RNA modifications in cancer
Abstract
RNA modifications are emerging as critical cancer regulators that influence tumorigenesis and progression. Key modifications, such as N6-methyladenosine (m6A) and 5-methylcytosine (m5C), are implicated in various cellular processes. These modifications are regulated by proteins that write, erase, and read RNA and modulate RNA stability, splicing, translation, and degradation. Recent studies have highlighted their roles in metabolic reprogramming, signaling pathways, and cell cycle control, which are essential for tumor proliferation and survival. Despite these scientific advances, the precise mechanisms by which RNA modifications affect cancer remain inadequately understood. This review comprehensively examines the role RNA modifications play in cancer proliferation, metastasis, and programmed cell death, including apoptosis, autophagy, and ferroptosis. It explores their effects on epithelial–mesenchymal transition (EMT) and the immune microenvironment, particularly in cancer metastasis. Furthermore, RNA modifications’ potential in cancer therapies, including conventional treatments, immunotherapy, and targeted therapies, is discussed. By addressing these aspects, this review aims to bridge current research gaps and underscore the therapeutic potential of targeting RNA modifications to improve cancer treatment strategies and patient outcomes.
1 INTRODUCTION
Cancer is a significant social, public health, and economic challenge in the 21st century.1 In 2022, nearly 20 million new cancer cases were reported globally, resulting in 9.7 million deaths. An estimated one in five individuals will be diagnosed with cancer in their lifetimes, and approximately one in nine men and one in 12 women will succumb to the disease.2 Each year, cancer leaves more than a million children orphaned.3 It is a leading cause of premature death in many countries.4
The association between RNA modifications and cancer was first discovered in 1971, marking the beginning of a long journey in epigenetics research.5 However, limited detection methods retarded progress for many years. With advancements in RNA sequencing and quantitative fluorescence techniques, the role of RNA modifications in cancer has gained increasing attention, becoming a research focus.6 RNA modifications involve chemical changes to RNA nucleotides that profoundly affect RNA structure and function. To date, more than 170 RNA modifications have been identified across all RNA molecule categories.7 These modifications typically affect RNA splicing, stability, localization, translation, and RNA–RNA and RNA–protein interactions, thereby regulating various biological processes.8-10
The recent surge in research into RNA modifications highlights their potential to address the limitations of traditional cancer therapies, such as surgery, radiotherapy, chemotherapy, immunotherapy,11 and biotherapy. Despite these promising findings, many patients continue to face poor prognoses. Therefore, an in-depth exploration of the molecular mechanisms driving cancer development is essential for early detection and the development of novel therapies. This review consolidates recent findings about the role of RNA modifications in cancer and cancer therapies over the past 3 years, providing insights into the current state of cancer research.
This review begins by categorizing the main types of RNA modifications, including N6-methyladenosine (m6A), 5-methylcytosine (m5C), N1-methyladenosine (m1A), N7-methylguanosine (m7G), pseudouridine (Ψ), and adenosine-to-inosine editing. Each modification is discussed in terms of its chemical nature and biological significance. Then, the role of RNA modifications in cancer proliferation, including how they influence metabolic reprogramming, biosynthetic pathways, signaling pathway regulation, and cell cycle control, is investigated. In addition, the impact of RNA modifications on cancer metastasis is explored, focusing on mechanisms such as epithelial–mesenchymal transition (EMT) and the regulation of the immune microenvironment.
This review also addresses the involvement of RNA modifications in programmed cancer cell death, including apoptosis, autophagy, and ferroptosis, highlighting their influence on cancer cell survival strategies and the tumor immune microenvironment. Finally, the potential of RNA modifications to improve cancer therapy is discussed, particularly in the context of targeted and immunotherapies, as well as in conventional therapies and metabolism-related treatments.
In conclusion, this review outlines the significant impact of RNA modifications on cancer proliferation, metastasis, and programmed cell death and emphasizes their potential as therapeutic targets. The types of RNA modifications are introduced first, followed by their roles in cancer proliferation, metastasis, and cell death, and then their implications for cancer therapy, to provide new insights and potential cancer treatment pathways. The majority of the referenced articles were collected from PubMed over the past 3 years to ensure its relevance and timeliness.
2 TYPES OF RNA MODIFICATIONS
RNA modifications are a type of epigenetic modification that can cause heritable phenotypic changes without altering the nucleotide sequence of an organism's genetic material.12 To date, more than 170 types of RNA modifications have been identified, and all classes of RNA contain modification sites. Ribosomal RNA (rRNA) and transfer RNA (tRNA) are particularly prone to these modifications. Over 60% of RNA modifications are methylation modifications, including m6A,13 m1A,14 m7G, and m5C.15 Other modifications include Ψ16 and adenosine-to-inosine editing,17 highlighting their widespread presence and significance in complex cancer regulation18, 19 (Figure 1).

Extensive research indicates that RNA modifications are crucial in the development of various cancers, including breast,20 lung,21 and colorectal22 cancer, hepatocellular carcinoma (HCC),23 gastric,24 esophageal,25 oral,26 prostate,27 bladder,28 ovarian,29 kidney,30 and pancreatic cancer (PC).31
2.1 N6-methyladenosine
Discovered in 1974, m6A is the predominant RNA modification in eukaryotes.32 Its broad influence manifests in biological growth, development, and cancer.33, 34 m6A plays a crucial role in influencing RNA stability, transport, splicing, and translation, affecting overall RNA expression.13, 35 m6A modification involves the catalysis of RNA methyltransferase (the “writer”), the removal of demethylase (the “eraser”), and interaction with m6A-binding protein (the “reader”). The writer includes various proteins,36 such as methyltransferase-like 3 (METTL3),20 methyltransferase-like 14 (METTL14),37 Wilms’ tumor 1 associated protein (WTAP), zinc finger CCCH-type containing 13 (ZC3H13), RNA-binding motif protein 15 (RBM15), vir-like m6A methyltransferase associated, zinc finger protein 217 (ZFP217), and Hakai E3 ubiquitin–protein ligase (HAKAI).38 The primary m6A writer complex includes METTL3 as the catalytic subunit, METTL14 as the stabilizer, and WTAP as the regulator. This complex binds to messenger RNA (mRNA) and increases the methylation of adenosine residues.38, 39
In various cancer tissues, the METTL3 and METTL14 complexes play diverse roles, functioning as both oncogenes and tumor suppressors.40-42 m6A demethylases, including fat mass and obesity-associated protein (FTO) and AlkB homolog 5 (ALKBH5), can dynamically, rapidly, and signal-dependently influence the function of m6A modifications.
FTO, the first demethylase discovered, has high-efficiency oxidative demethylation activity.43 FTO was initially discovered due to its role in regulating obesity and diabetes.44 FTO-mediated RNA m6A demethylation is not specific. FTO binds multiple RNA species, including mRNA, small nuclear RNA (snRNA), and tRNA, and can demethylate internal m6A and cap N6,2′-O-dimethyladenosine (m6Am) in mRNA, internal m6A in U6 RNA, internal and cap m6Am in snRNAs, and m1A in tRNA.45 The FTO gene is a multifaceted regulator, orchestrating a complex array of cellular processes.46 It functions by demethylating the m6A modification on cyclin D1 mRNA. This demethylation leads to the degradation of cyclin D1 mRNA, which impairs the progression of the G1 cell cycle phase and, ultimately, causes a decline in cancer cell proliferation.47 Furthermore, FTO is instrumental in regulating apoptosis, serving as a critical arbiter of cell survival and programmed cell death.48 The gene's influence extends to cellular motility, where it governs migration.48 These diverse roles underscore FTO's significance as a molecular nexus in cancer biology, with far-reaching implications for both tumor development and potential therapeutic intervention strategies.
Meanwhile, ALKBH5 can demethylate m6A sequences in single-stranded RNA (ssRNA). ALKBH5 has been employed in anti-PD-1 immunotherapy to regulate lactic acid levels in the tumor microenvironment (TME) and manage the accumulation of Tregs and myeloid-derived suppressor cells (MDSCs). ALKBH5 knockdown in a 4T1 mouse tumor model improved immunotherapy efficacy and mouse survival.49
The identified m6A readers include the YTH domain family (YTHDF)1/2/3, YTH domain-containing protein 1/2 (YTHDC1/2), heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1), heterogeneous nuclear ribonucleoprotein C (HNRNPC), heterogeneous nuclear ribonucleoprotein G (HNRNPG), eukaryotic translation initiation factor 3 (eIF3), staphylococcal nuclease domain 1 (SND1), and insulin-like growth factor 2 mRNA-binding protein 1–3 (IGF2BP1–3). HNRNPA2B1 is a key regulator of proliferation, migration, and invasion in oral squamous cell carcinoma (OSCC), influencing tumor growth and metastatic potential. Its role in these processes makes it a significant therapeutic target and a potential marker for disease prognosis.50 YTHDF2, an m6A reader, is pivotal in many cellular processes, including but not limited to migration, invasion, metastasis, proliferation, apoptosis, cell cycle regulation, viability, adhesion, differentiation, and inflammation, across various neoplastic diseases.51-55 YTHDF2 exhibits differential affinities for mRNAs with m6A modifications; the C-terminus specifically targets these modified mRNAs, while the N-terminus directs the YTHDF2–mRNA complex to the cellular RNA decay checkpoint for accelerated degradation. YTHDF2's recognition of m6A modifications in mRNAs is essential for regulating mRNA translation and stability.56
2.2 5-Methylcytosine
The m5C modification, which is catalyzed by S-adenosylmethionine (SAM) at the carbon-5 position of cytosine in RNA, is common in both mRNA and noncoding RNA (ncRNA). Sulfite sequencing has identified m5C within the coding regions of mRNA, especially near its translation initiation site.57 Another study discovered that m5C modification is also present in the untranslated region (UTR) of mRNA transcripts.58 m5C significantly influences many biological processes, such as cell proliferation, differentiation, migration, and apoptosis.59, 60
m5C modifications are primarily facilitated by writer proteins (methyltransferases), eraser proteins (demethylases), and reader proteins (binding proteins). m5C methyltransferases include NOP2/Sun RNA Methyltransferase Family (NSUN) methyltransferase and DNA methyltransferase 2 (DNMT2),61-63 which transfer a methyl group from adenosylmethionine to cytosine.
NSUN2 is a crucial RNA methyltransferase that introduces m5C into RNA. It methylates the majority of expressed tRNAs, as well as other ncRNAs and certain mRNAs.15, 64 In addition, NSUN2 functions as a direct glucose sensor, promoting tumorigenesis and resistance to immunotherapy by sustaining 3′ repair exonuclease 2 (TREX2) expression, which inactivates the cyclic GMP–AMP synthase (cGAS)/STING pathway in response to glucose activation.65 In colorectal cancer (CRC), NSUN2 reprograms glucose metabolism m5C-dependently, increasing lactate production. Lactate accumulation in CRC cells, in turn, activates NSUN2 transcription through histone H3K18 lactylation (H3K18la) and induces NSUN2 lactylation at Lys356. This positive feedback loop of metabolic reprogramming promotes CRC progression. NSUN2 also plays a pivotal role in the progression of HCC.67
The principal demethylases for m5C belong to the ten-eleven translocation (TET) enzyme family. Quantitative analysis via liquid chromatography–tandem mass spectrometry (LC–MS/MS/MS) indicates that TET overexpression can significantly increase the level of 5-hydroxymethylcytosine in human embryonic kidney 293T cells.68, 69 m5C readers can recognize and bind to m5C sites on RNA to enact biological functions. Two-dimensional liquid chromatography (2D-LC) analysis of m5C-modified RNA identified two m5C mRNA readers: ALY/REF export factor (ALYREF) and Y-box-binding protein 1 (YBX1).70, 71
Studies have shown that ALYREF can recognize and bind to m5C sites in mRNA, facilitating its export from the nucleus to the cytoplasm. The overexpression of ALYREF increases bladder cancer (BCa) cell proliferation by promoting pyruvate kinase M2 (PKM2)-mediated glycolysis.28 Similarly, YBX1 binds to m5C, regulating its presence in both coding and ncRNAs and influencing rRNA maturation.72
2.3 N1-methyladenosine
m1A is an ancient RNA modification found in bacteria, archaea, and eukaryotes. It involves three components: a writer, an eraser, and a reader.
The writer complex mainly comprises various methyltransferases, including the tRNA methyltransferase (TRMT)6–TRMT61A complex (for mRNA and mitochondrial tRNA),73 TRMT61B (for mitochondrial tRNA and rRNA),74 TRMT10B (for tRNA), TRMT10C (for mitochondrial tRNA and mRNA),75 and nucleomethylin (NML, also known as RRP8, for rRNA).76, 77 As a modification that occurs after transcription, m1A plays a crucial role in RNA stability by affecting base pairing.78
The m1A erasers include demethylases, such as AlkB homolog 1 (ALKBH1), AlkB homolog 3 (ALKBH3), AlkB homolog 7 (ALKBH7), and FTO, that can remove methyl groups.79 The enzymes FTO, ALKBH1, and ALKBH7 specifically target tRNA, while ALKBH3 acts on both tRNA and mRNA.80, 81 Although these m1A erasers share some functional similarities with m6A erasers, proteins that specifically recognize m1A in RNA have not yet been identified. The reader comprises various binding proteins, including YTHDF1, YTHDF2, YTHDF3, and YTHDC1.82
The m1A modification is essential in shaping the immune microenvironment and adds to the complexity of the TME. Changes in m1A modification patterns have been detected in several cancers, including ovarian and colon cancer, HCC, and OSCC; these changes are linked to poorer prognoses.83, 84
The m1A methyl group, which carries a positive charge, affects RNA base pairing, altering the structure and function of the modified RNA.85 During translation, m1A modifications affect the initiation and elongation processes by regulating tRNA, mRNA, and rRNA.85 Furthermore, these modifications increase the thermal stability of tRNA structures and contribute to the processing of nascent polycistronic mitochondrial RNA.86, 87
Although m1A is one of the most common RNA modifications in humans, its mechanisms and biological functions remain poorly understood. As m1A shares some regulators, such as YTHDF1–3, with m6A, studying m6A may provide insights into m1A. Due to its effect on RNA base pairing, m1A is expected to influence RNA interactions, including those involving mRNA, microRNA (miRNA), long ncRNA (lncRNA), and circular RNA (circRNA).
2.4 N7-methylguanosine
m7G, the methylation of guanine at the N7 position in RNA, occurs in roughly 0.4% of all guanosines.88 These m7G modifications are commonly located at the 5′ cap and internal sites of mRNA, as well as within rRNA, tRNA, and miRNA.89, 90 They actively affect biological and pathological processes via the metabolism of various RNA molecules.91
The primary enzyme responsible for this modification is METTL1, which works with the WD repeat domain 4 (WDR4) complex to enact m7G modifications in tRNA, miRNA, and mRNA, thereby influencing miRNA structure and biogenesis.92 The expression of WDR4 is closely correlated with METTL1 levels, underscoring WDR4's role as a crucial cofactor for METTL1.93 Reduced levels of METTL1 and WDR4 are strongly linked to neurological disorders, including brain ischemia94 and Alzheimer's disease.95 The METTL1/WDR4 complex modifies a specific subset of tRNAs with m7G, stabilizing these mRNAs against decay, increasing translation efficiency, reducing ribosome stalling, and increasing the expression of growth-promoting proteins, which facilitates cancer development.91, 96, 97
RNA guanine-7 methyltransferase (RNMT) and RNMT-activating mini-protein (RAM) are essential for effective mRNA cap methylation via m7G modifications.92 During T cell activation, stimulation of the T cell receptor induces RNMT, which coordinates the production of the mRNA, small nucleolar RNA (snoRNA), and rRNA necessary for ribosome biogenesis, improving translation capacity.98 Williams-Beuren syndrome chromosome region 22 (WBSCR22) and tRNA methyltransferase 112 (TRMT112) mediate m7G methylation in rRNA.99 WBSCR22, which was originally identified as one of the 26 genes associated with Williams syndrome, contains a nuclear localization signal and a highly conserved S-adenosyl-l-methionine binding motif.100 As a ribosome biogenesis factor, WBSCR22 has been reported in various human cancers,101 significantly contributing to tumor proliferation, migration, and development.102, 103
The m7G cap is recognized by eukaryotic translation initiation factor 4E (eIF4E) and the cap-binding complex (CBC), influencing mRNA maturation, nuclear export, and translation.104
m7G is commonly found in mRNA, where it plays a crucial role in regulating the translation process. Its role varies across different RNA types and diseases. m7G promotes some cancers, such as BCa,105 lung cancer,93 liver cancer,106 and gliomas,103 but has opposite effects in teratomas107 and PC.107 Our knowledge of m7G regulators remains quite limited. To date, no specific demethylase has been discovered that can control m7G levels. The interactions between m7G and other posttranscriptional modifications are gaining greater attention and their underlying mechanisms invite further exploration.
2.5 Pseudouridine
Discovered 70 years ago, Ψ is the C5-glycoside isomer of uridine, as well as the earliest and most abundantly modified nucleoside in RNA.108 Normal pyrimidine nucleosides form glycosidic bonds between the N-1 atom of the heterocycle and the C-1′ atom of the pentose. However, Ψ nucleosides bond the C-5 atom of the heterocycle to the C-1′ atom of the pentose.109 Ψ is found in nearly all types of RNA, both coding and noncoding, and is highly conserved across species.110-112
In humans, 14 Ψ writers have been identified that are responsible for catalyzing Ψ formation through either RNA-dependent or RNA-independent mechanisms. The RNA-dependent process involves dyskerin Ψ synthase 1 (DKC1), which is the catalytic subunit of the H/ACA snoRNA complex that catalyzes rRNA pseudouridylation.113 The remaining 12 writers are the RNA-independent single Ψ synthases (PUSs) PUS1/2/3/4/6/7/7L/9/10 and the RNA Ψ synthase domain-containing genes 1–4 (RPUSD1–4), each with specific cellular localization and RNA targets.114-118
Currently, no specific “readers” or “erasers” for Ψ have been identified. The lack of erasers might reflect the inertness of the C−C bond between the base and ribose in Ψ compared with the C−N bond, making pseudouridylation irreversible.
When Ψ is incorporated into RNA, it increases the thermodynamic stability and spatial conformation of the RNA by increasing base stacking, improving base pairing, and rigidifying the sugar–phosphate backbone.119, 120 tRNA contains numerous pseudouridylation sites, including in the anticodon stem and loop, TΨC loop, and the D stem. These modifications contribute to tRNA's structural stability, codon–anticodon recognition, and translation efficiency and accuracy.121, 122 In rRNA, Ψ is found in critical regions such as the decoding site, mRNA channel, peptidyl transferase center, tRNA binding sites, and ribosomal subunit interface. These sites play crucial roles in ribosome assembly, function, and protein synthesis.123, 124 In mRNA, Ψ can improve precursor mRNA splicing, facilitate the conversion of nonsense codons to sense codons and improve base pairing at the ribosome decoding center, contributing to protein diversity.111, 117, 125 Other in vitro studies have reported that mRNA with Ψ translates more slowly and affects mRNA decoding more than unmodified mRNA.126
Nucleoside modifications can effectively increase mRNA stability and translation efficiency while reducing immunogenicity in vivo. The benefits conferred by Ψ make mRNA a promising tool for gene replacement and vaccination.127 Ψ deposition can endow modified RNA with distinct molecular properties, altering its fate or activity. In addition, as a prevalent RNA modification, Ψ plays a crucial role in various cancers.128-130
2.6 Adenosine-to-inosine editing
Discovered over 30 years ago, adenosine-to-inosine (A-to-I) editing was initially identified for its role in introducing a premature stop codon in mRNA, resulting in the production of apolipoprotein B.131, 132 Recently, A-to-I editing has been linked to tumorigenesis and cancer progression. This process is facilitated by the adenosine deaminase acting on the RNA (ADAR) family of proteins, which deaminate adenosine to produce inosine in double-stranded RNA (dsRNA).133, 134
In humans, two catalytic forms, ADAR1 and ADAR2, mediate dsRNA-specific A-to-I editing. These enzymes comprise deaminase domains and dsRNA-binding domains (dsRBDs), functioning as both writers and editors of A-to-I modifications.135 In contrast, ADAR3 lacks editing capacity and functions as a negative regulator of ADAR1-mediated editing. It competitively binds to dsRNA, reducing the efficiency of ADAR1 and ADAR2.136
In 1995, researchers reported that ADAR1 comprises two main isoforms: the interferon-inducible ADAR1 p150 and the constitutively expressed ADAR1 p110. ADAR1 p150 is located in both the cytoplasm and the nucleus, while ADAR1 p110 primarily exists in the nucleus.137 In addition, ADAR1 contains a z-DNA binding domain.138 Both ADAR1 and ADAR2 are ubiquitously expressed, whereas ADAR3 is predominantly found in the brain. In UTRs, A-to-I editing can regulate various RNA processes, including transport, translation, and degradation.139-142
A-to-I editing facilitates the recruitment of the RNA-binding protein human antigen R to the 3ʹ UTR of cathepsin S (CTSS) mRNA, improving the stability and translation of CTSS mRNA.143 In miRNAs, A-to-I editing can affect miRNA biogenesis and function.144, 145 Furthermore, A-to-I editing inhibits the formation of dsRNA structures in Alu elements, promoting canonical linear mRNA splicing and suppressing circRNA formation.146, 147 A-to-I editing influences lncRNAs’ secondary structure, stability, and interactions with other molecules.148 Adenosine deaminase acting on tRNA 2 (ADAT2) and adenosine deaminase acting on tRNA 3 (ADAT3) are closely associated with tRNA decoding capabilities.145
3 RNA MODIFICATIONS’ ROLE IN CANCER PROLIFERATION
RNA modifications play a crucial role in cancer proliferation. By regulating various metabolic pathways, biosynthetic pathways, signaling pathways, and the cell cycle, they significantly affect tumor cell growth and survival.
3.1 Metabolic reprogramming
During the metabolic reprogramming of tumor cells, the glycolytic pathway is particularly crucial (Figure 2A). Due to the Warburg effect, tumor cells preferentially use glycolysis even under aerobic conditions.149 This metabolic strategy not only provides the energy required for rapid proliferation but also generates precursors for biosynthesis.150 Glycolysis metabolites are essential for tumor cell growth and survival.151
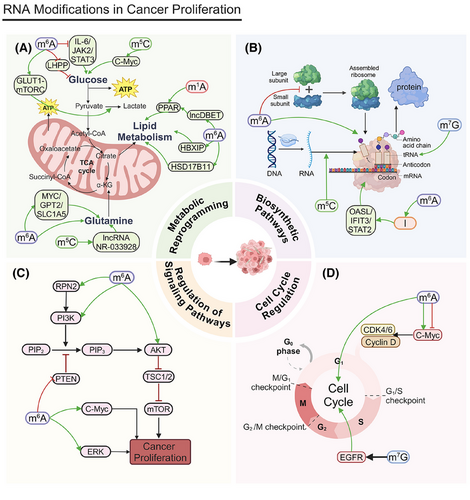
Mitochondrial dysfunction and the activation of glycolysis are key characteristics of HCC. Nucleolar protein 2 (NOP2) promotes HCC progression by regulating Myc proto-oncogene protein (c-Myc) expression through m5C modification, increasing glycolysis.152 FTO suppresses apolipoprotein E (APOE) expression by reducing its m6A modification. This regulates the interleukin 6 (IL-6)/Janus kinase (JAK2)/STAT3 signaling pathway, inhibiting glycolysis and tumor growth in papillary thyroid carcinoma (PTC) cells.153
In CRC, METTL3 directly induces the m6A–glucose transporter type 1 (GLUT1)–mechanistic target of the rapamycin complex 1 (mTORC1) axis, promoting glucose uptake, lactate production, and CRC progression. Inhibiting mTORC1 increases the anticancer effects of METTL3 silencing in CRC patients’ tissues and METTL3 transgenic mouse models.154 In PC, YTHDF3 interacts with lncRNA DICER1-AS1, promoting its degradation under glucose depletion. This degradation reduces the inhibitory effect lncRNA DICER1-AS1 imposes on glycolysis, resulting in more available energy and metabolic intermediates for rapid cancer cell proliferation.155 In lung adenocarcinoma (LUAD), m6A modification via METTL3 upregulation and ALKBH5 downregulation increases ENO1 translation, promoting glycolysis and providing energy for cancer cell proliferation. Increased ENO1 expression directly fosters tumor cell proliferation.156 LHPP, a histidine phosphatase, inhibits glycolysis and proliferation in gastric cancer cells by suppressing glycogen synthase kinase 3 beta (GSK3b) phosphorylation and mediating hypoxia-inducible factor 1 alpha (HIF1A). METTL14 adds m6A modifications to LHPP mRNA, inhibiting its expression, which may increase GSK3b activity and reduce HIF1A activity to promote cancer cell proliferation.157
Glucose not only promotes tumor progression through glycolysis but also acts as a signaling molecule to regulate tumor proteins. In prostate, liver, colon, and breast cancer (BC) cells, as well as melanoma, glucose binds to the N-terminal region of methyltransferase NSUN2, promoting its oligomerization and activation. Activated NSUN2 maintains overall m5C RNA methylation levels, stabilizing TREX2 and limiting dsDNA accumulation at the cell membrane and cGAS/STING activation, which promotes tumorigenesis.65
In addition to glycolysis, glutaminolysis is a crucial component of the metabolic reprogramming of tumor cells. Through glutaminolysis, tumor cells obtain energy and carbon sources that are essential for biosynthesis and contribute to antioxidation. Glutaminolysis is vital for tumor cell growth and survival.158, 159
NSUN2 methylates lncRNA NR-033928, stabilizing glutaminase (GLS) mRNA and promoting glutamine metabolism reprogramming, which increases gastric cancer proliferation.160 Methyltransferase-like 16 (METTL16) influences gene expression by regulating m6A modification, promoting genes associated with cell proliferation and supporting leukemia cell survival and proliferation.161 IGF2BP2 binds to m6A-modified mRNA, increasing its stability and translation and thus regulating key genes involved in the glutamine metabolic pathway, such as MYC, GPT2, and solute carrier family 1 member 5 (SLC1A5), promoting acute myeloid leukemia (AML) cell proliferation and leukemia stemness/self-renewal.162
The regulation of lipid metabolism also plays a vital role in the metabolic reprogramming of tumor cells. Tumor cells increase fatty acid synthesis and β-oxidation to meet the demands of rapid proliferation. Lipid metabolism is essential not only in the construction of tumor cell membranes but also in signal transduction.163
The TRMT6/TRMT61A complex boosts the m1A methylation of specific tRNAs, increasing peroxisome proliferator-activated receptor (PPAR) translation. This process stimulates cholesterol synthesis, activates Hedgehog signaling, and, ultimately, promotes the self-renewal and tumorigenesis of liver cancer stem cells (CSCs).164 Cancer cells persistently engage in elevated glycolysis and glutaminolysis by reprogramming their internal metabolism, which drives the progression of HCC. Understanding these mechanisms can provide new insights for cancer therapies. Hepatitis B virus X-interacting protein (HBXIP) is upregulated in liver cancer tissues and mediates the METTL3-induced metabolic reprogramming and malignant behavior of HCC cells, accelerating tumor progression.165 The elevated expression of FTO correlates with poor prognoses in patients with esophageal cancer (EC). FTO promotes lipid droplet (LD) formation by regulating hydroxysteroid 17-beta dehydrogenase 11 (HSD17B11) expression, affecting cell proliferation.166 In advanced BCa, m6A modification regulated by methyltransferase METTL14 increases the expression of lncDBET, which activates the PPAR signaling pathway and leads to increased lipid metabolism and cancer proliferation.167
3.2 Biosynthetic pathways
In tumor cell biosynthetic pathways, nucleic acid synthesis and ribosome synthesis are critical processes (Figure 2B). Tumor cells increase purine and pyrimidine synthesis to support rapid DNA and RNA replication and boost ribosome synthesis to meet high protein production demands.168, 169 These biosynthetic activities provide the necessary genetic material and proteins for rapid cell proliferation, promoting the cancer's growth and spread. This surge in nucleic acid and ribosome synthesis is closely tied to the swift proliferation of tumor cells, playing a pivotal role in their growth mechanisms.
The m7G methyltransferase RNMT regulates ribosome synthesis, making it essential for T cell activation. m7G in tRNAs maintains tRNA structural integrity by promoting stability and translation efficiency and reducing ribosome stalling, thus influencing the mRNA translatome.98 WBSCR22 is a ribosome biogenesis factor. The protein TRMT112 interacts with and promotes the overproduction of WBSCR22, which reduces ISG15 levels. This decrease in ISG15 expression reduces PC cells’ ability to migrate to and invade surrounding tissues.102
m5C RNA modification regulators, such as TRDMT1, NSUN1, and NSUN4, are differentially expressed in LUAD, potentially affecting RNA translation and stability and, thus, regulating tumor cell proliferation. Different m5C modification clusters are associated with differences in patient survival, supporting this interpretation.170 NOP2/NSUN1, an oncogene, is overexpressed in various cancers. By regulating rRNA processing and function, NOP2/NSUN1 may influence ribosome synthesis and promote cancer cell proliferation.171
In the nucleus, METTL1 regulates gene expression by modulating m6A modifications to mRNA. METTL16 operates in the cytoplasm, where its methyltransferase domain facilitates interactions with the eukaryotic initiation factors eIF3a and eIF3b, along with rRNA. These interactions aid in the formation of the translation initiation complex and promote mRNA translation, and they collectively promote HCC proliferation.172 The depletion of methyltransferase-like 5 (METTL5)-mediated m6A modification on 18S rRNA impairs 80S ribosome assembly, affecting mRNA translation and, consequently, protein synthesis related to cell proliferation.173 In gastric cancer, METTL3 associates with PABPC1, stabilizing the attachment of the eIF4F complex and preferentially boosting the translation of specific epigenetic factors. This mechanism enables cancer cells to more efficiently synthesize proteins related to proliferation, thereby promoting tumor growth.174 In glioblastoma, upregulated METTL3 methylates ADAR1 mRNA, increasing the resulting protein levels and establishing a protumor mechanism that involves METTL3, YTHDF1, and ADAR1. ADAR1 promotes cancer by binding to CDK2 mRNA independently of deaminase activity, and ADAR1 knockdown significantly inhibits glioblastoma growth in vivo. The METTL3/ADAR1 axis establishes a connection between m6A modification and A-to-I RNA editing during posttranscriptional regulation, which reveals a novel pathway involved in cancer progression.175
ADAR is markedly upregulated in BC tissues and may drive the progression of the disease by interacting with OASL, STAT2, and IFIT3. In vitro experiments show that ADAR knockdown hinders BC cell proliferation, invasion, and migration, while ADAR overexpression promotes these activities.176 The ADAR1 enzyme performs A-to-I edits, preventing the sensing of endogenous dsRNA. In triple-negative BC (TNBC) cells, ADAR1 knockdown inhibits cell proliferation and tumorigenesis, causing strong translational suppression.
TNBC cell lines that rely on ADAR1 also demonstrate increased levels of genes activated by interferon.177, 178
3.3 Regulation of signaling pathways
The signaling pathway comprising phosphoinositide 3-kinase (PI3K), protein kinase B (AKT), and mammalian target of rapamycin (mTOR) is essential for tumor cell communication, influencing cell growth, division, and maintenance (Figure 2C). The dysregulation of this pathway is strongly linked to the augmentation and persistence of numerous tumor types. Modulating this pathway can effectively influence tumor cell behavior.179
FTO facilitates pancreatic cancer cells’ growth and resistance to gemcitabine by modulating phosphatase and tensin homolog (PTEN) expression and affecting the PI3K/AKT signaling cascade.180 Meanwhile, YTHDF1 stabilizes m6A-modified mRNA, boosting the expression of its downstream target, RPN2, which then activates the PI3K/AKT/mTOR pathway, thereby promoting BCa cells’ growth and resistance to cisplatin. Suppressing METTL3 and YTHDF1 expression decreases RPN2 levels, which inhibits this pathway and affects both cancer cell proliferation and chemotherapy efficacy.181 High YTHDF2 expression promotes diffuse large B-cell lymphoma (DLBCL) cell proliferation and supports tumor growth by inhibiting apoptosis. YTHDF2 also influences ceramide metabolism and related pathways (e.g., extracellular signal-regulated kinase [ERK] and PI3K/AKT), further driving tumor progression.182 IGF2BP2 augments cellular survival and proliferation by upregulating the activity of p-AKT and c-Myc. The activation of the p-AKT pathway is intimately connected with cellular growth, metabolic processes, and survival.183
3.4 Cell cycle regulation
Cell cycle regulation is fundamental to tumor cell proliferation (Figure 2D). Cell cycle advancement is driven by cyclins and cyclin-dependent kinases (CDKs), which together facilitate tumor cell growth. Abnormal cell cycle regulation drives the uncontrolled proliferation of tumor cells.184 Understanding this regulatory process is essential for elucidating tumor cell growth mechanisms.185
Serine hydroxymethyltransferase 2 (SHMT2) influences c-Myc mRNA stability and expression by regulating its m6A modification, inhibiting c-Myc expression through METTL3/FTO/ALKBH5/IGF2BP2 and, thereby, blocking EC cell proliferation.186 The oncogene c-Myc is instrumental in advancing the cell cycle from the G1 to the S phase by upregulating genes such as Cyclin D and CDK4/6, which are essential for cellular proliferation.187 The tRNA m7G modification that is mediated by METTL1 and WDR4 is linked to unfavorable prognoses in HCC. Silencing METTL1 or WDR4 curtails HCC cell proliferation, migration, and invasion; conversely, elevated METTL1 expression facilitates HCC progression.97 METTL1 regulates epidermal growth factor receptor (EGFR)/EFEMP1 translation by modifying certain tRNAs, leading to BCa proliferation, migration, and invasion.105 FTO, an m6A demethylase, regulates RNA methylation status, affecting the cell cycle and proliferation-related gene expression. The upregulation of FTO may promote uterine leiomyosarcoma cell proliferation.188 ALKBH5 demethylates m6A modifications, influencing gene expression and cell processes, including proliferation. Inhibiting ALKBH5 increases m6A levels, which generally decreases mRNA stability and translation efficiency, thereby affecting cell cycle regulation.189 This inhibition can lead to reduced cancer cell proliferation by altering mRNA dynamics.190 IGF2BP1 promotes HCC proliferation by regulating c-Myc expression.191
In summary, RNA modifications play pivotal roles in metabolic reprogramming, biosynthetic pathways, signaling pathway regulation, and cell cycle control in tumor cells. These mechanisms collectively affect tumor cells’ rapid proliferation, driving cancer progression. A thorough investigation of RNA modification mechanisms and functions deepens our comprehension of tumor biology and offers potential targets for new cancer treatment strategies.
4 RNA MODIFICATIONS IN CANCER METASTASIS
The role of RNA modifications in cancer metastasis is also garnering increasing attention. These modifications influence tumor cell migration and invasion through various mechanisms. This section will consider the effects of RNA modifications in EMT, immune microenvironment regulation, signaling pathway modulation, and metabolic reprogramming.
4.1 Epithelial–mesenchymal transition
EMT plays a critical role in normal embryonic development and tissue repair (Figure 3A). However, its abnormal reactivation is linked to tumor cells’ malignant traits during cancer advancement and metastasis. EMT equips tumor cells with increased migratory and invasive properties by downregulating epithelial markers, such as E-cadherin, and upregulating mesenchymal markers, including N-cadherin and vimentin.192
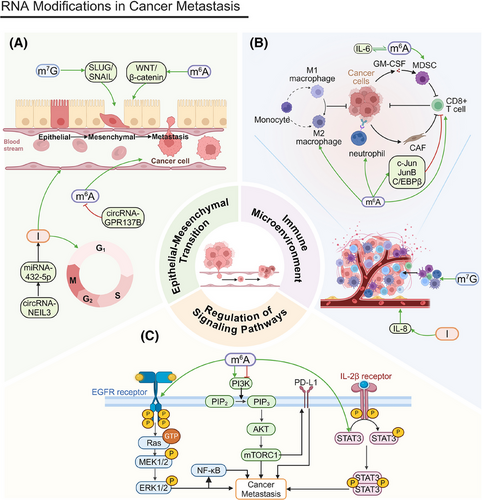
YTHDF2 overexpression triggers EMT in lung squamous cell carcinoma (LUSC), promoting greater cell migration and invasion. This phenomenon has been substantiated by Transwell invasion assays and wound healing assays.193 In pancreatic ductal adenocarcinoma (PDAC), circNEIL3 enhances proliferation and metastasis through the circNEIL3/miR-432-5p/ADAR1/GLI1 axis, affecting both the cell cycle and EMT processes. Its expression is modulated by ADAR1 through a negative feedback mechanism.194 In nasopharyngeal carcinoma (NPC), METTL1 facilitates proliferation and EMT by activating the WNT/β-catenin signaling pathway.195 In HCC, m7G modification increases EMT by promoting the translation of SLUG and SNAIL, key EMT regulators, increasing cell invasion and metastasis, especially after radiofrequency ablation (RFA).196
FTO knockdown significantly inhibits the invasion and stemness of EC cells, while FTO overexpression promotes these traits. Invasion and stemness are crucial for cancer cell migration and metastasis, and FTO enhances EC cell migration and invasion.166 circGPR137B inhibits distant metastasis in HCC by upregulating FTO.197 MIR100HG is a positive regulator of EMT; m6A modification strengthens the association between MIR100HG and hnRNPA2B1, stabilizing TCF7L2 mRNA. This activation of the Wnt/β-catenin signaling pathway promotes CRC cell invasion and metastasis.198 METTL14 regulates m6A modification of lncRNA–NEAT1_1, affecting renal cell carcinoma (RCC) cell migration. YTHDF2 selectively recognizes m6A modifications on NEAT1_1 and accelerates its degradation, inhibiting cell metastasis and demonstrating the critical role m6A modification plays in cancer metastasis.199
4.2 Regulation of the immune microenvironment
Another crucial factor in cancer metastasis is the regulation of the tumor immune microenvironment (Figure 3B). RNA modifications significantly affect tumor immune evasion and metastatic potential by altering immune cell function and infiltration. These modifications crucially mediate interactions between tumor cells and immune cells.200-202
The loss of METTL14 exacerbates the macrophage response to acute bacterial infections and drives CD8+ T cells toward dysfunction, impairing their ability to eliminate CRC tumors.203 FTO-mediated m6A demethylation increases the levels of the transcription factors c-Jun, JunB, and C/EBPβ in tumor cells, which alters glycolytic metabolism, impairs CD8+ T cell function, and inhibits tumor progression. Furthermore, m6A modifications alter the tumor immune microenvironment by modulating immune cell infiltration, contributing to cancer metastasis.204 The accumulation of MDSCs is intricately linked to tumor metastasis. METTL3 facilitates MDSC migration through the basic helix-loop-helix family member e41 (BHLHE41)–CXC motif chemokine ligand 1 (CXCL1)/CXCR2 axis, which may influence tumor cells’ metastatic potential.205 The subcellular localization of METTL3 plays a critical role in cancer metastasis. An IL-6-dependent positive feedback loop amplifies the function of nuclear METTL3, initiating BC metastasis. In addition, METTL3 deacetylation and nuclear translocation are associated with increased m6A modification levels, which may influence the expression of genes related to metastasis and, ultimately, promote it.206 ALKBH5 promotes cancer cell migration and invasion by modulating m6A modifications, a phenomenon confirmed by studies on glioma cells in vitro and in vivo. It also recruits M2 macrophages into the TME, potentially promoting tumor metastasis.207
m7G modification may increase cancer cells’ invasiveness and promote metastasis by altering the composition of immune cells within the TME. An immunosuppressive microenvironment can diminish the immune system's ability to monitor tumor cells, elevating the risk of cancer cell metastasis.208 Core m7G-modified genes FN1 and ITGB1 regulate immune cell infiltration (e.g., macrophage and neutrophil upregulation) by fostering interactions between the stroma and cancer cells, driving invasion and metastasis.209 Although glioma metastasis is less pronounced than that of other cancers, m7G can still influence tumor cell invasion via the SPP1 and PTN signaling pathways, facilitating its expansion into surrounding tissues.210
A-to-I editing of AZIN1 promotes tumor angiogenesis and increases tumor cell invasion and metastasis by upregulating IL-8.211 Having completed our discussion of the effects of RNA modifications on the immune microenvironment, we will next analyze their role in signaling pathway regulation.
4.3 Regulation of signaling pathways
The regulation of signaling pathways is essential for tumor cell migration and invasion (Figure 3C). RNA modifications affect tumor cell behavior through multiple signaling pathways, such as the PI3K/AKT pathway, the MAPK/ERK pathway, and others.
The PI3K/AKT pathway is fundamental for cell growth, proliferation, and survival. The upregulation of fibroblast growth factor 19 (FGF19) and its receptor, fibroblast growth factor receptor 4 (FGFR4), is associated with HCC cells’ invasiveness. By driving IGF2BP1 expression, FGF19/FGFR4 may foster metastasis. These proteins can promote programmed death ligand 1 (PD-L1) upregulation via the PI3K/AKT pathway, boosting tumor cells’ immune evasion and metastatic potential.212 METTL14 upregulation also inhibits RCC cell migration. The PI3K/AKT pathway is integral not only to cell proliferation but also to cell migration and invasion. By inhibiting this pathway via m6A modification, METTL14 indirectly suppresses cancer cell migration and invasion.213 FTO inhibitors may also alter tumor cell invasiveness by affecting cell proliferation and survival, influencing their metastatic capacity. Furthermore, FTO inhibitors can regulate the Wnt/PI3K–AKT signaling pathway, affecting cancer metastasis.214 The expression of EphA2 and vascular endothelial growth factor A (VEGFA) are closely linked to tumor metastasis. Research indicates that METTL3, through an IGF2BP2/3-dependent mechanism, inhibits the degradation of EphA2 and VEGFA mRNA by modulating the PI3K/AKT and ERK1/2 pathways, facilitating metastasis.215
The MAPK/ERK pathway facilitates tumor cell growth and migration by controlling genes related to the cell cycle and cell proliferation. RNA modifications are also crucial in this pathway. ALKBH5 boosts both the proliferation and metastasis of HCC cells by upregulating MAP3K8, which, in turn, activates the JNK and ERK signaling pathways that are essential for cell migration and metastasis.216 Deficiencies of the m6A methyltransferase METTL3 hinder YTHDF1-mediated translation of Sprouty-related EVH1 domain-containing protein 2 (SPRED2), augmenting the activation of nuclear factor kappa B (NF-κB) and STAT3. This augmentation promotes tumor growth and metastasis through the ERK pathway.217 METTL3 also plays a pivotal role in cancer metastasis through its regulation of the MAPK signaling pathway and related immune responses.218
In addition to these pathways, RNA modifications influence tumor cell migration and invasion through other signaling pathways.
METTL3 governs the m6A modification of Frizzled-10, increasing its expression in liver cancer stem cells and activating the β-catenin and yes-associated protein 1 (YAP1) signaling pathways. This increases tumor cell self-renewal and tumorigenicity. The upregulation of Frizzled-10 forms a positive feedback loop, further activating METTL3 and driving HCC metastasis and drug resistance.219
FTO not only promotes cell proliferation but also supports tumor metastasis by affecting cell migration and invasion. It regulates heat shock factor 1(HSF1)Heat Shock ProteinsHeat Shock Proteins expression through demethylation, altering HSP levels, which promotes the proliferation, survival, migration, and invasion of multiple myeloma cells.220 Carbon ion radiotherapy elevates METTL3 levels and its associated m6A modifications in NSCLC cells, influencing cancer cell migration and invasion. By regulating critical gene expression, m6A modifications can either promote or suppress cancer metastasis.221 The overexpression of WTAP markedly increases ovarian cancer cells’ invasive potential by modulating miR-200 expression and affecting genes involved in cell migration and invasion.222 The upregulation of METTL16 in HCC induces m6A modifications of lncRNA–RAB11B-AS1, decreasing its transcript stability and resulting in its downregulation. This process promotes the proliferation, migration, and invasion of HCC cells, inhibits apoptosis, and facilitates tumor growth in vivo.223
4.4 Metabolic reprogramming in cancer metastasis
Metabolic reprogramming is essential for tumor cells to adapt to their rapid proliferation and challenging environmental conditions. Because RNA modifications regulate metabolic pathways, affecting energy metabolism and biosynthesis, they can enhance migration and invasion.
YTHDF1 increases BC cell tumorigenicity and metastasis by upregulating PKM2, promoting glycolysis. Inhibiting YTHDF1 eliminates YTHDF1-dependent tumor growth and metastasis.224 Targeting METTL3/14 increases ACLY and SCD1 protein levels, along with the production of triglycerides and cholesterol and the accumulation of LDs. This change in membrane lipid composition fosters tumor cell migration and invasion.225 Mitochondrial m5C modification is essential for cancer cell metastasis. Tumor cells that are dependent on CD36 rely on m5C modifications to initiate invasion and dissemination. Cells deficient in m5C exhibit impaired metastasis, underscoring the significance of RNA modifications in modulating mitochondrial oxidative phosphorylation and metabolic pathways.226
In conclusion, RNA modifications drive numerous mechanisms underlying cancer metastasis. A comprehensive investigation of the specific functions and mechanisms of these modifications will improve our understanding of the intricate processes driving tumor metastasis and offer novel insights for future therapeutic strategies.
5 RNA MODIFICATIONS IN PROGRAMMED CANCER CELL DEATH
RNA modifications’ role in cancer programmed cell death is gaining increasing attention as these modifications influence tumor cell survival and death through various mechanisms. This section will detail how RNA modifications affect different types of programmed cell death through signaling pathway regulation and altering the tumor immune microenvironment.
5.1 Types of programmed cell death
Programmed cell death is an active cellular process that occurs under particular conditions, encompassing apoptosis, autophagy, and ferroptosis. Apoptosis is triggered by internal and external signals involving enzymes, such as caspases, and various signaling pathways (Figure 4A). Many anticancer drugs kill tumor cells by inducing apoptosis.
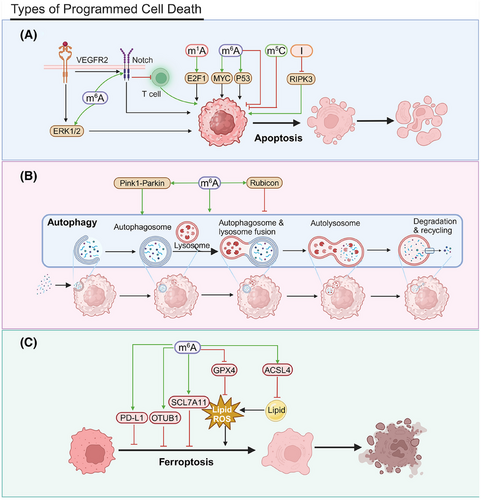
The m1A modification influences the stability of E2 promoter binding factor 1 (E2F1) mRNA, a key regulator of the cell cycle and apoptosis. By regulating E2F1 expression, m1A can indirectly affect programmed cell death in cancer cells.227 The m5C reader ALYREF is notably upregulated in HCC tissues and cell lines. ALYREF knockdown markedly suppresses the proliferation of Huh7 and HepG2 cells and elevates apoptosis rates, demonstrating tumor-suppressive effects in vivo.228
YTHDF1 upregulation may inhibit antitumor immune responses, affecting apoptosis and programmed cell death. FTO inhibitors, including Compound 18097, augment the m6A modification in suppressor of cytokine signaling 1 (SOCS1) mRNA, which increases its stability and activates the P53 pathway, facilitating apoptosis.229 FTO inhibitor 44/ZLD115 upregulates RARA and downregulates MyC in leukemia cells, inducing apoptosis.230 IGF2BP2 regulates m6A modifications, affecting mitochondrial activity and gene expression in hematopoietic stem cells HSCs and, thereby, influencing cell survival and programmed death. Mitochondrial activity is closely linked to both energy metabolism and apoptosis.231 IGF2BP1 knockdown induces cancer cell apoptosis; this effect highlights its role in programmed death. Cucurbitacin B (CuB), a chemogenetic small molecule, promotes apoptosis by blocking IGF2BP1's recognition of m6A-modified mRNA, which affirms m6A's critical role in cancer cell survival and death regulation.191 High FTO expression inhibits apoptosis and promotes leukemia cell survival by affecting the PDGFRB/ERK signaling axis.232, 233 IGF2BP2 knockdown reduces T-ALL cell proliferation and increases apoptosis by modulating m6A modifications and interacting with NOTCH1.234
ADAR1 prevents the accumulation of Z-RNA, inhibiting ZBP1 activation and RIPK3-mediated necroptosis and helping cancer cells evade programmed cell death.235
Autophagy is a cellular process that involves the degradation and recycling of intracellular components to maintain homeostasis (Figure 4B). Under specific conditions, autophagy can result in cell death. m6A modifications can affect cell viability by altering the expression of genes associated with autophagy.236 The m6A methyltransferase METTL3 targets DCP2, influencing the regulation of the Pink1-Parkin pathway, which, in turn, affects mitochondrial autophagy and damage in small-cell lung cancer (SCLC) cells. This interaction contributes to chemotherapy resistance in SCLC patients.237 METTL3 and YTHDF1 facilitate the m6A modification of Rubicon mRNA, increasing transcript stability. Rubicon, in turn, inhibits the fusion process between autophagosomes and lysosomes, obstructing the clearance of LDs.238
Ferroptosis is an emerging form of programmed cell death characterized by the accumulation of intracellular iron and subsequent peroxidation of lipids239 (Figure 4C). FTO promotes OTU deubiquitinase, ubiquitin aldehyde binding 1 (OTUB1) expression by demethylating its m6A, inhibiting ferroptosis and increasing radioresistance.240 FTO acts as a tumor suppressor in PTC by downregulating the cystine/glutamate antiporter solute carrier family 7 member 11 (SLC7A11) via the ferroptosis pathway.241 METTL3 engages the m6A reader YTHDF1 to boost m6A modification and the translation of SLC7A11, facilitating LUAD cell proliferation and suppressing ferroptosis.242 The METTL3/IGF2BP1/m6A axis stabilizes SLC7A11 mRNA, upregulating its expression by preventing deadenylation. This upregulation facilitates hepatoblastoma (HB) cell proliferation and mitigates ferroptosis in vitro and in vivo.243 The upregulation of m6A–SLC7A11 in glioblastoma confers resistance to ferroptosis, suggesting that targeting the m6A–SLC7A11 axis could, instead, promote cancer cell ferroptosis.244
Hypoxia-induced lncRNA–CBSLR interacts with YTHDF2, forming a signaling axis that reduces cystathionine beta-synthase (CBS) mRNA stability. This affects the methylation and degradation of ACSL4 protein, lowering proferroptotic phosphatidylethanolamine levels and leading to ferroptosis resistance.245 CD8 T cells stimulate ACSL4 expression via IFNγ, altering tumor cell lipid profiles and promoting the incorporation of arachidonic acid (AA) into phospholipids, inducing ferroptosis. Low-dose AA further promotes this mechanism and boosts immune checkpoint blockade (ICB)-induced antitumor immunity. Targeting the ACSL4 pathway could be an anticancer strategy.246 The inhibition of protein arginine methyltransferase 3 (PRMT3) leads to the upregulation of METTL14, which increases m6A–YTHDF2-dependent methylation. This process diminishes the stability of glutathione peroxidase 4 (GPX4) mRNA, heightens lipid peroxidation, and accelerates ferroptosis in endometrial cancer.247 YTHDF1 suppresses CD8+ T cell-induced ferroptosis in PCa, affecting programmed cell death. YTHDF1 upregulation increases PD-L1 expression, diminishing T cell cytotoxicity and ferroptosis; this effect affirms YTHDF1’s critical role in tumor immune evasion.248
5.2 Tumor immune microenvironment
The regulation of the tumor immune microenvironment is another key factor in cancer programmed cell death. CD8+ T cells serve as key effector cells that can directly eliminate tumor cells, and their infiltration levels within the TME are strongly linked to prognoses (Figure 5A). Boosting the infiltration and activity of CD8+ T cells is a primary objective of numerous immunotherapy approaches.
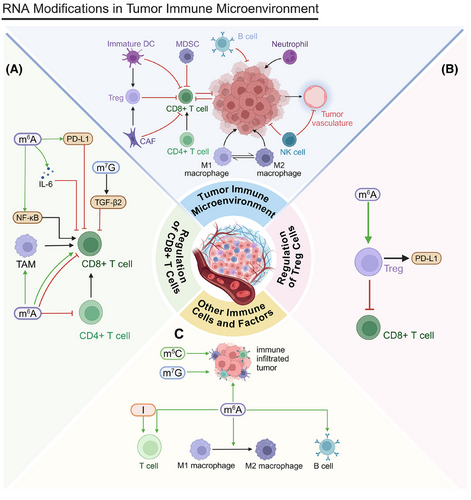
After RFA in HCC, METTL1 upregulation induces TGF-β2 translation, creating an immunosuppressive environment and inhibiting CD8+ T cell activity. Fewer CD8+ T cells decrease tumor cells’ sensitivity to programmed cell death. METTL1 influences tumor cell survival and programmed cell death by regulating the immune microenvironment.249 Within the TME, a deficiency in YTHDF2 augments the antigen-presenting capacity of tumor-associated macrophages (TAMs), facilitating the activation and proliferation of CD8+ T cells. Activated CD8+ T cells effectively recognize and kill tumor cells, promoting programmed cell death. Therefore, YTHDF2 affects tumor cell survival and death by regulating TAM function.250 Elevated YTHDF2 expression in SCLC is associated with decreased immune infiltration, manifesting as reductions in cluster of differentiation 4 (CD4)+ and cluster of differentiation 8 (CD8)+ T cells, affecting immune-related treatments and resulting in poor prognoses.251 ALKBH5 promotes CD8+ T cell infiltration in the CRC microenvironment by inhibiting the NF-κB pathway and reducing C-C motif chemokine ligand 5 (CCL5) expression. Increased CD8+ T cell infiltration may promote tumor cell programmed cell death.252
circCCAR1 induces CD8+ T cell dysfunction by stabilizing programmed cell death protein 1 (PD-1), potentially reducing tumor cells’ sensitivity to programmed cell death. IGF2BP3 increases circCCAR1 stability, indirectly affecting tumor cell survival and death.253 YTHDF1 inhibits CD8+ T cell cytotoxicity by inducing IL-6 secretion, allowing tumor cells to evade immune surveillance and inhibiting programmed cell death. This immunosuppressive environment facilitates tumor cells’ survival and proliferation.254 The reduction of YTHDF1 impedes tumor growth by reinstating CD8+ T cell infiltration, suggesting its significant role in programmed cell death.255 IGF2BP1 affects programmed cell death by reducing CD8+ T cell-mediated tumor cytotoxicity. The upregulation of IGF2BP1 increases PD-L1 stability, inhibiting CD8+ T cell function and reducing apoptosis in CRC.256 Retinoic acid-inducible gene I (RIG-I) (encoded by DDX58 mRNA) influences immune cell activity through the interferon alpha (IFNα) signaling pathway. The upregulation of ALKBH5 inhibits this mechanism, possibly enabling tumor cells to evade programmed cell death and, thereby, fostering tumor growth.257
Conversely, regulatory T (Treg) cells help tumor cells evade immune attack by inhibiting effector T cell activity (Figure 5B). Targeting Treg cells can improve antitumor immune responses.258 In tumors with high glycolytic activity, Treg cells actively uptake lactate through monocarboxylate transporter 1 (MCT1), which facilitates the translocation of nuclear factor of activated T-cells 1 (NFAT1) to the nucleus and increases PD-1 expression. Effector T cells, however, have suppressed PD-1 expression. Lactate in the high-glycolytic TME significantly affects cancer programmed cell death and immunotherapy efficacy by affecting Treg cells’ PD-1 expression.259 YTHDF2 deficiency increases apoptosis and impairs the suppressive function of Treg cells in the TME. Reduced Treg cells may increase tumor cells’ sensitivity to programmed cell death as Treg cells typically protect tumor cells by inhibiting effector T cell activity.260
Beyond CD8+ T cells and Treg cells, numerous other critical immune cells and factors populate the tumor immune microenvironment (Figure 5C). Specifically, in head and neck squamous cell carcinoma (HNSCC), the overexpression of ALKBH5 suppresses RIG-I-mediated IFNα secretion via the IκB kinase epsilon/TBK1/interferon regulatory factor 3 (IRF3) signaling pathway, which is closely related to tumor cell programmed cell death. In addition, the overexpression of ALKBH5 diminishes the population of tumor-infiltrating lymphocytes, which could reduce immune surveillance and programmed cell death.257 METTL14-mediated m6A modification may influence tumor cell programmed cell death by regulating the mRNA decay of negative immune regulators. By promoting positive selection, METTL14 may increase B cells’ resistance to immune attacks.261
The immunosuppressive function of tumor-infiltrating myeloid cells (TIMs) influences both tumor cell survival and the process of programmed cell death, primarily through METTL3 regulation. The presence of TIMs may inhibit T cell activity, affecting tumor cell programmed cell death.262 YTHDF3 may influence programmed cell death mechanisms by promoting M1 macrophage polarization, increasing the immune response against tumor cells, and increasing apoptosis rates. Conversely, M2 macrophage polarization may lead to immunosuppression and reduced tumor cell apoptosis.263 IGF2BP1 is linked to immune checkpoint expression and tumor mutational burden (TMB), potentially affecting programmed cell death mechanisms through immune pathways and altering cancer cell survival and death rates.264
m6A/m5C modifications can influence tumor cell sensitivity to programmed cell death by affecting the immune microenvironment. Early-stage LUAD with high m6A/m5C expression presents with elevated immune checkpoint gene and immune cell expression, potentially enabling tumor cells to evade immune surveillance and reducing their sensitivity to programmed cell death.265 m5C modification regulates the immune microenvironment by modulating immune cell infiltration and function, which can alter cancer cells’ sensitivity to programmed cell death (e.g., immune-mediated apoptosis), further aiding their survival.266, 267 m7G modification can affect tumor cells’ response to programmed death signals. Cancer patients with a high m7G risk also exhibit higher TMB and immunosuppressive states.268-270 ADAR1's two isoforms (p150 and p110) have dual roles in the immune response, indicating their potential regulation of cancer cell programmed death. The p150 isoform may increase immune surveillance in the TME by promoting T cell infiltration, facilitating tumor cell clearance. Conversely, silencing the constitutive p110 isoform reduces T cell chemotaxis, potentially allowing cancer cells to evade immune-mediated programmed death and fostering their survival.271
5.3 Signaling pathway regulation
The regulation of signaling pathways is essential to orchestrate programmed cell death. RNA modifications affect tumor cell survival and death through various pathways, including the PD-L1/PD-1 and mTOR/AKT pathways. The PD-L1/PD-1 pathway is significant in immune evasion and tumor survival272; meanwhile, the mTOR/AKT pathway contributes crucially to governing cell growth and metabolic regulation.273
Seven in absentia homolog 2 (Siah2), a RING E3 ubiquitin ligase, plays a vital role in tumorigenesis and cancer progression. In cholangiocarcinoma (CCA), METTL14 increases m6A modifications in the 3′UTR region of Siah2 mRNA, leading to its degradation via a YTHDF2-dependent mechanism. This degradation of Siah2 is crucial to sustaining PD-L1 expression on tumor cells, which, in turn, inhibits T cell proliferation and cytotoxicity, modulating programmed cell death in CCA.274
METTL3 posttranscriptionally augments PD-L1 expression in an IGF2BP3-dependent fashion, stabilizing PD-L1 mRNA. By inhibiting either METTL3 or IGF2BP3, antitumor immunity can be improved as this modification affects PD-L1-mediated T cell activation, exhaustion, and infiltration in vitro and in vivo.20, 275-277 METTL3 is essential for BCa cells to evade CD8+ T cell cytotoxicity as it controls PD-L1 expression. In addition, JNK signaling facilitates tumor immune evasion through a METTL3-dependent pathway.278 In HCC, LPS induces the upregulation of METTL14, which, in turn, increases the m6A methylation of MIR155HG. MIR155HG acts as a competing endogenous RNA, regulating PD-L1 expression via the miR-223/signal transducer and activator of transcription 1 (STAT1) axis, promoting immune evasion in HCC.279 In HCC, ALKBH5 increases the expression of MAP3K8, which then activates the JNK and ERK signaling pathways, regulating IL-8 expression and promoting PD-L1+ macrophage recruitment.216 METTL1 promotes PMN–MDSC accumulation via m7G, inhibiting tumor-specific T cell activity and reducing ICC tumor cell sensitivity to PD-1 therapy. PMN–MDSCs secrete inhibitory cytokines and express suppressive molecules, decreasing T cell function and affecting tumor cell survival and death.280 ALKBH5 affects T cell activity by regulating PD-L1 expression. PD-L1 upregulation typically inhibits T cell cytotoxicity, increasing tumor cell survival.281
Hypoxic conditions significantly elevate YTHDF2 expression, which activates the mTOR/AKT signaling pathway in LUSC, increasing its antiapoptotic capabilities and reducing programmed cell death. The mTOR/AKT pathway facilitates cell proliferation and suppresses apoptosis via multiple mechanisms.193 mTORC1, a crucial element of the mTOR signaling pathway, governs cellular growth and metabolism, especially in response to nutrient availability and growth factor cues. Postchemotherapy, the upregulation of L-amino acid transporter 2 increases amino acid uptake, activating mTORC1. This activation is associated with c-Myc-mediated cluster of differentiation 47 (CD47) transcription, where CD47 upregulation may inhibit macrophage phagocytosis of tumor cells, inhibiting programmed cell death.282 The inhibition of YTHDF1 results in the overexpression of the interferon-gamma (IFN-γ) receptor and activation of the JAK/STAT1 signaling pathways, which increase tumor cells’ sensitivity to the immune response.283 In HCC, m6A-modified circMDK is upregulated, increasing cell proliferation, migration, and invasion. This is achieved by sponging miR-346 and miR-874-3p and upregulating autophagy-related 16 like 1 (ATG16L1), which activates the PI3K/AKT/mTOR signaling pathway.284
In conclusion, RNA modifications regulate various mechanisms of programmed cell death in cancer. A comprehensive exploration of the specific roles and mechanisms of RNA modifications can improve our understanding of tumor cell survival and apoptosis, providing new perspectives for developing therapeutic strategies.
6 RNA MODIFICATIONS IN CANCER THERAPY
The application of RNA modifications to cancer therapies is another idea that has garnered increasing attention. These modifications affect tumor cell responses through various mechanisms. In the following sections, we investigate the roles of RNA modifications in conventional therapies, immunotherapies, molecular targeted therapies, and metabolism-related treatments.
6.1 Conventional therapies
Traditional cancer treatments include chemotherapy and radiotherapy. However, tumor cells often develop resistance to these therapies through various mechanisms. RNA modifications are essential to modulate tumors’ resistance to both chemotherapy and radiotherapy. By affecting drug metabolism, DNA repair, and apoptosis, RNA modifications can significantly alter tumor cells’ treatment sensitivity.
Chemoresistance presents a significant obstacle in cancer treatment (Figure 6A). RNA modifications affect tumor cells’ tolerance of chemotherapeutic agents by modulating the expression of drug-metabolizing enzymes and drug efflux transporters.285, 286
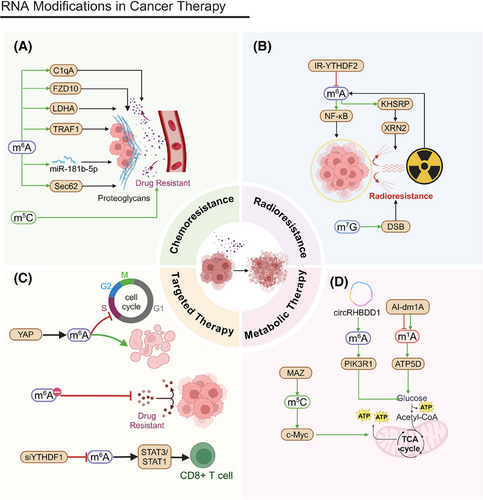
Chemoresistance to 5-fluorouracil (5-FU) significantly contributes to poor prognoses in CRC patients. Resistant cells bolster 5-FU resistance through the METTL3/lactate dehydrogenase A (LDHA) pathway. Inhibiting METTL3 can restore chemosensitivity in CRC cells.287 In CRC, METTL3-dependent m6A methylation upregulates miR-181b-5p in Cancer-Associated Fibroblasts (CAFs), reducing 5-FU sensitivity.288 METTL3-mediated m6A modification also upregulates Sec62, promoting CRC stemness and chemoresistance through β-catenin and Wnt signaling.289 Complement C1q subcomponent subunit A (C1qA) modulates rituximab resistance in DLBCL cells. The methylation of C1qA is regulated by METTL3 and YTHDF2, and knocking out these factors decreases rituximab resistance.290 METTL3 also increases lenvatinib resistance in liver cancer by regulating frizzled class receptor 10 (FZD10) m6A modification, activating the β-catenin and YAP1 pathways.219 TNF receptor-associated factor 1 (TRAF1) expression is linked to sunitinib resistance, and METTL14-dependent m6A modification stabilizes TRAF1. Targeting METTL14 and TRAF1 may present new intervention strategies.291 METTL16 expression correlates with PDAC cell sensitivity to Poly(ADP-ribose) Polymerase (PARP) inhibitors, especially when combined with gemcitabine.292 NSUN2 overexpression correlates with a poor prognosis in prostate cancer and decreased sensitivity to chemotherapeutic agents, such as docetaxel and cisplatin, underscoring the significance of the m5C modification to the chemotherapeutic response.226 RNA modification might alter treatment outcomes by regulating drug metabolism-related genes or affecting immune infiltration in the TME.293
Elevated METTL1 expression in tumor cells increases their sensitivity to specific chemotherapeutic drugs that target chromatin histone methylation, as well as the ERK–MAPK and WNT signaling pathways, suggesting METTL1 as a potential biomarker for drug sensitivity. Elevated METTL1 expression is linked to effective responses to anti-PD-L1 therapy, highlighting its potential to guide immunotherapy.209 Beyond common RNA modifications, such as m6A and m5C, ncRNA itself is a crucial regulatory mechanism, playing key roles in various diseases, including cancer.294 Research on ncRNA combined with drug therapy represents a development of traditional cancer treatments.295, 296
As a novel approach to combat chemotherapy-resistant tumors, an increasing number of clinical studies are being conducted utilizing RNA modification and RNA sequencing, offering promising prospects for cancer treatment (Table 1). Current RNA-focused clinical research primarily targets two areas: Due to the minimally invasive nature and lower risk of RNA detection, which only requires blood or bodily fluids for extraction, RNA is predominantly studied as a biomarker for precancerous conditions and postoperative monitoring. Given the complexity of RNA regulatory mechanisms, inherent medical risks, and ethical considerations, direct RNA-based therapeutic approaches remain limited. At present, RNA therapies are primarily employed in combination with traditional chemotherapeutic agents or monoclonal antibodies as RNA vaccines. In addition, most research is concentrated in Phase I/II clinical trials to assess the safety of RNA therapies. Much research remains before RNA therapies become a standard clinical treatment modality.
| Study identifier | Phase | Disease condition | Intervention | Status |
|---|---|---|---|---|
| NCT03206047 | I/II | Platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal carcinoma | Atezolizumab ± guadecitabine ± CDX -1401 vaccine + DNMT | Recruiting |
| NCT02901899 | II | Recurrent platinum-resistant ovarian cancer | Guadecitabine + pembrolizumab + DNMT | Completed |
| NCT03480152 | I/II | Metastatic melanoma, Gastrointestinal and genitourinary cancers | Personalized mRNA vaccine | Completed |
| NCT04847050 | II | Solid tumor malignancy, hematologic malignancy, leukemia, lymphoma, multiple myeloma | mRNA-1273 vaccine, mRNA-1273 vaccine booster | Completed |
| NCT06575452 | Not applicable | Glioma | RNA diagnostic test: blood, urine, and tumor tissue samples; plasma samples | Not yet recruiting |
| NCT04724239 | II | MSS/pMMR advanced colorectal cancer | Sintilimab, chidamide (histone deacetylase inhibitor; HDACi), bevacizumab | Completed |
| NCT05508399 | Observational | Locally advanced gastric adenocarcinoma, G/GEJ adenocarcinoma | DNA panel and RNA sequencing | Recruiting |
| NCT03253107 | Observational | Gastric cancer | mRNA and miRNA expression analysis, qRT-PCR validation | Recruiting |
| NCT02190227 | II | Invasive breast cancer | Tumor RNA disruption assay biopsy | Completed |
| NCT04683315 | II | Pancreatic cancer | RNA expression profiling for PurIST subtyping; Drug: mFOLFIRINOX, gemcitabine/nab-paclitaxel; Radiation: chemoradiation | Recruiting |
| NCT05916755 | Observational | Triple-negative breast cancer | Neoadjuvant chemotherapy (NACT) ± immune checkpoint inhibitor (ICI), multiomics analysis | Recruiting |
| NCT05854030 | Observational | Lung neoplasm, squamous cell carcinoma | Serum exosomal miRNA analysis | Recruiting |
| NCT03997747 | I | Recurrent glioblastoma | pp65 RNA-loaded lipid particles (pp65 RNA-LPs, DP1); RNA-loaded lipid particles (RNA-LPs, DP2) | Recruiting |
| NCT01663285 | II | Urothelial cancer, bladder cancer | Neoadjuvant cisplatin and gemcitabine; exploratory integrative tumor sequencing | Terminated |
| NCT03410693 | II/III | Carcinoma, transitional cell | Drug: rogaratinib (BAY1163877); chemotherapy; RNA in situ hybridization (RNA-ISH) for FGFR testing | Completed |
| NCT04367025 | II | Gastric cancer | Drugs: camrelizumab, oxaliplatin, S1; single-cell RNA sequencing for T cell expression analysis | Ongoing |
| NCT01374672 | Observational | Localized osteosarcoma, metastatic osteosarcoma, osteoblastic osteosarcoma, recurrent osteosarcoma | Laboratory biomarker analysis (DNA and RNA methylation and transcription changes) | Completed |
| NCT03127111 | Observational | Stage III colorectal cancer | Gene mutation analysis; gene methylation analysis; gene expression analysis; whole-genome bisulfite sequencing; RNA-sequencing (RNA-seq); genome-wide association study (GWAS) | Not yet recruiting |
| NCT04914026 | Observational | Testicular germ cell cancer, seminoma, nonseminoma testicular cancer, stage I testicular cancer | Biomarker analysis (miR371) | Recruiting |
| NCT05839834 | Observational | Neoplasms, cancer | Diagnostic test: blood test (RNA-based model) | Recruiting |
| NCT05660408 | I | Recurrent pulmonary osteosarcoma, recurrent high-grade glioma | pp65 RNA LP (DP1); pp65/tumor mRNA RNA-LP (DP2) | Recruiting |
| NCT06604130 | Not applicable | Prostate cancer | Diagnostic test: plasma exosome RNA combination for prostate cancer screening | Enrolling by invitation |
| NCT00672542 | I | Metastatic melanoma, absence of CNS metastases | Proteasome siRNA and tumor antigen RNA-transfected dendritic cells | Completed |
| NCT02316457 | I | Triple-negative breast cancer | RNA-based IVAC_W_bre1_uID; RNA-based IVAC_W_bre1_uID/IVAC_M_uID | Completed |
| NCT00108264 | I | Prostate cancer | Tumor RNA transfected dendritic cells | Completed |
| NCT04335890 | I | Melanoma, uveal metastatic | Vaccination with IKKb matured dendritic cells loaded with autologous tumor-RNA + RNA coding for defined antigens and driver mutations | Ongoing |
| NCT05202561 | I | Advanced solid tumor | RNA tumor vaccine/RNA tumor vaccine + navuliumab | Ongoing |
| NCT05697224 | Not applicable | Bladder cancer | Diagnostic test: urinary microRNA detection | Not yet recruiting |
| NCT04765410 | Observational | Pancreatic adenocarcinoma | qRT-PCR analysis of tissue microRNA profile via EUS-FNA | Ongoing |
| NCT06432413 | Observational | Colorectal cancer | Quantitative real-time PCR for SNHG3 and LUNAR1 in serum | Completed |
| NCT05708209 | Observational | Oral squamous cell carcinoma | Quantitative real-time PCR for MALAT1 and miRNA-124 in saliva | Completed |
| NCT03000764 | Not applicable | Breast carcinoma, fibrosis | RNA expression analysis; skin biopsies; blood samples | Completed |
| NCT06459895 | Observational | Bronchial asthma | Diagnostic test: Long noncoding MALAT1 gene expression assay | Completed |
| NCT03524430 | Not applicable | Breast neoplasm female | Procedure: core needle biopsy for RNA disruption assay (RDA) | Recruiting |
| NCT05264974 | I | Melanoma | Autologous total tumor mRNA-loaded DOTAP liposome vaccine | Recruiting |
| NCT04573140 | I/II | Adult glioblastoma, high-grade glioma, WHO grade III or IV malignant glioma | RNA-LP vaccines (autologous total tumor mRNA and pp65 full length LAMP mRNA-loaded DOTAP liposome vaccine) | Recruiting |
- Data source: Clinical data obtained from https://clinicaltrials.gov.
| RNA modification type | Regulator | Cancer type | Inhibition/promotion | Data source | Citation |
|---|---|---|---|---|---|
| m1A | ALKBH1 | CRC | Promotion | GenomicScape (177) | 81 |
| TRMT6/61A | Bladder cancer | Promotion | Organization (5) | 328 | |
| TRMT6/DNMT3B/DNMT1/WTAP | GMA | Promotion | CGGA (693) | 329 | |
| YTHDC1 | Glioma | Inhibition | TCGA (674) | 330 | |
| CGGA (657) | |||||
| TRMT6/10C/61B | Glioma | Promotion | TCGA (674) | 330 | |
| ALKBH1/3 | CGGA (657) | ||||
| YTHDF1/2/3 | |||||
| m5C | NSUN4 | HCC | Promotion | TCGA (787) | 331 |
| NSUN2 | HCC | Promotion | Organization (55) | 332 | |
| NSUN2 | GC | Promotion | TCGA (449) | 333 | |
| TRDMT1/NSUN6 | CRC | Promotion | TCGA (547) | 334 | |
| /ALKBH1 | |||||
| NSUN6 | PC | Inhibition | Organization (744) | 335 | |
| NSUN5 | Glioma | Inhibition | GSEA (1001) | 336 | |
| NSUN3/NSUN4 | LUSC | Promotion | TCGA (551) | 337 | |
| m6A | METTL3 | CC | Promotion | Organization (60) | 338 |
| METTL14 | TSCC | Inhibition | Organization (25) | 339 | |
| METTL14 | BC | Promotion | Organization (332) | 340 | |
| METTL3 | CRC | Inhibition | Organization (136) | 341 | |
| ALKBH5 | HNSCC | Promotion | Organization (138) | 257 | |
| ALKBH5 | NSCLC | Inhibition | Organization (60) | 342 | |
| YBX1 | AML | Promotion | TCGA (1068) | 343 | |
| YTHDC1 | PDAC | Inhibition | Organization (90) | 344 | |
| FTO | PTC | Inhibition | Organization (86) | 241 | |
| m7G | METTL1/WDR4 | HNSCC | Promotion | Organization (209) | 345 |
| TCGA (546) | |||||
| CCLE (1478) | |||||
| METTL1/WDR4 | ICC | Promotion | Organization (83) | 346 | |
| METTL1 | BC | Promotion | Organization (174) | 105 | |
| METTL1 | ESCC | Promotion | Organization (120) | 347 | |
| METTL1 | GBM/AML | Promotion | Mouse (27) | 96 | |
| TCGA (33) | |||||
| WBSCR22 | PDAC | Inhibition | TCGA (103) | 102 | |
| WBSCR22 | GBM | Promotion | GEPIA (162) | 103 | |
| WDR4 | HCC | Promotion | TCGA (680) | 106 | |
| WDR4 | LC | TCGA (571) | 348 | ||
| ψ | DKC1 | CRC | Promotion | Organization (130) | 349 |
| DKC1 | CRC | Promotion | Organization (411) | 350 | |
| DKC1 | HCC | Promotion | Organization (332) | 351 | |
| PUS10 | LC | Inhibition | NJMU (5543) | 352 | |
| FLCCA (8881) | |||||
| PUS7 | Glioblastoma | Promotion | REMBRANDT (247) | 353 | |
| TCGA (160) | |||||
| Gravendeel (167) | |||||
| PUS7 | OV | Promotion | TCGA (593) | 354 | |
| I | ADAR1 | GC | Promotion | Organization (76) | 355 |
| ADAR1 | PTC | Promotion | Organization (6) | 356 | |
| ADAR1 | ESCC | Promotion | TCGA (89) | 357 | |
| ADAR1 | Pancreatic cancer | Promotion | Organization (104) | 194 | |
| ADAR1/METTL3 | Glioblastoma | Promotion | Organization (16) | 175 | |
| ADAR1 | Prostate cancer | Promotion | Organization (28) | 358 | |
| ADAR1 | Melanoma | Inhibition | Organization (36) | 359 | |
| ADAR1 | Melanoma | Inhibition | TCGA (212) | 360 |
- Abbreviations: ADAR1, adenosine deaminase acting on RNA 1; ALKBH1, alkB homolog 1; ALKBH5, alkB homolog 5; AML, acute myeloid leukemia; BC, breast cancer; CC, cervical cancer; CRC, colorectal cancer; DKC1, dyskerin pseudouridine synthase 1; ESCC, esophageal squamous cell carcinoma; FTO, fat mass and obesity-associated protein; GBM, glioblastoma; GC, gastric cancer; HB, hepatoblastoma; HCC, hepatocellular carcinoma; hnRNPA2B1, heterogeneous nuclear ribonucleoproteins A2/B1; HNSCC, head and neck squamous cell carcinoma; ICC, intrahepatic cholangiocarcinoma; IGF2BPs, insulin-like growth factor 2 mRNA-binding proteins; LC, lung cancer; LUAD, lung adenocarcinoma; METTL14, methyltransferase-like 14; METTL3, methyltransferase-like 3; METTL5, methyltransferase-like 5; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma; OV, ovarian cancer; PDAC, pancreatic ductal adenocarcinoma; PTC, papillary thyroid carcinoma; PUS10, pseudouridylate synthase 10; PUS7, pseudouridylate synthase 7; TNBC, triple negative breast cancer; TSCC, tongue squamous cell carcinoma; WDR4, WD repeat domain 4; WTAP, Wilms’ tumor 1 associated protein; YTHDC1, YTH domain-containing protein 1; YTHDC2, YTH domain-containing protein 2; YTHDFs, YTH domain-containing family proteins.
Radiotherapy resistance affects cancer treatment outcomes (Figure 6B). RNA modifications regulate DNA re2pair and antioxidant genes, influencing the tumor response. MDSCs expand after radiotherapy, causing radioresistance and immunosuppression. YTHDF2 inhibition, via the ionizing radiation (IR)-YTHDF2-NF-κB circuit, enhances antitumor immunity and overcomes radioresistance, making it a promising target for RT and RT/immunotherapy combinations.297 Carbon ion radiotherapy increases the METTL3 and m6A levels in NSCLC cells. Targeting m6A and METTL3 may offer new strategies by affecting cancer cell proliferation, migration, and survival.221 Bone-specific m6A-modified eRNA is essential for mediating radiotherapy resistance in metastatic prostate cancer (mPCa) by averting XRN2 degradation through KHSRP. Disrupting the MLXIPe/KHSRP/PSMD9 complex suppresses tumor growth and heightens radiotherapy sensitivity.298 METTL1 increases radiotherapy resistance in HCC by mediating m7G tRNA modifications and promoting Double-Strand Break repair. High METTL1 expression correlates with poor radiotherapy outcomes in HCC patients.299
6.2 Immunotherapy
Immunotherapy represents a major advancement in cancer treatment. RNA modifications modulate the efficacy of checkpoint inhibitors, facilitating tumor immune evasion, and transforming cold tumors into hot tumors. In addition, these modifications affect the immune response within the TME by regulating inflammatory mediators and the infiltration of immune cells.
Immune checkpoint inhibitors (ICIs) boost antitumor immunity by relieving the immune system's suppression of tumor cells (Figure 7A). RNA modifications control the expression levels of checkpoint molecules, influencing the effectiveness of these inhibitors.
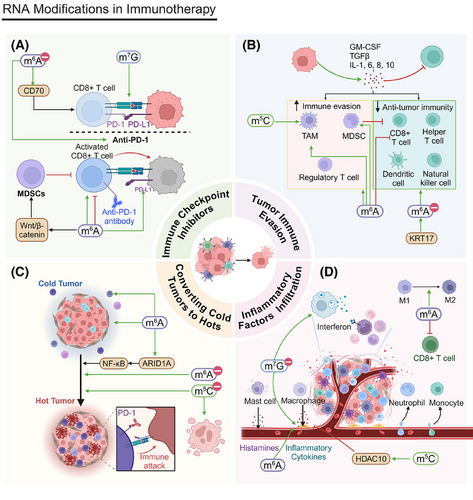
Many CRC patients respond poorly to ICI therapy. Reduced METTL16 and elevated PD-L1 were observed in CRC tissues and cell lines. The overexpression of METTL16 in CRC cells reduces the presence of PD-1-positive T cells. The METTL16/PD-L1/PD-1 axis is a promising therapeutic target.300 ALKBH5 demethylates axis inhibition protein 2 (AXIN2) mRNA, leading to its degradation and Wnt/β-catenin overactivation, inducing Dickkopf-related protein 1 (DKK1) and recruiting MDSCs and, therefore, driving CRC immunosuppression and tumorigenesis. Targeting ALKBH5 could sensitize CRC to immunotherapy.301 The depletion of YTHDF1 suppresses tumor growth by promoting CD8+ T cell infiltration, and this effect increases when combined with PD-1 blockade. Clinically, high YTHDF1 expression is linked to poor prognoses and suboptimal immunotherapy outcomes.255 IGF2BP1 influences antitumor immunity by modulating PD-L1 expression in colon and liver cancers. Targeting IGF2BP1 could improve the effectiveness of PD-1/PD-L1 blockade therapies.212, 256 The reduced expression of METTL3 is associated with resistance to anti-PD-1 therapy. METTL3 downregulation stabilizes CD70 mRNA, promoting an immunosuppressive microenvironment. Inhibiting CD70 using cusatuzumab can counteract M2 macrophage-induced resistance to anti-PD-1 therapy, highlighting METTL3's role in regulating the immunotherapy response.302
In EC cells and CD8+ T cell coculture, elevated METTL3 expression suppresses the proliferation and migration of endometrial carcinoma (EC) cells while encouraging the proliferation of CD8+ T cells. Conversely, the depletion of METTL3 yields the opposite effect. METTL3 bolsters immune surveillance by hindering the YTHDF2-mediated degradation of NLRC5 mRNA, indicating the METTL3/YTHDF2/NLRC5 axis as a potential target for EC immunotherapy.303 The arginine methylation of METTL14 by PRMT3 facilitates the progression of EC and contributes to treatment resistance. Blocking PRMT3 sensitizes EC to immunotherapy and increases anti-PD-1 efficacy by accelerating ferritin deposition.247 The m7G risk score (MRS) can be used to identify CRC patients who may benefit from ICIs. To optimize treatment strategies, m7G-related gene expression can be assessed.304
Tumor immune evasion refers to the strategies employed by tumor cells to escape attacks from the immune system (Figure 7B). RNA modifications influence immune evasion by regulating relevant gene expression.200 m6A modifications affect cancer immunotherapy by regulating immune cell functions. The aberrant expression of m6A methyltransferases or demethylases is linked to CD8+ T cell infiltration, as well as tumor immune evasion.204, 305 METTL3 mediates m6A modifications on Jak1 mRNA in TIMs, increasing JAK1 translation and STAT3 phosphorylation. Elevated METTL3 expression in TIMs is associated with a poor prognosis in colon cancer patients. Conversely, a deficiency of METTL3 in myeloid cells reduces tumor growth in mouse models.262 Targeting METTL3 could present new avenues for cancer immunotherapies. METTL3-induced circMYO1C promotes PDAC proliferation and migration in vitro and increases immune evasion by stabilizing PD-L1. Regulating m6A modifications may offer new strategies for PDAC immunotherapies as well.306 METTL3 knockdown in CRC cells diminishes the accumulation of MDSCs and sustains the activation and proliferation of CD4+ and CD8+ T cells. Inhibiting METTL3 could counteract immunosuppression and bolster antitumor immunity.205 Keratin 17 KRT1 increases T cell infiltration in CRC by promoting YTHDF2 degradation via the ubiquitin–proteasome system. This increases CXC motif chemokine ligand 10 (CXCL10) expression, improving tumors’ response to immunotherapy and highlighting RNA modifications’ role in immune evasion.307 NSUN5 increases TAM phagocytosis in glioma by regulating m5C-modified RNA and inhibiting β-catenin. It interacts with and degrades CTNNB1 caRNA, linking its function to DNA methylation and emphasizing RNA modifications’ importance in the tumor immune microenvironment.308
Cold tumors lack immune cell infiltration and are often unresponsive to immunotherapy (Figure 7C). RNA modifications can convert cold tumors to hot tumors by upregulating immune cell recruitment and activation, increasing immunotherapy efficacy.309, 310
In HCC, m6A methylation and ferroptosis-related lncRNA (mfrlncRNA) signatures have significant prognostic value, predicting both survival and risk stratification. High-risk patients show increased immunosuppressive cell infiltration and reduced cytotoxic immune cell infiltration, suggesting mfrlncRNA's role in converting cold tumors to hot tumors.305 In PCa, m6A-identified immune microenvironment subtype 2 (IMS2) shows a cold phenotype and a poor prognosis. HNRNPC serves as a biomarker for IMS2 and is also a promising therapeutic target to increase the efficacy of immunotherapy.311 NF-κB, a key transcription factor, regulates apoptosis and survival signals. Regulating the ARID1A/NF-κB pathway can improve the immunotherapy response.312
YTHDF1 deficiency inhibits lysosomal gene translation, limiting major histocompatibility complex class I (MHC-I) and antigen lysosomal proteolysis and, thereby, restoring tumor immune surveillance. It also converts cold tumors to hot ones, increasing ICI efficacy.313 YTHDF2 targets Treg cells in the TME, boosting antitumor immunity without affecting peripheral immune homeostasis, reducing unnecessary inflammation, and improving immunotherapy outcomes.260 NSUN2, a glucose sensor, maintains TREX2 expression upon glucose activation, promoting tumor cell proliferation by inactivating cGAS/STING. Deleting the glucose/NSUN2/TREX2 axis inhibits tumorigenesis and increases apoptosis and CD8+ T cell infiltration, overcoming resistance to anti-PD-L1 therapy in cold tumors.65 The thermal ablation (TA) of HCC has high recurrence and metastasis rates. Xiao et al.314 created a nanoparticle platform that releases tumor-associated antigens and FTO inhibitors to dendritic cells (DCs). In vivo, postintratumoral administration of these nanoparticles, they facilitate DC maturation, boost T cell infiltration, and establish immune memory. This synergizes with ICB therapy to suppress the growth of distant tumors and prevent metastasis.314
Inflammatory factors and the infiltration of immune cells are essential components of the TME (Figure 7D). RNA modifications modulate the expression of genes associated with these factors, affecting the immune response within the TME.315 The METTL1-mediated m7G modification of tRNA protects them from stress-induced cleavage. The loss of tRNA m7G methylation activates the interferon pathway, making tumor cells stress-sensitive.316 Reducing METTL1 expression in prostate cancer models results in increased proinflammatory immune cell infiltration and improves the response to immunotherapy, suggesting METTL1 as a potential target to improve chemotherapy sensitivity.317 IGF2BP3, an m6A reader, facilitates macrophage infiltration and M2 polarization by upregulating the expression of CCL5 and transforming growth factor beta 1 (TGF-β1), which, in turn, inhibits CD8+ T cell activation and contributes to the progression of liver cancer. The combination of IGF2BP3 inhibition with anti-CD47 therapy markedly suppresses liver cancer growth.318 In the BCa TME, NSUN6 mediates m5C methylation to promote histone deacetylase 10 (HDAC10) expression, inhibiting macrophage-related chemokine transcription and M2 macrophage recruitment. This provides insights into epigenetic regulation in TME and potential immunotherapy strategies.319
6.3 Molecular targeted therapy
Molecular targeted therapy offers effective treatment with fewer side effects by targeting key molecules in tumor cells (Figure 6C). RNA modifications affect these therapies by regulating key molecules’ expression and function.
In DLBCL, Hippo–YAP pathway inactivation via carbohydrate sulfotransferase 11 (CHST11) and KIAA1429/YTHDF2 repression inhibits cell proliferation and tumor growth and induces cell cycle arrest and apoptosis.320 METTL3 inhibitors are potential treatments for myeloid leukemia, according to Yankova et al.321 Increased METTL7B in drug-resistant LUAD cells induces EGFR-TKI resistance, but METTL7B knockdown resensitizes cells to gefitinib and osimertinib.322 A photosensitive, dual-targeting nanoparticle system (M.RGD@Cr-CTS)–small interfering RNA-targeting YTHDF1 (siYTHDF1) nanoparticles target TAMs to deliver the drug, altering the STAT3/STAT1 balance, reducing IL-10, increasing IL-12 and IFN-γ, and promoting CD8+ T cell infiltration, highlighting the potential of RNA interference in cancer immunotherapy.323
6.4 Metabolism-related therapies
Metabolic therapies target tumor cell metabolic pathways, offering a novel treatment approach (Figure 6D). RNA modifications are essential in the regulation of tumor metabolic reprogramming, as they affect the expression of metabolic enzymes and pathways. This, in turn, alters both energy metabolism and biosynthetic processes within the tumor.
m1A regulates cancer cell glycolysis.227 Xie et al.324 developed an abscisic acid-induced reversible m1A demethylation tool (AI-dm1A) to facilitate photoinduced m1A methylation or demethylation. AI-dm1A specifically reduces m1A levels in ATP synthase subunit delta (ATP5D), promoting ATP5D expression and increasing glycolysis. This m1A editing tool offers a flexible approach to studying m1A's effects on tumors and holds therapeutic potential.324 circRHBDD1 increases glycolysis and recruits the m6A reader YTHDF1 to PI3K regulatory subunit 1 (PIK3R1) mRNA, limiting the efficacy of anti-PD-1 therapy. Targeting circRHBDD1 may improve anti-PD-1 therapy's effects in HCC patients.325 NOP2 promotes HCC progression via MAZ/NOP2/c-Myc-mediated aerobic glycolysis. NOP2 knockdown combined with sorafenib increases sorafenib sensitivity, significantly inhibiting tumor growth.152
In summary, RNA modifications play a critical role in various cancer therapy mechanisms. By thoroughly investigating the specific functions and mechanisms of these modifications, we can better understand tumor cells’ responses to different treatments and develop novel therapeutic strategies.
7 CONCLUSION AND PROSPECTS
This review explored RNA modifications, such as m6A, m5C, m7G, and m1A, and their regulatory factors: writers, erasers, and readers. We also introduced the writers involved in Ψ and adenosine-to-inosine changes. These regulators participate in key biological processes, including metabolic reprogramming (e.g., glycolysis, glutaminolysis, and lipid metabolism), biosynthetic pathways (e.g., ribosome and nucleic acid synthesis), signaling pathway regulation (e.g., PI3K/AKT/mTOR), and cell cycle control, which all affect tumor proliferation. In addition, these RNA modifications influence metabolism and the microenvironment, providing energy and a foundation for tumor metastasis.
A critical mechanism of cancer metastasis is EMT, which increases cancer cell migration by converting epithelial cells to mesenchymal cells, promoting tumor metastasis. RNA modifications regulate EMT-related signaling pathways, altering gene expression and affecting cancer proliferation, metastasis, and stemness.326 Furthermore, RNA modifications play a crucial role in programmed cell death, including apoptosis, autophagy, and ferroptosis. These modifications influence cancer cell survival strategies by regulating pathways such as PD-L1/PD-1 and mTOR/AKT, as well as the immune microenvironment (e.g., CD8+ T cell infiltration and Treg cells), which presents new possibilities for cancer immunotherapies.
RNA modifications show great potential usefulness in cancer therapy, especially in targeted and immunotherapies. We summarized their roles in traditional treatments, such as chemotherapy and radiotherapy resistance. RNA modifications also regulate immune cell development, differentiation, proliferation, infiltration, activation, and apoptosis, affecting tumor immunotherapy. They also mediate immune checkpoint expression, promoting tumors’ immune evasion. Developing ICIs to target these RNA modification regulators could improve immunotherapy outcomes.
The conversion of “cold” tumors to “hot” tumors is crucial in cancer treatment as it reactivates the immune system through ICIs and immune suppressive cells, increasing the antitumor response.327 RNA modifications play a significant role in this conversion, offering new strategies to overcome immune evasion and develop more effective cancer therapies.
Given that RNA modifications significantly affect cancer proliferation, metastasis, and programmed cell death, they present promising targets for cancer treatment.
RNA modifications play crucial roles at various stages of cancer, exerting broad and complex effects. RNA modifications can reduce the stability of tumor suppressor genes, overcoming their inhibitory effects and fostering cancer progression. Meanwhile, these modifications can increase the stability of oncogene transcripts, which further accelerates tumor development. However, certain RNA modifications may exhibit opposite effects in different cancer types or, even, within the same cancer type; this underscores the complexity and significance of RNA modifications in cancer (Table 2). Current research has only begun to uncover the basis of epigenetic regulation in cancer.
The diversity and complexity of RNA modifications indicate their immense potential as therapeutic targets. RNA modifications play key roles not only in cancer proliferation, metastasis, and programmed cell death but also, potentially, in immunotherapy. Developing small molecule inhibitors that target RNA modification sites and their associated enzymes could provide novel, more specific cancer treatments. Future research should focus on elucidating specific RNA modification mechanisms to develop more effective therapeutic strategies and improve patient survival and quality of life.
AUTHOR CONTRIBUTIONS
Han Wu and Shi Chen conceived and designed the study. Han Wu wrote the manuscript. Han Wu constructed the figure. Dongxu Wang revised and edited the manuscript. Weiwei Liu revised the manuscript. Xiang Li, Yuyang Li, He Shi, and Yiwen Qing assisted in organizing the references. Bohe Shi, Yifei Tang, Zhuoyi Yan, and Hao Yang helped with spelling and formatting checks. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank BioRender for helping us in our drawing process. Thanks to the Jilin University Youth Faculty-Student Interdisciplinary Project (2022-JCXK-10, 2023-JCXK-08), the Jilin Province Scientific and Technological Development Program (grant no. 20240305037YY, YDZJ202301ZYTS166), the Jilin Provincial Development and Reform Commission (ZKJCFGW2023015), the Wenzhou Science & Technology Bureau Basic Public Welfare Research Program (Grant Y20240006)for funding and support. Thanks to all the authors for reviewing the review.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.
Not applicable.