The crosstalk between senescence, tumor, and immunity: molecular mechanism and therapeutic opportunities
Abstract
Cellular senescence is characterized by a stable cell cycle arrest and a hypersecretory, proinflammatory phenotype in response to various stress stimuli. Traditionally, this state has been viewed as a tumor-suppressing mechanism that prevents the proliferation of damaged cells while activating the immune response for their clearance. However, senescence is increasingly recognized as a contributing factor to tumor progression. This dual role necessitates a careful evaluation of the beneficial and detrimental aspects of senescence within the tumor microenvironment (TME). Specifically, senescent cells display a unique senescence-associated secretory phenotype that releases a diverse array of soluble factors affecting the TME. Furthermore, the impact of senescence on tumor–immune interaction is complex and often underappreciated. Senescent immune cells create an immunosuppressive TME favoring tumor progression. In contrast, senescent tumor cells could promote a transition from immune evasion to clearance. Given these intricate dynamics, therapies targeting senescence hold promise for advancing antitumor strategies. This review aims to summarize the dual effects of senescence on tumor progression, explore its influence on tumor–immune interactions, and discuss potential therapeutic strategies, alongside challenges and future directions. Understanding how senescence regulates antitumor immunity, along with new therapeutic interventions, is essential for managing tumor cell senescence and remodeling the immune microenvironment.
1 INTRODUCTION
Cellular senescence is a stress-inducible state with four interdependent hallmarks: (1) an irreversible cell-cycle arrest, (2) a senescence-associated secretory phenotype (SASP), (3) alterations in morphology and physiology, and (4) a reprogrammed metabolism.1 In 1961, Hayflick and Moorhead first described cellular senescence in human diploid fibroblasts to explain the finite lifespan of normal cells, as these cells have achieved their maximum replicative capacity.2 This phenomenon was later shown to result from telomere shortening and defined as replicative senescence (RS).3 Therefore, cellular senescence plays a broad physiological role to maintain genome stability and avoid damage accumulation, especially during embryonic development, wound healing, tissue remodeling, and resolution of fibrosis.4-7 Senescence can be triggered as a defense mechanism by various internal and external stressors. These include oncogenic, genotoxic, metabolic, lysosomal, and oxidative stress, which may result in different types of cellular senescence.8-10
Although much of our comprehension regarding the pathophysiological functions of senescent cells is grounded in experimental murine models and human somatic cells, emerging studies highlights the importance of cellular senescence in cancer biology and human disease. Specifically, several subsets of senescent cells might be associated with tumorigenesis, including oncogene-induced senescence (OIS) and therapy-induced senescence (TIS). Owing to their tumor-promoting properties, cellular senescence is recently recognized as a hallmark of cancer.11
While both senescent cells and tumor cells involve the progressive accumulation of damaged cells, they exhibit diametrically opposed biological behavior. More specifically, tumor cells display the potential for hyperproliferation and elevated metabolic rates, whereas senescent cells exhibit change of functionality and a halt in cell division.9, 12 Additionally, emerging studies have proposed that cellular senescence may be a double-edged sword in cancer, exerting pro- or antitumorigenic effects that depend on different contexts.13 Thereby, this dual effects of senescence significantly influences tumor progression or suppression.
The SASP, where senescent cells secrete a diverse set of proinflammatory cytokines, chemokines, growth factors, and proteinases, also plays an important role in the tumor microenvironment (TME).14 By releasing SASP factors, senescence can modulate pathways in neighboring cells and those at remote sites.3 Moreover, SASP factors are typically varied based on the type of senescent cells and the cellular milieu.15 Studies have shown that SASP factors can promote or inhibit tumor progression by altering the TME, which in turn affect the production of SASPs. This intricate interplay significantly influences how tumors develop. Though the mechanisms underlying this complex crosstalk are not completely clear, extensive preclinical studies have shown that modulating senescence or SASP holds promise for optimizing cancer therapy (more details in Section 5).
Immunity is characterized by the activation of various immune components, including T cells, B cells, natural killer (NK) cells, and antigen-presenting cells (APCs), which work collectively to identify and destroy malignant cells. The antitumor immune response is initiated when tumor-associated antigens, which are unique proteins expressed by cancer cells, are presented to the immune system. This recognition triggers a cascade of immune reactions aimed at eradicating the tumor.16, 17 The roles of immunity in the context of cellular senescence are ambiguous and even controversial. Senescence is a natural physiological process whereby organ function gradually deteriorates. When senescence occurs in the immune system, it is termed immunosenescence, characterized by immune dysfunction and lymphoid organ remodeling.18 It is a multifactorial phenomenon leading to high susceptibility to malignancy.18-20 Conversely, recent publications highlight that senescence might induce phenotypic changes in tumor cells, which subsequently stimulates the immune system and boosts the antitumor immunity.21 Given the complexity of immunity and tumor evolution, it is necessary to exceptionally convolute how senescent cells modulate antitumor immune response. Therapies that target tumor cell senescence or reverse immune cell senescence hold the potential to extend lifespan and are currently undergoing clinical trials.
In this review, we first introduce the induction process of cellular senescence, discuss the context-dependent roles of senescence with both tumor-suppressive and tumor-promoting features, explore the complicated effects of senescence on tumor–immune crosstalk, provide an overview of therapies that inhibit the SASP (senomorphics) or selectively attack senescent tumor cells (senolytics), highlight promising strategies to prevent T-cell senescence for boosting cancer immunotherapy, and conclude potential challenges and future directions in this field.
2 INDUCTION OF CELLULAR SENESCENCE
Cellular senescence could occur in response to various stressors, including DNA damage, oxidative stress, and telomere shortening. There are several types of cellular senescence, primarily classified based on their triggering stimuli and associated biological contexts. The most recognized forms involve RS and stress-induced premature senescence. Senescence-associated cell cycle arrest functions as a tumor suppressor, with its induction arising from the engagement of various signaling and downstream cell cycle inhibitor pathways.
2.1 Replicative senescence
RS is a fundamental biological process characterized by irreversible cessation of cell division, which occurs after a finite number of cell divisions in somatic cells.2, 22-24 Notably, a cell cycle arrest is required only for dividing cells, while nondividing and postmitotic cells can be senescent as well.25 RS is primarily driven by telomere shortening, a consequence of the end-replication problem during DNA replication. Telomeres, the protective caps at the ends of chromosomes, shorten with each cell division due to the inability of DNA polymerase to fully replicate the ends of linear DNA. When telomeres reach a critical length, they are identified as DNA DSB, triggering a DNA damage response (DDR). The initial checkpoint kinases, ATM (ataxia-telangiectasia mutated) and ATR (ataxia-telangiectasia and Rad 3-related) are subsequently activated to phosphorylate several proteins, with CHK2 being one of them. CHK2 transmits DDR signals by phosphorylating the tumor suppressor protein p53, which then activated the downstream protein p21. P21 prevents the phosphorylation of retinoblastoma protein (RB), leading to the cell cycle arrest at the G1 phase and enter cellular senescence.26, 27 In addition to the p53/p21 pathway, p16INK4a/RB could also induce RS.28
RS is also influenced by various molecular pathways and environmental factors. For instance, oxidative stress, often a byproduct of cellular metabolism, can induce DNA damage and contribute to the senescence phenotype. Reactive oxygen species (ROS) leads to genomic instability and further activates the DDR, exacerbating the senescence process.29 Furthermore, metabolic alterations, such as changes in serine metabolism, have been shown to regulate RS through epigenetic modifications like histone methylation, further complicating the molecular landscape of this process.30
Recent studies have also highlighted the role of RNA modifications, particularly m6A methylation, in the regulation of RS. Dynamic alterations in m6A methylation patterns have been observed during senescence, suggesting a regulatory mechanism that could influence gene expression and cellular aging.31 Additionally, the interaction of various signaling pathways, including the TGF-β and mTOR pathways, has been implicated in the modulation of senescence, indicating that RS is not merely a result of telomere shortening but a complex interplay of genetic, epigenetic, and environmental factors.32, 33
2.2 Stress-induced premature senescence
Numerous stressors could induce early senescence that can be classified into different categories based on the trigger. The following sections describe various forms of cancer-related senescence.
2.2.1 Oncogene-induced senescence
OIS is primarily caused by the activation of oncogenes, leading to a state of permanent growth arrest, which was first demonstrated in vivo in 1997.34-36 Subsequently, the concept of OIS was expanded to multiple tumorigenesis models, including lung adenomas, lymphoma, prostate cancer, and melanocytic naevi.34, 37-39 OIS acts as a barrier to tumorigenesis by preventing the proliferation of cells that harbor oncogenic mutations. Notably, this process can be triggered by various oncogenes, such as RAS, BRAF, and MYC, each contributing to the complex landscape of cancer biology. For example, melanocytic nevi caused by oncogenic BRAF mutations keep senescent for decades, thus impeding their progression into melanoma.39 PTEN inactivation induces growth arrest through the p53-dependent cellular senescence pathway in primary prostate epithelium.37
The mechanisms underlying OIS have been clarified. The activation of an oncogene triggers the production of ROS, introducing DSBs and DDR, which subsequently initiates cellular senescence.40 The activation of oncogenes results in DNA damage, which upregulates the expression of functional tumor suppressor proteins like p53 and p21. This upregulation induces a stable cell cycle arrest by inhibiting the downstream cyclin-dependent kinase (CDK)–cyclin complexes. Thereby, hyperphosphorylation of RB protein blocks S-phase entry and induces cellular senescence.35, 41, 42 Of note, only oncogene expression does not drive DDR in the absence of DNA replication; instead, OIS originates from DDR activation triggered by oncogene-induced DNA hyper-replication.43
2.2.2 Therapy-induced senescence
TIS is considered a reaction in tumor cells to an array of anticancer therapies, including chemotherapy and radiotherapy, which introduce DSBs and activate DDR.44
The mechanisms of TIS are complex and involve various signaling pathways and cellular responses. One of the key pathways implicated in TIS is the activation of the p53 and p16INK4a tumor suppressor pathways, which play critical roles in mediating cell cycle arrest and cellular senescence. Following DNA damage induced by chemotherapy or radiation, p53 is activated, leading to the transcription of genes that inhibit the cell cycle, thereby promoting senescence.45 For instance, cisplatin triggers the p53 pathway to induce cellular senescence.46 Additionally, an elevated level of ROS during therapy can further activate these pathways, exacerbating the senescence response.
Several studies have provided insights into the role of TIS in the context of specific cancer therapies. For instance, in the treatment of T-cell acute lymphoblastic leukemia with chemotherapy, the induction of senescence was found to suppress disease progression, suggesting that TIS might be beneficial in this context.47 Research has demonstrated that targeting senescent cells can enhance the efficacy of cancer therapies. For example, the use of senolytic drugs, which selectively eliminate senescent cells, has shown promise in reducing the side effects associated with chemotherapy while improving overall treatment outcomes.48, 49 These findings underscore the importance of understanding TIS not only as a byproduct of cancer treatment but also as a potential target for therapeutic intervention.
3 EFFECTS OF SENESCENCE ON TUMOR CELLS
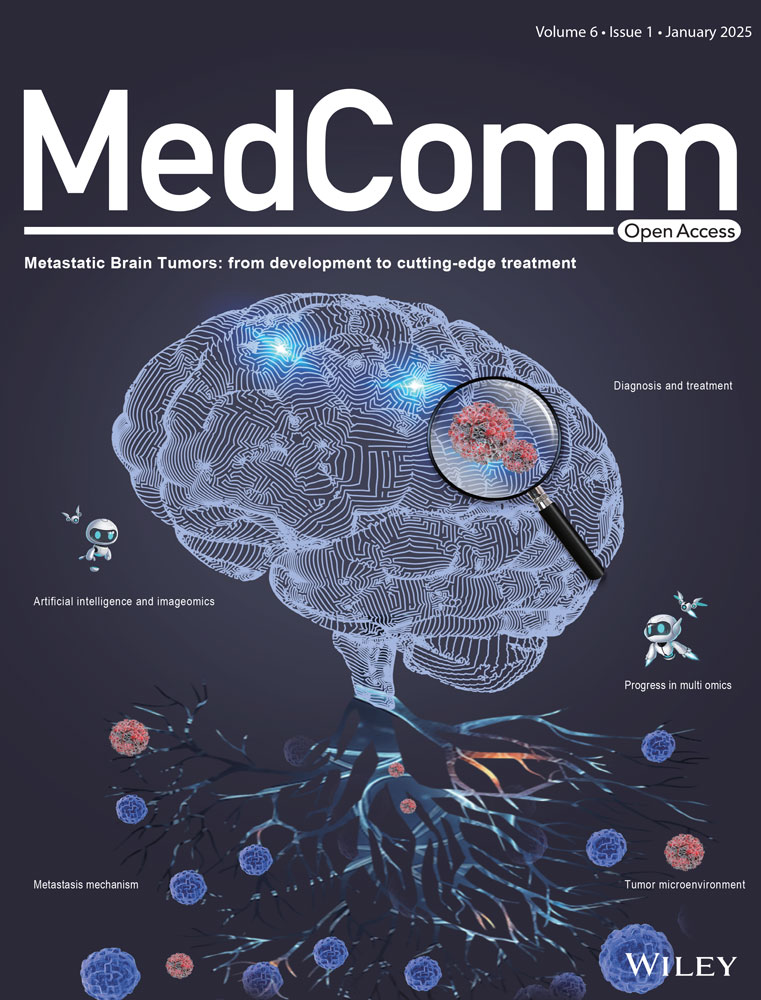
Cellular senescence is a double-edged sword in cancer, exerting both protumorigenic and antitumorigenic effects (Figure 1). It is noted that senescent cells triggered by different stress stimuli may secrete diverse SASP components.50 On the one hand, SASP factors can reinforce cell cycle arrest in an autocrine scenario and mediate paracrine senescence to adjacent cells.51-53 Meanwhile, SASP factors are essential contributors to favor immune clearance via recruiting immune effectors.54, 55 On the other hand, the SASPs could remodel the TME beneficial for tumor cells to proliferate, invade, and metastasize while increasing immunosuppressive cell infiltration contributing to immune evasion.15, 56 Whether the effects of senescent cells are protumorigenic or antitumorigenic partly depends on the complicated interplay between various SASP factors and the tumor immune microenvironment (TIME). Table 1 summarizes the roles of different SASP factors in the context of specific cancer types.
| SASP factors | Cancer type | Senescent cell | Major roles of the SASP | References | |
|---|---|---|---|---|---|
| Antitumorigenic SASP | IL-1α | Liver cancer | Hepatocyte | Immune clearance of senescent tumor cell | 55 |
| IL-6 | Osteosarcoma | Osteoblast | NKT cell recruitment | 57 | |
| CCL5 | Melanoma | Melanocyte | Lymphocyte recruitment | 58 | |
| CCL2 | Liver cancer | Hepatocyte | Macrophage activation | 54 | |
| VEGF | Pancreatic cancer | Pancreatic ductal cell | Enhanced vascularization; improved drug delivery efficacy | 59 | |
| Protumorigenic SASP | CCL2 | Liver cancer | Hepatocyte | MDSC differentiation | 54 |
| PGE2 | Liver cancer | Hepatic stellate cell | Impaired antitumor function of CD8+ T cell | 59 | |
| MMPs | – | Fibroblast | Tumor invasion promotion | 60 | |
| CXCL1, CXCL2 | Prostate cancer | Prostate epithelial cell | Myeloid cell recruitment | 61 | |
| CXCL12 | Thyroid cancer | Thyroid follicular cell | Anoikis resistance | 62 |
- Abbreviations: MDSC, myeloid-derived suppressor cells; NK T cell, natural killer cell; SASP, senescence-associated secretory phenotype.
3.1 Cellular senescence in tumor suppression
3.1.1 Senescence-mediated growth arrest
Entry of cells into senescence is a natural barrier to tumorigenesis, essential for eliminating cells from proliferation, particularly those with a heightened risk of malignant transformation.23 OIS operates as a cell-intrinsic mechanism that induces growth arrest of premalignant cells following activated oncogenes (such as N-RASG12V and BRAFV600E), which could be further reinforced by CDK inhibitors (commonly known as p21 and p16INK4a).34, 63, 64 Senescence also mediates tumor suppression in a cell-extrinsic fashion. SASP factors limit the propagation of precancerous cells or fully malignant cells in their vicinity through autocrine or paracrine manners.51, 65, 66 IL-1 triggers an autocrine inflammatory response via activating NF-κB signaling, driving the production of IL-6 and IL-8 that could reinforce growth arrest through increased ROS production and sustained DDR.67, 68 IL-1 also stimulates paracrine senescence in adjacent cancer cells to prompt tumor suppression.51, 65, 66 Moreover, some SASP factors could induce apoptosis or necrosis in neighboring cells. TNF- triggers ROS-dependent apoptosis in cancer cells.69 Conversely, IL-6 drives apoptosis in tumor-infiltrating T cells.70
3.1.2 Senescence-mediated immune surveillance
Provided evidence suggests that SASP is an important contributor to cancer immunosurveillance. Explicitly, IL-6, IL-8, and CCL2 facilitate the recruitment of M1-type macrophages, T helper 1 cells, and NK cells to the TME, driving the immune clearance of senescent cancer cells.54, 55 Both OIS and TIS could trigger SASP-mediated immunosurveillance against tumors.
Oncogene-induced senescent cells have the capacity to promote cancer immunosurveillance. For instance, N-RASG12V-driven senescent premalignant hepatocytes secrete SASP factors and are subject to immune elimination that depends on CD4+ T cell-mediated adaptive immunity.55 When NRASG12V-induced senescent human fibroblasts are injected into mice, a global remodeling of their super-enhancer landscape occurs. Super-enhancers are particularly powerful enhancer regions in the genome that significantly boost gene expression. This reconfiguration, particularly through the recruitment of chromatin reader bromodomain-containing protein 4 (BRD4) to SASP-gene-adjacent super-enhancer loci within the genome, plays a pivotal role in immunosurveillance. Given that BRD4 is essential for the SASP factors and downstream paracrine signaling, BRD4 inhibition would destroy the immune clearance of premalignant OIS cells.71 Importantly, except for participating in the induction of cellular senescence, the tumor suppressor p53–p21 axis also modifies the interplay between senescence and immunity.72, 73 As a tumor suppressor, p53 is frequently mutated in various tumors, manifesting the “proliferating state” tumor with the worst prognosis.74, 75 P53-restoration in mouse models contributes to tumor suppression with senescent phenotype, revealing that p53-mediated senescence paves the way for immune clearance triggered by the SASP factors, such as CSF1, CCL2, CXCL1, and IL-15.72, 73 By coordinating with NF-κB signaling, p53 regulates SASP secretion that triggers macrophage activation contributing to a tumor-suppressive microenvironment.76, 77 p21-induced senescence also activates immunosurveillance with a particular secretory phenotype involving CXCL14 and IGFBP3 that prompt the recruitment of macrophages and lymphocytes into the TME.78 Moreover, oncogenic RAS was found to drive immune surveillance against tumorigenesis via p21-dependent senescence.78
TIS also contributes to tumor suppression via immune cell-mediated clearance. In the KRAS-mutant lung cancer model, combinatory inhibitors of MEK and CDK4/6 suppress cancer cell proliferation while inducing RB-mediated cellular senescence and activating immunomodulatory SASP. SASP components, TNF- and ICAM1, are needed for NK cell surveillance leading to tumor regression.79 Similarly, SASP factors lead to vascular remodeling that facilitates cytotoxic CD8+ T cell accumulation following dual inhibitors of MEK and CDK4/6 in KRAS-mutant pancreatic ductal adenocarcinoma.68 Furthermore, CDK4/6 inhibitor-mediated senescence induces potent immunosurveillance via regulatory T (Treg) cell suppression and CD8+ T cell enhancement.67, 80
3.2 Cellular senescence in tumor promotion
Though the SASP initially functions in tumor-suppressive roles by reinforcing growth arrest and facilitating immunosurveillance, this phenotype exhibits paradoxical tumor-promoting properties during ageing.3, 81, 82
3.2.1 SASP-driven carcinogenesis
Several studies have demonstrated protumorigenic effects of senescent cells mediated by individual SASP factors. Matrix metalloproteinases (MMPs) can mediate the degradation of extracellular matrix (ECM) components associated with growth factor release while prompting tumor-driven angiogenesis, operating as a switch favoring tumor growth and invasion.60, 83, 84 Accordingly, GDF-15, FGF1, and IGFBP3 also facilitate tumor dissemination to secondary sites.85 Moreover, hepatocyte growth factor (HGF) is reported to collaborate with MMPs to further promote tumor progression.60 SASP factors IL-6 and IL-8 are important drivers of cancer proliferation via creating a chronic inflammatory microenvironment, facilitating ECM cleavage by MMPs, and driving epithelial-to-mesenchymal transition (EMT), thereby collectively contributing to tumor invasiveness.56, 86, 87 Furthermore, VEGF, IL-6, and CXCL5 stimulate angiogenesis to support tumor metastasis. Overall, different SASP factors mediate tumor growth, proliferation, invasion, and metastasis via multiple mechanisms.
3.2.2 SASP-mediated immune evasion
As a negative effect, SASP factors are critical mediators of TIME suppression leading to immune evasion. For example, IL-6 is proven to recruit myeloid-derived suppressor cells (MDSCs) to the tumor site, which are reported to block immune surveillance by suppressing CD8+ T cells and NK cells.54, 88 Meanwhile, MDSCs are found to inhibit IL-1 signaling and consequently hinder the induction of senescence in tumor cells.61 IL-6 and IL-8 lead to the upregulation of HLA-E that interacts with Natural Killer Group 2 Member A (NKG2A), which blunts the effector activity of NK cells and CD8+ T cells.89
3.2.3 Senescence-associated stemness
Pioneering studies pointed out that TIS can induce stemness properties in malignant cells, driving them to escape proliferation arrest and re-enter the cell cycle.90, 91 For instance, chemotherapy-induced senescence in transgenic mice with B-cell lymphomas where WNT signaling is activated ultimately showed distinct stem-cell markers.90 Oncogene-induced senescent cells also acquire stemness characteristics driving tumor aggressiveness.92 In an OIS mouse model of breast cancer, RANK-induced senescence is required for RANK-triggered stemness; despite initially displaying a delayed tumor onset, tumor progression and aggressiveness are observed in the long term.93 Moreover, overexpression of CIP1 enables p53-null tumor cells to gain enhanced stem cell properties following a transient senescent state.94 Growing evidence indicates that senescence-associated stemness is triggered by WNT signaling activation depending on SASP factors rather than WNT ligands.90 Therefore, senescent tumor cells that regain proliferative capacity exhibit WNT-dependent growth, a process that is particularly common in recurrent tumors.90
4 EFFECTS OF SENESCENCE ON TUMOR–IMMUNE CROSSTALK
The interaction between cellular senescence and antitumor immunity is complex. Explicitly, immune system senescence may reshape the TME by reducing the infiltration of immune effector cells and diminishing immune functions, facilitating tumor cells’ ability to evade immune surveillance. On the contrary, tumor cell senescence might trigger a switch from immune evasion to immune elimination.
4.1 Senescence-related immune cells inducing an incompetent TIME
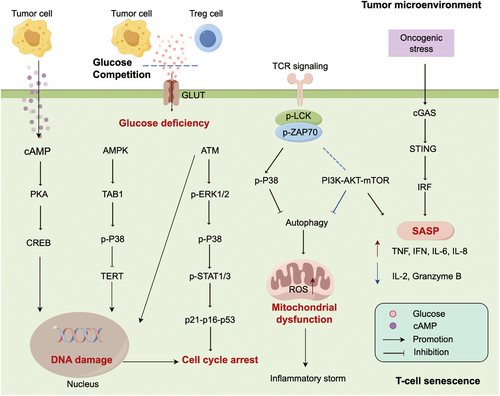
Immunosenescence proposed by Roy Walford in 1964,18 reflects the senescence of various immune cell subsets essential for antitumor activity. Since then, scientists have devoted a deeper insight into the mechanisms and effects of immunosenescence. In 2000, inflammaging was first introduced by Claudio Franceschi, a state of low-grade, chronic damage resulting from high levels of proinflammatory markers within the body.95, 96 Inflammaging could result from immunosenescence-mediated immune dysfunction but also engenders a feedforward process driving immunosenescence.97, 98 Emerging evidence supports that changes in immune cell subpopulations may trigger a shift toward an incompetent TIME.
4.1.1 Abnormal molecular mechanisms underlie immunosenescence
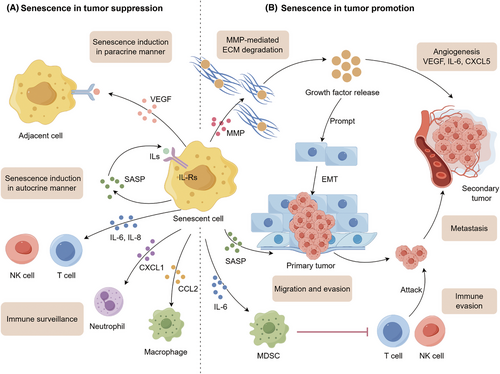
Recent studies have underscored the significance of immunosenescence in tumor development.99 Many factors within the TME possess the capability to trigger immunosenescence. As the most powerful immune cells in eradicating tumor cells, T-cell senescence has attracted the most interest. Given the potential of T cells to be continuously activated by antigens and affected by inflammatory cytokines, it is plausible that the TME may be the source of senescent T cells.100 Signaling pathways involved in T-cell senescence are displayed in Figure 2.
Tumor-derived cyclic adenosine monophosphate (cAMP), driven by hypoxia, has the potential to inhibit tumor-specific effector T cells by inducing DNA damage and senescence.101, 102 Glucose competition between T cells and Treg cells could initiate a series of events, including ATM-related DNA damage, activation of ERK1/2 and P38 pathways, and interaction with STAT1/3 accompanied by upregulation of p21–p16–p53, eventually leading to T cells withdraw from the cell cycle.103, 104 Furthermore, the activation of AMPK pathways due to glucose deficiency could downregulate the telomerase reverse transcriptase gene binding to TAB1, followed by autophosphorylation of P38. The activation of P38 leads to the downregulation of TERT, thereby inducing DNA damage.105 The downregulation of T cell receptor (TCR) signaling could activate the P38 pathway and hinder the PI3K/AKT/mTOR signaling pathway, which in turn inactivates autophagy and accelerates ROS production in senescent T cells. Of note, high levels of ROS contribute to inflammaging and immunosenescence.106
4.1.2 Cellular level mechanisms in immunosenescence
Immune system senescence, also referred to immunosenescence, reflects the senescence process observed in diverse subpopulations of immune cells (Figure 3). Innate immune senescence is characterized by a reduced ability to process and present antigens, leading to a diminished responsiveness to stimuli. Meanwhile, adaptive immune senescence encompasses a decline in the diversity of the TCR repertoire and an impaired formation of immunological memory. Therefore, immune cell senescence dramatically impacts the efficacy of antitumor responses.
T cells
Senescent T cells, with defects in effector function, accumulate in the context of ageing, chronic inflammation, and autoimmune disorders where antigen stimulation persistently exists.107, 108 Numerous cytokines and signals that coexist in the TME could induce T cell senescence, paving the way for tumorigenesis and cancer progression.100
Various T-cell subpopulations display heterogenous changes as the immune system ages.109 This process is characterized by a decline in naïve T cells but an increase in highly differentiated CD28− memory T cells, a phenomenon known as naïve-memory ratio imbalance. T cell replenishment mainly relies on thymus output. Thereby, thymus degeneration reduces the output of naïve T cells and destroys T cell homeostasis, which explains the age-related failure of adaptive immunity.110, 111 Conversely, memory T cells gradually accumulate with ageing.112-114 Emerging evidence indicated that CD4+ T cells could adapt to the challenges of senescence and maintain a slight balance between naïve and memory cells, while CD8+ T cells exhibit a significant imbalance in this regard.115 In healthy elderly individuals, a decrease in circulating naïve CD8+ T cells is a crucial and consistently noted sign of immunosenescence.
Senescent T cells lose the capacity for antigen-specific killing. In the TME, T-cell activation needs TCR (signal-1) and costimulatory signaling (signal-2) to be present simultaneously. However, important components of TCR signaling (CD3) and costimulatory molecules (CD27 and CD28) are decreased in senescent T cells.105, 116 Moreover, the diversity of TCR declines with age, particularly in senescent CD8+ T cells.117, 118 Senescent T cells display decreased expression of functional molecules, such as perforin, granzyme B, and SA--gal, leading to a weakened antitumor activity in mice or patients.119-121 Senescent T cells develop a hypersecretory, proinflammatory phenotype to remodel the TME. SASP is both a consequence of cellular senescence and a driver for further senescence.122 On the one hand, SASP factors, like IL-6 and TNF-, accelerate T cell senescence in an autocrine or paracrine manner.123, 124 On the other hand, senescent T cells secrete SASP factors leading to the TME with features of an “immune desert,” also known as an immune cold microenvironment, refers to a specific state of tumors characterized by a limited immune response.125-127 The CCL5/CCR5 axis facilitates the induction and recruitment of M2-type macrophages and MDSCs that impede effector T cell function.128 CXCL1 not only prompts adjacent cell senescence but also induces immune evasion via paracrine signaling.51 IL-6 also recruits MSDCs into the TME and drives EMT, triggering tumor growth and invasiveness.61, 86, 129 Also, it has been proven that IL-18 increases the generation of MDSCs and augments their immunosuppressive activity in multiple myeloma.130 A high level of IFN-γ induced by senescent T cells, could inhibit T cell cytotoxicity by upregulating several immunosuppressive factors, such as IDO, PD-L1, and CTLA-4.131 Therefore, SASPs reshape the TIME via regulating immunosuppressive cell infiltration. In addition to remodeling the TIME by SASP factors, Treg cells, a unique subpopulation of T cells, directly impede effector T cell function. Given that the change of Treg cells in age-related pathology is hotly debated, Treg cells may play a context-specific role in different tumors.132, 133 Focusing on T-cell senescence, stress facilitates a general dysfunction of ETC (electron transport chain) components and OXPHOS (oxidative phosphorylation) subunits, leading to elevated ROS generation, dysfunctional mitochondria synthesis, and impaired one-carbon metabolism.82, 106, 134 Moreover, excessive glucose consumption by tumor cells inevitably results in responder T-cell senescence, a new strategy for tumor immune evasion.104 Overall, the accumulation of senescent T cells in the TME contributes to immune evasion and tumor progression.
Monocytes/macrophages
Senescent macrophages, major contributors to immunosenescence, present deterioration in phagocytic capacity and defects in the ability to fight external threats.135, 136 A large amount of evidence indicates that the immunosuppressive M2 tumor-associated macrophages display a high infiltration in the spleen, bone marrow, and lymphoid tissues of aged mice, facilitating tumor progression in an ageing context.137-139 Furthermore, macrophages from old individuals exhibit weakened antigen-presenting ability attributed to declined expression of MHC-II and coreceptors.140-142 Significant downregulation of glycolysis and mitochondrial OXPHOS has been demonstrated in aged macrophages, giving rise to an energy-depleted state that impedes normal macrophage function.143
Neutrophils
Neutrophils undergo immunosenescence in a low-grade inflammatory environment, with deterioration in their metabolism and immune function that prompt tumor progression.144, 145 The age-related neutrophils exhibit a decline in phagocytic capacity, abnormalities in adhesion, chemotaxis trap network release, increased apoptosis, and aberrant Toll-like receptor function.146-151 N2-type tumor-associated neutrophils (TAN) represent a distinct subpopulation that has been described in various types of tumors.152, 153 Studies have shown that the infiltration level of N2-TANs in the TME is higher in older mice than in young mice. And these suppressive N2-TANs share similar functions with MDSCs.154 However, the direct involvement of senescence-induced N2-TAN in the TIME still needs further investigation.
MDSCs
MDSCs are a population of immunosuppressive cells that accumulate in patients with cancer and establish a premetastatic niche in distant organs.155 MDSCs hamper immune surveillance by impairing the function of NK and CD8+ T cells, both affecting innate and adaptive immunity.129, 156 Young mice implanted with breast cancer cells present an increased infiltration of effector T cells, whereas aged mice exhibit a consistent increase in MDSC number.157, 158 In senescent MDSCs, upregulated expression of the chemokine receptor CX3R1 induced by p16 and p21 overexpression could enhance MDSC recruitment into tumor sites to mediate a protumorigenic effect.159
NK cells
NK cells are basic components of innate immunity and act as the first defense mechanism in humans.160 The ageing process leads to an elevated number of NK cells but compromises their ability to kill targets.161, 162 Specifically, age-related NK cells exhibit reduced effector function, with reduced secretion of perforin, granzyme, and IFN-γ.163, 164
4.2 Tumor cell senescence triggering a switch from immune evasion to immune elimination
Cellular senescence is a damage-induced response characterized by a stable cell cycle arrest and a secretory program. In some contexts, senescence serves as an endogenous homeostatic mechanism to drive the immune clearance of senescent cells (Figure 4).165
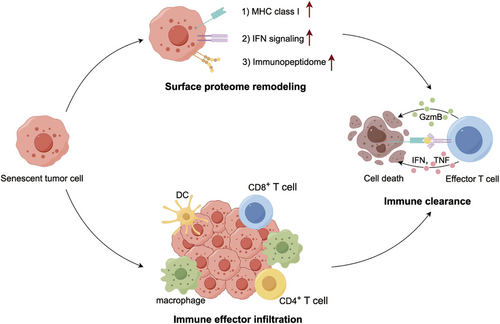
As discussed above, senescence-associated immune surveillance exerts potent antitumor roles.71, 79 Importantly, the molecular basis by which senescent tumor cells become visible to the immune system will facilitate novel treatment strategies to boost antitumor immunity.166, 167 On the one hand, tumor cell senescence directs an abrupt switch from immune evasion to immune elimination mediated by TIME remodeling, laying the basis for productive antitumor immunity. In an immune-competent liver cancer model, p53-driven tumor senescence moderately declined the percentage of immune suppressors (MDSCs and neutrophils) while prominently increasing the infiltration of immune effectors (macrophages, CD4+ T cells, and CD8+ T cells), driving a shift from an immune-incompetent to an immune-competent TME.166 On the other hand, senescence reshapes the cell-surface proteome to rewire how tumor cells sense microenvironment signals.166, 167 First, senescent cells augment the IFN-γ receptor IFNGR1 and become hypersensitive to microenvironmental IFN-γ signaling.166 Secon, senescent cells upregulate MHC class I expression to enhance their antigen-presenting capacity, which can be explained by their hyperactivated IFN-γ signaling.167 Senescent cells present an enhanced capacity to send and receive environmental signals necessary for effective adaptive immunity. Destroying the integrity of IFN-γ signaling in senescent cells will blunt their immune-dependent clearance. Additionally, senescent cells generate a secretome containing immune-modulatory factors, increase antigen presentation, and express costimulatory receptors such as CD137L and OX40L to facilitate T cell activation.168
The TME remodeling and sensing programs engaged by senescence allow tumor cells to be more visible to immune surveillance. These immediate-early senescent tumor cell responses facilitate immune-mediated tumor cell eradication. However, in the late stages of senescence induction, senescent tumor cells may express immune checkpoints that engage coinhibitory receptors on immune cells.169, 170 Overall, senescent cells have an immunogenic potential that can be harnessed to prime the immune system toward tumor clearance but needs to be carefully wielded to avoid their immunosuppressive effects.167, 168
5 TARGETING SENESCENCE FOR ANTITUMOR THERAPY
Activation of the host immune system is considered an attractive way to eliminate senescent cancer cells.171 Existing drugs targeting senescence that selectively eliminate senescent cells (senolytics) or inhibit the SASP (senomorphics) are promising anticancer therapies. Alternatively, therapeutic strategies for reversing senescence of tumor-specific T cells have emerged to enhance cancer immunotherapy. Potential interventions targeting senescence are summarized in Table 2.
| Intervention | Type | Example | References |
|---|---|---|---|
| Senolytic therapy | BCL-2 family inhibitor | Navitoclax, ABT-737, A1155463, A1331852, EF24, venetoclax | 172-174 |
| mTOR inhibitor | AZD8055, temsirolimus, sertraline | 175-177 | |
| p53 activity modulator | FOXO4-DRI peptide, UBX0101 | 178, 179 | |
| BET family degrader | ARV825 | 180, 181 | |
| Cardiac glycosides | Digoxin, ouabain | 182, 183 | |
| Immunotherapy | PD1/PD-L1 blocking antibodies, CAR-T cells | 184-186 | |
| Senomorphic therapy | SASP regulator | Metformin, Rapamycin, ruxolitinib | 187-189 |
| SASP antibody | Siltuximab, canakinumab | 190, 191 | |
| Hormone | Melatonin, androgens, estrogens, estradiol, glucocorticoids | 192-195 | |
| Senescent T cells | Metabolic regulator | Rapamycin, metformin, BIRB 796, HBOT, 7-ddA, H89, ssRNA40 | 103, 125, 196-200 |
| Signaling pathway regulator | MAPK inhibitors, ATM inhibitors | 201-203 |
5.1 Modulating senescence-related tumor cells and SASPs
5.1.1 Senolytic therapies
The persistence of therapy-induced senescent cells can lead to adverse outcomes, including an increased incidence of secondary tumors, the recurrence of more aggressive cells, and complications related to treatment.204, 205 This argues for a strategy to eliminate senescent tumor cells by senolytic agents, which can selectively induce apoptosis in senescent cells but spare other nonsenescent cells.206 Notably, a key feature of senescent cells is a change in chromatin structure that alters gene expression. These changes can affect crucial processes like apoptosis regulation, creating new vulnerabilities in senescent cells that can be specifically targeted by senolytic drugs.8 Therefore, applying senolytic agents in combination with or following senescence-inducing therapy may generate unexpected clinical benefits.
BCL-XL-modulating drugs have shown the greatest promise in obliterating senescent tumor cells by targeting antiapoptotic BCL-2 pathways.207 For example, navitoclax (ABT-263), selectively targeting BCL-2, BCL-XL, and BCL-W proteins, could effectively eliminate senescent cells by inducing programmed cell death.172, 208 As reported, navitoclax could kill breast and ovarian cancer cells after application of PARP inhibitors in aged animal models and in vitro culture.209 Exposure to navitoclax following etoposide or doxorubicin showed strong effectiveness in slowing tumor progression.204, 210 Consistently, in a phase-II study, the combination of navitoclax and rituximab showed higher effectiveness than rituximab monotherapy accompanied by well tolerance in patients with chronic lymphocytic leukemia.211 Other BCL-2 family inhibitors, such as ABT-737, A1155463, A1331852, and EF24, have been developed as promising senolytic drugs.165, 173, 174
However, the side effects of navitoclax primarily arise from its off-target effects on hematological cells, including thrombocytopenia and neutropenia, which significantly limits its broader use.212 Several attempts have been made to address this challenge. Galacto-conjugated navitoclax prodrug (nav-gal) has been designed to improve delivery accuracy.213 This prodrug is processed by SA--gal in senescent cells, thereby specifically killing senescent cells and limiting off-target effects in normal cells. The combination of nav-gal and cisplatin has demonstrated efficient clearance of lung cancer cells. Moreover, nanocarrier-encapsulated doxorubicin is another strategy to induce specific cytotoxicity in senescent cells, which has been validated in various studies.214, 215 Alternatively, the proteolysis-targeting chimera (PROTAC) drug PZ15227 is reported to limit platelet toxicity of navitoclax by hijacking the cereblon (CRBN) E3 ligase for BCL-XL degradation. Given the minimal expression of CRBN in platelets, PZ15227 could eliminate senescent tumor cells without inducing severe thrombocytopenia.216
The mTOR inhibitor AZD8055 also showed potent senolytic effects against cancer cells with a senescent phenotype induced by DNA-replication kinase CDC7 inhibitors.177, 217 Similarly, the dual treatment of docetaxel and mTOR inhibitor temsirolimus results in a remarkable reduction of tumor growth in prostate and breast cancer animal models.175 Sertraline, a selective serotonin reuptake inhibitor, is frequently used to treat psychological disorders. By suppressing mTOR signaling, sertraline was identified as a senolytic agent to kill hepatocellular carcinoma cells that have been rendered senescent through CDC7 inhibition.177 Nevertheless, the senolytic effect of mTOR inhibitors still requires further exploitation and tests in more tumor types.
Moreover, pharmacological interventions that regulate p53 activity have senolytic effects. Given that FOXO4 can retain p53 in the nucleus, the FOXO4-DRI peptide could disrupt the p53–FOXO4 interaction, leading to p53-mediated apoptosis in senescent cells.178 Despite the FOXO4-DRI peptide displaying well tolerance in mice, its clinical use remains lacking enough evaluation. Notably, compounds that modulate p53 activity are only effective in tumors with p53-wild-type, significantly limiting their therapeutic scope.218, 219
The BET family protein degrader ARV825 provokes senolysis via attenuating nonhomologous end-joining (NHEJ) DNA DSB repair and upregulating autophagic gene expression.180, 181 In mice undergoing doxorubicin-induced senescence, ARV825 treatment effectively eliminates senescent hepatic stellate cells, leading to delayed development of liver cancer.181
Cardiac glycosides, including widely used digoxin, could selectively kill senescent cells in multiple cancer models.182, 183 Cardiac glycosides that inhibit the Na+/K+ ATPase pump could drive cell depolarization and acidification, ultimately leading to senescent cell death.183 Besides, these compounds can activate the proapoptotic BCL-2 family protein NOXA to trigger intrinsic apoptosis.182
Although numerous small molecules have demonstrated encouraging efficacy against cellular senescence, these agents exhibit a deficiency in sensitivity and may result in significant side effects. During the past decades, immunotherapy has gained breakthroughs in treating patients with advanced or drug-resistant malignancies.220 Immunotherapy could mediate endogenous senolysis to prompt senescent cell eradication. Antiprogrammed cell death protein 1 (PD1) blockades can elicit senolytic effects following TIS. The combination of trametinib and palbociclib induced senescence in a mouse model of KRAS-mutant PDAC, accompanied by increased tumor vascularization and endothelial cell activation, ultimately improving the efficacy of anti-PD1 therapy.68 Notably, chimeric antigen receptor T-cell (CAR-T) therapy directed at senescence-specific surface antigens represent a viable treatment strategy, ablating senescent cells in vitro and in vivo. The urokinase-type plasminogen activator receptor (uPAR) as a cell membrane protein is significantly upregulated following senescence induction. uPAR-specific CAR-T cells extended the tumor growth in mice with lung adenocarcinoma previously treated with a combination of MEK and CDK4/6 inhibitors.184 Other cell surface proteins might be potential targets for cancer immunotherapy, such as DEP1, DPP4, NKG2D, ICAM1, NOTCH1, and NOTCH3.221-226 Given these promising findings, more clinical studies are required to explore whether the benefits of senolytics outweigh their potential side events and to determine the optimal dose schedule. We have summarized relevant clinical trials in Table 3.
| Targeted tumor type | Agents | Targets | Identifier | Phase | Enrollment |
|---|---|---|---|---|---|
| B-cell CLL | ABT-263 | BCL-2, BCL-XL | NCT01087151 | Phase-2 | 118 |
| DLBCL | Temsirolimus | mTOR | NCT01653067 | Phase-2 | 88 |
| Breast cancer | Anti-PD-L1 | PD-L1 | NCT04360941 | Phase-1 | 45 |
| Advanced solid tumors | Sym023 | Tim-3 | NCT03489343 | Phase-1 | 24 |
| Liver cancer | TSR-022, TSR-042 | Tim-3, PD-1 | NCT03680508 | Phase-2 | 42 |
| Melanoma | TSR-022, TSR-042 | Tim-3, PD-1 | NCT04139902 | Phase-2 | 56 |
| Cervical cancer | BGB-A317, BGB-A1217 | TIGIT, PD-1 | NCT04693234 | Phase-2 | 178 |
| ESCC | BGB-A317, BGB-A1217 | TIGIT, PD-1 | NCT04732494 | Phase-2 | 125 |
| Multiple myeloma | CD3/CD28 | KLRG-1 | NCT01426828 | Phase-2 | 40 |
| HIV-associated HL | Ipilimumab, nivolumab | PD-1, CTLA-4 | NCT02408861 | Phase-1 | 96 |
- Abbreviations: CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; ESCC, esophageal squamous cell carcinoma; HL, Hodgkin lymphoma (https://clinicaltrials.gov/).
5.1.2 Senomorphic therapies
Senomorphic SASP inhibitors could function as efficacious alternatives to senolytics. Metformin, a widely recognized medication, could prevent the translocation of NF-κB pathway components to the nucleus and inhibit their subsequent activation, thus leading to a decrease in the expression of diverse SASP factors. This underlying mechanism could plausibly elucidate the antiaging and antitumor effects of metformin in both murine models and diabetic patients.187 mTOR inhibitor, such as rapamycin, could reduce NF-λB activity, suppress inflammatory SASPs at the translational level, and constrain the tumor-prompting effect of senescent bystander fibroblasts.52, 188 Inhibitors of the JAK signaling pathway in aged mice, such as ruxolitinib, could reduce inflammation and ameliorate frailty by suppressing SASP factors. Currently, several JAK inhibitors are being tested in clinical trials including patients with acute myeloid leukemia or lymphomas.189, 227
Antibodies against SASP factors also have senormophic effects. For example, siltuximab, an antibody inhibiting IL-6 approved for the treatment of multicentric Castleman disease, has shown activities in diverse oncological contexts.190 Canakinumab, an anti-IL-1 monoclonal antibody approved for pyrexia-featured inflammatory syndromes, is evaluated in clinical trials involving patients diagnosed with non-small cell lung cancer.191
Some hormones may hold the potential to limit detrimental SASP factors. As a novel SASP suppressor, melatonin suppresses SASP gene expression via modulating poly (ADP-ribose) polymerase 1 (PARP1).195 Other hormones, such as androgens, estrogens, and glucocorticoids, also possess the ability to regulate the secretion of proinflammatory SASP factors.192-194 Notably, further investigation is necessary to thoroughly examine the anticancer efficacy of these senescence-dependent, SASP-suppressing agents in suitable model systems and clinical trials.
5.2 Therapeutic strategies for preventing T-cell senescence
The failure of immunity to eradicate tumor cells is mainly attributed to T-cell dysfunction, with flaws in proliferation and effector function. Increasing evidence has shed light on T-cell senescence used by malignant cells to achieve immune evasion. Therefore, novel strategies to prevent the occurrence of senescence and control the fate of tumor-specific T cells hold the potential to hinder tumor development and optimize cancer immunotherapy.
5.2.1 Targeting metabolic reprogramming
Senescence is the culmination of the common effect of internal and external stimuli. Metabolic stresses, such as glucose deprivation, mitochondrial dysfunction, imbalanced lipid metabolism, hypoxia, and tumor metabolites, are critical to regulating T-cell senescence.
Excessive glucose consumption by tumor cells and Treg cells leads to a deficiency of glucose supply for effector T cells, ultimately triggering responder T-cell senescence.103, 104 The addition of glucose could avoid effector T-cell senescence induced by glucose competition.228 Molecular inhibitors targeting mTORC1 or p53 hold the potential to rejuvenate mitochondrial fitness, thereby preventing T-cell senescence.125, 198, 199 There is a mechanistic link between T-cell senescence and lipid metabolic reprogramming in the TME. Findings identify that senescent T cells display hyperactive glycolysis but imbalanced lipid metabolism.229 The latter changes the expression of lipid metabolic enzymes, which, in turn, affects the species and amount of lipid droplets accumulated in T cells.230 Specifically, over-expression of group IVA phospholipase A2 in malignant and Treg cells could induce T-cell senescence. Inhibition of group IVA phospholipase A2 activity is reported to alter T-cell lipid metabolism, prevent T-cell senescence in vitro, and boost antitumor immunity in in vivo models of melanoma and breast cancer.231 The hypoxic TME may also induce T-cell senescence.197 Consequently, hyperbaric oxygen therapy (HBOT) is confirmed to exert senolytic effects by increasing telomere length and reducing immunosenescence in peripheral blood cells.200 Alternatively, downregulating tumor-derived cAMP in the TME by cAMP pharmacological inhibitors or activating the TLR8 signaling pathway by synthetic poly-G3 and natural TLR8 ligand can prevent the induction of senescence in responder T cells while reversing the suppressive effect of senescent T cells.103, 196, 197 Ample evidence supports that TLRs directly participate in metabolic reprogramming to interfere with malignant behavior in several solid tumors.197, 232-234 Therefore, TLR agonists might be effective agents or adjuvants for cancer immunotherapy.
5.2.2 Manipulating signaling pathways
The importance of the mitogen-activated protein kinase (MAPK) signaling pathway in mediating T-cell senescence has recently been exploited.105, 235 Molecular inhibitors targeting ERK, p38, and STAT signaling pathways may reverse or prevent T-cell senescence.90, 236-239 Meanwhile, the MAPK signaling pathway plays a vital role in T-cell activation and effector functions.235, 240 Hence, it is urgently needed to select the optimal MAPK inhibitors for restraining senescence induction without concession in self-renewal and cytotoxicity of tumor-reactive T cells.
Selective MAPK inhibitors already available for clinical trials are preferential candidates for treating melanoma patients, with improved T-cell recognition of tumor cells without affecting lymphocyte function.201, 202 Moreover, the CRISPR/Cas9 gene editing technology could modulate the MAPK signaling pathway more stably and precisely. Similarly, selective ATM inhibitors have been tested in clinical trials for cancer patients.203, 241 Furthermore, the role of sestrins in regulating immunosenescence is multifaceted. Increasing evidence supports that sestrins coordinate ERK, JNK, and P38 phosphorylation in CD4+ T cells. Sestrin knockdown restores T-cell responsiveness and cytokine production in vivo during ageing.242 Notably, sestrins may induce the programming of senescent CD8+ T cells to obtain NK-like killing ability but lose TCR-dependent signaling activity.116 More mechanisms of sestrin-mediated T-cell senescence need further elaboration.
6 CHALLENGES AND FUTURE DIRECTIONS
A two-step therapy, consisting of prosenescence therapy followed by senolytic intervention, seems theoretically sound. This is because senescence is a stable cellular condition that continues even after the inducing factor is eliminated. Therefore, a primary unmet need is the lack of drugs that are highly effective in inducing senescence in a high percentage of cancer cells. Meanwhile, such medications must be highly selective to target cancer cells instead of healthy cells, as triggering senescence in normal tissues can lead to harmful side effects.
Another potential challenge lies in the lack of a reliable quantitative assessment of the unique contribution of each SASP factor and the absence of gold standard biomarkers for detecting the senescence state in clinical settings. Currently, no single indicator could reliably distinguish senescence from other arrested states. Noninvasive imaging methods would be ideal for measuring the effectiveness of senescence induction in tumors of patients on therapy. Besides, liquid biopsy could be used to detect senescent cells. For example, oxylipin production rises significantly during senescence.243 The intracellular prostaglandin dihomo-15D-PGJ2, a specific type of oxylipin, notably accumulates in senescent cells and is discharged during senolysis. As such, dihomo-15D-PGJ2 could be detected in urine and blood samples from patients. Alternatively, noninvasive detection of SASP factors in plasma might uncover senescence burdens.14
A further issue is tumor heterogeneity that may limit the efficacy of senescence induction within tumors. Appropriate preclinical models, such as organoids, can better imitate the TME and resemble a more physiological human cancer model. Also, single-cell and spatial sequencing help to map the location of distinct cell types and subpopulations in the TME, thereby clarifying the complicated interplay of cellular senescence, immunity, and tumor evolution. Additionally, senescent cells can disseminate the senescence phenotype through the SASP to the adjacent nonsenescent cells within tumors; however, this has yet to be substantiated. These bystander effects in the context of senescence-inducing therapies hold the potential to overcome tumor heterogeneity related to treatment responsiveness.
Using senolytic therapies in the old people requires careful assessments. Clearly, senescent cells constitute a large percentage of the number of cells, which could compromise tissue structural integrity or impact vascular endothelial cells, resulting in blood–tissue barrier dysfunction. Therefore, this issue highlights the urgent need to develop tumor cell-specific senolytic agents.
Overall, although numerous open questions still linger unresolved, significant benefits of senescence-based therapies warrant further investigation in this field.
7 CONCLUSION
Cellular senescence has traditionally been regarded as a tumor-suppressive mechanism whereby the proliferation of dysfunctional cells susceptible to malignant transformation is halted. However, a more intricate perspective has risen regarding the involvement of cellular senescence in tumorigenesis and response to cancer therapy over the past decades. Given the highly complicated and context-dependent effects of cellular senescence in cancer, weighing the balance of its “bright” and “dark” sides is inevitably critical. Similarly, the intersection of immune surveillance and tumor evolution harbors considerable complexities as well. Increasing evidence suggests the TME drives immunosenescence through multiple pathways. The accumulation of senescent immune cells potentially contributes to progressive tumors and limits the efficacy of cancer treatment. More importantly, senescent tumor cells possess an immunological capacity that can be harnessed to provoke immune surveillance. Of note, wielding this potential should be cautious to mitigate the immunosuppressive, protumorigenic capacities of senescent cells. From the perspective of translational medicine, a one-two-punch sequential therapy is feasible, whereby tumor cells are treated with senescence-induction therapy followed by senolytics or senomorphics. Activating the host immune system presents a highly appealing way to eliminate senescent cells. Preventing T-cell senescence might be a novel strategy to enhance cancer immunotherapy. Future studies should continue to conduct research on senescence and immunotherapy synergies. Therefore, exploiting cellular senescence as an antitumor therapeutic strategy will hold great interest in the future.
AUTHOR CONTRIBUTIONS
Zehua Wang contributed to study design, performed literature search, and drafted the original manuscript. Chen Chen contributed to study design, performed literature search, and confirmed the final manuscript. Jiaoyu Ai, Yaping Gao, Lei Wang and Shurui Xia critically revised of the manuscript for important intellectual content. Yongxu Jia and Yanru Qin critically supervised the whole study as the expert and confirmed the final manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (No. 82273381), National Natural Science Foundation of China (No. 82203852), and Science and Technology Department of Henan Province (Grand No. 232102311053). We thank Figdraw (www.figdraw.com) for the assistance in creating figures and Home for Researchers (www.home-for-researchers.com) for their help with language.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.