Manganese boosts natural killer cell function via cGAS–STING mediated UTX expression
Qianyi Ming Jiejie Liu and Zijian Lv contributed equally to this study.
Abstract
Natural killer (NK) cells play a crucial role in both innate immunity and the activation of adaptive immunity. The activating effect of Mn2+ on cyclic GMP-AMP(cGAS)–stimulator of interferon genes (STING signaling has been well known, but its effect on NK cells remains elusive. In this study, we identified the vital role of manganese (Mn2+) in NK cell activation. Mn2+ directly boosts cytotoxicity of NK cells and promotes the cytokine secretion by NK cells, thereby activating CD8+ T cells and enhancing their antitumor activity. Furthermore, Mn2+ can simultaneously activate NK-cell intrinsic cGAS and STING and consequently augment the expression of ubiquitously transcribed tetratricopeptide repeat on chromosome X (UTX to promote the responsiveness of NK cells. Our results contribute to a broader comprehension of how cGAS–STING regulates NK cells. As a potent agonist of cGAS–STING, Mn2+ provides a promising option for NK cell-based immunotherapy of cancers.
1 INTRODUCTION
In recent decades, cancer treatment has shifted toward using immune checkpoint blockers, adoptive immune cell infusion, and other tumor immunotherapies as the primary options. These treatment strategies primarily harness the endogenous cytotoxicity of CD8+ T cells to destroy cancer cells, this frequently leads to the development of drug resistance in the cancer cells, hence diminishing the treatment's effectiveness. The identification of innate immune sensing as a crucial trigger for innate and adaptive antitumor immune responses has been established in both natural and therapeutic biological scenarios.1 Natural killer (NK) cells are a type of lymphocytes that belong to the innate immune system and be regarded as the innate counterparts of CD8+ cytotoxic T cells.2
NK cells are “first responders,” possessing the ability to rapidly identify and eliminate infected, transformed, allogeneic, or stressed cells without prior encounter.3-9 They function as both direct and supporting contributors in antitumor immune responses by swiftly eradicating cancer cells through targeted cytotoxicity mechanisms and effectively producing proinflammatory cytokines and chemokines that recruit and activate other immune cells to generate an adaptive response.10-13 NK cells possess these characteristics that allow them to enhance adaptive immune responses against malignancies and directly eliminate cancers that have evaded T cell responses. This makes NK cells very promising candidates for immunotherapy. The cyclic GMP-AMP (cGAS)–stimulator of interferon genes (STING) cascade is crucial in the innate immune system's detection of cytoplasmic DNA14-16 and in promoting the cancer-immunity cycle. The activation of this pathway results in the production of type-I interferons (IFN-I) (IFNα and IFNβ),17 as well as proinflammatory cytokines and chemokines.18-24 2′,3′-cyclic GMP–AMP (cGAMP) is produced when double-stranded DNA (dsDNA) is cleaved by cGAS, which then binds to STING to recruit protein kinase TANK binding kinase 1 (TBK1).18, 25-29 The cGAMP–STING complex activates the transcription factors interferon regulatory factor 3 (IRF3) and nuclear factor-κB (NF-κB), which in turn create type-I IFN, IFN-responsive genes, and other cytokines and chemokines.30, 31 Extrinsic cGAS–STING activation of NK cells has been previously described in tumor models. Tumor-derived second messenger cGAMP can activate NK cells’ endogenous STING. It can also activate STING in nearby myeloid cells, which in turn causes type I IFN production and increases NK cells’ antitumor activity. Nevertheless, the precise mechanism by which NK cell–intrinsic cGAS signaling and its downstream critical genes regulate NK cell activity remains unclear.
Furthermore, intratumoral administration of STING agonists, such as cGAMP, has demonstrated the enhancement of the antitumor activity of NK cells through their effects on both NK cells and myeloid cells. Nevertheless, the feasibility of intratumoral delivery is restricted. Manganese (Mn2+) can directly activate cGAS, raise cGAS's sensitivity to double-stranded DNA (dsDNA, and increase STING's affinity for binding to cGAMP. As a result, Mn2+ can function as a potent agonist of the cGAS–STING cascade at multiple levels, thereby enhancing INF-I production.32-34 Notably, as an essential metal element, Mn2+ has been extensively studied for its toxicity and can be safely administered systemically.35-38 Given its easy availability and extensively researched toxicity, Mn2+ could be an attractive option to augment NK cell-based cancer immunotherapy as it acts as a pan-agonist of cGAS and STING.
In this study, we show that the intrinsic cGAS signaling enhances the antitumor ability of NK cells after Mn2+ administration, independent of cGAMP from tumor cells. Moreover, Mn2+ can improve the efficacy of NK cells in eliminating cancer cells and influence the secretion profile of NK cells, leading to boosted activation of T cells and remodeling of the tumor microenvironment. Mechanistically, Mn2+ facilitates the activation of NK cells by inducing the production of ubiquitously transcribed tetratricopeptide repeat on chromosome X (UTX), a crucial molecular mediator involved in the effector responses of NK cells, through the cGAS–STING pathway. Hence, our results propose alternate strategies for enhancing NK cell-based cancer therapies.
2 RESULTS
2.1 Antitumor effect of Mn2+ depends on NK cells
To verify the antitumor effect of systemic administration of Mn2+, we established tumors by injecting murine melanoma cells B16F10 subcutaneously in C57/BL6 mice. The mice were then treated with MnCl2 intraperitoneally on four times (Figure 1A). Administration of Mn2+ severely impaired tumor growth (Figure 1B). Compared with the control tumors, the increased accumulation of NK cells, innate lymphoid cells (ILCs), CD8+ T cells, CD4+ T cells, dendritic cells (DCs), and natural killer T cells (NKT cells) were observed within the Mn2+-treated tumors (Figures 1C and S1A,B). Furthermore, we demonstrated enhanced antitumor activity of NK cells characterized by CD107a, Interferon γ (IFNγ), granzyme B (GZM), and perforin within the Mn2+-treated tumors (Figures 1D and S2A). Consistent with the effects of systemic administration, Mn2+ injection also increased the concentration of the aforementioned immune cells and NK cell activation in the peripheral blood and spleen (Figures S1C–F and S2B–D).
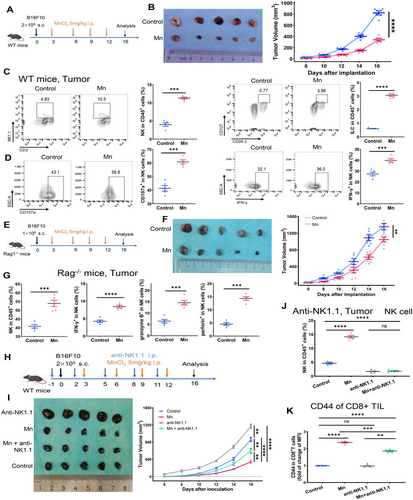
To gain a deeper understanding of the primary factors that contribute to the anticancer effect of Mn2+, we conducted experiments using Rag1−/− mice, which are deficient in T and B cells. This allowed us to eliminate the influence of T or B cells (Figure 1E). The anticancer effects of Mn2+ were partly diminished but remaining significant (Figure 1F). The treatment also induced robust activation of NK cells in the tumor, peripheral blood, and spleen (Figures 1G and S1G,H). The observations indicated that NK cells play a crucial role in the antitumor effect caused by the Mn2+ injection.
To further explore the role of NK cells in Mn2+ antitumor effect, we established a mouse tumor model with antibody-mediated depletion of NK cells (Figure 1H). NK cell depletion led to accelerated tumor growth and significantly attenuated the antitumor impact of Mn2+ (Figure 1I,J). Nevertheless, the growth of the tumor was only partially suppressed, since there was still some level of activation of CD8+ T cells observed following treatment with Mn2+ (Figure 1I,K). Collectively, these results indicate that Mn2+ mostly affects NK cells directly and may also modulate other immune cells either by activating NK cells or independently of NK cell activation.
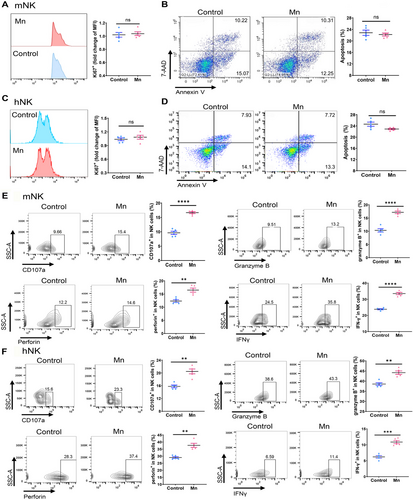
2.2 Mn2+ directly mediated the cytotoxic effect of NK cells
NK cells have a vital function in identifying and eliminating tumor cells. Our next objective was to explore the potential role of Mn2+ in the cytotoxicity of NK cells. We employed NK cells purified from murine spleens and human peripheral blood mononuclear cells (PBMCs) to validate the impact of Mn2+ on NK cell activation ex vivo. Mn2+ did not have a notable impact on the growth and apoptosis of both human and mouse NK cells, indicating that the enhanced antitumor effect of NK cells is a result of direct activation (Figure 2A–D). Exposure to Mn2+ could enhance the expression of CD107a and stimulate the production of IFNγ, granzyme B, and perforin in both murine and human NK cells in vitro (Figure 2E,F).
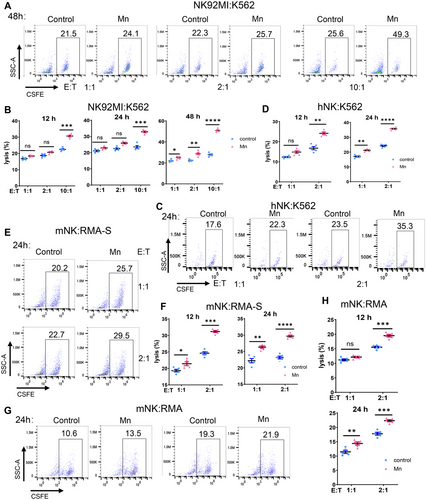
We further conducted analysis on the impact of Mn2+ on the cytotoxicity of NK cells by utilizing an in vitro coculture system of NK cells and target cells. Prolonged exposure to Mn2+ and enhanced effect-target ratio led to a considerable increase in the cytotoxicity against tumor cells in vitro by both the NK-92MI cell line and human primary NK cells (Figures 3A–D and S3A,B). The results clearly demonstrated a consistent and noticeable improvement in the killing capacity of primary murine NK cells against TPA2-deficient RMA-S lymphoma cells. Meanwhile, the elevated cytotoxicity of NK cells is noteworthy in wild-type RMS cells, although being somewhat abolished (Figures 3E–H and S3C,D). To summarize, Mn2+ enhances the anticancer effect of NK cells by directly increasing their ability to kill tumors.
2.3 Mn2+ boosts the activation of CD8+ T cells by functioning on NK cells
The aforementioned studies demonstrated a partial impairment in tumor rejection when Mn2+ was administered, namely in cases where T cells or NK cells were depleted (Figure 1F,I), indicating the direct and NK-mediated impact of Mn2+ on T cells. In order to assess the significance of Mn2+-induced NK cell activation on CD8+ T cell activation, we conducted a detailed analysis of the functional marker of CD8+ tumor-infiltrating lymphocytes (TILs) using NK cell depletion. The activation of CD8+ TILs was somewhat diminished after administering Mn2+ to the NK-depleted mice, but not to the same extent as in the control mice (Figures 1K and S4). Therefore, we postulated that Mn2+ enhances the activation of CD8+ T cells, to some extent, via acting on NK cells. Single-cell RNA sequencing (scRNA-seq) analysis was conducted on the CD45+ tumor infiltrating cells to investigate the alterations in NK and T cell following Mn2+ treatment. Following Mn2+ treatment, there was a notable concurrent rise in the infiltration of NK T and CD8+ cells, as shown by both the proportion of CD45 + cells and the absolute numbers (Figure 4A–C). The gene ontology (GO) and kyoto encyclopedia of genes and genomes (KEGG) analyses on NK cells revealed notable activation of chemokine pathways and leukocyte migratory signaling in the Mn2+-treated tumor, which aligns with the cell phenotyping and functional investigations (Figure 4D,E). Furthermore, the induction of Mn2+ resulted in improvements in the release of various cytokines from primary murine NK cells in vitro, including a notable enhancement of IFNγ, which has the potential to stimulate the activation of CD8+ T cells (Figure 4F).
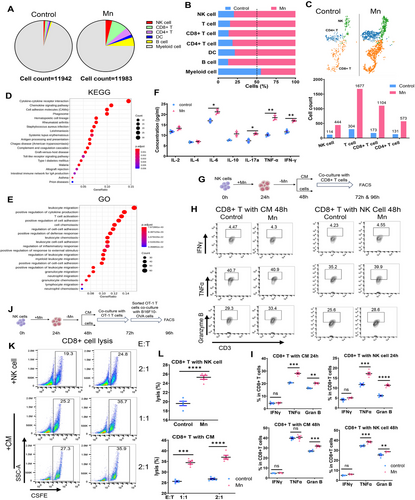
We further elucidated the effect of Mn2+-induced activation of NK cells on the functionality of T cells. Purified murine NK cells were subjected to a 24-h exposure to Mn2+, followed by a 24-h period without Mn2+. Subsequently, the CD8+ T cells were cultured with either NK cells or the conditioned medium for 24 or 48 h (Figure 4G). The activation of CD8+ T cells was greatly enhanced in both conditions of Mn2+-incubated NK cells, particularly when cocultured with NK cells (Figure 4H,I). We conducted an extensive examination of the impact of Mn2+-induced NK cells on the tumor-specific killing of CD8+ T cells using the coculture system (Figure 4J). The cytotoxicity of CD8+ T cells was dramatically boosted when managed with either Mn2+-activated NK cells or conditioned medium (Figure 4K,L). In conclusion, these data suggest that Mn2+ exert a dual role in boosting CD8+ T cell activation, both through NK cell-induced mechanisms and through direct effects.
2.4 Mn2+ activates NK cells through NK cell-intrinsic cGAS–STING
In agreement with the established function of Mn2+ as a potent agonist of cGAS–STING, we detected a substantial increase in the phosphorylation of STING, TBK1, and IRF3 in primary murine NK cells after Mn2+ treatment. Meanwhile, there was a significant elevation in the expression of IFNb1 mRNA, which is a primary downstream indicator of STING activity (Figure 5A,B). Previous study has demonstrated that internal STING is necessary for the antitumor activity of NK cells. We subsequently concentrated on confirmation of the involvement of STING in the activation of NK cells triggered by Mn2+ utilizing the STING deficient (STING−/−) mice. The proliferation and apoptosis of murine STING−/− NK cells were unaffected by the Mn2+ treatment, in accordance with NK cells from wild-type WT mice (Figure 5C,D). Notably, the stimulation of NK cell activity by Mn2+ was completely abolished in the absence of STING in NK cells (Figure 5E).
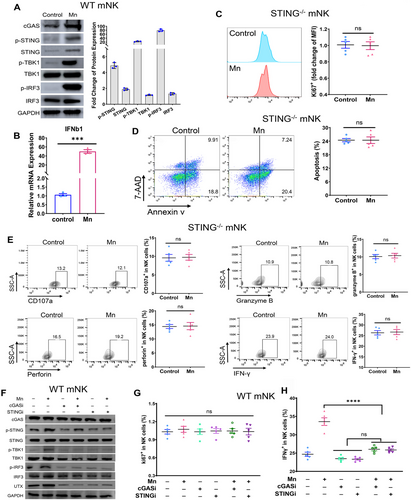
Prior work has demonstrated that tumor-specific cGAS can trigger STING signaling in NK cells via cGAMP transfer. Considering that Mn2+ can efficiently activate cGAS independent of dsDNA, we further explored whether Mn2+ enhances NK cell function by activating NK cell-intrinsic cGAS. Inhibitor of cGAS (cGASi) exhibited minimal impact on STING pathway activity, but effectively eliminated the Mn2+-induced elevation in phosphorylation of STING, TBK1, and IRF3, which are downstream targets of the cGAS signaling (Figure 5F). STING inhibitors have a comparable impact on STING signaling (Figure 5F). Although the proliferation of NK cells was not impacted by either cGASi or STINGi, the activation of NK cells, as indicated by the production of IFNγ, CD107a, and granzyme B, was substantially dampened by both inhibitors even in the presence of Mn2+ induction (Figures 5G,H and S5). Together, these results suggest that Mn2+ triggers the activation of the NK cell-intrinsic cGAS–STING and subsequently boosts NK cell responsiveness.
2.5 Mn2+ induces UTX depending on STING to stimulate NK cell activation
Subsequently, we endeavored to get a more thorough comprehension of the molecular mechanism responsible for the activating effects of intrinsic cGAS–STING on NK cells. UTX has evidently played a critical role in the effector responses of NK cells.39 To determine the effect of Mn2+-induced stimulation of cGAS–STING signaling on the expression of UTX, we assessed the relationship between cGAS–STING and UTX using our scRNA-seq data. Significant correlations were observed between the transcript levels of MB21D1 (cGAS), TMEM173 (STING), and KDM6A (UTX) in the scRNA-seq data of mice tumor infiltrating NK cells (Figure 6A). We conducted an exploration to identify the correlation between these genes using the publicly accessible data from the cancer genome atlas TCGA. The correlation between the expression of MB21D1, TMEM173, and KDM6A was once again verified using TCGA data, paralleling with our scRNA-seq data (Figure S6A). Moreover, the quantity of KDM6A expression is directly associated with the abundance of NK cell infiltration in various types of cancers (Figure 6B). Notably, the presence of Mn2+ augmented the levels of both UTX mRNA and protein in primary murine NK cells in vitro (Figure 6C,D). However, this elevated expression is diminished in STING−/− NK cells (Figure 6C,D). These data suggest that Mn2+ activates intrinsic cGAS–STING in NK cells to elevate UTX expression.
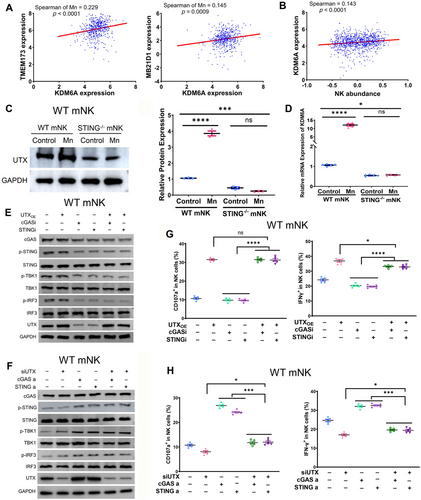
We further verified whether Mn2+-induced intrinsic cGAS–STING activation on NK cell responsiveness was carried out by the expression of UTX. Overexpression or knockdown of UTX in primary mouse NK cells did not have a substantial impact on the downstream signaling of cGAS–STING, indicated as the phosphorylation of STING, TBK1, and IRF3. This was observed regardless of the treatment of cGAS or STING inhibitors or agonists (Figure 6E,F). Furthermore, it has been observed that the presence of cGAS or STING inhibitors does not impede the ability of UTX to greatly boost NK cell activation, as evident by the increased production of IFNγ, CD107, granzyme B, and perforin (Figures 6G and S6B). Likewise, the repression of UTX during the administration of cGAS or STING agonists resulted in the eradication of cGAS–STING-induced activation of murine NK cells (Figures 6H and S6C). Collectively, these results indicated that Mn2+governs the expression of UTX by activating the cGAS–STING signaling, which subsequently boosts the responsiveness of NK cells.
3 DISCUSSION
As cytotoxic lymphocytes, NK cells can be a valuable complement to immunotherapies that modulate T-cell responses, especially crucial for cancers without T-cell-specific antigens or major histocompatibility complex (MHC)-class I molecules.40-42 The data indicate that STING signaling may play a vital role in regulating NK cell function.43 However, the underlying molecular mechanism still needs to be better understood. However, the unique characteristics of STING agonists pose multiple obstacles in their practical application. This study utilized Mn2+, a cGAS–STING agonist proven safe for clinical practice,44 to affect the direct cytotoxicity and cytokine secretion of NK cells in vitro, regardless of MHC expression. Furthermore, it augmented the antitumor efficacy of NK cells in vivo. Our findings offer a potentially promising option for immunotherapy that targets NK cells.
Early studies, which focused on observable characteristics, indicated the regulatory effect of Mn2+ on NK cells.45, 46 However, the underlying mechanism remains unclear. Mn2+ is an essential metal element in the human body that exerts function in multiple physiological functions by regulating various Mn-dependent enzymes. Mn2+ simultaneously stimulates the immune response through different mechanisms, including nutritional immunity, The NLR family pyrin domain containing 3 (NLRP3) inflammasome, ataxia-telangiectasia mutated (ATM)–p53 pathway, and cGAS–STING signaling.47-49 The exact process by which Mn2+ is involved in the regulation of NK cell has yet to be established. Our previous study indicates that Mn2+ could function as a cGAS–STING agonist, stimulating the augment of CD107a and Granzyme B in NK cells.44 However, further evidence is required to determine its regulatory effect and mechanism on NK cell activity. This work validated the cytotoxicity and responsiveness of NK cells, which were boosted by the activation of cGAS–STING induced by Mn2+. The experiments were conducted using STING−/− mice and inhibitors of cGAS or STING. Furthermore, further study demonstrated that the cGAS–STING signaling plays a vital role in controlling the activity of NK cells by regulating the expression of UTX. This brings further insight into the impact of Mn2+ on the regulation of NK cells.
We observed a simultaneous enhancement of the killing ability and cytokine secretion of NK cells due to the addition of Mn2+. Antitumor immunity is the result of the orchestrated modulation of different cells within the tumor microenvironment.50, 51 It is crucial to investigate whether Mn2+ affects other immune cells by modifying NK cells’ secretory function to facilitate antitumor responses. The boosting effect of Mn2+ on tumor-infiltrating T cells was significantly reduced in NK cell-depleted mice, indicating the vital role of NK cells in regulating the effect of Mn2+ on T cells. In addition to the known direct effects that Mn2+ has on T cells,44 a coculture in vitro model also showed that Mn2+ has indirect effects on T cells by activating NK cells. Our discovery broadens our comprehension of the mechanism underlying the anticancer effects of Mn2+-induced NK cells.
The cGAS–STING cascade activation can trigger the subsequent activation of the transcription factors IRF3 and type I IFN, resulting in the production of IFN-stimulated genes, which include numerous cytokines and chemokines. Our data demonstrated that Mn2+ can stimulate the cGAS–STING signaling, leading to the increased production of various cytokines by NK cells, including TFNα, IFNγ, interleukin 6 (IL-6), and IL-17a. tumor necrosis factor-alpha (TNFα) and IFNγ play a crucial role in the cytotoxicity of NK cells.43 Additionally, IFNγ has been indicated to contribute to the activation of CD8+ T cells.52 Meanwhile, despite being categorized as inhibitory cytokines, both IL-6 and IL-17a are pleiotropic cytokines.53, 54 They are able to stimulate CTL activation, which leads to the destruction of tumor cells. However, they also can attract suppressive immune cells like MDSCs to the tumor microenvironment, which hinders the function of CTLs.55 They primarily cooperate with other cytokines, such as TFNα and IFNγ, to delicately modulate the signaling in target cells according to the intracellular environment.
NK cell–intrinsic STING interacts with tumor cell-inherent cGAS via cGAMP transport to enhance the antitumor response of NK cells.30, 56 NK cell-based strategies, such as chimeric antigen receptor modified (CAR)-NK or combination regimens, are regarded as practicable options for tumor immunotherapy.57, 58 A crucial requirement for clinical applications is to explore NK cell activation approaches in a tumor-derived cGAMP-independent manner through activating NK cell intrinsic cGAS. As a novel cGAS–STING agonist, Mn2+ can improve the sensitivity of cGAS to dsDNA and the binding capacity of STING to cGAMP.44 Significantly, Mn2+ can directly activate cGAS independent of dsDNA.33 The utilization of Mn2+ as an agonist can directly stimulate the inherent cGAS–STING cascade in NK cells, without relying on tumor cells, and enhances the responsiveness of NK cells.
The activation of the STING signaling has emerged as a promising approach for cancer therapy, and STING agonists have shown remarkable benefits in various preclinical tumor models.59, 60 Nevertheless, the utilization of STING agonists in trials is primarily restricted to intratumoral injection due to concerns over drug stability and toxicity.61 Consequently, their application is limited in scope and their efficacy is suboptimal. Given its widespread accessibility and well-documented toxicity, Mn2+ has the potential to be a potent cGAS–STING agonist. The initial Phase I clinical trial has shown that when administrated systemically as a cGAS–STING agonist, Mn2+ is safe for clinical application and effective in multiple cancers.44 Mn2+ could function as a cGAS–STING agonist and contribute to innovative NK cell immunotherapies, such as CAR-NK.
Our findings validate that Mn2+ can enhance the responsiveness of NK cells by activating NK cell–intrinsic cGAS and STING, independent of the existence of tumor cells. Furthermore, the activation of NK cells induced by cGAS–STING signaling is facilitated by the regulation of UTX expression, which offers valuable insights into cGAS–STING activation in NK cells. Furthermore, Mn2+ not only boosts the cytotoxicity of NK cells, but also modulates the production of cytokines by NK cells, thereby affecting the activity of T cells. This offers a logical explanation for the antitumor response of Mn2+. Mn2+, a potent cGAS–STING agonist, can be effectively utilized as a regimen for NK cell immunotherapy.
4 MATERIALS AND METHODS
4.1 Mice
Female C57BL/6J mice, aged 4−6 weeks old, were obtained from SPF (Beijing) Biotechnology Co. Ltd [License No. SCXK (Beijing) 2016-0002]. The mice had a specific pathogen-free (SPF) grade and weighed between 24 and 26 g. They were housed in groups of five mice per cage at a temperature of 24–26°C and a relative humidity of 60%. The mice were exposed to a 12-h light-dark cycle, and were provided with ample food and water. The animal experiment received approval from the Animal Ethics Committee of the Chinese PLA General Hospital. The rearing and experimental environments complied with the national regulations on animal experimentation, health inspection, and quarantine.
4.2 Cells and cell culture
B16F10 melanoma cells and K562 tumor cells, acquired from ATCC, were cultured in DMEM (Gibco) with the addition of 10% FBS (Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin. K562 cells additionally received 10 mM HEPES. The cells were incubated at 37°C in a 5% CO2 atmosphere. Both mouse and human primary NK cells were grown in DMEM with identical supplements, including 10 mM HEPES. The NK-92MI cell line, obtained from ATCC, was cultured in MEM medium supplemented with 12.5% horse serum (Hyclone), 12.5% fetal bovine serum (Gibco), 0.02 mM folic acid (Sigma), 0.2 mM myo-inositol (Sigma), 1% penicillin/streptomycin, and 0.1 mM b-mercaptoethanol (Gibco). The absence of mycoplasma contamination was verified in all cell cultures.
4.3 Mouse primary NK cell isolation
Spleens were harvested from mice and placed on a 70-mesh grinding net. Then, the spleens were gently grinned using a syringe piston with a small amount of PBS. Then, the diluent was slowly mixed with splenocytes to the lymphocyte isolation solution along the wall of the centrifugal tube and centrifuged at 2000 rpm for 25 min. Further, the middle layer of single nucleated cells was collected. The cells were washed once with PBS and then NK cells were sorted with the mouse NK cell isolation magnetic beads (Miltenyi Biotec; cat 130-115-818). Primary NK cells were cultured with the addition of IL-2 and IL-15.
4.4 Human primary NK cell isolation
PBMCs were isolated from peripheral blood samples of healthy volunteers. Written informed consent was obtained from all participants. The blood sample was mixed with an equal volume of PBS, then layered with Ficoll (Solarbio) and subjected to centrifugation at 2000 rpm for 20 min at a room temperature. PBMCs were collect from the middle layer of single nucleated cell and sorted with the human NK cell isolation magnetic beads (Miltenyi Biotec; cat 130-092-657). Primary marine NK cells were cultured with the addition of IL-2 and IL-15.
4.5 Mouse subcutaneous tumor models
Either 2 × 105 or 1 × 105 B16F10 cells were resuspended in 100 µL of PBS and then injected subcutaneously to the flanks of wild-type or Rag1−/− C57BL/6J mice, respectively. Treatment of 5 mg/kg MnCl2 was started 3 days after tumor cell injection, as shown in Figure 1A,E,H. In the NK cell-depleted mice model, 400 µg of anti-NK1.1 antibody (clone PK136; BioXCell) was administered intraperitoneally every 3 days, starting 1 day before the injection of tumor cells. Flow cytometry of PBMC was used to confirm the depletion of NK cells.
4.6 In vitro cellular analysis
Preactivated primary murine or human NK cells were or not exposed to 100 µM MnCl2 for 24 h. Subsequently, the activated cells were gathered and subjected to flow cytometry to assess the quantity of cytotoxic factors. In order to assess the effectiveness of tumor destruction, the NK cells from mice were cultured with K562 targets, whereas the NK cells from humans were exposed to either RMA-S or RMA cells.
To evaluate T cell cytotoxicity, preactivated murine NK cells were cultivated for 24 h with or without 100 µM MnCl2, followed by an additional 24 h without Mn2+. Subsequently, the cells and supernatant (also known as conditioned medium) were collected separately. T cells were extracted from the spleen of mice and subjected to preactivation. Subsequently, they were cocultured either with NK cells or with conditioned medium. An analysis using flow cytometry was conducted to evaluate the cytotoxic factor and the ability to destroy tumor cells.
4.7 Flow cytometry
Samples of tumors and spleens were resected from mice at the time scheduled and mechanically smashed down to produce a single-cell suspension. PBMCs were extracted from the peripheral blood of the mouse tumor models. Ex vivo cultured cells were harvested at the time specified. Immune cells were identified and functional parameters were evaluated using flow cytometry after staining the cells. Cells were fixed for intracellular labeling using cell nucleus fixation/permeabilization buffer (eBioscience). Data acquisition was conducted using a Beckman DxFLEX flow cytometer (Beckman COULTER) and processed using the Flowjo Software (BD Biosciences). All the antibodies used are listed in Table S1.
4.8 Quantitative RT-PCR
The TRIzol Reagent (Thermo Fisher Scientific) was used to extract the total RNA. Reverse transcription was performed using the ReverTra Ace qPCR RT Master Mix (Toyobo) according to the recommended protocol. The qPCR analysis was conducted using the SYBR Green Real-time PCR Master Mix (Toyobo) on the 7500 Fast Real-Time PCR System with a 96-Well Block (Applied Bio-systems). The data were standardized based on the expression levels of GAPDH. The primer sequences are listed in Table S2.
4.9 Western blotting
The cells were rinsed with ice-cold PBS twice, then subjected to lysis using RIPA buffer (Bioss) containing protease inhibitors. The protein concentrations of the total lysates were determined using the BCA assay and adjusted using the extraction reagent. The Western blot experiments were conducted using the antibodies specified in Table S1. Band intensity was assessed using the ImageJ software (NIH). By employing GAPDH staining, the loading sample was standardized.
4.10 NK cell cytokine in vitro analysis
Primary murine NK cells were cultured in vitro with or without 100 µM MnCl2 for 24 h. The supernatant was collected for the cytokine secretion assay using LEGENDplex flow cytometry-based multiplex immunoassay (mouse anti-virus response panel, 7406024; BioLegend). Data acquisition and analysis were performed on Beckman DxFLEX flow cytometer (Beckman COULTER) and LEGENDplex Data Analysis Software (BioLegend), respectively.
4.11 scRNA sequencing and data analyses
Tumors derived from mice, both treated with and without MnCl2, were harvested and enzymatically digested to obtain single-cell suspensions. The 10× library preparation and sequencing were carried out in accordance with the standard procedure established by Shanghai Biotechnology Corporation. The cells were clustered together using graph-based clustering of the PCA-reduced data using the Louvain Method, after the calculation of a common closest neighbor graph. The identification of cell type was performed using SingleR and known marker genes. We performed differential expression analysis specifically on NK cells. Subsequently, we conducted GO and KEGG pathway enrichment analyses to identify the biological functions and signaling pathways associated with the genes that were elevated by Mn2+ treatment.
4.12 Statistical analysis
The data were reported as mean ± SEM. The statistical analyses were performed using GraphPad Prism 10.0. For data that follows a normal distribution, we used unpaired two-tailed Student's t-tests or one-way ANOVA with Bonferroni's multiple comparisons. The analysis of non-normally distributed data involved the use of Wilcoxon matched-pairs signed rank tests for two paired groups or Kruskal-Wallis tests for more than two groups. Tumor growth analysis using a repeated-measures ANOVA. Correlations were performed by Spearman correlation test.
AUTHOR CONTRIBUTIONS
Conception and design: Qian Mei and Weidong Han. Development of methodology: Qianyi Ming, Jiejie Liu, Zijian Lv, and Qian Mei. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Qian Ming, Jiejie Liu, Zijian Lv, Tiance Wang, Runjia Fan, Yan Zhang, and Meixia Chen. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Qianyi Ming, Jiejie Liu, Zijian Lv, and Tiance Wang. Writing, review, and/or revision of the manuscript: Qianyi Ming and Qian Mei. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Yingli Sun and Qian Mei. Study supervision: Yingli Sun, Weidong Han, and Qian Mei. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank anonymous reviewers for helpful comments on the manuscript. The authors are also grateful for the healthy volunteers. This work was supported by National Natural Science Foundation of China (82073183 to Q. Mei. and 82150108 and 82341208 to W. H.). Image of graphical image and mouse model was created with Figdraw.
CONFLICT OF INTEREST STATEMENT
The authors confirm that this article content has no conflict of interest.
ETHICS STATEMENT
The animal study underwent review and approval by the Animal Ethics Committee of the Chinese PLA General Hospital (A2020-066-01). Peripheral blood was obtained from the healthy volunteers after collecting signed informed consent and approval from the Institutional Review Board of the Chinese PLA General Hospital (S2018-182-01).
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the conclusions can be obtained from the corresponding authors upon reasonable request via email (Qian Mei: [email protected]). The scRNA sequence data have been deposited in National Genomics Data Center, China National Center for Bioinformation/ /Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA017689) (CNCB-NGDC, https://ngdc.cncb.ac.cn/gsa/).




