil is an appropriate first-line treatment for R/M HNSCC.174 Finotonlimab is an innovative recombinant human McAb against PD-1, which can specifically bind to PD-1, enhance the function of CD8+ T cells, and inhibit tumor growth. Four clinical trials of finotonlimab are in progress for R/M HNSCC (NCT04146181, NCT05552807, NCT04146402, NCT04146181).
Durvalumab is a high-affinity humanized McAb targeting PD-L1, restoring the immune response and killing tumor cells.175 Durvalumab showed significant efficacy and good safety in a phase I/II clinical trial (NCT01693562). Monotherapy of duvazumab showed good antitumor activity and safety in R/M HNSCC patients with PD-L1 overexpression (NCT02207530). However, neither Durvalumab alone or in combination with temlimumab significantly alleviate patients' OS (NCT02369874). Atezolizumab and avelumab are two novel PD-L1 inhibitors that have shown good activities in multiple clinical trials. Other clinical studies showed that patients with HPV+ HNSCC benefited more from immunotherapy (ORR: 26.5% in the HPV-positive group vs. 7.9% in the HPV-negative group)176 (Figure 5A). Recent research showed that PD-L1 expression is positively correlated with OS when treated with PD-1 or PD-L1 inhibitors.177
3.2.2 CTLA-4 antibodies
Ipilimumab and tremelimumab are mono-antibodies, which block CTLA-4 and are currently being tested for efficacy in HNSCC, despite promising results in other tumors.133 Wang et al.178 found that blocking the CTLA-4 receptor can activate CD8+ T cells, increase production of IFN-γ and TNF-α, activate the STAT1/IRF1 axis and trigger tumor cell heat death in HNSCC.
Furthermore, several antibodies and small molecules targeting other immune checkpoints are still in clinical development, such as LAG3, TIGIT, TIM3, B7H3, CD39, CD73, adenosine A2A receptors, and CD47.179 However, due to its shortcomings such as limited efficacy of single drug, large side effects and use plan to be optimized, it is mainly combined with PD-1 antibody to amplify the tumor inhibition effect of the latter. First-line nivolumab and ipilimumab was evaluated for the treatment of R/M HNSCC (NCT02741570). The results showed that Nivolumab combined with ipilimumab showed a good safety profile180 (Figure 5A).
3.2.3 New immunotherapy strategies
In addition to ICIs, new immunotherapy strategies include CAR-T therapy, OVs therapy, tumor vaccines, and immunomodulators.
Immune evasion of cancer cells is mainly related with a lack of MHC-associated antigen presentation in convention immunotherapy of HNSCC. CAR-T therapy has significant advantages without MHC association.181 However, due to the high tumor heterogeneity, complex tumor structure, lacking of tumor-specific antigens, TME immunosuppression, treatment-related toxicity, and the risk of off-target adverse events, the antitumor effect of CAR-T cell therapy on HNSCC is weak, and the side effect is large, which is the obstacle of clinical transformation.181, 182 Recently studies have shown that selecting a suitable CAR target protein is of great significance in enhancing the killing ability of T cells. A series of trials of CAR-T cells treatment have shown that MUC1, HER-2, EGFR, CD70, LMP1, CD44v6, CD276 (B7-H3), CD98hc, NKG2DL, FAP, HER3, and NKGD2 are promising CAR-targeted proteins for HNSCC immunotherapy180 (Figure 5B).
OV therapy has great potential in local tumor control, low incidence, and safety, and it has become the fourth generation of tumor immunotherapy after ICI therapy.183 OV is a kind of naturally mutated double-stranded enveloped DNA viruses or genetically engineered viruses, like adenovirus, vaccinia virus, reovirus, measles virus, herpes virus, parvovirus, coxsackie virus, VSV,184 and newcastle disease virus. OV exert toxic effects on tumor cells through multiple mechanisms including autolysis, immune cell homing, disruption of vascular supply, and enhancement of other adjuvant anticancer therapies.175, 185 Oncorine is the earliest OV used for HNC.186 Compared with conventional systemic CT, intratumoral injection of oncorine showed significant efficacy and good safety in a phase III clinical trial.133, 186 ONYX-015, an adenovirus attenuated E1B gene, selectively inactivates the p53 gene. Intratumorally injection of ONYX-015 improved survival of patients in a phase II clinical trial; however, the tumor is prone to rapid recurrence. And the phase III trial of ONYX-015 is under study.187 Besides, many other therapeutic OVs are currently investigated in clinical trials, including oncolytic measles virus (MV-NIS, NCT01846091) encoding the thyroid sodium iodide transporter, oncolytic herpesvirus carrying GM-CSF (T-VEC, NCT02626000), recombinant fowlpox virus expressing B7.1, ICAM-1, LFA-3, CEA, MUC1 (TRICOM, NCT00021424), and dioncolytic adenovirus (VISTA, NCT03740256).133 When used in combination with other immunotherapies, such as ICIs, CARs, and autologous tumor infiltrating lymphocytes, OV therapy significantly improved treatment for a range of tumor types, and reduced side effects and resistance, including HNSCC184 (Figure 5C).
With the emergence of tumor neoantigens, tumor vaccine therapies have broadened the immunotherapy landscape for patients with R/M HNSCC. DCs as antigen delivery carriers are a new focus in the development of HNSCC cancer vaccines.188 Current DC-based vaccination approaches mainly rely on in vitro production of monocytes or CD34+ cell-derived DCS loaded with antigens, which can be activated with various TLR ligands and cytokines, then activated DCs can be reinjected into HNSCC patients to promote cytotoxic T cell responses.175 Schuler et al.189 reported a phase I clinical trial (NCT00404339) of a selected wild-type p53 polypeptide vaccine based on autologous monocyte derived DC loading. The results showed that Treg levels continued to decline, and the 2-year survival rate was 88%.189 Recently, a recent study reported a vaccine administered with Wilms Tumor 1 (WT1) peptide-loaded DC. The results showed that no vaccine-related serious adverse reactions, extended survival, and enhanced WT1-specific immunity were observed in 11 patients with R/M HNSCC combined with conventional CT.175 Xu et al.190 also reported a hybrid nanovaccine (Hy-M-Exo) that inherits CCR7, a key protein of DCMV lymphatic homing, and shows higher LN targeting efficiency. At the same time, a robust T cell response was induced by retained tumor antigens and endogenous danger signals in the hybrid nanovaccine activated APCs and showed good therapeutic effect in HNSCC mouse model, which suggest a viable strategy for antitumor immunotherapy.190 In a completed phase 2 study with CPI-experienced R/M HNSCC, FLX475a selective CCR4 antagonist in combination with pembrolizumab was shown to be well tolerated and has good efficacy particularly in those with HPV-positive tumors.191 A neoantigen DC vaccine showed significant efficacy and good safety in a phase Ib clinical trial of ESCC (NCT 05023928).192 (Figure 5D).
Currently, immunomodulators widely studied include Toll-like receptor agonists TLR8 agonist motolimod (VTX-2337) and TLR9 agonists (EMD 1201081, SD-101, CMP-001), OX40 agonists (MEDI0562, INBRX-106, BGB-A445, PF-04518600), and cytokine modulators (IL-12, IRX-2, NT-I7, N-803). In addition, HB-201 and HB-202, an arenavirus-based immunotherapy, enhance antitumor immunity in HPV16+ HNSCC.193 Different subtypes of B cells and TLSs in the TME of HNSCC patients are revealed to impact the efficacy of ICIs.194 Therefore, new therapeutic strategies based on B cells can be developed to enhance immunotherapy responses in HNSCC.
3.3 Gene therapy
Gene therapy has been widely utilized for cancer treatment, significantly enhancing antitumor efficacy. Various gene therapy agents employing the rAd-p53 vector have been used in cancer treatment, with clinical gene therapy programs in China using rAd-p53 for HNC treatment since 1998. Oncorine, when combined with CT, has proven both safe and effective in Chinese clinical trials for HNSCC. Following oncorine, two additional oncolytic viral agents, H103 (developed by Shanghai Sunway Biotech Co., Ltd.) and KH901 (developed by Chengdu Kanghong Biotechnology Co., Ltd.), have also been explored in Chinese clinical trials.186
E10A, a human endostatin gene carried by a type-5 recombinant replication-deficient adenovirus vector developed by Guangzhou Double Bioproducts, Co., Ltd. (Guangzhou, China), can be directly introduced into tumor cells. Here, it is translated into endogenous endostatin protein to limit vascularization.195 Another similar adenovirus, EDS01, combined with CT for HNC, entered a multicenter randomized phase II clinical study in 2017. In the phase I clinical trial in China, intratumoral injection of EDS01 showed better therapeutic effects against metastatic tumors. This offers new insights into the treatment of advanced HNC.
Suicide gene therapy, such as adenoviral vector (AdV)-TK, is another form of gene therapy. AdV-TK is an engineered replication-incompetent adenovirus vector containing a suicide gene called HSV-TK. The protein product of HSV-TK converts the nontoxic antiviral drug GCV into a highly cytotoxic phosphorylated form. The HSV-TK/GCV system was adapted in a phase I Chinese clinical study. Intratumoral injection of AdV-TK followed by systemic administration of GCV proved to be both safe and effective.196 Additionally, another clinical study in China demonstrated significant efficacy when combining AdV-TK/GCV with photodynamic therapy for oral cancer.197
However, the success rates of AdV-based gene therapy for HNSCC have not been satisfactory, likely due to low AdV transduction efficiency. To address this, scientists have developed mutated AdV vectors incorporating the integrin-binding motif RGD, which have shown promising efficacy.198
Recently, a therapeutically relevant fusion transcript named UBE3C–LRP5 fusion has been identified in HNSCC. This represents a promising therapeutic target for HNC, and preliminary findings suggest that pyrvinium pamoate may effectively target this translocation.199
3.4 Epigenetic modification therapy
Epigenetic modifications play crucial role in the progression of HNC, and treatments targeting these modifications have shown great potential. Epigenetic drugs, including DNMT inhibitors and HDAC inhibitors, have been extensively researched in both preclinical and clinical HNC studies. Two DNMT inhibitors including azacytidine and decitabine are under clinical trials in HNC. Azacytidine can inhibit cell growth, induce cell death, downregulate the expression of HPV genes, and stabilize p53 and suppress the expression of MMPs in vitro and in vivo.200 Decitabine has been reported to reverse methylation and sensitive cisplatin in HPV-negative HNSCC. The preclinical data suggest that HDAC inhibition could sensitize HPV-negative HNSCC cells to cisplatin and suppress their proliferation, migration and invasive potential.201 HDAC inhibitors currently in clinical trials for HNSCC include romidepsin, panobinostat, vorinostat, valproic acid, abexinostat, and CUDC-101.202 A trial investigating romidepsin (NCT00084682) as a monotherapy for R/M HNSCC showed limited clinical efficacy and tolerability.203 However, combining vorinostat with cisplatin/RT has shown good tolerability and encouraging efficacy in HPV-negative HNSCC.204 Additionally, combining vorinostat with pembrolizumab has demonstrated higher response rates in advanced staged HNSCC.204
3.5 Combination therapy
HNC is a highly heterogeneous tumor, and the effectiveness of standalone treatments like surgery, radiation, or CT is often limited. In recent years, the advent of immunotherapy and targeted therapy has led to significant breakthroughs in treating R/M HNC.205 By searching the keywords “combination therapy of head and neck cancer” on ClinilcalTrials.gov, we found 2113 combined treatment strategies for HNC, including postoperative combined CT and McAb therapy, RT combined CT and CT combined McAb therapy, RT combined with targeted drug therapy, immunosuppressive therapy combined with CT, and so on.206 Among all relevant clinical trials, the research contents are shown in Table 3. Otherwise, a recent study showed that HSP90 inhibitor ganetespib could increase the therapeutic efficacy of RT for HNC.207 With the deepening of the pathogenesis of HNC, the advancement of precision medicine or the identification of biomarkers, such as HOXB9,208 iron death related gene CISD2,209 Hedgehog (HH),210 FUT6,211 cysteine-rich protein 2 (CSRP2),212 homeobox-D 1,213 variant transcription factor 5 (ETV5214), and the developing of neoadjuvant chemoimmunotherapy (NACI) and photoimmunotherapy,215 clinicians can develop personalized treatments and predict outcomes based on each patient's unique biochemical and genetic profile. This approach further broadens the scope of combination therapy available for HNC patients. For additional details on ongoing clinical trials, refer to Table 3.
| Combinations | Interventions | Target (s) | Clinical trials | Phase | Enrollment | Indication |
|---|---|---|---|---|---|---|
| ICIs + TriAd vaccine | M7824, N803, TriAd vaccine (ETBX-011, ETBX-051 & ETBX-061) |
PD-L1, TGF-β MUC1, IL-15 |
NCT04247282 | I/II | 21 (Actual) | Head and neck neoplasms, HNC |
| ICIs + Anti-B7-H3 antibody |
Enoblituzumab, retifanlimab, tebotelimab |
PD-1, LAG3, CD276 | NCT04634825 | II | 62 (Actual) | Head and neck neoplasms, HNC, HNSCC |
| ICIs + PI3K inhibitor | Duvelisib, pembrolizumab | PI3Kγ, PI3Kδ | NCT04193293 | I/II | 2 (Actual) | HNSCC |
| ICIs + AKT inhibitor |
Ipatasertib pembrolizumab |
AKT, PD-1 | NCT05172258 | II | 52 (Estimated) | HNSCC, R/M HNSCC, R/M OCSCC, R/M OSCC, R/M LSCC |
| ICIs + ATR inhibitor |
Elimusertib pembrolizumab |
ATR, PD-1 | NCT04576091 | I | 37 (Estimated) | HNSCC, R/M HNSCC, R/M OCSCC, R/M OSCC, R/M LSCC, recurrent salivary gland carcinoma |
| ICIs + Oncolytic virus | OBP-301, pembrolizumab | Telomerase, PD-1 | NCT04685499 | II | 1 (Actual) | HNSCC with inoperable recurrent or progressive disease |
| ICIs + Radiation |
Pembrolizumab, radiation |
PD-1 | NCT04318717 | II | 16 (Estimated) | MMHN |
| Nivolumab, radiation | PD-1 | NCT03758729 | II | 26 (Estimated) | Locally advanced, unresectable MMHN | |
| Ipilimumab, nivolumab, radiation | PD-1, CTLA-4 | NCT03799445 | II | 180 (Estimated) | HPV-mediated (p16-Positive) OC, HPV+ OSCC, oropharyngeal basaloid carcinoma, OTSCC, soft palate squamous cell carcinoma | |
|
ICIs+radiation +chemotherapy |
Carboplatin, cisplatin, fluorouracil, paclitaxel, pembrolizumab, radiation | PD-1, DNA | NCT05721755 | III | 290 (Estimated) | HPV-mediated (p16+) OC, metastatic HNSCC, metastatic LSCC, metastatic OCSCC, metastatic OSCC |
| ICIs + IL-2R agonists + radiation | Pembrolizumab, NKTR-214, palliative radiation | IL-2R | NCT04936841 | II | 5 (Actual) | HNC |
| ICIs+chemotherapy | Carboplatin, cemiplimab, paclitaxel | PD-L1 | NCT04862650 | II | 42 (Estimated) | HPV-mediated (p16+) OC, R/M HNSCC, R/M LSCC |
|
ICIs + EGFR inhibitor + chemotherapy |
Atezolizumab, bevacizumab, carboplatin, cisplatin, Cetuximab, docetaxel |
EGFR, PD-L1, DNA | NCT05063552 | II/III | 430 (Estimated) |
R/M HNSCC, R/M NSCC, R/M LSCC R/M lip and oral cavity carcinoma R/M NCSCC |
|
EGFR inhibitor + radiation + chemotherapy |
cetuximab, cisplatin, radiation |
EGFR | NCT01855451 | III | 189 (Actual) | HPV-positive OSCC |
- Abbreviations: HNC, head and neck cancer; HNSCC, head and neck squamous cell carcinoma; LSCC, laryngeal squamous cell cancer; MMHN, mucosal melanoma of the head and neck; MMHN, head and neck mucosal melanoma; NSCC, nasopharyngeal squamous cell carcinoma; OC, oral cavity; OCSCC, oral cavity squamous cell carcinoma; OSCC, oral squamous cell carcinomas; OTSCC, oral tongue squamous cell carcinoma; OCSCC, oral cavity squamous cell carcinoma.
- Sources: Data were obtained from ClinicalTrials.gov.
4 CONCLUSIONS AND PROSPECTS
Over the past three decades, despite advancements in understanding the pathogenesis, development of new detection methods, and the emergence of innovative targeted therapies and immunotherapies, challenges such as drug resistance and tumor recurrence or metastasis continue to complicate the treatment of HNC.13, 216 This comprehensive review delves into the innovative pathogenesis of HNC and summarizes various therapeutic strategies including molecular targeted therapy, immunotherapy, gene therapy, and epigenetic modifications for HNC.
Targeted therapy of HGF/MET may require a combination of EGFR inhibitor strategies.60 Studies have showed that novel combinations of inhibitors targeting EGFR, HER2, and c-Met are more effective against relapsed and resistant HNSCC compared with targeting these pathways individually.217 Related studies have shown that signaling pathways such as HER2118, HER3, ALK1, MEK, RAF, CDK, and IDO1 can also be used as targets for HNSCC clinical trials. Although ICIs therapy has improved outcomes for various cancers, only a small percentage of patients achieve a lasting response. About 20−30% of patients still develop primary or secondary resistance, resulting in tumor recurrence and metastasis.179 Factors such as PD-L1 expression, HPV status, tumor mutational burden, and interferon levels also influence the effectiveness of immunotherapy. Therefore, combining ICIs with other strategies is necessary to combat tumor immune escape.218 ICIs have been reported to improve sensitivity to salvage CT. As the tumor immune microenvironment changes after ICI administration, markers like the neutrophil-to-lymphocyte ratio and CRP levels can predict the efficacy of the treatment.219 Novel agents targeting immune checkpoints and costimulatory receptors, such as LAG-3, OX40, HLA-E, and 4-1BB, are currently evaluated in clinical trials for HNSCC. Additionally, combining ICIs with other anticancer strategies like radiation, surgery, and CT is advancing rapidly.168 In a phase II trial (NCT03799445), the combination therapy of anti-PD-1 and anti-CTLA-4 antibodies with RT is being investigated for patients with HPV-positive oral squamous cell carcinoma. HPV-associated HNSCC has shown greater sensitivity to radioimmunotherapy due to its unique genetic and immunogenomic landscape.220 CAR-T therapy in solid tumors, while promising, still faces numerous challenges that need to be addressed to benefit more HNSCC patients.
Despite these advancements, the OS rate for HNC patients remains low. There is a need to discover new prognostic targets and develop new targeted therapies, such as antibody–drug conjugates (ADCs), photodynamic therapy, radionuclide therapy, and mRNA vaccines, to enhance efficacy and minimize side effects. Clinical trials are ongoing for ADCs like ADRX-0706 (NCT06036121) and BAT8007 (NCT05879627).221 In 2020, Japan approved the photoimmunotherapy drug Akalux (ASP-1929, cetuximab conjugated with IRDye700DX) for use with laser system medical devices in treating unresectable locally advanced or recurrent HNC. Iopofosine I-131 (CLR 131), a novel targeted small molecular phospholipid ether drug conjugate, demonstrated good safety in a phase I study.222 mRNA cancer vaccines like Lipo-MERIT, encoding a fixed combination of shared cancer antigens for HNC, (BNT113/NCT04534205) are also in clinical trials.223
In the near future, mapping the specific genetic profiles of HNC will aid in discovering biomarkers for prognosis. Moreover, highly efficient and strongly immunogenic vaccines targeting neoantigens are rapidly developing.
AUTHOR CONTRIBUTIONS
Yan Liu performed the clinical trial retrieval, main text writing, and production of some figures and tables. Nannan Zhang contributed with the introduction writing and full text modification and supervision. Yi Wen performed the searching of clinical trials and adjusting article format. Jiaolin Wen contributed with the supplement and enhancement of main text writing, production of some figures and tables, and payment for publishing fee. All the authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We express our gratitude to Professor Li Chunjie and Dr Huang Guangzhao from the West China Hospital of Stomatology for their assistance. This work is supported by the grant from the Frontiers Medical Center, Tianfu Jincheng Laboratory (TFJC2024020005).
CONFLICT OF INTEREST STATEMENT
No potential conflict of interest were disclosed.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




