Rational Design, Synthesis, and Biological Assessment of Potential Indole-Capped HDAC6 Inhibitors for Gastric Cancer Suppression
Funding: This study was supported by the National Natural Science Foundation of China (Nos. U21A20416 and 82020108030 to H.M.L.), China Postdoctoral Science Foundation (No. 2023M743233 to X.H.Z.), Science and Technology Project of Henan Province (No. 252102311230 to Y.G.).
Dear Editor,
Gastric cancer and gastroesophageal junction cancer have long been among the top killers in the cancer realm [1]. Moreover, East Asian countries such as China, Japan, and South Korea are known as high-incidence regions for gastric cancer on a global scale. As the limited availability of precision medicine drugs for gastric cancer at present, there is still a need for the development of small molecule compounds targeting novel targets. Accumulating evidence suggests a link between dysregulated histone deacetylase 6 (HDAC6) activity and numerous oncological disorders [2, 3]. HDAC6 is frequently overexpressed in various malignancies, including primary acute myeloid leukemia, gastric cancer, breast cancer, ovarian cancer, and colorectal cancer, and is strongly associated with unfavorable prognosis [4]. These findings underscore the pivotal role of HDAC6 as a prominent therapeutic target in the pathogenesis and progression of diverse tumor types.
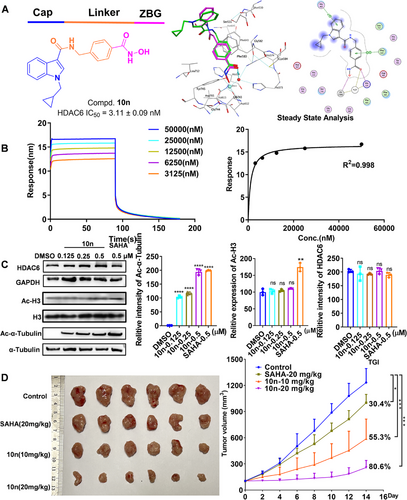
To develop small molecule inhibitors targeting the HDAC6 enzyme, we relied on the in-house library compounds screening strategy and obtained a lead compound. We then optimized the structure of the lead compound L9. Among them, enzyme activity assays revealed compound 10n exhibited excellent inhibitory effects on HDAC6 with an IC50 value of 3.11 nM. Simultaneously, 10n showed notable selectivity in inhibiting HDAC1, HDAC2, HDAC3, and HDAC8 compared to HDAC6, with selectivity ratios of 133.7-fold, 27.8-fold, 82.8-fold, and 622.22-fold, respectively. Ricolinostat (ACY-1215), one selective HDAC6 inhibitor in the clinical stage, exhibited IC50 values of 50, 42, 60, 120, and 5.3 nM for HDAC1, 2, 3, 6, and 8, respectively. The selectivity ratios of HDAC1/6, 2/6, 3/6, and 8/6 are 9.4-fold, 7.9-fold, 11.3-fold, and 22.6-fold, respectively. Compound 10n exhibits greater selectivity against HDAC1, HDAC2, HDAC3, and HDAC8 compared to HDAC6. This indicates that 10n is a significantly selective HDAC6 inhibitor.
Silico docking study revealed that 10n was capable of fitting into the substrate-binding pocket of HDAC6. Additionally, the C═O group was seen to form a hydrogen bond with a Zn2+-bound water molecule, with as O…H separation of 2.05 Å (Figure 1A). Furthermore, the N-benzylformamide-based linker portion nested in the hydrophobic tunnel and was sandwiched between P583 and P643, forming a clear π–π stacking interaction with P643 (Figure 1A). Additionally, BLI verified that 10n exhibits a high affinity for HDAC6, with a Kd value of 330 ± 180 nM (Figure 1B).
Furthermore, 10n exhibited enhanced cytotoxicity against MGC-803 and MKN-45 cells, with IC50 values of 1.067 ± 0.312 and 2.704 ± 0.935 µM at 72 h. EdU incorporation assays and plate clone formation assays were conducted to evaluate the antiproliferative effects of 10n. Upon treatment with 10n, there was a dose-dependent increase in the proportion of apoptotic cells and an increase in mitochondrial membrane potential ratios. Consistent with expectations, 10n could dose-dependently induce the expressions of the PARP, Caspase 3, and Caspase 9, while the expressions of active form cleaved-PARP, cleaved-Caspase 3, and cleaved-Caspase 9 were significantly decreased when cells were exposed to 10n. These findings indicated that 10n induced pro-apoptotic effects through mitochondrial-dependent apoptosis in gastric cancer cells.
Moreover, the interaction between 10n and HDAC6 within cellular environments was conducted. 10n notably stabilized HDAC6, suggesting the binding capability of 10n to HDAC6 at the cellular level by CETSA. Additionally, 10n induced a dose-dependent increase in acetylated α-tubulin levels while having no impact on acetylated H3 levels and HDAC6 (Figure 1C). The expression of PD-L1 acts as a pivotal prognostic marker for the responsiveness of antitumor T cell immunity [5]. It is noteworthy that the PD-L1 expression in MGC-803 cells exhibited a dose-dependent reduction in the 10n treatment cohort compared to the vehicle control group, potentially enhancing the antitumor immune response in gastric cancer cells.
After characterizing the antigastric cancer cell activity of 10n in vitro, a pharmacokinetic study was performed in mice. 10n exhibited a good half-life of 1.61 ± 0.34 h (i.v.) and 2.00 ± 0.44 h (p.o.), demonstrating acceptable systemic exposure with an area under the curve (AUC) value of 349.61 ± 51.93 h ng/mL (i.v.) and 729.98 ± 139.74 h ng/mL (p.o.). The oral bioavailability was calculated to be 41.8 ± 0.08%. Given these favorable pharmacokinetic parameters, 10n was selected for subsequent in vivo antitumor experiments. As indicated in Figure 1D, 10n significantly inhibited the growth of MFC cells at oral doses of 10 mg/kg and 20 mg/kg, achieving tumor growth inhibition rates (TGI) of 55.3% and 80.6%, respectively, surpassing that of pan-HDAC inhibitor SAHA. Notably, the body weight of all mice steadily increased, suggesting its minimal toxicity. Consistent with the in vitro results, treatment with 10n markedly elevated the levels of Ac-α-tubulin with no changes in HDAC6 protein expression were observed in animals treated with 10n. Furthermore, Ki-67 expression decreased upon 10n administration in mice. Histopathological examination using H&E staining demonstrated that 10n exerts a pro-apoptotic function, thereby inhibiting the progression of gastric cancer. The percentages of CD3+CD8+ cells in gastric cancer tumors from two treatment groups were higher than the control. Furthermore, the 10n-treated group exhibited no apparent toxicity, as evidenced by stable weight and absence of organic alterations.
In this study, we identified a novel HDAC6 inhibitor, 10n, which shows the strongest inhibitory effect on HDAC6 with an IC50 value of 3.11 ± 0.09 nM and a high binding affinity for HDAC6 (Kd = 330 ± 180 nM). Subsequent studies have substantiated the significant impact of 10n in inducing apoptosis and alleviating immunosuppression, leading to the inhibition of gastric cancer cells both in vitro and in vivo. Additionally, 10n exhibited promising pharmacokinetic characteristics and showed no significant side effects in vivo. These findings underscore the potential of compound 10n as a promising therapeutic intervention for impeding gastric cancer advancement (Supporting Information).
Author Contributions
Ya Gao: conceptualization, funding acquisition, investigation, methodology, supervision, writing – original draft. Hai-Qian Nie: investigation. Hong-Min Liu: conceptualization, funding acquisition. Xin-Hui Zhang: conceptualization, writing – review & editing, funding acquisition. Li-Ying Ma: conceptualization, writing – review & editing. All authors have read and approved the final manuscript.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. U21A20416 and 82020108030 to H.M.L.), China Postdoctoral Science Foundation (No. 2023M743233 to X.H.Z.), Science and Technology Project of Henan Province (No. 252102311230 to Y.G.).
Ethic Statement
The animal experimentation ethics number is ZZU-URIB-2024-O-109. All experimental animals adhered to the ARRIVE guidelines and were strictly in accordance with the National Research Council Guidelines for the Care and Use of Laboratory Animals.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




