STAT3 Signaling Pathway in Health and Disease
Funding: This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU), Jeddah, Saudi Arabia under grant no. (GPIP: 81-141-24).
ABSTRACT
Signal transducer and activator of transcription 3 (STAT3) is a critical transcription factor involved in multiple physiological and pathological processes. While STAT3 plays an essential role in homeostasis, its persistent activation has been implicated in the pathogenesis of various diseases, particularly cancer, bone-related diseases, autoimmune disorders, inflammatory diseases, cardiovascular diseases, and neurodegenerative conditions. The interleukin-6/Janus kinase (JAK)/STAT3 signaling axis is central to STAT3 activation, influencing tumor microenvironment remodeling, angiogenesis, immune evasion, and therapy resistance. Despite extensive research, the precise mechanisms underlying dysregulated STAT3 signaling in disease progression remain incompletely understood, and no United States Food and Drug Administration (USFDA)-approved direct STAT3 inhibitors currently exist. This review provides a comprehensive evaluation of STAT3's role in health and disease, emphasizing its involvement in cancer stem cell maintenance, metastasis, inflammation, and drug resistance. We systematically discuss therapeutic strategies, including JAK inhibitors (tofacitinib, ruxolitinib), Src Homology 2 domain inhibitors (S3I-201, STATTIC), antisense oligonucleotides (AZD9150), and nanomedicine-based drug delivery systems, which enhance specificity and bioavailability while reducing toxicity. By integrating molecular mechanisms, disease pathology, and emerging therapeutic interventions, this review fills a critical knowledge gap in STAT3-targeted therapy. Our insights into STAT3 signaling crosstalk, epigenetic regulation, and resistance mechanisms offer a foundation for developing next-generation STAT3 inhibitors with greater clinical efficacy and translational potential.
1 Introduction
Signal transducer and activator of transcription (STATs) were first identified in 1988 as proteins that bind to interferon (IFN)-stimulated response elements in DNA sequences and facilitate the transcription of type I IFNs. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway was designated following its discovery in three independent laboratories in 1992. The JAK/STAT signaling pathway function as a crucial regulatory network for various cellular processes. This pathway mediates diverse downstream processes, including apoptosis, tissue repair, inflammation, hematopoiesis, immune regulation, and adipogenesis [1-3]. STAT3, a member of the STATs family, was identified in 1994 and has been implicated in multiple biological processes, such as wound healing, immune response, tissue regeneration, carcinogenesis, cancer stem cell (CSC) regulation, and cell proliferation and differentiation [4].
STAT3 functions as a central regulator where multiple signaling pathways are activated by various molecules, including specific cytokines, peptide ligands, growth factors, and oncogenes [5, 6]. These proteins undergo activation through tyrosine phosphorylation in response to cytokine signals within the cytoplasm. Once activated, STATs translocate into the cell nucleus, where they bind to specific DNA sequences and function as transcription factors [7]. This transcription factor plays a critical role in numerous biological processes, including angiogenesis, cell proliferation, cell growth, and apoptosis [8, 9]. The activation of STAT proteins enhances the transcription of multiple target genes, leading to processes such as angiogenesis, antiapoptotic responses, and uncontrolled cell division [10]. The human body contains six STAT family members: STAT-1, STAT-2, STAT3, STAT-4, STAT-5A, STAT-5B, and STAT-6, each comprising 750–850 amino acids and playing a vital role in cytokine signaling [10].
Among them, STAT3, particularly through the interleukin-6 (IL-6)/JAK/STAT3 axis, has been extensively implicated in both physiological and pathological conditions [7]. Over the past two decades, research has linked persistent STAT3 activation to a wide range of diseases, including bone-related diseases [11], cardiovascular diseases [12], inflammatory diseases [13], autoimmune disorders [14], neurodegenerative diseases [15], and various cancers [16]. Given its widespread role in disease progression, STAT3 has emerged as a promising therapeutic target for drug development. However, despite significant research efforts, no United States Food and Drug Administration (US FDA)-approved direct STAT3 inhibitors currently exist, highlighting a critical gap in translating preclinical findings into clinical applications [16].
While several reviews have explored STAT3's role in individual diseases, a comprehensive review integrating its function in both health and disease is still lacking. Most studies focus on either molecular mechanisms or therapeutic strategies yet fail to bridge the gap between fundamental biology and clinical applications. Additionally, STAT3 activation is highly complex, involving crosstalk with multiple signaling pathways, which contributes to immune evasion and drug resistance [9, 17]. With advancements in targeted therapies, immunotherapies, and nanomedicine-based drug delivery, an updated review consolidating recent discoveries, therapeutic advancements, and future directions is essential for both researchers and clinicians.
This review aims to fill this gap by providing a systematic and in-depth discussion of STAT3's dual role in health and disease, with a particular focus on oncogenesis and targeted therapy. We explore mechanistic insights into STAT3 signaling, including canonical and noncanonical pathways [18], epigenetic modifications [19, 20], and its role in tumor progression, metastasis, and therapy resistance [16]. Additionally, we analyze emerging therapeutic approaches, such as JAK inhibitors (tofacitinib, ruxolitinib) [21, 22], Src Homology 2 (SH2) domain inhibitors (S3I-201, STATTIC) [23, 24], monoclonal antibodies (siltuximab, tocilizumab), antisense oligonucleotides (AZD9150) [25], and innovative nanomedicine-based drug delivery strategies [26]. By evaluating both preclinical and clinical studies, this review seeks to highlight the current challenges and future opportunities in STAT3-targeted therapies.
To ensure clarity and coherence, this review is structured systematically. It begins with a detailed overview of STAT3's molecular structure, activation mechanisms, and biological functions [27]. We then explore STAT3's involvement in multiple diseases, with a strong focus on cancer development and progression [16]. The latter sections highlight therapeutic approaches, including direct STAT3 inhibitors, immunotherapy-based interventions, and nanomedicine-enhanced drug delivery systems (DDSs) [27]. Finally, we discuss current limitations, potential biomarkers for patient stratification, and future research directions. By providing a comprehensive and translational perspective, this review serves as a valuable resource for researchers and clinicians working toward the development of next-generation STAT3-targeted therapies with improved clinical efficacy and specificity.
2 Overview of STAT3
The STAT3 gene is located on chromosome 17 at position 21 on the long arm (17q21). This gene encodes a protein with an approximate molecular mass of 92 kDa, consisting of 770 amino acids. The protein structure comprises several distinct domains, including the N-terminal domain, the coiled-coil domain (CCD), the DNA-binding domain (DBD), the SH2 domain, and the C-terminal domain, also known as the transactivation domain (TAD). The primary activation sites for STAT3 are the tyrosine and serine residues at positions 705 and 727, respectively, within the C-terminal region. Additionally, an alpha-helical linker domain, spanning amino acids 500–575, precedes the SH2 domain. The formation of the STAT3 dimer is dependent on a specific SH2 domain that selectively binds to phosphotyrosine motifs, a process essential for the proper regulation of gene expression [9, 17]. Six isoforms of STAT3 have been identified: STAT3α, STAT3β, STAT3γ, STAT3δ, STAT3ε, and STAT3ζ. While these isoforms perform distinct functions, the classical activities of STAT3 are primarily mediated by STAT3α [28, 29]. The STAT3 gene consists of 24 exons, with alternative splicing of exon 23 leading to the generation of STAT3β, a shorter isoform. This splicing event introduces a frameshift, resulting in the substitution of seven amino acids in the TAD of STAT3α, thereby altering its function [30]. STAT3β exhibits tumor-suppressive properties due to the absence of a specific activation domain present in STAT3α. Additionally, STAT3β plays a role in stabilizing the ternary complex, inhibiting self-renewal and proliferation, reducing chemotherapy resistance, attenuating invasion, and promoting apoptosis [31]. The STAT3γ and STAT3δ isoforms arise through proteolytic processing, which is associated with granulocyte and neutrophil development [32, 33]. In contrast, STAT3ε and STAT3ζ are newly identified truncated isoforms of acetylated STAT3α. Furthermore, the N-terminal region of STAT3ε and the C-terminal region of STAT3ζ, containing different segments of STAT3α, display structural similarities [33]. The domain organization and function of STAT3 are illustrated in Figure 1.

3 Role of STAT3 Signaling in the Development of Various Diseases
Aberrant STAT3 signaling has been linked to a broad spectrum of diseases, including bone-related disorders [11], cardiovascular conditions [12], inflammatory diseases [13], autoimmune disorders, [14] neurodegenerative diseases, [15] and various types of cancer [16]. Figure 2 provides an overview of STAT3 signaling involvement in multiple diseases.
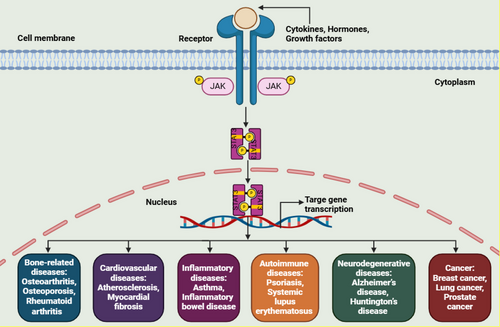
3.1 Modulation of STAT3 Signaling in Bone-Related Diseases
Bone-related diseases comprise a group of chronic conditions, including osteoarthritis (OA), osteoporosis (OP), rheumatoid arthritis (RA), and various bone abnormalities. These disorders are prevalent among elderly and obese individuals. STAT3 functions as an essential downstream signaling protein for numerous cytokines and plays a vital role in modulating cell proliferation and intercellular interactions within the bone microenvironment [11, 34]. Due to its involvement in immune responses and bone metabolism, STAT3 dysregulation has been associated with several bone-related disorders [35, 36]. By influencing mesenchymal stem cell development, osteoclast activation, macrophage polarization, angiogenesis, and cartilage degradation, STAT3 directly contributes to the progression of bone-related diseases [37-39].
The STAT3 signaling pathway play an essential role in cytogenesis and is implicated in the pathogenesis of OA [40, 41]. Persistent activation of STAT3 can disrupt chondrocyte metabolism, promoting catabolic processes that contribute to joint degradation and the formation of osteophytes or bone spurs, ultimately leading to OA-associated bone changes [38, 40]. Various in vitro and in vivo investigations have demonstrated that STAT3 activation in chondrocytes, rather than ERK1/2, induces OA, resulting in cartilage degradation and osteophyte formation [42, 43]. Liang et al. [44] identified a positive correlation between retinoic acid receptor-related orphan receptor α (RORα) expression and OA severity. Furthermore, RORα has been shown to counteract IL-6-induced elevations in p-STAT3, thereby restoring chondrocyte expression of type II collagen (Col-2) and aggrecan [44]. The synovial membrane secretes substantial amounts of inflammatory cytokines, including IL-1, IL-6, TNF-α, and IL-8, which diffuse into the cartilage via synovial fluid. This process activates chondrocytes, enhancing the production of additional proinflammatory cytokines, which in turn accelerates degradation of cartilage and progression of OA [45, 46]. The impact of these cytokines contributes to the initiation and advancement of OA through cytokine-mediated signaling and immune responses [47, 48].
STAT3 plays a crucial role in regulating the inflammatory microenvironment that contributes to OP-related bone loss. OP is often associated with elevated levels of inflammatory cytokines, particularly IL-6, which activates STAT3 [49, 50]. In OP, STAT3 modulates osteoclast activity, promoting bone resorption. Excessive osteoclast activation disrupts bone homeostasis, leading to OP, where reduced bone mineral density significantly increases the disability risk and mortality in older individuals [49, 51]. The receptor activator of nuclear factor κB ligand (RANKL) is a crucial mediator in the interactions between osteoclasts and osteoblasts. It plays a vital role in promoting the differentiation and growth of osteoclasts while simultaneously suppressing the osteogenic differentiation of mesenchymal stem cells [52, 53]. RANKL activates the STAT3 pathway, leading to a reduction in tartrate-resistant acid phosphatase-positive cells and an increase in the expression of NFATc1, a key osteoclast marker [54, 55]. STAT3 facilitates NFATc1 transcription by directly binding to its promoter. The RANKL–STAT3–NFATc1 axis may play a significant role in RANKL-induced osteoclast overactivation, further contributing to OP progression [49].
Similarly, STAT3 serves as a key regulator in the development of RA, and inhibiting its activity has been shown to suppress joint inflammation and osteoclast activation [56, 57]. In individuals with RA, inflammatory cytokines such as IL-6, TNF-α, and IL-1 are the primary activators of STAT3, which subsequently promotes IL-6 expression through a positive feedback loop [58, 59]. Activated STAT3 also upregulates the expression of RANKL, a critical factor involved in osteoclastogenesis [60]. Osteoclasts play a pivotal role in joint destruction associated with RA, contributing to disease progression [61, 62].
3.2 Modulation of STAT3 in the Development of Cardiovascular Diseases
Cardiovascular diseases encompass a group of disorders affecting the heart and blood vessels and represent the leading cause of mortality worldwide [63, 64]. STAT3 has been implicated in various cardiovascular conditions, including atherosclerosis [65], myocardial fibrosis [12], and other related disorders [66]. Atherosclerosis serves as the primary pathological foundation for ischemic and cerebrovascular diseases. Activation of the JAK2/STAT3 pathway is strongly linked to the IL-6 cytokine family, which plays a critical role in endothelial cell dysfunction associated with atherosclerosis [65, 67]. Furthermore, IL-6 functions as a key proinflammatory cytokine, significantly contributing to STAT3-mediated inflammation in the progression of atherosclerosis [68].
Upon activation of the JAK2/STAT3 pathway in vascular endothelial cells, IL-6 has been found to upregulate monocyte chemotactic protein-1 expression, leading to several proinflammatory effects [69]. In atherosclerotic plaques, IL-10 is primarily expressed in macrophages. Unlike the IL-6-induced STAT3 signaling pathway, which promotes inflammation, the IL-10/JAK/STAT3 signaling pathway exerts anti-inflammatory effects in macrophages [70, 71]. Depending on local microenvironmental signals, macrophages differentiate into either a proinflammatory (M1) or anti-inflammatory (M2) phenotype, playing a role in atherosclerosis progression [72, 73]. The JAK2/STAT3 pathway promotes macrophage polarization toward the M1 phenotype, increasing the production of inflammatory molecules such as tumor necrosis factor-alpha (TNF-α), thereby accelerating the development of atherosclerosis [74, 75].
Myocardial fibrosis is characterized by an excessive accumulation of extracellular matrix proteins, primarily collagen, within the myocardium. Research has demonstrated that the JAK/STAT3 pathway plays a crucial role in the cardiac fibrosis process [12, 76]. This pathway can be activated by various profibrotic mediators, including transforming growth factor beta 1 (TGF-β1), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), IL-6, Ang II, serotonin, and endothelin, ultimately contributing to fibrogenesis [12, 77]. Additionally, the JAK/STAT3 pathway serves as a key integrator of multiple profibrotic signaling cascades, leading to increased fibroblast activation and the upregulation of fibrosis-associated genes, such as α-smooth muscle actin, collagens, and fibronectin [78, 79]. Moreover, activated STAT3 can induce epithelial-to-mesenchymal transition (EMT), facilitating the transformation of epithelial cells into mesenchymal cells with enhanced migratory and invasive properties, thereby promoting fibrosis progression [80, 81].
3.3 Modulation of STAT3 in Inflammatory Diseases
STAT3 is activated by various cytokines, including IL-6, IL-10, IL-17, and TNF-α, which play a central role in the onset and progression of inflammatory diseases [82-84]. Persistent or dysregulated activation of STAT3 has been linked to multiple chronic inflammatory conditions, such as asthma [85], inflammatory bowel disease (IBD) [84], and other inflammatory disorders [83].
Asthma is characterized by airway inflammation, leading to increased airway sensitivity and structural remodeling of the airway wall. STAT3 activation, along with elevated Th2 and Th17 cytokine level in the lungs, has been associated with airway inflammation and remodeling [86, 87]. During allergic inflammation, STAT3 regulates the recruitment of immune cells, particularly Th2 cells, and contributes to the production of Th17 cells [88]. Th17 cell activation is mediated by cytokines such as TGF-1, IL-1, IL-6, and IL-23, resulting in increased expression of transcription factors specific to Th17 differentiation, including RORγ and RORα [85, 89]. Dysregulated production of IL-17 results in the generation of proinflammatory cytokines and chemokines, which subsequently recruit inflammatory cells to the affected site [90]. Several studies have indicated that prolonged IL-17 activation contributes to enhanced deposition of collagen, increased mass of airway smooth muscle, and enlarged mucous glands [91, 92].
IBD encompasses a group of chronic disorders affecting the colon and small intestine. STAT3 plays a critical role in inflammation, tissue repair, and immune regulation in IBD [93, 94]. More than 160 genetic loci have been associated with IBD susceptibility, including STAT3-related genes involved in intestinal mucosal immune responses [95]. The pathophysiology of IBD is characterized by elevated levels of cytokines such as IL-6, IL-22, and IL-23, which serve as ligands for cell surface receptors, leading to STAT3 activation [96, 97]. The effects of STAT3 activation are context-dependent, influenced by the cellular environment. STAT3 promotes regulatory T cells (Tregs) to modulate excessive immune responses while also facilitating Th17 cell development and survival, thereby contributing to chronic inflammation [98, 99]. Increased STAT3 expression in T-cells, macrophages, and epithelial cells has been strongly correlated with histological inflammation severity [100]. Additionally, STAT3 activation in T-cells has been implicated in the pathogenesis of colitis [101, 102].
3.4 Modulation of STAT3 in Autoimmune Diseases
STAT3 plays a crucial role in the early development and maturation of B-cells within the bone marrow. Additionally, it facilitates class-switch recombination in B-cells in response to specific cytokines, which is necessary for generating distinct antibody isotypes [103-105]. STAT3 also regulates multiple aspects of natural killer (NK) cell biology, including NK cell development, activation, cytotoxic function, and modulation of innate and adaptive immune responses [106, 107]. Dysregulated or excessive STAT3 signaling has been associated with the onset and progression of various autoimmune diseases, including psoriasis and systemic lupus erythematosus (SLE) [108-110]. Psoriasis is a chronic autoimmune disorder characterized by red, dry, itchy, and scaly skin patches. It is primarily driven by Th17 lymphocytes, which differentiate from naive T-cells upon IL-6 stimulation [111, 112]. Inflammatory interactions between Th1 and Th17 cells and keratinocytes further contribute to psoriasis pathogenesis [113, 114]. The skin of individuals with psoriasis exhibits elevated STAT3 expression [115, 116]. The topical application of a STAT3 inhibitor has been shown to reduce psoriatic lesions in both transgenic mice and clinical patients [117, 118].
SLE is a chronic autoimmune disorder marked by extensive inflammation and autoantibody production [119, 120]. Hyperactivity of immune cells, including T-cells, B-cells, and dendritic cells, has been associated with abnormal STAT3 activation in SLE [121, 122]. This dysregulated activation promotes the production of autoantibodies and immune complexes, contributing to tissue damage in the kidneys, skin, joints, and other organs [123]. STAT3 transactivates IL-10 in T-cells of SLE patients through epigenetic remodeling [124, 125]. An imbalance between Th17 and Tregs is believed to play a critical role in SLE progression, leading to an enhanced proinflammatory response, particularly during active disease phases [126, 127]. The use of STAT3 inhibitors in combination with immunosuppressive therapies has been shown to improve SLE by restoring this balance, while agents targeting STAT3 phosphorylation have demonstrated efficacy in treatment [109, 128]. Additionally, increased Th17 proliferation and an active IL-17/STAT3 axis have been implicated in SLE pathogenesis [129].
3.5 Modulation of STAT3 in Neurodegenerative Diseases
STAT3 plays a key role in the response to neurotrophic factors, such as nerve growth factor and brain-derived neurotrophic factor (BDNF), which support neuronal survival and development [130, 131]. Following injury, STAT3 activation in brain astrocytes contributes to scar formation and tissue repair in the central nervous system [132, 133]. Its activation leads to the upregulation of genes involved in neuroprotection, neuroregeneration, and neurodevelopment [134].
Alzheimer's disease (AD) is the primary contributor of dementia in elderly populations [135, 136]. STAT3 has been implicated in neuroinflammation and the progression of AD [137]. The JAK2–STAT3 signaling pathway influences astrocytes, hippocampal neurons, and microglia, contributing to disease pathology [138, 139]. Additionally, STAT3 has been shown to impact A-β42, beta-site APP cleaving enzyme 1 (BACE1), tau tangles, and other key components in the AD brain [140, 141].
Huntington's disease (HD) is a fatal neurodegenerative disorder characterized by striatal neurodegeneration, the accumulation of mutant huntingtin (mHTT), and the presence of reactive astrocytes. STAT3 activation plays a crucial role in regulating inflammatory responses in glial cells, particularly astrocytes, and microglia, which are essential for maintaining brain homeostasis [142, 143]. Persistent STAT3 activation in HD has been associated with increased neuroinflammation, a hallmark of the disease [131, 144]. The JAK2–STAT3 pathway regulates astrocyte reactivity and has been found to be activated in the putamen of individuals with HD [145]. Moreover, inhibition of the JAK2–STAT3 pathway in reactive astrocytes has been shown to reduce their reactive characteristics while increasing the accumulation of mHTT aggregates [146, 147].
3.6 Modulation of STAT3 in Different Cancer Types
Cancer remains one of the leading causes of mortality worldwide, with its incidence and prevalence increasing annually as the population ages [148, 149]. Several key signaling pathways, including the mammalian target of rapamycin, Wnt, STAT3, mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase, play essential roles in cell growth, survival, and proliferation, and their dysregulation is a hallmark of cancer [150]. STAT3, in particular, has been strongly linked to the progression of multiple cancer types, including malignancies of the head and neck, lungs, stomach, liver, colon, prostate, and breast, where it facilitates cancer cell proliferation, invasion, and metastasis [151, 152]. Research indicates that STAT3 influences DNA modification and chromatin remodeling in the nucleus through epigenetic mechanisms [5, 153]. Additionally, STAT3 contributes to the regulation of immune responses within various tumor microenvironments (TMEs). The existing scientific literature underscores the critical role of STAT3 in multiple human diseases, particularly cancer. The following sections will examine the involvement of STAT3 in cancer progression and explore its potential as a therapeutic target, highlighting both the opportunities and challenges associated with these approaches.
4 The Canonical and Noncanonical STAT3 Signaling Pathways in Cancer
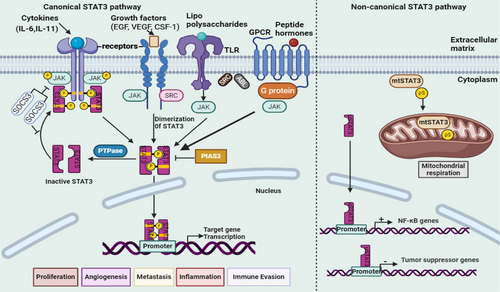
The cytoplasm contains an inactive monomeric STAT3 unit under normal conditions. Activation of STAT3 requires the phosphorylation of tyrosine 705, a critical step in the canonical STAT3 activation process. Once activated, STAT3 interacts with various receptors stimulated by cytokines and growth factors [18]. Following activation, two monomeric STAT3 units associate via the tyrosine 705 residue, forming either homodimers (composed of two STAT3 molecules) or heterodimers (a STAT3 molecule paired with another STAT protein). Upon nuclear translocation, these dimers regulate the expression of genes involved in multiple cellular functions, including cell division maintenance [154], metastasis promotion [16], angiogenesis facilitation [155], inflammation induction [156], apoptosis inhibition [157], immune response suppression [158], TME modulation [159], CSC maintenance [158], metabolic alterations [154], drug resistance development [160], and the mediation of cancer hallmark activities through exosomes [161]. This regulation occurs upon binding to the SH2 domain. In addition to tyrosine 705, serine 727 represents another critical functional site within STAT3. Serine and threonine kinases facilitate the phosphorylation of serine 727, thereby contributing to STAT3 activity [162]. STAT3 regulation is primarily controlled by protein tyrosine phosphatases (PTPases), which dephosphorylate STAT3, modulating its function. Furthermore, suppressor of cytokine signaling 3 (SOCS3) inhibits STAT3 at the receptor level, whereas the protein inhibitor of activated STATs 3 (PIAS3) suppresses STAT3 function at the gene transcription stage [70].
The canonical signaling pathway involves STAT3 activation through the phosphorylation of tyrosine 705. Research has demonstrated that STAT3 can shuttle between the cytoplasm and nucleus, influencing cellular processes even in its nonphosphorylated state [18]. Kinases such as MAPK, JNK1/2, GSK3α/3β, and CDKs phosphorylate STAT3 at serine 727, enhancing its mitochondrial functions without requiring nuclear localization. GRIM-19 facilitates the import of phosphorylated serine STAT3 (P-Ser-STAT3) into mitochondria, where it regulates electron transport chain complexes I, II, and V. Mitochondrial STAT3 (mtSTAT3) contributes to ATP production, reduces reactive oxygen species release, and enhances mitochondrial calcium uptake and mitochondrial permeability transition pore regulation. In addition to phosphorylation, STAT3 undergoes acetylation at lysine 685 (K685Ac), a modification that influences its stability, activity, and interactions with other proteins, thereby broadening its functional repertoire [33, 134, 163].
The noncanonical STAT3 pathway is characterized by β-catenin-independent mechanisms, encompassing intracellular signaling and target gene regulation. It is implicated in biological processes such as tissue repair, immune regulation, tumor progression, and metabolic adaptation [152, 164]. In the cytoplasm, STAT3 modulates signaling pathways through interactions with proteins such as NF-κB, thereby influencing survival and inflammatory responses. It also regulates microtubule dynamics and cytoskeletal organization, facilitating metastasis and cell migration [165]. Nonphosphorylated STAT3 dimers may participate in epigenetic regulation by directly or indirectly modulating gene expression through interactions with chromatin modifiers [18]. Additionally, in its unphosphorylated form, where phenylalanine replaces tyrosine 705, STAT3 regulates NF-κB transcription and genes associated with EMT by interacting with either unphosphorylated NF-κB or Jun activation domain-binding protein 1, contributing to tumor suppression [33]. Figure 3 illustrates the mechanism of action of canonical and noncanonical STAT3 signaling pathways.
5 Various Roles of STAT3 Signaling in Cancer
5.1 Oncogenic Role of STAT3 Signaling
Oncogenes such as K-ras and src are specialized genes capable of transforming normal cells into cancerous cells upon activation [166, 167]. These oncogenes may be introduced into cells through viral infection or arise from mutations in normal genes. Persistent STAT3 activation has been observed in breast cancer [168], suggesting a direct association with oncogenic signaling. Cells infected with Epstein–Barr virus and human T-lymphotropic virus 1 exhibit continuous STAT3 activity due to increased tyrosine kinase activity [169]. STAT3 functions as a central regulator in several well-established oncogenic pathways and plays a key role in activating Toll-like receptors (TLRs), G protein-coupled receptors (GPCRs), fibroblast growth factor (FGF), insulin-like growth factor, IL-6, IL-11, IL-10, and IFN-α [170]. By activating multiple downstream targets, angiotensin II receptor (AgtR2) and IFN contribute to the regulation of diverse cellular processes. These include cyclin D1, which governs the cell cycle; c-Myc, which promotes cell proliferation; matrix metalloproteinases (MMP)-2 and MMP-9, which facilitate tissue remodeling; and VEGF, which supports angiogenesis. Additionally, STAT3 influences cyclooxygenase-2 (COX-2), involved in inflammation; survivin, which inhibits apoptosis; and programmed cell death ligand 1 (PD-L1), which modulates immune responses [171-173]. Research has demonstrated that STAT3 remains persistently activated in v-Src-transformed cells, suggesting its potential as a therapeutic target in cancer. The introduction of a constitutively active STAT3 variant has been shown to be necessary for transforming immortalized fibroblasts and normal epithelial cell lines derived from the breast or prostate [7, 174]. These findings indicate that aberrant STAT3 activation may lead to persistent alterations in gene expression patterns, ultimately contributing to malignant transformation.
Inflammatory cytokines such as IL-6 play a key role in immune responses and can be produced following STAT3 activation. Activated STAT3 induces the expression of angiogenic proteins, including VEGF and hypoxia-inducible factor 1-alpha (HIF-1α), which are critical for new blood vessel formation. These processes contribute to a tumor-supportive microenvironment that facilitates cancer progression and metastasis [175]. Constitutive activation of STAT3 has been observed in both solid tumors and hematological malignancies, such as leukemia and lymphoma, and serves as a prognostic marker for disease progression [16, 33]. In gastric cancer, elevated levels of phosphorylated STAT3 (pY705 STAT3) have been associated with reduced overall survival [176, 177]. Similarly, ovarian and prostate cancers exhibit increased STAT3 activity [160, 178]. In colorectal cancer, heightened STAT3 expression correlates with tumor invasion, lymph node metastasis, and tumor progression [179, 180]. Additionally, elevated STAT3 levels have been linked to poor clinical outcomes in several malignancies, including cervical cancer [181], esophageal squamous cell carcinoma [182], and squamous cell carcinoma of the head and neck (HNSCC) [183]. These findings highlight the critical role of activated STAT3 in oncogenesis, supporting the potential of STAT3 suppression as a therapeutic strategy for specific cancer types.
The STAT3C construct has shown to enhance tumor growth in various cell types by upregulating key factors such as MMP-9, VEGF, and C-terminal Tensin-like [184, 185]. Elevated STAT3 expression contributes to tumor development and progression by inhibiting apoptosis in cancer cells [6, 186]. A study analyzing tissue samples from breast cancer patients, both with and without lymph node metastasis, reported a correlation between increased levels of phosphorylated STAT3 (phospho-STAT3) and improved short- and long-term survival rates [187]. According to Pascal et al. [188], STAT3 regulates the ARF–MDM2–p53 pathway, where ARF inhibits MDM2, a protein responsible for targeting p53 for ubiquitination and proteasome-mediated degradation. This suggests that STAT3 may influence the tumor-suppressive function of p53. A reduction in STAT3 signaling in a prostate cancer mouse model has been associated with an increased likelihood of metastasis and disease recurrence. These findings indicate that inhibiting the IL-6/STAT3 pathway may not be a viable therapeutic approach for prostate cancer and could potentially result in poorer clinical outcomes [188].
5.2 | Activation and Regulation of STAT3
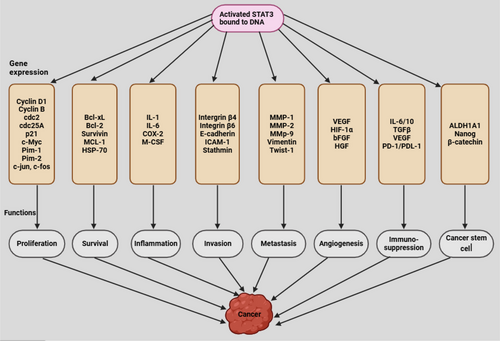
STAT3 regulates the initiation and progression of human cancer through multiple mechanisms [153, 160]. Activation of STAT3 by tyrosine kinase inhibitors, a class of cancer therapeutics, has been associated with the development of resistance to these treatments [189, 190]. Persistent STAT3 activation enhances the production of antiapoptotic proteins such as survivin, MCL-1, Bcl-2, and Bcl-xL while simultaneously downregulating the Fas signaling pathway, which plays a role in programmed cell death [9, 172, 191]. STAT3 directly interacts with the promoters of MMPs, including MMP-1, MMP-2, MMP-7, and MMP-9, leading to increased expression of these proteins in various aggressive malignancies [16]. Additionally, STAT3 promotes the generation of CSCs and facilitates EMT by upregulating transcription factors associated with EMT, such as N-cadherin, TWIST, ZEB1/2, Snail, and Vimentin, while suppressing E-cadherin, a key protein involved in cell-cell adhesion [192]. Emerging evidence suggests that STAT3 plays a crucial role in modulating immune responses related to tumor development and immune suppression [9, 165]. In lymphoma-associated macrophages, STAT3 regulates the expression of immune checkpoint proteins, including programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1), contributing to immune evasion in cancer [171]. Figure 4 demonstrates STAT3 activation leading to cancer development.
5.2.1 Regulation of STAT3 Signaling
Under normal physiological conditions, STAT3 is tightly regulated and functions as both an oncogene and a transcription activator. Scientific studies have indicated that STAT3 remains persistently activated in various cancers and plays a crucial role in tumor initiation and progression [164, 186]. STAT3 influences gene expression through epigenetic modulation. Acetylated STAT3 has been shown to facilitate DNA methylation-mediated silencing of tumor suppressor genes, while unphosphorylated STAT3 contributes to chromatin organization [19, 20]. Despite its association with multiple regulatory mechanisms and its significance in numerous biological processes, effective therapeutic strategies for STAT3 inhibition in clinical applications have not yet been fully developed. Further investigation into the complex roles of STAT3 in different cancer types is essential for the development of successful therapies targeting the STAT3 signaling pathway.
5.2.1.1 Positive Regulators of STAT3
Phosphorylation of the pY705 residue occurs through multiple pathways that activate the STAT3 signaling cascade. These pathways include nonreceptor tyrosine kinases (nRTKs), such as Src and Abl kinases, cytokine receptors that activate JAKs, and receptor tyrosine kinases (RTKs), including epidermal growth factor receptor (EGFR) and PDGF receptor [193, 194]. STAT3 undergoes phosphorylation at both S727 and Y705 residues, with kinases such as cyclin-dependent kinase 5 (CDK5) and MAPK mediating S727 phosphorylation. Additionally, phosphorylation at both pY705 and pS727 is required for complete STAT3 activation [195, 196]. Acetylation of STAT3 at the K685 residue enhances its dimerization capacity and increases transcriptional activity, thereby strengthening its role in gene regulation [19, 197]. A key mechanism through which STAT3 exerts its effects involves the secretion of growth factors and cytokines by the TME. This persistent stimulation of STAT3 occurs through paracrine (between adjacent cells) or autocrine (within the same cell) signaling mechanisms [198] Continuous exposure to regulatory molecules within the TME leads to sustained STAT3 activation. Activators such as IL-6, IFN-γ, and EGF stimulate the JAK/STAT pathway, which is critical for tumor progression and metastasis [199]. IL-6, a cytokine widely distributed in the TME, functions as a mediator of both pro- and anti-inflammatory responses. Upon binding to the IL-6 receptor (IL-6R) on cell membranes, IL-6 forms a complex with gp130 or IL-6Rβ, triggering STAT3 activation and promoting tumor development [200]. In the trans-signaling pathway, the soluble form of IL-6 receptor (sIL-6R) binds to IL-6, and the IL-6/sIL-6R complex subsequently interacts with gp130 to facilitate signaling [201]. Furthermore, activated STAT3 can enhance IL-6 expression, establishing a positive feedback loop that sustains STAT3 hyperactivation. In prostate cancer, IL-6-induced STAT3 activation accelerates tumor progression, a condition clinically identified as neuroendocrine-differentiated prostate cancer [202].
JAK1, JAK2, JAK3, and TYK2 are nRTKs belonging to the JAK family. These enzymes play a crucial role in transmitting signals from various cytokine receptors to the intracellular environment, where they regulate cell development and immune responses. TYK2, JAK1, and JAK2 are expressed in multiple cell types, whereas JAK3 expression is predominantly restricted to hematopoietic cells. Upon interaction with gp130, JAK activation occurs, leading to the phosphorylation of STAT3 [203]. Additionally, mutations in nRTKs contribute to the expression of the oncoprotein BCR–ABL, which has been associated with the development of hematological malignancies, including leukemia, lymphoma, and myeloma, through the STAT3 signaling pathway [204, 205].
Multiple signaling pathways can activate STAT3 through JAK/STAT3 activation, extending beyond the IL-6/JAK/STAT3 pathway. Receptors such as EGFR, GPCRs, CXCR, FGF receptor, and B7-H3 initiate these signaling cascades. Exposure to carcinogens that phosphorylate STAT3 can also trigger these pathways [206, 207]. Protein tyrosine kinases (PTKs), including Lyn, Fyn, Hck, Src, Lck, and Fgr, contribute to STAT3 activation. Furthermore, in the absence of JAK, viral Src induces constitutive STAT3 activation. Cellular Src tyrosine kinase, activated by ligands of the human EGFR family and PDGF, positively regulates STAT3 activity, enhancing its responsiveness to growth factors [27]. EGFR, frequently overexpressed in epithelial malignancies, promotes cancer cell survival and progression. It directly interacts with active STAT3 and phosphorylates it, thereby increasing its activity. Targeting both STAT3 and EGFR has been shown to disrupt the feedback loop between these proteins and inhibit pancreatic cancer progression [190]. Additionally, two well-characterized GPCRs, sphingosine-1-phosphate receptor and AgtR2, activate STAT3 via JAKs [20]. TLRs, including TLR2, TLR3, TLR4, TLR7, and TLR9, are expressed in various immune and epithelial cells, as well as in stromal compartments. These receptors play a critical role in regulating immune responses and influencing cancer progression [33]. TLR stimulation directly activates STAT3 during human B-cell IgG production and is crucial for both antibody and IL-10 production [208]. The classical TLR4 activator, lipopolysaccharide, significantly increases phosphorylated STAT3 levels in human bladder cell line T24, highlighting the involvement of TLR4 signaling in STAT3 activation [209].
Noncoding RNAs, including microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), also regulate STAT3 activity [210]. By interacting with specific mRNA targets, these ncRNAs either promote mRNA degradation or inhibit translation, thereby modulating STAT3 expression [211]. Several lncRNAs, such as HOTAIR, ITIH4-AS1, GACAT3, NEAT1, and FOXD2-AS1, have been identified as positive regulators of the STAT3 pathway. Additionally, miRNAs that enhance STAT3 activation include miR-629, miR-34a, miR-149, miR-495-3p, and miR-24 [27, 153]. Conversely, STAT3 can also be indirectly activated by miRNAs such as miR-182-5p, miR-203, miR-221-3p, and miR-4449. This activation occurs through the suppression of members of the SOCS family and PIAS [27, 33].
5.2.1.2 Negative Regulators of STAT3
To prevent excessive activation, several regulators tightly control STAT3 signaling in normal tissues, maintaining a balanced state. However, tumor cells often suppress these negative regulators, allowing sustained STAT3 activation. Targeting these regulators, either by directly inhibiting STAT3 or by disrupting the STAT3 signaling pathway, presents potential therapeutic strategies for cancer treatment.
Tyrosine phosphatases play a crucial role in the negative regulation of STAT3 through dephosphorylation [212]. The PTP family, which includes T-cell PTP (TC-PTP), SH2-domain-containing PTP1 (SHP1), SHP2, PTP-nonreceptor type 9 (PTPN9), PTP receptor-type D (PTPRD), PTP receptor-type T (PTPRT), and PTP receptor-type K (PTPRK), is essential for regulating the JAK–STAT3 pathway by dephosphorylating STAT3 [18, 32]. Reduced PTPRD expression has been reported in nasopharyngeal carcinoma (NPC), whereas increased PTPRD levels enhance the sensitivity of NPC cells to radiotherapy by decreasing STAT3 phosphorylation [213]. Similarly, reduced PTPRT expression is associated with elevated phosphorylated STAT3 levels and increased susceptibility to STAT3 inhibition in HNSCC [213]. PTPRK has been shown to suppress tumor growth by inhibiting EGFR signaling [214]. Additionally, in triple-negative primary breast cancer, TC-PTP deficiency enhances cell proliferation by strengthening Src family kinase (SFK) and STAT3 signaling pathways [215, 216].
The SOCS protein family consists of cytokine-inducible SH2-containing protein and SOCS1-7, which inhibit STAT3 activation through distinct mechanisms. These proteins can directly bind to specific regions of the JAK protein or interact with JAK-activated cytokine receptors, thereby preventing STAT3 activation [217]. Yoshikawa et al. [218] reported that silencing SOCS1 led to persistent activation of the JAK2/STAT3 pathway in liver cancer cells. Restoring SOCS1 function suppressed cell proliferation in a manner similar to AG490, a JAK2 inhibitor, indicating that SOCS1 plays a crucial role in the negative regulation of the JAK/STAT3 pathway [218]. Additionally, SOCS1 suppresses the expression of CDK2 and 4, along with cell cycle regulatory proteins such as cyclin D1 and cyclin E, thereby inhibiting prostate cancer growth and metastasis [27]. A study demonstrated that SOCS1 and SOCS3 facilitate myogenic differentiation by inhibiting JAK1 and gp130 signaling [219]. Reduced expression or mutations in SOCS1 and SOCS3 have been associated with sustained STAT3 activation, accelerating the progression of pancreatic ductal adenocarcinoma [220], prostate cancer [221], and glioblastoma [222].
A distinct class of proteins, known as PIAS, regulates STAT3 activity within the nucleus. Four PIAS genes—PIAS1, PIAS2, PIAS3, and PIAS4—have been identified in mammals. These proteins inhibit STAT3 function by binding to activated STAT dimers, thereby preventing their interaction with DNA and subsequent gene expression modulation [223]. Among the PIAS family, PIAS3 serves as a key negative regulator. Several studies have demonstrated that elevated PIAS3 expression can suppress cell proliferation and enhance tumor sensitivity to specific therapeutic agents [224]. Jiang et al. [225] reported that excessive activation of the JAK/STAT pathway, due to SOCS3 and PIAS3 abnormalities, contributes to the formation of early-stage breast cancer myeloid-derived suppressor cells. These cells suppress immune responses, facilitating cancer progression. PIAS1, which is overexpressed in human prostate cancer, downregulates p21 expression, thereby promoting cancer cell survival [223]. Conversely, PIAS3 overexpression has been shown to inhibit lung cancer cell proliferation and restore sensitivity to chemotherapeutic drugs [226]. Furthermore, increased PIAS3 expression induces apoptosis in cancer cells, highlighting its potential role in cancer suppression [227].
Several lncRNAs have been identified as enhancers of STAT3 expression. A correlation has been observed between tumor progression, poor patient prognosis, and a consistent reduction in the expression of these lncRNAs in cancerous tissues. Certain lncRNAs exhibit an inverse relationship with STAT3, suggesting their role as negative regulators of STAT3 signaling. Research has shown that specific miRNAs, including members of the miR-548d-3p and miR-17 cluster families, as well as lncRNAs such as PTCSC3, MEG3, and lncRNA-p21, can directly target STAT3 [27]. Given the diverse functions of STAT3, the development of novel small molecules capable of directly targeting STAT3 presents a promising approach for cancer therapy [8, 148]. Over the past three decades, multiple inhibitors targeting STAT3, either directly or indirectly, have been identified. Encouragingly, most of these inhibitors have demonstrated strong tumor-suppressive effects in both preclinical and clinical studies. However, a specific STAT3 inhibitor has yet to receive approval for clinical use.
6 STAT3: Therapeutic Target
6.1 Small Molecule Inhibitor-Based Therapies
Various small-molecule inhibitors that directly target STAT3 have shown promising results in different in vitro models. However, no STAT3-specific small-molecule inhibitors employed in clinical trials due to limitations in conventional drug design methods and inefficiencies in screening processes [228]. The testing of thousands to millions of compounds remains impractical due to high failure rates, extensive time requirements, and significant development costs [229]. Therefore, identifying STAT3 inhibitors from naturally occurring compounds or repurposing existing drugs presents a more efficient and feasible approach. The pharmacokinetics and safety profiles of clinical drugs and natural products have already been established through absorption, distribution, metabolism, and elimination (ADME) studies [230]. Tables 1, 2, and 3 provide a comprehensive list of commonly used drugs that inhibit STAT3 in preclinical and clinical trials, offering potential avenues for accelerating the development of STAT3-targeted therapies in clinical applications.
| Names of inhibitors | Target | Mechanism of action | Preclinical outcomes | References |
|---|---|---|---|---|
| BP-1–102 | Direct inhibitor, SH2 | Prevents nuclear translocation and STAT3 dimerization | Triggers apoptosis and suppresses development of tumor against glioma cells | [231] |
| Stattic | Direct inhibitor, SH2 | Stops dimerization process | Promotes apoptosis and inhibits DNA-binding capacity | [232, 233] |
| LLL12 | Direct inhibitor, SH2 | Inhibits phosphorylation at Tyr705 | Enhances the suppression of tumor growth, inhibits cell migration, triggers apoptosis | [234] |
| SG-1709 | Direct inhibitor, DBD | Reduces STAT3 phosphorylation | Inhibits breast cancer growth | [235] |
| Periplogenin | Direct inhibitor, SH2 | Suppresses Tyr705 phosphorylation of STAT3 | Induces apoptosis and inhibits the growth of prostate cancer and esophageal squamous cell carcinoma | [236] |
| HP590, HJC0152 | Direct inhibitor, DBD | Inhibits phosphorylation of STAT3 | Prevents the growth of gastric cancer | [177, 237] |
| MS3-6 | Direct inhibitor, CCD | Decreases nuclear translocation and DNA binding | Interferes STAT3's interaction with the IL-22 receptor and prevents STAT3-dependent transcriptional activation | [23] |
| TG101209 | Indirect inhibitor, JAK2 | Inhibits signaling axis of JAK2/STAT3/c-MYB | Prevents Burkitt lymphoma cell proliferation and trigger apoptosis | [238] |
| Cirsiliol | Indirect inhibitor, TYK2 | Reduces STAT3 nucleus localization and dimer formation | Prevents the proliferation of esophageal squamous cell carcinoma | [239] |
| SC-78 | Indirect inhibitor, SHP1 | Prevents STAT3 phosphorylation | Inhibits human colorectal cancer cells' stemness | [240] |
- Abbreviations: TYK2, tyrosine kinase 2; SHP1, Src homology region 2 (SH2) domain-containing phosphatase 1.
| Name of compound | Mechanism of action | Indication | Phases | Clinical trial identifier | References |
|---|---|---|---|---|---|
| Napabucasin/BBI608 | Phosphorylation inhibitor | NSCLC | 3 | NCT02826161 | [241] |
| Advanced malignancies | 1/2 | NCT01775423 | [242] | ||
| CRC | 3 | NCT01830621 | [243] | ||
| Metastatic colorectal cancer | 3 | NCT03522649 | [195] | ||
| Metastatic pancreatic ductal adenocarcinoma | 3 | NCT02993731 | [244] | ||
| Metastatic colorectal cancer | 2 | NCT03647839 | [245] | ||
| Celecoxib | Phosphorylation inhibitor | CRC | 3 | NCT00087256 | [246, 247] |
| C188-9 | Phosphorylation inhibitor | NSCLC, CRC, HNSCC, BC, HCC, melanoma, GAC, advanced cancer | 1 | NCT03195699 | [248, 249] |
| OPB-51602 | Phosphorylation inhibitor | Advanced solid tumors | 2 | NCT01423903 | [250] |
| Nasopharyngeal carcinoma | 1 | NCT02058017 | [251] | ||
| Hematological malignancies | 1 | NCT01344876 | [252] | ||
| OPB-111077 | Phosphorylation inhibitor | Solid tumors | 1 | NCT01711034 | [253] |
| Acute myeloid leukemia | 1 | NCT03197714 | [254] | ||
| Advanced HCC | 1 | NCT01942083 | [255] | ||
| Disulfiram | Phosphorylation inhibitor | Metastatic pancreatic cancer and refractory solid tumors | 1 | NCT02671890 | [250] |
| WP1220 | Phosphorylation inhibitor | Cutaneous T-cell lymphoma | 1 | NCT04702503 | [256] |
| TTI-101/C188-9 | Phosphorylation inhibitor | HCC, NSCLC, HNC, NSCLC, breast, gastric, colorectal melanoma | 1 | NCT03195699 | [257] |
| Pyrimethamine | Phosphorylation inhibitor | Small lymphocytic lymphoma, CLL | 1/2 | NCT01066663 | [258, 259] |
| AZD9150 | Reduces the STAT3 protein's expression by attaching to its mRNA | Lymphoma | 1/2 | NCT01563302 | [260] |
| Saracatinib | Targets SRC | Osteosarcoma | 2 | NCT00752206 | [33] |
| Momelotinib | Targets JAK1/2 | Non-small-cell lung cancer | 1 | NCT02258607 | [261] |
- Abbreviations: NSCLC, non-small cell lung cancer; CRC, colorectal cancer; HNSCC, head and neck squamous cell carcinomas; HCC, hepatocellular carcinoma; BC, breast cancer; GAC, gastric adenocarcinoma; HNC, head and neck cancer; CLL, chronic lymphocytic leukemia.
| Drugs | Name of material | Delivery routes | Cell/tissue specificity | References |
|---|---|---|---|---|
| Curcumin-loaded liposomes-STAT3 siRNA | Liposomes | Intratumorally administration | Skin cancer | [347] |
| DOX/CALP | Liposomes | Intratumorally administration | Ovarian cancer | [348] |
| Hyaluronic acid/TN-CCLP | Liposomes | Intravenous administration | Breast cancer | [27] |
| LP-R/C@AC NPs | Liposomes | — | Gastric cancer | [349] |
| Stattic | Liposomes | — | Melanoma cells | [350] |
| NP-Stattic-IL20RA | Liposomes | — | Breast cancer | [241] |
| HA/siSTAT3 PPLPTX | Polymer | Intravenous administration | Breast cancer | [351] |
| CSA/Gef-NPs | Polymeric micelles | — | Lung cancer | [352] |
| SVMAV | Polymeric micelles | — | Melanoma | [353] |
| Gel-NSC74859-ICG | Polymer | Intravenous administration | Head and neck squamous cell carcinomas (HNSCCs) | [27] |
| Ritonavir derivative | Polymer | Intravenous administration | HNSCC | [354] |
| Cucurbitacin-D; doxorubicin | Polymer | Intravenous administration | Breast cancer | [355] |
| siRNA-SS-PNIPAM | Polymeric micelles | — | Glioblastoma tumor | [356, 357] |
| Chol–DsiRNA Polyplexes | Polymeric micelles | Intravenous administration | Breast cancer | [358] |
| AuNP-NUAP–STAT3d | Inorganic material | — | HNSCC cells | [27, 260] |
| LbL-AuNP | Inorganic material | Intratumorally administration | Melanoma cells | [350] |
| AIRISE-02 siRNA–CpG–mesoporous silica nanoparticle | Inorganic material | Intratumorally administration | Breast cancer | [359, 360] |
| CaP@LDL | Inorganic material | — | Hepatocellular carcinoma | [241] |
| CaP@HA | Inorganic material | — | Breast cancer | [361] |
| SPION–TMC–ChT–TAT–H NPs | Inorganic material | Intratumorally administration | Colorectal cancer | [362] |
| ZnAs@SiO2 | Inorganic material | — | Hepatocellular carcinoma (HCC) | [363] |
| NPs-αIL6R Ab–CD44 | Biomimetic material | Intravenous administration | Breast cancer | [364] |
| CaP-cored low-density lipoprotein nanovehicle-STAT3 decoy ODNs | Biomimetic material | Intravenous administration | HCC cells | [27] |
| Exo-JSI124 | Biomimetic material | Intranasal delivery | Glioblastoma tumor | [365] |
| EVs-L-PGDS | Exosome | — | Gastric cancer | [366] |
| Exo-An2-siRNA | Exosome | — | Glioblastoma tumor | [367] |
| CDNVs | Nanovesicles | — | Lung cancer | [368] |
6.2 Targeting JAK
The activation of STAT3, primarily induced by various cytokines, is largely dependent on JAKs [262]. Multiple JAK inhibitors are currently under investigation and clinical evaluation for the treatment of cytokine release syndrome and chronic inflammatory diseases [263, 264]. Additionally, certain orally administered small-molecule JAK inhibitors, which target ATP-binding sites, are being explored for potential use in treating solid tumors [265, 266]. However, recent applications of JAK inhibitors have been predominantly directed toward hematological malignancies and inflammatory disorders [265, 267, 268].
Baricitinib and Tofacitinib are orally administered JAK inhibitors approved by the US FDA for the treatment of autoimmune diseases [21, 22]. Among the most extensively studied US FDA-approved JAK inhibitors are Paclitaxel, Ruxolitinib, and Tofacitinib. Several related compounds are currently in early-stage laboratory development before progressing to clinical evaluation. Tofacitinib effectively inhibits JAK1 and JAK3 but has a weaker inhibitory effect on JAK2 [269, 270]. Initially developed as an inhibitor of FMS-like tyrosine kinase 3 (FLT3), lestaurtinib (CEP-701) has also demonstrated JAK2 inhibition. It exhibits the potential to suppress cancer cell proliferation, limiting metastasis, and preventing colony formation in malignancies such as acute myeloid leukemia (AML), human neuroblastomas, and anaplastic thyroid carcinoma [271, 272]. Similarly, AZD1480 has shown antitumor activity in an HPV-associated HNSCC animal model and inhibits IL-6-induced STAT3 phosphorylation [273, 274]. However, AZD1480 treatment in patients with solid tumors led to dose-limiting toxicities in phase 1 clinical trials [27, 275].
WP1066 inhibits JAK2 phosphorylation and has demonstrated effectiveness in the treatment of AML, melanoma, and bladder cancer [196]. This inhibition enhances tumor sensitivity to chemotherapy across various cancer models. WP1066 specifically targets the STAT3/miR-21 axis, increasing the susceptibility of oral squamous cell carcinoma cells to cisplatin [196]. The combination of WP1066 with a dexamethasone derivative (DX10) has shown potential in melanoma treatment [276, 277]. In a xenograft tumor model using Tca8113/DDP cells, the combined administration of WP1066 and cisplatin significantly reduced tumor growth [27]. Additionally, studies have reported that AG490, a JAK2 inhibitor, suppresses angiogenesis and reduces MDSCs within the HNSCC TME by inhibiting the JAK2/STAT3 pathway [27, 278].
Rufolitinib (INC424), a JAK1/2 inhibitor, has been approved by the US FDA for the treatment of inflammatory diseases [279]. As a repurposed drug, it has demonstrated good tolerance and an overall hematologic response rate of about 32% in patients with abnormal chronic myeloid leukemia and chronic neutrophilic leukemia [280]. Similarly, pacritinib (SB1518) is an orally administered inhibitor that competes with ATP to block both JAK2 and FLT3, thereby suppressing the growth of various cancer types. Currently, pacritinib is in the first phase of clinical trials for potential lymphoma treatment [281].
Several natural compounds have been identified as inhibitors of the JAK/STAT3 pathway. Arctiin has been shown to deactivate JAK and Src while inhibiting excessive pSTAT3 expression [18]. Additionally, 8α-tigloyloxyhirsutinolide-13-O-acetate, a bioactive compound derived from Vernonia cinerea, inhibits the JAK/STAT3 pathway and exhibits antitumor effects in an HNSCC mouse model [27]. The indirubin derivative E738 competes with ATP to inhibit JAKs and SFKs, effectively reducing STAT3 expression in malignant cells [282].
6.3 Targeting IL-6 and IL-6R
The initial step in STAT3 activation involves the interaction between cytokines and their respective receptors. Three primary strategies are employed to inhibit IL-6-mediated signaling: the use of fusion proteins, such as sgp130, to target the IL-6-soluble IL-6R complex; the direct neutralization of IL-6 with antibodies like siltuximab; and the blockade of the IL-6R with antibodies such as tocilizumab [283, 284]. Targeting the IL-6–sIL-6R complex with sgp130 fusion proteins selectively inhibits trans-signaling, while direct inhibition of IL-6 or IL-6R effectively suppresses both classical and trans-signaling pathways [201].
Siltuximab (CNTO-328), a chimeric mouse-human monoclonal antibody, selectively binds to IL-6, preventing its interaction with IL-6R and thereby disrupting the IL-6/STAT3 signaling pathway. This inhibition has demonstrated efficacy in reducing tumor growth and invasion in non-small cell lung cancer (NSCLC) and cholangiocarcinoma [285, 286]. Following multiple clinical trials, siltuximab received US FDA approval in 2014 for the treatment of multicentric Castleman disease [287]. Additionally, it has shown antitumor activity in ovarian [288], prostate [289], and lung cancer [290]. In a phase I–II clinical trial, siltuximab treatment led to a reduction in active STAT3 and MAPK levels in prostate cancer patients [291]. Similarly, in a separate phase I–II trial, over 50% of individuals with metastatic renal carcinoma exhibited disease stabilization following siltuximab administration [292]. However, no significant clinical efficacy was observed in advanced-stage cancers, including head and neck, lung (NSCLC), colorectal, pancreatic, or ovarian cancers [293]. While preclinical studies have shown promising outcomes, clinical trials have yielded limited success in solid tumors. These findings suggest that targeting IL-6 alone may not be sufficient for solid tumor treatment, highlighting the need for combination therapies and the identification of reliable predictive biomarkers.
Clazakizumab, olokizumab, MEDI5117, and sirukumab are anti-IL-6 antibodies currently under evaluation for cancer treatment; however, these agents remain in the early stages of development [82, 294]. These antibodies inhibit IL-6-mediated signaling cascades involving JAK and STAT3 across various cancer types. Olokizumab specifically interferes with the interaction between the gp130 signal-transducing component and IL-6–IL-6R and IL-6 dimers, thereby preventing the formation of hexamers [295].
Tocilizumab binds to IL-6R and inhibits both trans-signaling and classical signaling pathways [201]. The US FDA has approved its use for managing cytokine-release syndrome in patients with B-cell acute lymphoblastic leukemia undergoing chimeric antigen receptor T-cell therapy, as well as for the treatment of RA and systemic juvenile idiopathic arthritis [296]. Tocilizumab has also demonstrated efficacy in ovarian [288], pancreatic [297], and colorectal cancers associated with colitis [298]. In a phase I clinical study, the combination of tocilizumab with carboplatin and/or doxorubicin (Dox) in ovarian cancer patients showed promising results [299, 300]. Additionally, early-phase trials are being conducted to evaluate the efficacy and safety of tocilizumab in pancreatic cancer, breast cancer, and B-cell chronic lymphocytic leukemia [301, 302].
Selective inhibition of the trans-signaling pathway may be beneficial for tumor patients with limited or no IL-6R expression. Proteins containing the sgp130 sequence selectively inhibit trans-signaling by binding to and blocking the IL-6R and IL-6 complex [201]. Preclinical studies have demonstrated that the sgp130-Fc fusion protein suppresses the growth and metastasis of pancreatic cancer, colitis-associated premalignant colorectal cancer, and KRAS-driven NSCLC [27]. IBD and RA patients are currently being treated with olamkicept, an sgp130-Fc fusion protein, in phase I–Ib clinical trials. However, targeted inhibition of IL-6 signaling may reduce STAT1 activity, which possesses tumor-suppressive properties, presenting a potential challenge for cancer treatment [294].
Bazedoxifene, a third-generation selective estrogen receptor modulator, inhibits GP130, IL-6, and IL-6R complexes, thereby preventing subsequent STAT3 activation [303]. It has shown promising effects against pancreatic cancer by reducing cancer cell proliferation and migration. Additionally, its therapeutic efficacy is enhanced when combined with other chemotherapeutic agents [304, 305].
6.4 SH2 Domain Inhibitors
The SH2 domain of STAT3 enables its binding to tyrosine-phosphorylated residues on cell surface receptors, which is crucial for its activation. Additionally, the SH2 domain is essential for the formation of STAT3 dimers, where one SH2 domain binds to a phosphorylated tyrosine residue on another STAT3 molecule. Inhibiting the SH2 domain effectively blocks STAT3 activation and phosphorylation. Targeting the SH2 domain allows direct STAT3 inhibition through two primary mechanisms: first, by preventing Tyr705 phosphorylation on STAT3 at the cell membrane through RTKs or nonreceptor kinases, and second, by interfering with the formation of functional STAT3 dimers. Two classes of inhibitors, peptides, and small molecules, target the SH2 domain and have demonstrated the ability to limit tumor cell proliferation [24, 195]. Peptidomimetics mimic a specific protein sequence, pTyr–Xaa–Yaa–Gln, and bind to the STAT3 SH2 domain, competing with its natural binding partners to prevent dimerization [306]. A notable example is the phosphopeptide inhibitor derived from the PY*LKTK sequence, where Y* represents phosphorylated tyrosine. This small molecule directly binds to STAT3, interfering with dimerization and inhibiting its activity. Other compounds, including C-pTyr–Leu–Pro–Gln–Thr–Val–NH2, BP–PM6, BP–PM7, and PMM-172, have been shown to suppress STAT3 by reducing its persistent phosphorylation in HNSCC and breast cancer through SH2 domain inhibition [27].
Phosphorylation of the SH2 domain at Tyr-705 is essential for STAT3 dimerization and DNA binding. Therefore, inhibitors targeting the STAT3 SH2 domain also disrupt its interaction with DNA [16]. An oxazole-based peptidomimetic, S3I-M2001, has been reported to specifically inhibit STAT3 dimerization, thereby suppressing transcription, transformation, survival, and migration in both mouse and human cells [196, 307]. Another peptidomimetic, S3I-1757, derived from benzoic acid, directly interacts with the Tyr-705 binding region within the STAT3 SH2 domain, limiting hyperactivation and reducing malignant transformation. By preventing STAT3 dimerization and DNA binding, this inhibition leads to apoptosis and reduced cell proliferation through the suppression of key STAT3 target genes, including cyclin D1, Bcl-xL, MMP-9, and survivin [308]. Similarly, S3I-201, a salicylic acid-derived compound, and its analogs inhibit STAT3 DNA binding by interacting with the SH2 domain. This inhibition induces apoptosis in cancer cells by downregulating proteins essential for cell survival and proliferation, such as survivin, Bcl-xL, and cyclin D [23, 24]. Additionally, S3I-201 has been shown to inhibit STAT3 activation in a mouse model of anal squamous cancer negative for HPV, suppressing cancer cell growth and reducing their ability to evade immune responses [27].
Phosphopeptides exhibit limited cell permeability, prompting the investigation of a new class of small molecules capable of inhibiting the STAT3 SH2 domain. One such compound, STA-21, a naturally occurring deoxytetrangomycin, selectively binds to the SH2 domain, preventing STAT3 dimerization and nuclear translocation, thereby significantly reducing the proliferation and progression of breast cancer cells [260]. Another small molecule, STATTIC, specifically disrupts STAT3 dimerization and DNA binding while also inhibiting enzymes responsible for STAT3 activation, leading to apoptosis in breast cancer cells [309]. Similarly, BP-1–102 targets the STAT3 SH2 domain, effectively suppressing cell survival, growth, migration, and invasion in lung and breast cancer [310].
Several nonpeptide small molecules have also been identified as STAT3 inhibitors [196, 311]. OPB-31121 has demonstrated strong antitumor activity, particularly in multiple liver cancer models [312]. Likewise, OPB-51602 interacts with the SH2 domain, interfering with intradomain interactions and causing STAT3 aggregation. This disruption affects mitochondrial function, ultimately inducing cancer cell death [24].
Curcumin, a natural compound-derived inhibitor, targets the SH2 domain of STAT3. When modified with proline, curcumin effectively inhibits STAT3 dimerization [23]. Similarly, cryptotanshinone, a naturally occurring compound, interacts with the SH2 domain to suppress STAT3 phosphorylation and prevent dimer formation, thereby reducing the expression of cell survival genes such as Bcl-xL, survivin, and cyclin D1 [313]. Additionally, several other natural compounds, including cucurbitacin E [191], alantolactone [314], piperlongumine [315], and silibinin [316], have demonstrated the ability to inhibit STAT3 by binding to the SH2 domain, highlighting their potential as therapeutic agents targeting STAT3 signaling.
Celecoxib, a COX-2 inhibitor, also binds to the SH2 domain of STAT3. By competitively inhibiting native peptide binding, celecoxib reduces tyrosine phosphorylation, leading to decreased cell motility and viability [317, 318]. A separate class of STAT3 dimerization antagonists, derived from salicylic acid, exhibits enhanced membrane permeability, offering an advantage over peptidomimetics. These compounds effectively disrupt STAT3–phosphopeptide interactions, thereby preventing STAT3 dimerization. Additionally, they induce apoptosis and inhibit intracellular STAT3 phosphorylation [319]. The SH2 peptide inhibitor (SPI), a 28-amino acid peptide, blocks the interaction between the STAT3 SH2 domain and phosphorylated tyrosine on IL-6R. SPI suppresses STAT3 activation and promotes apoptosis, demonstrating potential as a therapeutic agent for STAT3-driven malignancies [320]. Furthermore, ODZ10117, another SH2 domain inhibitor, has been shown to prevent tyrosine phosphorylation and STAT3 dimer formation, ultimately reducing tumor progression [321, 322].
6.5 STAT3 DBD Targeting
Targeted gene promoter sites interact with the DBD of STAT3, which exhibits relatively high specificity. STAT3 plays a crucial role in cell proliferation, migration, and invasion by binding to DNA within the cell nucleus. Its activity can be reduced by inhibitors that target the STAT3 DBD, thereby preventing its interaction with DNA [18]. C468 is the first identified small-molecule inhibitor of the STAT3 DBD, binding to cysteine (C468) on glutathione sulfhydryl within the DBD region. This interaction prevents activated STAT3 from accumulating in tumor cell nuclei, leading to a significant reduction in tumor growth [323]. The platinum (IV) compound IS3-295 disrupts the DNA-binding ability of STAT3 through a noncompetitive mechanism [308]. Additionally, apoptosis in human cancer cells is induced by various platinum (IV) compounds, including CPA-1, CPA-7, and platinum (IV) tetrachloride, which inhibit STAT3 DNA binding [16]. A study synthesized (E)-2-methoxy-4-(3-(4-methoxyphenyl) prop-1-en-1-yl) phenol (MMPP), a novel small molecule that effectively inhibits cancer progression by targeting the STAT3 DBD. MMPP exhibits selective binding, reducing the likelihood of nonspecific interactions and associated side effects [324]. Using a STAT3 decoy to target activated STAT3 has been shown to inhibit cancer cell growth, induce apoptosis, and suppress STAT3-mediated gene expression in head and HNSCC cells [325]. One study reported about a 7.4-fold increase in programmed cell death in an HNSCC xenograft model treated with a combination of cisplatin and a STAT3 decoy. Additionally, a series of G-quartet oligodeoxynucleotides (GQ-ODNs) have been synthesized to inhibit the DNA-binding activity of STAT3. Treatment of xenograft HNSCC tumors with GQ-ODN in combination with paclitaxel for 21 days resulted in a 35% reduction in average tumor size [27, 326]. The STAT3 DBD can be selectively targeted using DBD-1, a small peptide aptamer. In a murine model, the interaction between the DBD of STAT3 and DBD-1 was weak. However, in murine carcinoma B16 cells, DBD-1 significantly induced apoptosis [327]. Figure 5 depicts the targeting of STAT3 by several substances.

6.6 Challenges and Limitations of Different STAT3 Inhibitors
The therapeutic targeting of STAT3 presents several challenges and limitations across different classes of inhibitors. Small-molecule inhibitors often exhibit poor selectivity and specificity, leading to systemic toxicity, off-target effects, and the development of resistance through the activation of alternative signaling pathways. Additionally, these inhibitors face issues related to cell permeability and bioavailability, which limit their overall therapeutic potential [196, 308]. Similarly, JAK inhibitors, which target upstream kinases, exert broad effects on multiple STAT proteins. Their inhibition of the JAK–STAT pathway can lead to immune suppression and hematological toxicity, along with the activation of compensatory pathways that may reduce their effectiveness [328, 329].
IL-6 and IL-6R inhibitors also encounter significant challenges due to the pleiotropic functions of IL-6 in metabolism, inflammation, and immune regulation [68, 330]. These inhibitors may cause systemic adverse effects and only partially suppress STAT3, as other pathways, such as those activated by growth factors, can also contribute to STAT3 activation [82, 331]. Similarly, SH2 domain inhibitors may only partially block STAT3 and often struggle with achieving high affinity and selectivity due to structural similarities with other STAT proteins. Mutations in the SH2 domain and suboptimal pharmacokinetics further compromise their efficacy [195, 230]. Inhibitors targeting the DBD of STAT3 face additional challenges, including limited accessibility due to the nuclear localization of the target and the complexity of designing inhibitors that can selectively bind to the large, flat surface of the DBD. Furthermore, inhibiting the DBD may disrupt the transcription of genes essential for normal cellular function, increasing the risk of off-target effects [332, 333]. To address limitations related to specificity, efficacy, and safety, novel approaches such as combination therapies and advanced DDSs, including nanoparticle (NP)-based platforms, may enhance the effectiveness of STAT3-targeted treatments.
7 Combination Strategies With STAT3 Inhibitors
The combination of STAT3 inhibitors has the potential to enhance tumor suppression and counteract resistance mechanisms through a synergistic approach. Modulating the TME using this strategy may improve the efficacy of immune checkpoint inhibitors and facilitate the elimination of resistant CSCs. Additionally, combination therapies could allow for reduced drug dosages, thereby minimizing systemic toxicity and adverse effects [190, 334].
The combination of immune checkpoint inhibitors, such as anti-PD-1 or anti-PD-L1 antibodies, with STAT3 inhibitors represents a promising strategy for cancer therapy. Preclinical studies in lung cancer and melanoma models have shown that STAT3 inhibition enhances antitumor immunity by modulating the TME and reducing PD-L1 expression [335, 336]. Chemotherapeutic agents, such as gemcitabine and Dox, also demonstrate synergistic effects when combined with STAT3 inhibitors. For instance, apabucasin enhances the efficacy of gemcitabine in pancreatic cancer, while FLLL32, a curcumin derivative, increases apoptosis in triple-negative breast cancer (TNBC) when used in combination with Dox [235]. STAT3 inhibitors have also been evaluated in combination with targeted therapies, such as EGFR inhibitors (e.g., gefitinib) or JAK inhibitors (e.g., ruxolitinib), for malignancies driven by these pathways [308]. In glioblastoma models, STAT3 inhibition has been found to enhance the effects of radiation therapy by disrupting DNA repair and survival pathways. Additionally, the combination of cyclophosphamide with natural compounds such as arctigenin has exhibited enhanced anticancer activity, particularly against TNBC [315]. Similarly, combining STAT3 inhibitors with epigenetic modulators, such as HDAC inhibitors or DNA methyltransferase inhibitors, has demonstrated efficacy against both solid tumors and hematological malignancies [337]. The STAT3 SH2 domain inhibitor YHO-1701 has shown significant synergy with alectinib in NCI-H2228 xenografts, reducing body weight loss while avoiding systemic toxicity. Furthermore, YHO-1701 has improved the anticancer effects of sorafenib in an SAS xenograft model that secretes IL-6 [307]. In medulloblastoma xenografts, the combination of cisplatin and LLL12B has effectively suppressed tumor growth in D283 and D425 models [338].
While these strategies highlight the versatility of STAT3 inhibitors, several challenges remain, including overlapping toxicities, potential resistance mechanisms, and tumor heterogeneity, which necessitate personalized treatment approaches. Clinical trials are essential for optimizing these combination therapies, with a focus on establishing their efficacy and safety in diverse patient populations.
8 Decreasing STAT3 Expression
Antisense oligonucleotides (ASOs) inhibit STAT3 mRNA translation by binding to complementary single-stranded RNA sequences [260]. A specific type of ASO, modified with 2-O-methoxyethyl, has been shown to reduce circulating VEGF levels, suppress neovascularization, and inhibit cancer cell proliferation and metastasis [339]. Oweida et al. [340] reported that the combination of STAT3 ASOs with radiotherapy enhanced antitumor effects and reduced radiation resistance. In LY2 and MOC2 tumor-bearing mice, the average tumor volumes following combined radiation and STAT3 ASO treatment were 53.0 ±5.6 and 254.8 ±81.6 mm3, respectively. In contrast, tumor volumes in mice treated only with STAT3 ASOs were 277.4 ±53.8 and 1042.9 ±326.8 mm3, respectively [340]. Posttranscriptional inhibition of STAT3 is commonly achieved using RNA interference, such as siRNA. The STAT3 inhibitor STX-0119 has exhibited cytotoxic effects against various pancreatic cancer cell types, particularly those with low PD-L1 expression, highlighting its potential therapeutic relevance [341].
AZD9150 is a specialized ASO designed to bind to the 3′-untranslated region of the STAT3 gene. In lung cancer and lymphoma models, AZD9150 has been shown to effectively reduce STAT3 activity and its downstream targets by decreasing STAT3 mRNA levels [25]. When combined with cisplatin, AZD9150 significantly enhanced tumor sensitivity and improved survival rates compared with monotherapy with either drug alone [342]. In addition to ASOs, miR-124-3p has been identified as a regulatory molecule that interacts with the 3′ untranslated region of STAT3, leading to its transcriptional downregulation. This mechanism induces apoptosis in NPC cells and inhibits their proliferation, migration, and invasion [343]. A distinct approach to inhibiting STAT3 activity involves the use of ODNs, which capture active STAT3 dimers in the cytoplasm and prevent their interaction with importin, thereby blocking nuclear translocation [260, 344]. Another effective strategy for suppressing STAT3 involves siRNA, which degrades STAT3 mRNA. By targeting STAT3 mRNA, siRNA reduces the levels of antiapoptotic proteins such as Bcl-xL and Bcl-2, ultimately triggering cell death [260].
9 Targeted Delivery of STAT3 Inhibitors
The clinical application of STAT3 inhibitors presents several challenges, including low oral bioavailability, nonspecific targeting, and potential toxicity to healthy cells [308, 345]. To address these limitations, research efforts have focused on developing advanced DDSs that encapsulate STAT3 inhibitors using nanomaterials such as polymers, liposomes, inorganic materials, and biomimetic carriers. Nanomaterial-based drug delivery approaches offer several advantages over conventional STAT3 inhibitors. These systems exhibit high biocompatibility and improved tumor-targeting capabilities, thereby minimizing damage to healthy tissues. Additionally, their high drug-loading capacity allows for the simultaneous delivery of multiple therapeutic agents. Furthermore, nanomaterials protect encapsulated drugs from rapid clearance in the bloodstream, thereby enhancing their stability and prolonging therapeutic efficacy [241, 346].
Liposomes are nanoscale, spherical vesicles composed of lipid bilayers, typically ranging from 50 to 500 nm in diameter. They form when natural or synthetic lipids are dispersed in water, creating one or more lipid layers surrounding an aqueous core [135, 369]. Due to their structural similarity to cell membranes, high drug-loading capacity, and biocompatibility, liposomes effectively encapsulate hydrophobic drugs within their lipid bilayers [370, 371]. A calcium phosphate-core low-density lipoprotein nanocarrier was utilized by Shi et al. [372] to develop a Trojan horse strategy for delivering STAT3 decoy ODNs, effectively overcoming resistance to TNF-related apoptosis-inducing ligand. Additionally, PEGylated liposomal FLLL32, a specialized STAT3 inhibitor targeting the SH2 domain, has demonstrated enhanced antitumor activity while reducing systemic toxicity [373, 374]. Recent advancements in liposomal drug delivery have led to the development of a dual-drug-loaded liposome for gastric cancer treatment, where a hybrid membrane (R/C) (LP-R/C@AC NPs) is coated with cinobufagin and apatinib. By inhibiting the VEGFR2/STAT3 pathway, LP-R/C@AC NPs effectively suppress cancer cell proliferation and reverse immune suppression by downregulating MMP-9 and PD-L1 expression. This approach has shown a two-fold increase in tumor targeting and immune system modulation [241]. Exosomes, a type of liposome-like vesicle containing antigens, mRNAs, and miRNAs, have emerged as promising drug delivery vehicles capable of overcoming therapeutic barriers in cancer treatment. Intranasal administration of exosome-packaged STAT3 inhibitors has been explored as a noninvasive therapeutic strategy. Studies have shown that microglial cells can absorb these inhibitors, leading to a 44.5-day increase in survival in GL26 tumor-bearing animals compared with control groups. Exosomes secreted by brain endothelial cells can penetrate the blood–brain barrier, allowing targeted drug delivery for lung cancer-associated brain metastases. This capability presents a promising approach for effective cancer therapy [365, 375].
To achieve sustained drug release, Zheng et al. [376] utilized polylactic-co-glycolic acid (PLGA) nanomaterials for the co-delivery of the STAT3 inhibitor nifurate and Dox. Another study employed HPMA-based copolymers containing Dox and the STAT3 inhibitor cucurbitacin D for gradual drug release, demonstrating effectiveness in breast cancer treatment [355]. Additionally, DDSs such as PLGA-JSI-124 conjugation, PEO-b-P(CL–JSI-124) conjugates, and PEO-b-PBCL micelles have exhibited improved therapeutic efficacy in cancer models [27]. A novel drug delivery approach has been proposed for direct pulmonary administration using fine droplets (FM@PFC/siRNA) composed of perfluorocarbon (PFC). This system delivers anti-STAT3 siRNA alongside a C-X-C motif chemokine receptor 4 (FM) antagonist (FM). The FM@PFC/siRNA nanoemulsions effectively inhibit STAT3 and CXCR4 signaling pathways, leading to increased apoptosis and reduced cancer cell invasion. Furthermore, in a lung metastatic tumor model, this method successfully counteracted the immunosuppressive TME, highlighting its potential in targeted lung cancer therapy [377, 378].
Over the past two decades, inorganic NPs, including magnetic, mesoporous silica, and gold/silver NPs, have been extensively studied as potential cancer therapeutics. These NPs offer several advantages, including ease of synthesis, high biocompatibility, and the ability to functionalize their surfaces for targeted drug delivery [379-381]. In one study, imatinib mesylate and anti-STAT3 siRNA were encapsulated within layer-by-layer assembled gold NPs (LbL-AuNPs) for melanoma treatment. These positively charged NPs were constructed through the sequential adsorption of sodium alginate, chitosan, and natural polyelectrolytes. The positive charge facilitated the use of iontophoresis therapy, which enhances skin penetration for localized melanoma treatment. When applied topically by iontophoresis, these dual-drug-loaded LbL-AuNPs significantly suppressed tumor growth and reduced STAT3 expression in melanoma mouse models [350, 382]. Additionally, ZnAs@SiO2 NPs have been shown to downregulate stemness markers (Oct-4, Sox-2, and CD133) and EMT markers (slug, vimentin, and E-cadherin). The inhibition of the STAT3 signaling pathway by these NPs also contributed to a reduction in tumor spheroid formation in a three-dimensional model [363].
The combination of STAT3 inhibitors with other therapeutic agents enhances both the safety and efficacy of cancer treatment while improving the therapeutic potential of STAT3 blockade through advanced drug delivery modalities. Immunotherapy targeting immune checkpoint proteins has emerged as a promising strategy for cancer management. However, NSCLC has demonstrated resistance to tremelimumab (anti-CTLA4 antibody) and durvalumab (anti-PD-L1 antibody) due to STK11 gene alterations, which impair immune system efficacy. STAT3 ASOs can counteract this inhibitory effect, significantly improving the antitumor response when administered in combination with immune checkpoint inhibitors [383]. Additionally, DDSs integrated with anti-STAT3 inhibitors have demonstrated superior outcomes in cancer treatment compared with the use of inhibitors alone, highlighting the potential of NP-based approaches to enhance drug targeting, reduce systemic toxicity, and improve overall therapeutic efficacy [308].
The development of DDSs for STAT3 represents an advanced strategy aimed at addressing the limitations of conventional therapies. These systems enhance therapeutic efficacy by reducing systemic toxicity, improving bioavailability, and optimizing targeted drug delivery. Various DDSs, including NPs, liposomes, micelles, dendrimers, and hydrogels, have been explored to increase the accumulation of STAT3 inhibitors at tumor sites. Recent advancements in stimuli-responsive DDSs, such as pH-, temperature-, or enzyme-sensitive systems, have facilitated the controlled release of STAT3 inhibitors within the TME. These approaches enhance local drug concentrations while minimizing damage to healthy tissues [384-386]. Additionally, DDSs improve drug transport and targeting, maximizing tumor site concentration while reducing systemic exposure and associated toxicity. Furthermore, these systems can effectively penetrate physiological barriers, including intracellular compartments and the TME. DDSs can also be engineered to respond to specific signals within the TME, further enhancing the precision of drug delivery [387, 388].
Despite promising results, the development of DDSs for STAT3 inhibitors still presents several challenges. One of the primary difficulties is designing systems that can selectively target and penetrate tumor tissues while minimizing off-target effects on healthy cells. Although functionalization enhances targeting, tumor heterogeneity and the dynamic nature of the TME complicate the consistent and specific delivery of drugs to STAT3-overexpressing cells. Additionally, the scalability and stability of these delivery platforms for clinical applications require further investigation. The potential toxicity and immunogenicity of certain DDS components, along with the capacity of cancer cells to develop resistance mechanisms, may reduce long-term therapeutic efficacy [389, 390]. While DDSs offer significant advantages for STAT3-targeted cancer therapy, further research is necessary to address these limitations and ensure clinical feasibility.
10 Conclusion and Future Prospective
STAT3 is an essential protein present in all tissues, playing a crucial role in cellular processes and influencing the growth and function of multiple physiological systems. Upon activation by growth factors or cytokines, STAT3 enhances the expression of genes that regulate the cell cycle and promote cell proliferation. Dysregulated STAT3 activity, whether through hyperactivation or inactivation, has been implicated in various human diseases, including atherosclerosis, bone-related disorders, neurological conditions, autoimmune diseases, and cancer. Despite its potential as a therapeutic target in cancer, existing STAT3 inhibitors exhibit limited potency and provide only modest clinical benefits. Therefore, exploring novel strategies for targeting STAT3 in cancer therapy remains a priority. Since STAT3 signaling is regulated by molecules such as miRNAs, TLRs, and GPCRs, small-molecule inhibitors that selectively target these pathways may effectively suppress STAT3 activity, thereby reducing tumor progression and inflammation. Furthermore, targeting STAT3 epigenetic modifications presents an emerging and promising approach for cancer treatment.
Despite advancements in STAT3-targeted therapies, direct inhibition of STAT3 remains challenging due to its ubiquitous expression in the body. To date, only a limited number of STAT3 inhibitors have been approved by the USFDA for cancer treatment, and several concerns persist. One major issue is the need for specificity, as these inhibitors must selectively target STAT3 without affecting structurally similar proteins, such as STAT1. Additionally, adverse effects, including hematological toxicity, remain a significant challenge. Further research should focus on improving the specificity and safety profile of STAT3 inhibitors to minimize off-target effects. Current inhibitors primarily target STAT3 dimerization, but assessing their interactions with other proteins remains difficult. Therefore, beyond the SH2 domain, additional regions of STAT3 should be investigated as potential therapeutic targets. Moreover, reducing the dosage of STAT3 inhibitors and combining them with immunotherapy, chemotherapy, or personalized treatment approaches may enhance therapeutic efficacy while mitigating adverse effects. Additionally, the development of advanced DDSs is essential to overcome the limitations of conventional therapies. DDSs offer the potential to enhance the therapeutic profile of STAT3 inhibitors by improving bioavailability, reducing systemic toxicity, and ensuring targeted drug delivery. The integration of STAT3 inhibitors with other anticancer agents and optimizing DDSs could significantly improve treatment outcomes in cancer therapy [27].
Advancements in understanding the STAT3 signaling pathway and the development of STAT3 inhibitors have underscored their potential in cancer therapy. Future research may focus on integrating STAT3-targeted therapies with conventional treatments in a multimodal approach to enhance therapeutic outcomes. Although various strategies have been explored to identify small molecules capable of inhibiting STAT3 signaling, further investigations are required to optimize their efficacy and clinical applicability.
Author Contributions
M. A. S., I. A., A. A., and A. H. conceived, designed, and performed the literature survey, and drafted the article's first draft. F. A., M. H. A., A. K., and S. T. provided the critical feedback, finalized the content, and made the final draft. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU), Jeddah, Saudi Arabia under grant no. (GPIP: 81-141-24). The authors, therefore, acknowledge with thanks DSR for technical and financial support. All the images inserted in this article was made using Biorender.com.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




