Liquid–liquid phase separation in diseases
Abstract
Liquid–liquid phase separation (LLPS), an emerging biophysical phenomenon, can sequester molecules to implement physiological and pathological functions. LLPS implements the assembly of numerous membraneless chambers, including stress granules and P-bodies, containing RNA and protein. RNA–RNA and RNA–protein interactions play a critical role in LLPS. Scaffolding proteins, through multivalent interactions and external factors, support protein–RNA interaction networks to form condensates involved in a variety of diseases, particularly neurodegenerative diseases and cancer. Modulating LLPS phenomenon in multiple pathogenic proteins for the treatment of neurodegenerative diseases and cancer could present a promising direction, though recent advances in this area are limited. Here, we summarize in detail the complexity of LLPS in constructing signaling pathways and highlight the role of LLPS in neurodegenerative diseases and cancers. We also explore RNA modifications on LLPS to alter diseases progression because these modifications can influence LLPS of certain proteins or the formation of stress granules, and discuss the possibility of proper manipulation of LLPS process to restore cellular homeostasis or develop therapeutic drugs for the eradication of diseases. This review attempts to discuss potential therapeutic opportunities by elaborating on the connection between LLPS, RNA modification, and their roles in diseases.
1 INTRODUCTION
An organelle is a specific subcellular structure, and maintaining organelle homeostasis plays a critical role in inhibiting carcinogenesis and cancer progression. Deregulations have been found in several tumor cell organelles, including mitochondria, endoplasmic reticulum, Golgi apparatus, proteasomes, and lysosomes.1 Most common organelles are separated from the internal and external environment by biological membranes, forming sites for specific physiological functions called membranous organelles. There are also a variety of membraneless chambers in cells, including stress granules (SGs), processing bodies (P bodies), promyelocytic leukemia (PML) protein bodies, Balbiani bodies, Cajal bodies, centrosomes, germ granules, heterochromatin, nucleoli, nuclear speckles, paraspeckles, super-enhancers, and signaling puncta (Figure 1).2-4 The sophisticated crosstalk between membranous and membraneless organelles is beneficial for maintaining homeostasis. For biomolecules, phase separation exists in the form of liquid droplets.5, 6 The emerging field of “liquid–liquid” phase separation (LLPS) focuses on the presence, formation, biological functions, and disease associations of membraneless bodies in cells2, 7 and is associated with cell fate determination, signal transduction, endocytosis, regulation of gene expression and protein translation, and regulation of RNA metabolism.8-10
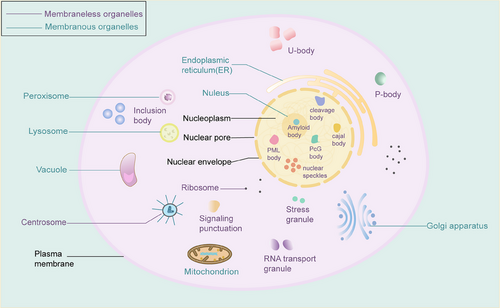
Plenty of membraneless chambers are an emerging paradigm of the cellular organization of RNA and RNA-binding proteins. RNA–RNA interactions and RNA condensation are critical in LLPS, especially for ribonucleoprotein granule formation.11, 12 Ribonucleoproteins are RNA–protein complexes in prokaryotes and eukaryotes that transport, store, or degrade messenger RNA, thereby indirectly regulating protein synthesis and the more important of these are the SGs.13 Various RNA modifications, such as N6-methyladenosine (m6A), N1-methyladenosine (m1A), and N7-methylguanosine (m7G), are involved in LLPS, including but not limited to the formation of SGs and editing of RNA sites.14-16 Multiple lines of evidence have shown that RNA modification with the m6A mark regulates the LLPS and their accumulation in membraneless chambers, such as SGs and P bodies.17, 18
Various mechanisms function in orchestrating transcriptional and translational regulation to modulate pathological events and therapeutic effects. Among them, m6A is the most common transcript chemical modification involved in many pathological processes, including tumorigenesis.19 Studies have revealed that m6A can dynamically and reversibly regulate all stages of RNA fate by controlling RNA splicing, exportation, degradation, and translation initiation.20 Dysregulation of m6A on RNAs would lead to the occurrence and development of diseases. Modulating LLPS is an alternative mechanism for m6A to maintain RNA stability and control disease progression and therapeutic outcomes.
LLPS is involved in a wide range of diseases, affecting a range of basic cellular pathways, and is most widely engaged in neurodegenerative diseases and cancer. In this review, we introduce the underlying genesis of LLPS in great detail and summarize the role of LLPS in immune signaling pathways, Wnt/β-Catenin signaling, and other crucial disease-related signaling pathways. Additionally, the amyloid aggregation has been associated with many neurodegenerative diseases, and aberrant LLPS has been observed in pathologic inclusion bodies in a variety of diseases, including Alzheimer's disease (AD), progressive supranuclear palsy, amyotrophic lateral sclerosis (ALS), and frontotemporal dementia (FTD).21-23 Cancer-associated proteins can drive aberrant gene transcription and tumorigenesis through phase separation.24 Therefore, this review emphasizes the role of LLPS in neurodegenerative diseases and cancers. Importantly, we would analyze the potential of RNA modifications in regulating LLPS to search for new therapeutic targets and strategies. Approach-wise, we draft the article with an extensively broad eyesight, from relevant concept to recent progress, from physiological function to pathological implication, from pathogenesis to therapeutic strategy, trying to spark deep thinking and rational analysis of LLPS in the future disease treatment.
2 LIQUID–LIQUID PHASE SEPARATION
LLPS transforms weakly interacted macromolecules, such as proteins and RNA, into separate cell droplets. Multiple modular structural domains of functionally similar proteins, intrinsically disordered regions (IDRs) of proteins (Table 1), and RNAs mediate multivalent interactions within and between biomolecules.25 Individual droplets can move freely in the solution, gradually collide, and fuse to form a bigger-sized structural entity.26 The main driver of LLPS may involve multivalent interactions. Molecules with multivalent interactions can form aggregates that are constantly enriched with each other, leading to an increment in the concentration of molecules in the aggregates. Until the solubility limit, aggregates would precipitate from the solution as LLPS. Various factors, including concentration and phosphorylation, contribute to LLPS, and different triggers determine different fates and physiological functions.27 Physical modulators like temperature and pressure can also regulate LLPS behavior. Scaffold protein theory uses scaffold proteins as driver molecules to form a protein–RNA network to drive the formation of phase separation.28 Two protein types are involved in facilitating the formation of such interaction networks: one class is characterized by multiple folded structural domains, such as the SH3 structural domain in Nck proteins, where interactions between the SH3 structural domains in Nck and proline-rich motifs (PRMs) in N-WASPs assemble into higher-order oligomers; another class of proteins is characterized by IDR.29 IDR-containing proteins are enriched in many condensates and have different phase transition capabilities depending on the length and number of IDR and the characteristics of the IDR sequences.24 A common feature of IDRs is that they consist of low-complexity sequence regions (LCRs), such as repeated sequences of a single amino acid.30 In summary, the two proteins have similarities and interact through multiple structural domains or modules.
| Genes (abbreviation) | Number residues disordered | Disordered/predicted residues (%) | Function |
|---|---|---|---|
| Tumor protein 53 (P53) | 193 | 49.11 | Encoding tumor suppressor proteins containing transcriptional activation, DNA binding, and oligomerization domains |
| Retinoblastoma (Rb) | 356 | 38.36 | Negative regulator of the cell cycle |
| Adenomatous polyposis coli (APC) | 1838 | 64.65 | It encodes a tumor suppressor protein that acts as an antagonist of the Wnt signaling pathway. |
| Nonmetastatic protein 23 (NM23) | 22 | 12.22 | A housekeeping enzyme and mainly considered as a tumor suppressor gene |
| Neurofibromin 1 (NF-1) | 547 | 21.86 | Function as a negative regulator of the Ras signal transduction pathway |
| Synaptotagmin binding cytoplasmic RNA interacting protein (SYNCRIP) | 268 | 43.02 | Member of the cellular heterogeneous nuclear ribonucleoprotein family |
| G3BP stress granule assembly factor 1 (G3BP1) | 10 | 9.52 | DNA-unwinding enzyme prefers partially unwound 3′-tailed substrates and can also unwind partial RNA/DNA and RNA/RNA duplexes in an ATP-dependent fashion. |
| DEAD-box helicase 3 X-linked(DDX3X) | 97 | 20.42 | Member of the DEAD-box protein family |
| YTH N6-methyladenosine RNA binding protein F2 (YTHDF2) | 246 | 46.5 | Member of the YTH (YT521-B homology) superfamily |
| Ataxin 2 like (ATXN2L) | 351 | 87.53 | The Nod1/Apaf-1 family members encode a protein with two caspase recruitment (CARD) domains and six leucine-rich repeats (LRRs) |
| Argonaute2 (AGO2) | 104 | 12.61 | Member of the Argonaute family of proteins that play a role in RNA interference |
| Insulin like growth factor 2 mRNA binding protein 1 (IGF2BP1) | 157 | 19.03 | Similar to insulin in function and structure, it is a member of a family of proteins involved in mediating growth and development. |
| Mov10 RNA helicase (MOV10) | 158 | 15.75 | Enable RNA helicase activity and RNA binding activity to participate in the defense response to viruses; negative regulation of translocation, RNA-mediated; posttranscriptional regulation of gene expression |
| Cell cycle-associated protein 1 (CAPRIN1) | 390 | 62.10 | Produces several transcriptional variants that act as CDK4 kinase inhibitors |
| G3BP stress granule assembly factor 2 (G3BP2) | 84 | 42.42 | DNA-unwinding enzyme that prefers partially unwound 3′-tailed substrates and can also unwind partial RNA/DNA and RNA/RNA duplexes in an ATP-dependent fashion |
| FMR1 autosomal homolog 1 (FXR1) | 338 | 55.59 | An RNA binding protein that interacts with the functionally similar proteins FMR1 and FXR2 |
| FMR1 autosomal homolog 2 (FXR2) | 428 | 63.60 | An RNA-binding protein containing two KH domains and a RCG box, which is associated with polysomes |
| UPF1 RNA helicase and ATPase (UPF1) | 349 | 30.91 | Part of a postsplicing multiprotein complex involved in both mRNA nuclear export and mRNA surveillance |
| Poly(A) binding protein cytoplasmic 4 (PABPC4) | 294 | 44.48 | Poly(A)-binding proteins (PABPs) bind to the poly(A) tail present at the 3-prime ends of most eukaryotic mRNAs. |
| Ubiquitin specific peptidase 10 (USP10) | 194 | 36 | Member of the ubiquitin-specific protease family of cysteine proteases |
In cells, LLPS triggers the formation of membraneless chambers. Membraneless chambers are involved in cellular physiological responses through specific proteins and RNAs within them. In addition, membraneless chambers facilitate gene regulation through different mechanisms and play critical roles in a variety of biological processes, including RNA metabolism, translation, protein modification, and signal transduction.31, 32 LLPS, an organizational pattern of biological macromolecules, is essential for signal transduction and regulation of gene expression. Once the formation or dynamics of normal LLPS is changed, it can lead to the development of diseases.33 A typical characteristic of LLPS is a saturation concentration, which means the LLPS structure is formed only when the concentration of components exceeds a given threshold. LLPS structures can exhibit different properties under different factors and environmental influences, such as salt concentration, temperature, and other ions. Material properties, such as viscoelastic liquids, network fluids, liquid-crystalline, micellar, or semi/para-crystalline condensates, have been proposed to be risk factors in several diseases.34 In Parkinson's disease (PD), tau proteins may act as scaffolding proteins for neuronal cohesion, and full-length but not carboxy-terminally truncated α-synuclein is concentrated within tau droplets under the regulation of tau phosphorylation.35, 36 In summary, scaffold molecules constitute the driving factor for phase separation and occur under specific physicochemical properties. At the same time, modifications, such as phosphorylation, sumoylation, ubiquitination, and methylation, can alter the course and extent of LLPS.
There are two sides, good and bad, to any matter, and phase-separated structures play different roles in various biological processes and the pathogenesis of protein aggregation diseases. From the beneficial side, LLPS can locally concentrate molecules in the condensate to activate cytoskeletal structural responses, signaling processes, nucleation and RNA processing reactions. For example, miRNA-induced silencing complex (miRISC) is a multiprotein assembly in which microRNAs (miRNAs) recognize target-repressed mRNA and functions in an LLPS-dependent manner. Argonaute2 (Ago2) and TNRC6B, the two core protein components of miRISC, isolate target RNAs from bulk solution in living cells via structural domains separated by multivalent interactions with tryptophan and condense miRISC droplets to recruit a deadenylation factor.37 Ago2 and TNRC6B condensed into phase-separated droplets are essential for mRNA translation and stability in eukaryotes. miRISC is a multiprotein assembly that uses microRNAs to recognize mRNAs targeted for repression.38 LLPS is also manifested in a variety of diseases.39, 40 Protein aggregation is a significant cause of many neurodegenerative diseases, attacking the brain and killing neurons. These proteins (e.g., α-synuclein, FUS, tau, and TDP-43) undergo LLPS independently through electrostatic interactions, increasing protein concentration. This process of molecular self-assembly is present in a variety of pathological inclusions.41-43 miRISC might implicate cancer initiation and progression in specific cellular contexts. Aberrant formation and regulation of LLPS can lead to malignant transformations to acquire cancer hallmarks, such as evading growth suppressors, resisting cell death, sustaining proliferative signals, and inducing genome instability.3, 4 Plenty of proteins with IDR regions have irreplaceable roles in multiple signaling pathways,44-46 and their overexpression, mutation, or fusion can lead to under- or overactivation of pathways.
3 LLPS IS AN IMPORTANT HUB IN SEVERAL SIGNALING PATHWAYS
Studies have demonstrated the role of LLPS in the immune response, not limited to immune cell maturation and activation, immune signaling, and immunomodulation.47, 48 In addition, several important signaling pathways functioned in the accompanied with LLPS.
3.1 T-cell receptor and B-cell receptor immune signaling
Immune signaling pathways alter the conformation of immune receptors by ligand binding of pathogenic stimuli, inducing spatial reorganization and, thus information transfer. LLPS is found in a variety of immune signaling pathways, including the T-cell receptor (TCR)49 and the B-cell receptor (BCR)50 pathway, as well as cytoplasmic signaling pathways, such as cGAS–STING.51 Normally, LLPS is recognized as 3D structures formed in the cytoplasm and nucleus, but 2D membrane-associated condensates formed along the cell membrane are well represented in immune signaling.52 Membrane LLPS restricts protein movement to higher concentrations and induces a lower protein concentration threshold in condensates than in membraneless organelles.53 On the surface of the cell membrane of immune cells, there are membrane clusters54 that LLPS regulates and thus proceed the immune signaling cascade.55, 56 To decipher how LLPS drives the formation of immune signaling condensates to establish immunity could contribute to developmental research and disease treatment.
The TCR signaling pathway depends on T cell microclusters on the plasma membrane,57 which contain multiple transmembrane receptors.58 Components of clusters typically have high densities and low mobilities. High densities allow more frequent contact between molecules within the cluster and low mobilities mean less time for interactions to occur.59 In addition to the effects of microclusters, the combined effects between proteins and lipids drive LLPS between TCR-associated proteins in the proximal part of the membrane. For example, cholesterol enhances protein condensate TCR clustering, whereas downstream TCR triggering TCR clustering promotes cholesterol LLPS.60, 61 It has been shown that LAT, GRB2, and SOS1 can form oligomers through multivalent interactions.62 In the signal transduction system of the T-cell junction protein LAT, stimulation induces phosphorylation of specific residues to generate multiple binding sites to promote LLPS of the signal transduction protein.49, 63, 64 Transmembrane proteins and their cytoplasmic binding partners (GRB2 of LAT) induce LLPS, which in turn prompts the multivalent SH3 structural domain-PRM to bind to a downstream binding partner (SOS1 of GRB2).55, 65 In addition, the dependence of LLPS on phosphorylation by harmful feedback regulatory mechanisms means that it will be significantly reduced or eliminated without a stimulus.66 LAT condensate promotes tyrosine phosphorylation and marks the activation of the TCR signaling pathway. However, LAT can be bypassed in the microcluster formation of chimeric antigen receptors (CARs) by establishing multivalent interactions between CARs and the LAT-binding chaperone GADS.67 Distinct physical and biochemical compartments LLPS create can facilitate different TCR signaling.
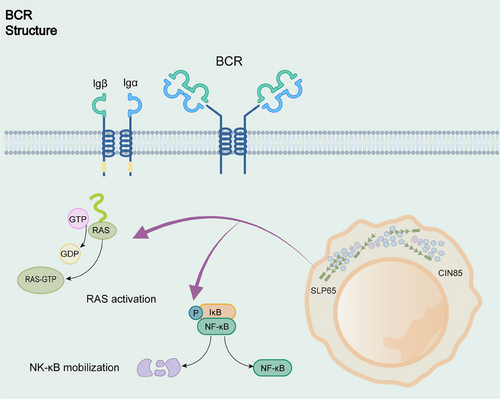
The scaffolding protein SLP65 (also known as BLNK) possesses a critical function in the BCR signaling pathway in driving LLPS, similar to LAT in the TCR signaling pathway (Figure 2). SLP65 with its binding partner CIN85, undergoes LLPS via multivalent interactions between the SH3 structural domain of CIN85 and the proline motifs of SLP65.68 Liposomes play a crucial role in facilitating cohesion formation. SLP65 contains an amino-terminal lipid-binding domain that binds to small, highly curved vesicles.50 SLP65 mutants lacking the N-terminal vesicle-binding domain fail to undergo LLPS at the cytoplasm or plasma membrane, leading to defects in BCR signaling, such as calcium inward flow.50 Higher-order assembly provides a theme in sensitivity control, signal transduction, innate immunity and adaptive. BCR downstream has a ternary complex called CBM signalosome, generated by CARM1, BCL10, and MALT1 assembly, which mediates NF-κB activation.69-71 These higher-order assemblies with solid-like behaviors or phase-separated liquid-like droplets can inhibit unnecessary immune activation and enable activation of proximity-driven protein and spatial control of immune signaling.
3.2 cGAS–STING signaling
In response to stimulation by pathogens or damaged cytoplasmic dsDNA, cGAS would be activated and binds to dsDNA.72 Cyclic GMP–AMP (cGAMP) synthesizes and activates STING, which transduces downstream signals and induces the expression of proinflammatory cytokines.73 Sensing DNA, cGAS–STING signaling triggers innate immune responses and is an important axis of autoimmunity, sterile inflammatory response and cellular senescence.74 Besides the role of immune defense, the GAS–STING DNA-sensing pathway has also been shown to be connected with antitumor immunity, exhibiting paradoxical function in both immune surveillance for tumor inhibition and immune-suppressive tumor microenvironment for metastasis-promoting.
cGAS consists of a positively charged disordered N-terminal and a structured C-terminal region. Depending on the positively charged residue at the N-terminal and the DNA-binding site in the C-terminal, cGAS can induce LLPS upon DNA binding.48, 75, 76 Long DNA strands, free zinc ions and DNA-binding structural domains in the catalytic core of cGAS are significant factors contributing to LLPS formation.77 Factors affecting the occurrence of LLPS in cGAS have now been found to be multiple.51, 78 It has been reported that the binding of streptavidin to cGAS enhances the interaction of cGAS with DNA and thus promotes the formation of its condensation complex.79 However, ORF52/VP22-type tegument proteins, as a family of inhibitors against cGAS–DNA phase separation, restrict cGAS–DNA LLPS. ORF52/VP22-mediated restriction of cGAS–DNA LLPS is implemented by self-condensation with viral genomic DNA without strong direct interaction between viral proteins and cGAS.80 This method of altering one's structure to undergo LLPS with DNA to inhibit phase-separated polymers cleverly evades head-on counteracting cGAS–STING immunization. Also, it demonstrates the impact of LLPS on the way biomolecules interact with each other. It has been shown that the percentage of cells containing cGAS condensates is significantly lower in G3BP1-deficient cells81, 82; however, the underlying mechanism remains obscure. LLPS could also modulate cGAS–STING cascades. For example, LLPS of the oncogene neurofibromin 2 (NF2) mutant inhibits the cGAS–STING pathway.83 NF2 promotes innate immune-sensing of cytoplasmic nucleic acids and has a missense mutant NF2m. NF2m aggregates into dynamic condensates in the cytoplasm induced by IRF3 in its activated state. Aggregates of NF2m can restore average tumor growth in a mouse model of STING-initiated antitumor immunity and hinder STING-initiated antitumor immunity.
3.3 Wnt/β-Catenin signaling
The Wnt/β-Catenin pathway regulates the cellular transcriptional program and plays a vital role in the organism's homeostasis and development.84, 85 APC and Axin are key multidomain scaffolding proteins in the signaling pathway. The G protein signaling domain of Axin has an intrinsically disordered intermediate region that contains multiple kinases and APC binding sites.50, 86, 87 When Axin is expressed in different cell types, it spreads into a “dot” to increase its concentration, recruiting APCs and other destructive complex proteins.88, 89 There is evidence that the complex controlling Wnt/β-catenin is formed precisely by the protein LLPS driven by Axin.90 The complex facilitates phosphorylation of β-catenin by GSK3β, which is essential for the regulation of β-catenin protein stability to maintain Wnt/β-catenin signaling.91, 92 The β-catenin destruction complex and its receptor signalosome can robustly and precisely regulate Wnt/β-catenin signaling. Assembly of Wnt signalosome is driven by Dishevelled and Axin copolymerization.93, 94 Dishvelled-2 (Dvl2) has an IDR at the N-terminus and can undergo LLPS in vitro and cells.95, 96 The formation of Dvl2 condensates depend on ubiquitylation of Dvl2 through K63 linkage by WWP2.95 LLPS of Dvl2 has been found to be facilitated by signalosome components (e.g., Fzd5).96 In addition, Dvl2 LLPS weakens LLPS of Axin that is organized into the signalosome and displays high mobility at low concentration.96 Wnt signalosome and phase-separated β-catenin destruction complex emphasize the distinct mechanism LLPS provides in signal transduction.
3.4 RAS/MAPK signaling
The RAS/MAPK signaling pathway is involved in a variety of cellular processes, including differentiation, proliferation, and survival, and their mutations occur frequently in many tumors making it one of the most critical pathways associated with cancer.97-99 The nonreceptor protein tyrosine phosphatase SHP2 is crucial in RAS–MAPK signaling during normal development.100 The NH2-terminal end of SHP2 has two tandem SH2 structural domains, a central PTP-catalyzed structural domain, and a C-terminal tail.101 SHP2’ folded PTP structural domain mediates its LLPS by intermolecular electrostatic interactions.102 Wild-type SHP2 LLPS could be recruited and promoted by disease-related mutation of SHP2 to induce MAPK hyperactivation.102 In another study, ERK signal responsive repressor ERF has been determined to form condensates vis LLPS, and ERF condensates can be attenuated using ERK inhibitor U0126.103 Generally, some components of RAS/MAPK signaling pathway display LLPS behaviors, and RAS/MAPK signaling could also participate in the modulation of LLPS to adjust cellular organization.
3.5 Hippo/YAP signaling
The Hippo–YAP signaling network integrates signals with its transcription factor YAP protein to control cell proliferation and differentiation.104, 105 Activation of this pathway promotes regeneration of damaged organs and is associated with tumorigenesis.106-108 Several components of Hippo–YAP signaling include low-complexity domains and can undergo LLPS. Hippo signaling complexes undergoing LLPS to produce biomolecular condensates in the cytoplasm has been documented.109 LLPS of the Hippo–YAP signaling pathway is closely related to super-enhancers. YAP LLPS occurs near chromatin compartmentalizes YAP from other relevant coactivators to induce transcription of YAP-specific proliferative genes.110 YAP coalescence occurs in the super-enhancer region, regulates cell proliferation and survival, and activates target genes.111 It has been shown that hyperactivated YAP promotes super-enhancer condensates in embryonic stem cells through LLPS, leading to gene-specific and restrictive regulation to control lineage differentiation.112 Glucose in tumor cells tends to be consumed in increased amounts to support tumor proliferation, and it has been found that accumulated glycogen can undergo LLPS.113 Glycogen droplets segregate Hippo kinase from YAP so that inhibition of the latter is deregulated, the Hippo pathway is inactivated, and the activity of the downstream proto-oncoprotein YAP is increased, ultimately promoting tumor growth.114, 115 Destroying YAP LLPS prevents tumor growth, increases immune response, and enhances the sensitivity of anti-PD-1 therapy,116 indicating that YAP LLPS can be applied as a therapeutic target for anti-PD-1 therapy. In addition, DDR1, a collagen-binding receptor tyrosine kinase, has been found to counteract the Hippo/YAP pathway in a LLPS-dependent manner.117 Therefore, LLPS can regulate the Hippo/YAP pathway and can also be controlled by the Hippo/YAP pathway to exert different functions.
4 LLPS PHENOMENON IN NEURODEGENERATIVE DISEASES
Neurodegenerative diseases tend to have one thing in common: protein aggregates. These aggregated proteins have a propensity for LLPS.42 Currently, some studies have now observed the link between LLPS and neurodegenerative diseases, including ALS,118, 119 AD, and some forms of FTD.120-122 Here, we discuss the LLPS of four major proteins in neurodegenerative diseases: α-synuclein, fused in sarcoma (FUS), tau, and TAR DNA-binding protein 43 (TDP-43).
4.1 TAR DNA-binding protein 43
As a significant nuclear RNA/DNA-binding protein with a prion-like structural domain, TDP-43 regulates various RNA processing steps. It accumulates in the cytoplasm of patients with a variety of neurodegenerative diseases.123, 124 TDP-43 consists mainly of two RNA recognition motifs, a structured N-terminal domain with a C-terminal glycine-rich low-complexity structural domain.125, 126 Recognition motifs of TDP-43 are primarily involved in RNA translocation, stability, and splicing,127 whereas the disordered C-terminal structural domains126, 128, 129 with highly conserved N-terminal structural domains130 are connected to LLPS. TDP-43 can undergo LLPS, and liquid phase-separated droplets containing TDP-43 can be converted into gel/solid structures under prolonged stress and even to amyloid fibril structures in vitro.131 In normal cells, phase separation of these RNA-binding proteins is ordered and controlled, with TDP-43 acting as a liquid shell and encapsulating the HSP70 family chaperone.132 As molecular chaperones, the HSP70 family maintains proteostasis to protect proteins from misfolding and aggregation133, 134 and is reported to be transcriptionally downregulated in AD.135 Furthermore, under oxidative stress, HSPB1, along with HSP70 chaperone activity, is central to the maintenance of TDP-43 protein homeostasis through the interaction of the low-complexity structural domains with TDP-43.136 Similar to the C-terminal domain, N-terminal domain interactions are also important for cellular function. In addition to ensuring that the expansion of the aggregation-prone region of the C-terminal structural domain drives endogenous TDP-43 aggregates and sequesters,137 LLPS is also affected by the expansion of the aggregation-prone region of the structural domain. Wang et al.130 reported that disrupted TDP-43 N-terminal domain polymer by phosphomimetic substitution at S48 can block LLPS and disrupt RNA splicing activity in vitro.
4.2 Fused in sarcoma
Similar to TP-43, FUS is a widely expressed RNA-binding protein involved in multiple RNA metabolic pathways.130, 138-140 The N-terminal domain of FUS is a highly conserved low-complexity structural domain that mediates protein–protein interactions and drives the aggregation of FUS into protein inclusion bodies. This region can undergo reversible LLPS.141, 142 Furthermore, it has been shown that cation–π interactions between tyrosine in the low-complexity structural domain and arginine in the structured C-terminal structural domain promote LLPS and gelation under the regulation of methylation.143 ALS is the most common motor neuron disease in adults, and cytoplasmic mislocalization and aggregation of FUS in neurons and glial cells in affected individuals is the cause of ALS.144, 145 Transient SGs are formed when neurons encounter cellular stress, sequestering untranslated mRNAs and associated proteins to reduce energy requirements.146 Overexpression of FUS induces the spontaneous formation of SGs and is recruited to this membraneless organelle.147 The persistence of SGs provides an environment for mutations in RNA-binding proteins such as FUS,148, 149 and SGs may be a locus for disease biogenesis.43 Also, there may be transmission factors in diseases such as ALS/FTD. It has been found that prion-like mechanisms in neurodegenerative diseases can transmit protein misfolding and aggregation.150 TDP-43 and FUS in ALS have low-complexity structural domains similar in amino acid composition to yeast PrLDs (prion-like domains; PrLDs).151 Prion-like propagation mechanisms are active in ALS and FTD, and preformed recombinant TDP-43 fibers in neuronal cell lines can trigger overexpression and aggregation of endogenous TDP-43.152, 153
4.3 Tau
Tau consists of four distinct regions: the N-terminal projection domains, the proline-rich domains, the microtubule-binding domains, and the C-terminal domains.41 This structure facilitates binding to microtubules and allows for an intrinsically disordered and inhomogeneous charge distribution over the entire length of the tau.154, 155 Tau is a soluble neuron-specific microtubule-binding protein that is a significant component of inclusion bodies in various neurodegenerative diseases, including AD and FTD.22, 156, 157 Currently, one critical factor for the proteotoxicity of tau is the formation of neurofibrillary tangles, the abundance of which correlates with disease progression.22, 157 Proteins are susceptible to a large number of posttranslational modifications such as phosphorylation, acetylation, and ubiquitination, which are involved in the pathogenesis of neurodegenerative disorders.158 neurons can release and take up hyperphosphorylated tau, triggering templated tau misfolding in neurons, leading to cytotoxicity.159 It has been repeatedly observed that tau forms droplets via LLPS under physiological conditions, regulates microtubule assembly and function, and may be converted to amyloid aggregates upon aging.160, 161 It is currently believed that tau undergoes LLPS driven by attractive intermolecular electrostatic interactions between the negatively charged N-terminal and positively charged middle/C-terminal structural domains of the protein.160, 162 Also, aberrant LLPS may occur when tau binds to other molecules. For example, tau undergoes complex coalescence after binding to RNA.163, 164
4.4 α-Synuclein
α-Synuclein is structurally and functionally highly distinct from TDP-43, FUS, and Tau and is intimately associated with Lewy body formation.165, 166 Lewy bodies are a characteristic hallmark of PD, leading to neuronal death.167 α-Synuclein consists of a membrane-bound structural domain consisting of a positively charged N-terminal structural domain, a hydrophobic nonamyloid component, and an acidic structural domain containing the C-terminal structural domain, which is involved in its controversial nuclear localization and various interactions.168, 169 The C-terminal region of α-synuclein undergoes long-range interactions with the N-terminal region and can remain monomeric, highly flexible, and disordered after the occurrence of LLPS.170 The α-synuclein LLPS is driven by electrostatic interactions in the amphiphilic N-terminal structural domain and hydrophobic interactions between nonamyloid components.171 High concentrations of α-synuclein trigger the process of LLPS and the formation of amyloid hydrogels containing oligomeric and fibrillar material.171, 172 Unstructured proteins of α-synuclein form partially folded intermediates, then oligomerize and protofibrillate, ultimately giving rise to amyloids.173, 174 Furthermore, it has been shown that abnormally aggregated α-synuclein molecules induce normal molecular misfolding to form polymers and are taken up by neurons, leading to neurodegenerative alternations.175 α-Synuclein aggregates are also closely related to mitochondrial autophagy. Aggregates interfere with the normal function of mitochondrial autophagy-associated proteins, thereby affecting mitochondrial autophagy processes.176
5 LLPS IN CARCINOGENESIS AND CANCER DEVELOPMENT
Cancer has traditionally been regarded as a genetic illness, and subsequently, epigenetic abnormalities and tumor microenvironment are all found to be critical for tumorigenesis.177, 178 Genetic alterations in dysregulated transcriptional programs contribute to cancer development, and modulation of gene expression may also help improve the outcome of cancer treatment. Gene expression is a biological process in which genetic information guides the synthesis of RNA molecules that code for proteins or build noncoding RNAs (ncRNAs), and any failure in its overall expression, such as abnormal protein expression and epigenetic alterations, may promote the proliferation and metastasis of cancer cells. Various mechanisms orchestrate transcriptional and translational regulation to modulate tumorigenesis events and therapeutic effects. m6A is the most prevalent transcript chemical modification and is involved in many pathological processes, including tumorigenesis.19 Studies have revealed that m6A can dynamically and reversibly regulate all stages of RNA fate via controlling RNA splicing, exportation, degradation, and translation initiation.20 Exploiting the role of transcriptional machinery and posttranscriptional network in cancer occurrence and therapy has the potential for searching for new therapeutic targets and strategies.
Abnormal gene expression profiles, or dysregulation of oncogene or tumor suppressor gene activity following degradation of proteins required for average cell growth, drive many abnormal responses, such as aberrant gene amplification, missense mutations, malfunctioning chromosomal translocations, cellular conditions and external signals, and cell acquiring uncontrolled proliferation.179 LLPS of cancer-related proteins involved in epigenetic and transcriptional regulation, translation, signal transduction, and protein degradation plays a crucial role in cancer development (Table 2).180 Generally, LLPS might participate in all cancer biological events, including tumorigenesis, cancer development and therapy.
| Dysregulated process | Protein | Impact of LLPS on cancer development (reference) |
| Signal transduction | T cell receptor (TCR) | Cancer-associated antigens; tumor immune mediators181, 182 |
| Beta-catenin | Mutated Wnt pathways lead to a variety of growth-related pathologies and cancers.93 | |
| Zona occludens (ZO) | Fusion of dense protein polymerization and linker bands are driven by LLPS transitions.183 | |
| Tumor protein p53 binding protein 1 (TP53BP1) | 53BP1 LLPS increases p53 target gene expression and repairs DNA damage.184 | |
| Son of sevenless (SOS) | Activation of Ras, a cofactor involved in RAS signaling in tumor development185 | |
| Epigenetics | Heterochromatin protein 1 (HP1α) | LLPS leads to gene silencing of HP1 in heterochromatin.186 |
| Bromodomain-containing protein 4 (BRD4) | LLPS-mediated transcriptional dysregulation renders cancer cells highly dependent on the transcriptional regulator BRD4.187 | |
| Chromobox protein homolog2(CBX2) | IDR of LLPS-regulated cbx2 is involved in chromatin regulation of testis development.188 | |
| Heat shock factor 1 (HSF1) | LLPS segregation downregulates HSF1 function, and chaperone gene induction is reduced.189, 190 | |
| Transcription | m6A-related proteins | Modulation of RNA transcripts through LLPS-mediated deregulation, thereby affecting tumorigenesis191 |
| Yes1 associated transcriptional regulator/WW domain-containing transcription regulator 1 (YAP/TAZ) | TAZ increases activity in various cancers by forming nuclear condensates through LLPS.189 | |
| Octamer-binding transcription factor 4(OCT4) | Mediator enhances LLPS, activates genes, and controls gene transcription.192 | |
| Heterogeneous ribonucleoprotein particle | LLPS improves transcriptional response and synergizes chromosomal enhancer assembly in breast cancer.193 | |
| Mediator complex subunit 1 (MED1) | Coactivators overexpressed and modified in cancer194, 195 | |
| Polypyrimidine tract-binding protein 1 (PBP1) | LLPS promotes p62 recruitment and Nrf2-mediated stress response.196 | |
| Protein degradation | C/EBP homologous protein (CHOP) | LLPS degradation of carcinogenic substrates197 |
| Fused in sarcoma (FUS) | Recruitment of DNA damage sites, assembly of damaged DNA-rich compartments, transcriptional coupling repair effectors198 | |
| RNA repair | RAD52 homolog, DNA repair protein (RAD52) | LLPS induces the formation of DNA double-strand breaks and colocalization with Rad52, which increases the mobility of damaged chromatin.199 |
5.1 LLPS contributes to aberrant transformation via gene fusion and mutation
Disordered structures of various proteins form droplets through LLPS, repeated fusions among genes that bind proteins, and chromosomal translocations that produce multiple chimeras.200 SPOP–DAXX bodies, PML vesicles, and FET fusion proteins formed via multivalent interactions affect various cancers regarding gene fusion and mutation.
5.1.1 SPOP–DAXX body
Spotted POZ (poxvirus and zinc finger protein) protein (SPOP), a broad complex, tram-track and small curio (BTB) complex protein, acts as a substrate articulator for Cullin 3 RING E3 ubiquitin ligases to target various proto-oncoproteins for ubiquitination and their proteasomal degradation.201 Normally, SPOP assembles with substrates and recruits to the nucleosome, but cancer-associated mutations disrupt the SPOP/substrate colocalization mechanism and substrate-mediated LLPS of ubiquitin ligases. Substrates are candidates that mediate separation from SPOP and regulate its subcellular localization and function, including death domain-associated proteins (DAXX), androgen receptors (AR), and other critical signaling cascade effectors.202, 203 DAXX has potent oncogenic properties and regulates multiple processes, including transcription, DNA damage response, cell signaling and cell death.204 The SPOP-mediated degradation of DAXX induces apoptosis and extracellular matrix degradation.205 Thus, SPOP mutants disrupt substrate binding and LLPS, resulting in the inability to colocalize and flip with the substrate.197 Disruption of interaction among substrates by SPOP mutations may inhibit tumor formation through uncontrolled DAXX ubiquitination activity.
5.1.2 PML vesicles
PML vesicles, 0.1–1.0 µm in diameter, are membraneless nuclear substructures that function as tumor suppressors via regulating cell cycle, senescence, programmed cell death and DNA damage response.206, 207 PML vesicles are biomolecular condensates assembled by LLPS of members of a protein superfamily containing a tripartite motif and identified initially as fusion partners with the retinoic acid receptor alpha (RARα) generated by the t(15;17) genomic translocation in acute promyelocytic leukemia (APL).208 Both nuclear receptor-induced differentiation and PML-triggered apoptosis can be interfered with PML–RARα proteins. The loss of function of PML and RARα is implicated in APL pathogenicity, and inactivation of the tumor suppressor activity of PML might promote cancer development by inducing genome instability.
5.1.3 FET fusion proteins (EWS–FLI, FUS–CHOP)
FUS, Ewing sarcoma breakpoint region 1 (EWSR1, also known as EWS), and TBP-associated factor 15 (TAF15) make up FET (FUS/EWSR1/TAF15) RNA-binding protein family, which is involved in multiple steps of RNA metabolism, including transcription, RNA processing, and the cytoplasmic fates of mRNAs.209, 210 FUS participates in RNA transport, splicing and translation and has been shown to undergo LLPS to form granules in various types of cells.211 CHOP (DDIT3/GADD153), a transcription factor family member, can be upregulated by ER stress, growth arrest, and DNA damage.142, 212 FUS–CHOP fusion oncoprotein is a genetic hallmark of the myxoid/round cell type and promotes myxoid liposarcoma tumorigenesis and metastasis.213, 214 EWSR1 is a multifunctional RNA-binding protein often fused to various partner genes due to chromosomal translocation. EWSR1–Friend leukemia integration one transcription factor (FLI1) acts as a potent tumor-specific chimeric oncoprotein, and high EWSR1–FLI1 expression exponentially endows cancer cell proliferation.215 EWSR1–FLI1 fusion protein is found in approximately 85% of sarcomas and is required for Ewing's sarcoma tumorigenicity.216, 217 FUS–CHOP and EWSR1–FLI1 are highly localized to the nucleus and drive aberrant transcriptional programs in Ewing's and liposarcoma, respectively.218
LLPS aggregates FUS and EWSR1 to enhance transcriptional activity.219 FUS has been shown to undergo LLPS both in vivo and in vitro to generate functional RNA granules and aggregates. In addition, the LLPS of FUS can be enhanced by adenosine triphosphate (ATP), indicating that its LLPS behavior can be fine-tuned with proper strategy.220 Fusion transcription factors generated by genomic translocations have been recognized as important cancer drivers, especially for sarcomas and leukemias. FUS–CHOP, a FET oncofusion protein, undergoes PrLD-mediated LLPS into liquid-like condensates. It is well known that the N terminus of FUS is necessary for its LLPS, therefore, transcriptional activation by FUS–CHOP can account for the N terminus driving nuclear LLPS transition.
Furthermore, phase-separated condensates of FUS–CHOP are colocalized with BRD4, a marker of super-enhancer condensates,221 and FUS–CHOP recruits the chromatin remodeler SWI/SNF to forward global transcriptional reprogramming.222 It would be possible to target the LLPS and epigenetic remodeling complexes engagement capacities of FUS–CHOP confusion proteins to implement precision medicine for cancer therapy. EWSR1 also contains PrLDs to undergo LLPS, and O-linked β-D-N-acetylglucosaminylation (O-GlcNAcylation) has been found to decrease the LLPS propensity of N-terminal low complexity region of EWSR1.223 In addition, BRG1/BRM-associated factor (BAF) with a PrLD has been found to interact with EWSR1. Following the LLPS transition, the BAF complex is recruited to tumor-specific GGAA microsatellite repeat enhancers to activate target gene transcription.216 The formation of EWSR1 condensates can be intervened under different conditions. As for EWSR1–FLI1 takes the low complexity domain from EWSR1 and a DNA binding domain from FLI1. The low complexity domain of EWSR1–FLI1 is required for its undergoing LLPS and formation of protein granules in cells.224 EWSR1–FLI1 LLPS transition properties require retargeting the BAF chromatin remodeling complex and activating enhancers that drive the transcriptional program promoting cancer progression.216 This information sheds light on fine-tuning the LLPS of FET fusion proteins with additional molecules.
5.2 Involvement of LLPS in heterochromatin formation in tumor cells
LLPS affects the formation of chromatin structure, which might ultimately contribute to the development of cancer.225 The chromatin of eukaryotes consists of positively charged histones and negatively charged DNA. Heterochromosomes are chromosomes in a cohesive state with tight morphology, gene deletions, high duplication of genomic regions, and transcriptional inertia.226 Heterochromosomes are primarily in the perisynaptic and telomeric regions, allowing for flexible gene silencing at multiple levels. Methylation of Histone H3 lysine 9 (H3K9me), a vital disability for posttranslational modification of histones, is a marker of heterochromatin formation. The heterochromatin protein family HP1 is an essential component of the dynamic nuclear response that senses and corrects defects in epigenetic information encoded in chromatin through histone modifications and DNA methylation. Defects in this crucial “chromatin repair” response in transformed human cells can be ideal targets for killing cancer cells. IDR-containing HP1 and its homolog Swi6 can trigger LLPS on chromatin by recognizing H3K9me and forming heterochromatin.227 In contrast, the conformation of HP1 molecules altered by N-terminal chromatin phosphorylation exposes the binding site, disrupts the molecular network structure, and inhibits LLPS.228 LLPS occurs based on multivalent interactions. Therefore, the forces and associated networks between multivalent molecules affect the structure and function of the related chromatin on the basis of LLPS.229, 230 Inhibition of HP1 LLPS by phosphorylation provides preferential elimination of cancer cells.186 In cancer cells, the nuclear morphology is frequently altered, a phenomenon known as atomic heterogeneity.231 Alternation in nucleoli's composition, number, size, and activity can provide a diagnostic basis for cancer. Because LLPS controls the integrity of part of the nuclear structure and the integrity of the microenvironment, modulating LLPS may play a key role in cancer therapy.
In eukaryotes, heterochromatin maintains genomic integrity and stability and is organized into distinct nuclear domains. DNA damage proteins can access heterochromatin by specific mechanisms, and erosion of heterochromatin by abnormal factors is linked to carcinogenesis. Tethering LLPS condensates with heterochromatin adds complexity to regulating the nuclear microenvironment. Heterochromatin binding protein HP1 has three homologs termed HP1α, HP1β, and HP1γ that function in DNA repair, nuclear organization, chromosome segregation, telomere maintenance, and gene silencing. HP1 might participate in many types of cancers and can serve as a potential biomarker for cancer prognosis and therapeutic target. Growing evidences have supported that HP1 proteins act as key modulators in micro-LLPS and the segregation of heterochromatin-like domains/complexes.232 HP1α and HP1β have been reported to form phase-separated droplets to contribute to forming heterochromatin compartments.233, 234 Besides HP1, 53BP1 is another important player in regulating heterochromatin formation. It has been shown that 53BP1, together with HP1α undergoes LLPS at heterochromatin in a mutually dependent manner.235 Modulating the LLPS of factors, such as HP1 and 53BP1, to maintain heterochromatin integrity and genome stability can aid in preventing tumorigenesis and improve cancer therapy outcomes.
5.3 LLPS regulates gene expression to induce carcinogenesis and malignant behavior
Phase-separated regulation of gene expression is involved in both transcription and translation processes. LLPS plays a critical role in both regulating DNA damage response pathway and RNA transcription process. LLPS regulates DNA-responsive injury pathways to maintain genomic stability. DNA damage response and repair occur when signaling factors are activated and repair proteins are concentrated. 53BP1 is a scaffold for LLPS to bind DNA damage recognition and repair factor assembly to cell fate decisions.184 p53-binding protein1 (53BP1) is a significant player in the DNA damage responding and repairing pathway and accumulates at DNA lesions, creating a nuclear environment called “53BP1 nuclear bodies” to scaffold factors for downstream signal cascades.236 p53 (a transcription factor) and USP28 (a deubiquitinase stabilizing p53) are assembled in 53BP1 by dynamic fusion and fission, symbolizing LLPS to encode an oncogenic factor.237 Generally, the 53BP1 nuclear body coordinates the DNA damage recognition with gene expression. Besides 53BP1, RAP80 LLPS has also been found to enhance the recruitment of BRCA1, a well-known tumor suppressor gene, to DNA double-strand breaks.238
Three different types of enzymes mainly regulate transcription, Pol I, Pol II, and Pol III.239 Pol I synthesizes preribosomal RNAs (rRNAs), Pol II produces messenger RNAs (mRNAs) and a variety of precursors for ncRNAs, and Pol III is responsible for transfer RNAs (tRNAs). LLPS differentially phosphorylates 239 RNA polymerase II at transcription initiation, elongation and termination to generate transcription factory or condensates.194 Upon entering the extension phase, RNA polymerase II dissociates from the transcriptional condensate in response to differential phosphorylation and relocates to another condensate used for splicing factors.240 These findings imply that LLPS is critical for transcription processes, including initiation and the switch to elongation, by concentrating factors and conferring dynamics with phosphorylation.241
In the first step of gene transcription, Pol II is a scaffold for the preinitiation complex, recruiting other factors to interact with itself and coordinate the transcription initiation. The CTD is a critical element of the scaffolding of Pol II and is highly structurally disordered, which is essential for the development of LLPS.242 CTD can promote LLPS through interactions with other regulators, and these binding sites enrich multiple transcriptional regulators in the condensate of CTD to achieve efficient activation of transcription initiation.243 CTDs can also be recruited to transcription centers formed by specific transcription factors to enhance transcription initiation.192, 244, 245 After escaping the promoter, Pol II is in a suspended state near the proximal region of the promoter.246, 247 To release from this proximal paused state, the cell cycle protein-dependent kinase CDK9 phosphorylates residues of the Pol II CTD thereby removing the inhibitory effect of paused Pol II.248 CycT1 possesses an IDR and can fold into a structural domain through its N-terminal region that binds tightly to CDK.249 CycT1 concentrates and compartmentalizes transcription factors into phase-separated environments by LLPS through histidine-rich structural domains within its IDR, leading to hyperphosphorylation of Pol II CTD and continued transcription elongation.250, 251 At the end of the regulatory transcription cycle, the Arabidopsis RNA-binding protein FCA undergoes LLPS, in which the curly helix protein facilitates to reduce transcriptional read-through.252-254 It can be concluded that LLPS participates in the whole process of gene transcription.
As a flexible and variable molecule, RNA is a potentially powerful provider of polyvalency in LLPS.255 lncRNA SLERT acts as an RNA modulator to regulate LLPS of fibrillar center/dense fibrillar component to facilitate Pol I transcription.256 Complex network structures formed by RNA–RNA interactions may directly trigger LLPS or provide a platform for protein coalescence and undergoing LLPS.257 Posttranscriptional modification of RNA regulates the properties and metabolism of RNA.258, 259 Numerous membraneless chambers promote gene regulation through various mechanisms, such as concentrating the activity of chelating factors to facilitate biochemical reactions or constructing interchromosomal hubs to promote the occurrence of macromolecules associated with gene expression.31, 260 Recently, several groups have reported that multivalent m6A-modified RNAs act as scaffolds to gather YTHDF proteins and thus lead to LLPS both in vitro and in vivo.261 In addition, a membraneless organelle, the P-body is involved in the regulation of translational repression and mRNA decay mechanisms. Changes in P-body mediators affect mRNA metabolism and may lead to alternations in expression profiles and epigenetic regulation. P-body mediators, such as miRNA and m6A, are associated with cancer.18 LLPS in RNA modification regulation would be a new research perspective because RNA biology is widely associated with various biological processes.
Abnormal transcriptional machinery is a widely accepted cancer hallmark. Therefore, based on the above description, it is easy to reach a consensus that LLPS holds a dominant role in cancer development. LLPS of RNA-binding protein triggers the phosphorylation and release of Pol II to enhance active transcription.262 Many RNA-binding proteins, such as TIA1, LIN28, ZEB1/2, RBM38, and TRBP, have been shown to be aberrantly expressed in cancers.263, 264 The transcriptional regulators’ YAP/TAZ can mediate cancer cells reprogramming into cancer stem cells, inciting carcinogenesis and cancer metastasis.265 LLPS of YAP fusions, YAP-MAMLD1 and C11ORF95-YAP have been considered the leading mechanism in initiating ependymoma from neural progenitor cells.266 Furthermore, inhibiting transcriptional coactivator activity mediated by condensates can prevent tumorigenesis,266 confirming the important role of YAP LLPS in oncogenic activity. SNHG9, a cancer-promoting lncRNA, drives the formation of a liquid-like droplet of LATS1 and downregulates the Hippo signaling.267 SNHG9 together with phosphatidic acids binds to LATS1 C-terminal domain to trigger LAST1 LLPS, thus blocking LATS1-mediated YAP phosphorylation.267 These results indicate a novel regulatory strategy to modulate cancer-promoting transcription apparatus by facilitating LLPS.
In addition, the nuclear microenvironment of cancer is altered in response to LLPS.268 Super-enhancers, which act as enhancer clusters to mediate dysregulation of transcriptional programs, are essential oncogenic drivers that sustain cancer cells.269, 270 Super-enhancer-rich transcriptional coactivators BRD4 and MED1 can form LLPS droplets at the super-enhancer IDR, concentrating the factors in specific genomic regions.270 LSD1, also named KDM1A, is involved in the progression of multiple cancers. It has been demonstrated that LSD1 interacts with BRD4 and FOXA1 and is enriched at super-enhancer regions, showing LLPS behavior.271 NUP98–HOXA9, a homeodomain-containing transcription factor chimera in leukemias, contains IDR and can establish LLPS puncta of chimera.200 Phase-separated NUP98–HOXA9 generates a “super-enhancer”-like binding pattern to potentiate transcriptional activation and induce leukemic transformation.200 NUP98–HOXA9 LLPS leads to the formation of proto-oncogenes enriched CTCF-independent chromatin loops.200 HOXB8 and FOSL1 are core regulatory circuitry components that control chromatin accessibility in super-enhancers. HOXB8 and FOSL1 form dynamic phase-separated droplets to incite the release of RNA Pol II from the promoter of super-enhancer-driven genes and the condensates can be specifically destroyed by GSK-J4, a H3K27 demethylase inhibitor, to suppress metastasis and re-establish sensitivity to chemotherapy treatment.272 LLPS remodels nuclear microenvironment provide mechanistic and therapeutic insights for targeting epigenetic factors to regulate chromatin accessibility in cancer therapy.
5.4 LLPS maintains cellular homeostasis to regulate cancer progression
The cell is separated from its surroundings by a cell membrane, and the internal conditions of the cell are very different from the fluid surrounding the cell. The cell constantly exchanges substances with the surrounding fluid and maintains its internal constancy, which is called cellular homeostasis. Cellular homeostasis includes proteostasis, redox homeostasis, calcium homeostasis, and so on. Cellular homeostasis has many essential roles in cancer therapy. For example, cellular metabolism can modulate the effector T cells to influence the adaptation of cellular functions in cancer immunity.273
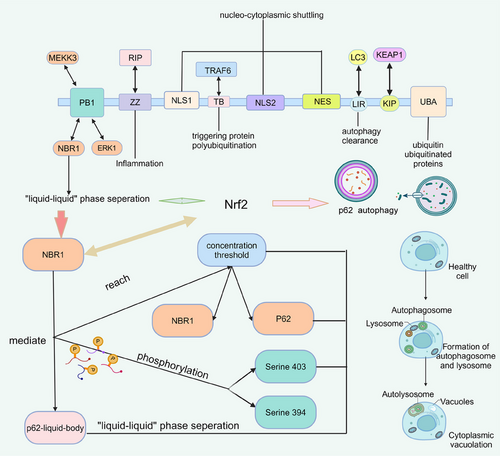
Degradation of misfolded proteins is essential for cellular homeostasis. Selective autophagy mediates the degradation of harmful substances by separating these proteins into larger cohesions in stages through multivalent interactions and tethering them to the autophagosomal membrane.274 Membraneless particles(such as postsynaptic densities, signaling granules at DNA damage repair sites, and p62 granules involved in selective autophagy) have been found to assemble in membraneless compartments in several biochemical pathways and cellular pathways.49, 275, 276 During proliferation and spread, the intracellular environment of cancer cells, including the homeostasis of proteins, is altered. Gene activation or genomic instability impedes protein folding in cancer cells. Inadequate amino acid supply, glucose deprivation, and so on challenge protein processing in ER.277 p62 is a scaffolding and stress-inducible protein. By regulating autophagy and apoptosis, this multifunctional protein controls cell viability in response to cytotoxic stress (Figure 3).278-280 Selective autophagy mediates the degradation of harmful substances by segregating these protein stages into larger endosomes through multivalent interactions and binding them to the autophagosome membrane. P62-dependent hepatic differentiation is activated by autophagy inhibition. When the mTOR pathway is activated, p62 autophagy would be inhibited to cause protein accumulation. p62 is also closely associated with causing resistance to cancer therapy. Reduced p62 levels through autophagy upregulation in cisplatin-resistant ovarian epithelial cancer have been observed to increase tumor sensitivity to the drug.278, 281
The oxidation and reduction reactions within the cell change the properties of the cellular components and provide energy to the organism. Redox imbalances in cells can lead to oxidative stress that impairs intracellular functions, for example, the susceptibility of the purine nucleotide guanine to oxidation, leading to DNA mutations that may initiate and propagate cancer.282 LLPS enables efficient energy production, promotes cell survival under acute hypoxia, and protects cells from apoptosis during cellular translational remodeling.282, 283 Nuclear factors erythroid 2-related factor 2 (NRF2)-mediated redox-regulated pathways can sense cellular damage through LLPS and activate autophagy to degrade these damaged organelles. NRF2 has been shown to be a key activator of cancer-supporting anabolism, and NRF2 activation has significant and protumor solid effects, particularly in the reprogramming of cancer cell metabolism.284
Calcium homeostasis controls autophagy in cancer cells. Ca2+ is a multifunctional second messenger, and as one of the most important factors regulating processes such as cell death, Ca2+ activates various regulatory proteins, including enzymes and transcription factors.285 Alterations in Ca2+ concentration in the cytoplasm and endoplasmic reticulum are integrated into the core through different regulatory mechanisms of autophagic activity.286 Ca2+ triggers the FIP200 complex to drive assembly via LLPS, triggering the formation of FIP200 sites, after which the autophagosome complex assembles in the endoplasmic reticulum.287 As a survival-promoting and death-inducing factor, autophagy boasts an excellent scope for tumorigenesis and prevention regulation.
5.5 LLPS remodels microenvironment to control cancer progression
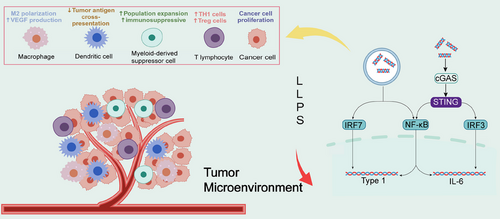
The tumor microenvironment consists of tumor cells and their surrounding immune and inflammatory cells, tumor-associated fibroblasts, nearby mesenchymal tissues, microvasculature, part of the neuronal regulatory circuits, as well as a variety of cytokines and chemokines, and is a complex integrated system.288, 289 In recent years, there is increasing evidence for the diversity of LLPS in the regulation of immune regulation, including the maturation and activation of immune cells, immune signaling, and so on.47 LLPS is also involved in the regulation of intracellular immune signaling, such as the cGAS–STING pathway (Figure 4).290 Cancer-associated fibroblasts are essential components of the tumor microenvironment, and are activated to promote tumor growth, angiogenesis, invasion and metastasis. In addition, cancer-associated fibroblasts can interact with tumor-infiltrating immune cells and other immune components within the tumor microenvironment by secreting various factors and other effector molecules, resulting in immune effector cell dysfunction and suppression of antitumor immunity.291 Then, whether modulation of immune cells by LLPS and attenuation of their action with tumor-promoting factors by immune components can inhibit cancer growth and invasion in the tumor microenvironment.
6 SYNERGY OF LLPS AND RNA MODIFICATIONS IN DISEASES
To date, more than 150 different types of chemical modifications of RNA have been identified. Common RNA modifications include m6A, m1A, 5-methylcytidine (m5C), 5-hydroxymethylcytosine (hm5C), adenosine-to-inosine editing (A-to-I), m7G, pseudouridine (Ψ), N4-acetylcytidine (ac4C), and 2ʹ-O-methylation. Modifications of different RNA species, also named epi transcriptome, have emerged as a critical regulator of transcript expression, molecular function and homeostasis. All these RNA modifications might be involved in cancer pathogenesis (Figure 5). RNA modifications are involved in various signaling pathways, and several of these proteins play a role in regulating disease progression.292-294 RNA modifications (mainly m6A) have been reported for their protein LLPS,255 and they in turn, are strongly associated with pathological processes. Therefore, it is possible to fight against diseases by regulating LLPS of RNA-modified related proteins.
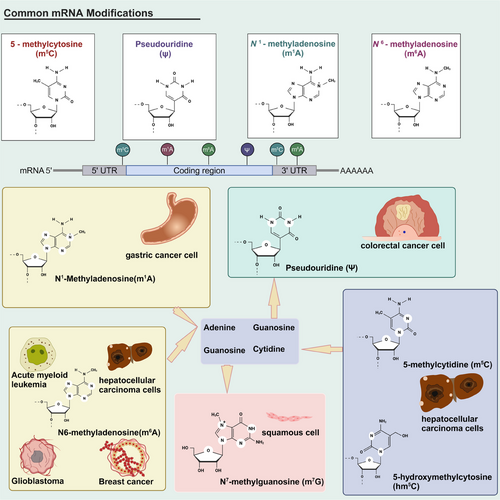
6.1 N6-methyladenosine
6.1.1 Introduction of m6A
Discovered in the 1970s, m6A is the most abundant internal modification of mRNA and long-stranded ncRNA (lncRNA) in most eukaryotes.295 Mostly, m6A modification occurs at the RRACH motif (R denotes A or G, H denotes A, C, or U). The formation of m6A is a reversible process involved in all aspects of RNA metabolism, including pre-mRNA splicing, 3′-end processing, nuclear export, translational regulation, mRNA decay, and ncRNA processing.296 Besides regulating the RNA cycle, m6A is widely involved in the metabolic reorganization of tumor cells and is an important influencing factor in many cancers. The principal function of m6A has been indicated to regulate the stability of targeted mRNAs. m6A-associated proteins use different mechanisms to recognize and bind m6A. For example, YTH structural domain-containing proteins (YTHDF1/2/3 and YTHDC1/2) use the YTH structural domain to recognize m6A, and LLPS regulates these proteins.261, 297 The effects of m6A are dynamically and reversibly determined by methyltransferases (writers), demethylases (erasers), and m6A binding proteins (readers).
m6A writers: Methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14) and Wilms’ tumor 1-associated protein (WTAP) form the m6A methyltransferase complex, and their knockdown reduces m6A levels in polyadenylated RNA.298 Results of the experiment have shown that METTL3 and METTL14 form a stable METTL3–14 complex at a 1:1 stoichiometric ratio, and the dimer of the METTL3–14 complex induces m6A deposition on nuclear RNA. WTAP binds to the METTL3–14 complex, which has no methyltransferase activity but interacts with the METTL3–14 complex to influence m6A methyltransferase activity in vivo and location in nuclear spots.299
m6A erasers: Fat mass and obesity-associated protein (FTO) and AlkB homolog 5 (ALKBH5) have been reported to exhibit demethylation activity.300 FTO and ALKBH5 belong to the α-ketoglutarate-dependent dioxygenase family and catalyze m6A demethylation in a Fe(ii) and α-ketoglutarate dependent manner. Overexpressed FTO and ALKBH5 significantly reduce m6A levels in mRNA. ALKBH5 strictly selects substrates in the catalytic process, and the loss of ALKBH5 damages RNA metabolism, mRNA output, and assembly.300
m6A readers: To exert biological function, m6A needs to be identified by variable “readers” to exercise different downstream effects.301 Members of the YT521-B homologous (YTH) domain family, including YTHDF1, YTHDF2, YTHDF3, YTH N6-Methyladenosine RNA Binding Protein C1 (YTHDC1), and YTHDC2, recognize m6A and function as readers with certain members of the heterogeneous nuclear ribonucleoprotein (HNRNP) family. YTHDF1 promotes the translation of m6A-methylated mRNA, YTHDF2 accelerates the decay of m6A-methylated mRNA, and YTHDF3, together with YTHDF1 and YTHDF2, noticeably enhances the metabolism of m6A-methylated mRNA in the cytoplasm.302 For HNR family, HNRNPA2B1 promotes primary miRNA processing and maturation by binding to m6A methylated transcripts, and HNRNPC and HNRNPG also regulate mRNA abundance and splicing by processing m6A modified RNA transcripts.295, 303, 304
6.1.2 Dual role of m6A modification in cancer development and therapy
m6A methylation might act as a potent regulator of tumor metabolism (Table 3). In metabolic reprogramming in tumor cells, m6A methyltransferase and corresponding signaling pathways are essential modulators. m6A modification can play an active role in multiple pathways of tumor inhibition. However, for some signaling pathways, transcription factors, and enzymes associated with metabolic recombination, dysregulation of m6A methylation can damage cells or lead to treatment failure.305
| Type | Proteins | LLPS | Cancer | Mechanism (reference) |
|---|---|---|---|---|
| m6A Writers | METTL3 | METTL3 phase separation regulates m6A methyltransferase complex assembly.306 | Glioblastoma | High levels of METTL3 promote m6A transcription and increase ADAR1 protein levels, leading to cell cycle acceleration. Targeting ADAR1 in glioblastoma tumors completely inhibited tumor growth in vivo.307 |
| Glioblastoma | Overexpression of METTL3 significantly promoted the growth and invasion of bladder cancer cells.308 | |||
| Breast cancer | METTL3 promotes aberrant expression of mammalian hepatitis B X-interacting proteins driving breast cancer aggressiveness.309 | |||
| Acute myeloid leukemia | METTL3 is a regulator of chromatin-based pathways that is necessary for the maintenance of leukemic cells.310, 311 | |||
| METTL14 | WTAP/WTAP- Vir like m6A methyltransferase associated (VIRMA)312 interacts with the METTL3–METTL14 condensate to influence methylation.299, 313 | Hepatocellular carcinoma | METTL14 suppresses the metastatic potential of hepatocellular carcinoma by regulating m6A-dependent primary microRNA processing.314 | |
| WTAP | Acute myeloid leukemia | WTAP is important in the abnormal proliferation and differentiation arrest of leukemic cells.315 | ||
| VIRMA | Nasopharyngeal carcinoma | VIRMA enhances mRNA stability and transactivates integrin subunit alpha (ITGA2), promoting nasopharyngeal carcinoma progression.316 | ||
| m6A Erasers | ALKBH5 | The disordered C-terminus of ALKBH5 promotes LLPS.317 | Breast cancer | ALKBH5-dependent approach induces increased NANOG mRNA and protein expression and breast cancer stem cell phenotype.318 |
| Glioblastoma | ALKBH5 is highly expressed in glioblastoma stem cell-like cells.319 | |||
| m6A readers | YTHDF2 | YTHDF2 enhances the phase separation potential of mRNA.261 | Acute myeloid leukemia | YTHDF2 inhibits the proliferation of stem cells into targeted mRNA decay.320 |
| Hepatocellular carcinoma | YTHDF2 inhibits the ERK/MAPK pathway to suppress cell proliferation.321 | |||
| YTHDF1 | YTHDF1 promotes mRNA degradation via and LLPS.297 | Ovarian cancer | YTHDF1 enhances EIF3C translation in an m6A-dependent manner by binding to m6A-modified EIF3C mRNA, thereby promoting tumorigenesis and metastasis in ovarian cancer.322 | |
| YTHDF3 | LLPS and proapoptotic effects of YTHDF3 can be modulated.261 | Breast cancer | YTHDF3 promotes the interaction of breast cancer cells with brain endothelial cells and astrocytes, blood–brain barrier extravasation, angiogenesis, and growth.323 | |
| YTHDC1 | YTHDC 1 can undergo LLPS and form YTHDC1–m6A condensates.324 | Bladder cancer | YTHDC1 regulates the positive expression of the PTEN/PI3K/AKT signaling pathway and enhances chemoresistance in bladder cancer.325 |
m6A modification inhibits tumor progression
Many studies have shown that downregulation of m6A promotes the progression of multiple tumors. Glioblastoma is the most-deadly primary brain tumor. Glioblastoma stem cells can promote tumor growth and invasion, resist radiotherapy and chemotherapy, and drive a poor prognosis of glioblastoma. It has been found that decreased m6A levels in ADAM19 enhance its expression, promoting glioblastoma stem cell growth and self-renewal and ultimately leading to tumorigenesis.326 Studies have shown that the progression of hepatocellular carcinoma is associated with abnormal m6A modification.326 METTL3 is frequently upregulated in hepatocellular carcinoma patients, and the upregulated METTL3 inhibits the expression of tumor suppressor SOCS2 through m6A methylation and promotes the development of hepatocellular carcinoma.327 METTL14-mediated m6A modification of PTEN mRNA suppresses the progression of clear-cell renal-cell carcinoma.328 Pri-miR126 is a direct substrate of METTL16. In hepatocellular carcinoma, the decreased m6A level on pri-miR126 influences its maturation and disrupts the tumor suppressor function of miR126, accelerating tumor progression.314 In certain circumstances, restoration of METTL3 and METTL14 expression to re-establish m6A distribution can inhibit cancer cell progression.
m6A modification promotes tumor progression
Some studies also reveal that m6A modification might exhibit tumor-promotion properties. Acute myeloid leukemia is one of the most common hematological malignancies with apparent genetic aberrations and unsatisfactory therapeutic outcomes. METTL3 has been reported to play a carcinogenic role in acute myeloid leukemia. CEBPZ recruits METTL3 to the promoter region of SP1 to enhance the m6A modification of SP1 and stimulate its translation.310 SP1 then activates the oncogene c-MYC, leading to the development of acute myeloid leukemia.310 Recently, METTL3 has been found to mediate the m6A modification of PTCH1 and GLI2 to control their RNA stability and translation to activate Sonic hedgehog signaling, resulting in the promoted progression of SHH subgroup medulloblastoma.329 METTL3-mediated m6A modification decreases the expression of IFFO1, a novel tumor suppressor, via affecting mRNA stability to promote tumor development and cisplatin resistance in ovarian cancer.330 In addition to METTL3, METTL14 has also been shown to enhance tumorigenesis and be associated with poor prognosis in several types of cancers.331, 332 Knockdown of METTL14 inhibits the malignant progression in vitro and in vivo.331, 333 METTL3 and METTL14 may thus be a potential prognosis marker and a therapeutic target in certain types of cancers. Proper regulation of METTL3 and METTL14 may help restore m6A landmarks to halt cancer progression. Acetylation in METTL3 has been found to impede lung cancer metastasis by impaired m6A methylation.334
6.1.3 m6A in other diseases
In addition to its role in cancer, m6A is also widely involved in diseases, including cardiovascular and neurological. Some research has found that mRNAs encoding protein kinases and many signaling molecules are hypermethylated in hypertrophied hearts.335 METTL3 has been reported to be involved in the regulation of epilepsy. Activation of METTL3-mediated downregulation of Vim expression attenuates hippocampal neuronal damage and apoptosis and prevents epilepsy progression.336 Overexpression of METTL3 leads to hypermethylation of genes involved in cardiac hypertrophy.337 Also, m6A methylation plays an essential role in cardiac fibroblast differentiation, and METTL3 overexpression leads to enhanced differentiation of fibroblasts into myofibroblasts.338 Recently, the role of METTL14 has also been shown in vascular complications. METTL14 knockdown significantly reduces atherosclerotic plaque development.339 The expression of FTO and ALKBH5 is reduced in the failing heart and hypermethylation of m6A of these two genes regulates the translation of particular mRNA transcripts.340, 341 FTO gene expression is significantly reduced in both mouse and human failing hearts, and alterations in its expression may contribute to many cardiovascular abnormalities, including hypertrophic cardiomyopathy, arrhythmias, and coronary artery disease.340 Knockdown of ALKBH5, on the other hand, significantly reduced the expression of autophagy significant factors, which may be involved in autophagy and apoptosis in hypoxia-treated cardiomyocytes.342 Besides writers and erasers, m6A readers also play a critical role in gene expression. Altered YTHDF1–3 expression may alter the translational efficiency of methylated transcripts.302 YTHDF2 inhibition significantly reduces inflammatory cytokines possibly involved in cardiovascular disease.343 m6A is increased in overall abundance in the brain and is involved in nerves related to embryonic brain development,344 learning and memory.345, 346 Deletion of METTL3 or METTL14 has a strong effect on glial cells, leading not only to prolonging the cell cycle of radial glial cells,347 but also a decrease in the number of glial cells.348 In the nervous system, FTO also has important effects with ALKBH5. FTO is highly expressed in adult neural stem cells and neurons and affects brain size, body weight, learning and memory.346 Under hypoxic conditions, the deletion of ALKBH5 disrupts many vital genes.349 YTHDF1 is increased in the hippocampus350 and promotes protein translation in response to neuronal stimulation.351
6.2 N1-methyladenosine
m1A, another type of RNA methylation, is a crucial internal RNA modification that controls gene expression, appearing on the first nitrogen atom of adenosine in RNA.352 Similar to m6A, m1A can be installed by “writer” methyltransferases, removed by “eraser” demethylases and recognized by m1A-binding proteins called “reader.”353 m1A is very abundant in tRNAs and is most conserved and common in bacteria, archaea and eukaryotes.354 m1A at position 58 is the first m1A modification of the initial transcripts of tRNA in the nucleus, whose methyl methyltransferase complex has been first discovered in Saccharomyces cerevisiae.355 m1A plays an important role in the regulation of pre-RNA processing, RNA structure, translation and stability.356 It should be noted that the biological function and modification sites of m1A modification vary in different species.
m1A is involved in regulating disease initiation and progression. m1A methylation upregulates microfibril-associated protein 2 (MFAP2) expression and promotes colorectal cancer metastasis by blocking autophagic degradation of CDC Like Kinase 3 (CLK3).356 In gastric cancer cells, m1A regulates the PI3K/AKT/mTOR pathway and ErbB pathway. Knockdown of ALKBH3, the “writer” protein of m1A, has been observed to result in poor prognosis and metastasis in gastric cancer.357 In addition, m1A is also involved in the prognosis of gliomas.358 m1A-related proteins, including ALKBH1, TRMT6, TRMT10C, and YTHDF1, are significantly overexpressed in gliomas. TRMT6 regulates cell cycle distribution and increases glioma cell proliferation and death.358, 359 m1A methylation in mitochondria and cytoplasm has been reported to be dysregulated in AD.360, 361 For example, aberrant m1A deposits lead to complex I dysfunction in AD.362 In sum, there has been increasing evidence of alterations in m1A occurring in various diseases, and these m1A modifications may be potential drug targets.
6.3 m5C and hm5C
In eukaryotes’ RNA, m5C and hm5C are the prominent modifications and are thought to contribute to epigenetic regulation through various mechanisms.363 NOL1/NOP2/NSUN family and DNMT2 catalyze m5C, which covalently transfers methyl from S' adenosine methionine to the cytosine pyrimidine ring.363, 364 m5C methylation is a reversible epigenetic modification with a constant turnover of cytosine modifications.365 This dynamic regulation of reversible chemotaxis is important in cell fate determination and can act as an epigenetic barrier by limiting the developmental potential of cells and restricting their differentiation.366
The methyltransferase of m5C has been observed in a variety of cancers, including glioma, gastric cancer and leukemia.364 In hepatocellular carcinoma cells, the methyltransferase NSUN2 of m5C can target the tumor-associated gene lncRNA H19 to promote tumorigenesis and progression. G3BP1 regulates a variety of tumorigenesis-related pathways, including Ras, Wnt/β-catenin, PI3K/AKT, and NF-κB/Her2 signaling pathways. RNA purification and mass spectrometry analyses indicate that m5C modifications regulate the binding of G3BP1 protein to H19 RNA.367 In addition, NSUN2 is upregulated in gastric cancer and promotes cancer cell proliferation, migration and invasion. SUMOylation is a significant regulatory posttranslational modification, and its covalent bond interaction with NSUN2 to form the SUMOylation–NSUN2–m5C axis is a novel mechanism and therapeutic target for gastric cancer cells.368 The methyltransferase DNMT2, in turn, can interact with RNA-binding proteins to mediate the 5-azacytidine response in leukemia.369 Azetidine acrylamides, stereoselective covalent inhibitors of human NSUN2, can cause a global reduction in tRNA m5C content in cancer cells.370 Growing studies are currently undergoing to unveil the role of m5C modification in cancer development.
NSUN2 are all enriched in the developing brain371 and are knocked down to delay or block late differentiation in specific tissues including skin and testis.372 Several mutations in NSUN2 may cause growth and mental retardation, unusual facial and skin abnormalities.373 And mutations in NSUN3 have been reported to be detected in patients with mitochondrial-deficiency disorders.374, 375 NSUN5 is located in the deletion genome of a neurodevelopmental disorder called Williams-Beuren syndrome,376 and the deletion of NSUN5 may be one of the pathogenic causes of the disorder.377 NSUN7 is widely expressed in testicular cells and may lead to reduced sperm viability under induced mutations.378 In summary, m5C is commonly involved in a variety of diseases.
As the hydroxylated form of m5C, hm5C is now widely recognized as the “sixth base” of the mammalian genome.379, 380 The hm5C modification has also been identified in RNA, and ten-eleven translocation (TET) enzymes are responsible for the oxidation of m5C into hm5C in RNA.381, 382 It has been found that the deposition of hm5C on tRNA depends on TET2, and TET2 knockout impairs the abundance of several ncRNAs.383 In a recent study, it has been confirmed that ALKBH1 is the primary enzyme for hm5C modification in RNA other than TET2.384 It is well known that both TET2 and ALKBH1 are essential for cancer initiation and progression. Besides hm5C, there are also different types of oxidative metabolite of m5C in RNA, including 5-Formylcytidine (f5C)385 and 2′-O-methyl-5-hydroxymethylcytidine (hm5Cm).384, 386 However, the biological function of these modifications in RNA remains largely unknown.
6.4 Adenosine-to-inosine editing
Irreversible deamination reaction A-to-I converts adenosine to inosine in the dsRNA region, thus functionally acting as an A-to-G mutation.387, 388 Adenosine deaminase acting on the RNA (ADAR) family is the most prominent A-to-I modifying enzyme and is dysregulated in many human diseases.389
ADAR1, which is much more enriched than ADAR2, plays the most important role in the family and is closely associated with cancer development.390 ADAR1 recognizes the hairpin structure of dsDNA and edits it, leading to inactivation or activation of the final translation product and disrupting miRNA binding sites to alter mRNA stability.391 Knockdown of ADAR1 results in mice exhibiting massive apoptosis, defective erythropoiesis, and abnormal innate immune responses.392 ADAR2 enzyme targets the Q/R locus in the mammalian central nervous system in the mouse brain, and defects at this locus may result in cell death of excitotoxic neurons, leading to excitotoxicity.393, 394 As an important target of ADAR1, Antizyme inhibitor 1 (AZIN1) is involved in forming various cancers, including colorectal and lung cancer. Takeda et al.395 proposed a new mechanism by which AZIN1 promotes the accumulation of proteins necessary for polyamine synthesis, activating and increasing fibroblast invasion into the tumor microenvironment. AZIN1 promotes tumor angiogenesis in colorectal cancer by upregulating IL-8, and the IL-8 receptor profoundly affects the tumor microenvironment, increasing the proliferation, survival, and migration of vascular endothelial cells and the permeability of endothelial cells.396
6.5 N7-methylguanosine
m7G is the RNA methylation of guanine at the N7 position, which primarily occurs in the cap region of mRNAs with RNAs and tRNA internals.397 The METTL1/WDR4 complex appears to be the “writer” in m7G modification.398 Although METTL1 is mainly responsible for mediating m7G methylation, WDR4 has more favorable binding sites on RNA for stabilization.399 In cancer, METTL1 is frequently overexpressed in inducing oncogenic cell transformation. Arg-TCT-4-1, the m7G-modified tRNAs, can increase translation of AGA codon-rich mRNAs and control tumorigenesis and growth, and the upregulated phenotype of tRNA–Arg–TCT-4-1 has been shown to be a METTL1/WDR4 overexpression phenotype.400, 401 A missense mutation in WDR4 may be the causative variant in microcephalic primordial dwarfism, a neurological disorder with facial deformities and brain malformations.394 Furthermore, WDR4 expression levels were significantly reduced in hippocampal tissues of Down syndrome mice,402 and overexpression of WDR4 promoted cognitive function and hippocampal plasticity.403 Mutations in METTL1/WDR4 cause neurodevelopmental disorders through multiple mechanisms.404 Furthermore, in head and neck squamous cell carcinoma, METTL1 accelerates cancer progression by promoting the translation of cell cycle proteins and related oncogenes through the PI3K/AKT/mTOR signaling pathway.405 The METTL1/WDR4 complex continues to promote oncogene translation and regulates the progression of cell cycle-related proteins in lung cancer through a codon-dependent manner.406 Hence, targeting m7G to inhibit oncogene translation would be a potential antitumor strategy. WBSCR22/TRMT112 is another significant m7G methyltransferase complex.407, 408 As an m7G writer, WBSCR22 has been reported to be involved in organ regeneration and enhancing glucocorticoid receptor function in lung cancer.404, 409, 410 In addition, WBSCR22 has been shown to affect tumor cell survival and drug resistance in a variety of cancers, including breast cancer, melanoma, and colorectal cancer.411-414 TRMT112 is a cofactor of WBSCR22, maintains its stability and aids in m7G modification installation.407, 415 The information concludes that m7G modifications and related methyltransferases function in regulating disease development and drug resistance.
6.6 Pseudouridine
The C5-glycoside isoform of uridine, Ψ, deposits in both coding and noncoding regions of RNA and is highly conserved among species.416 Ψ in tRNA contributes to its structural stabilization with inhibition of protein synthesis.417, 418 Possibly, since C-C is more inert than C-N, Ψ’s “reader” and “writer” proteins are less detectable than other modifications.∖Mutations in the DKC1 enzyme in the Ψ-mediated catalytic mode by RNA-dependent mechanisms are associated with aberrant protein translation and have been reported to be upregulated in a variety of cancers.407, 419 Impaired telomerase activity and gene expression in the absence of DKC1 activity leads to decreased proliferation and survival of cancer cells.420 DKC1 is significantly upregulated in colorectal cancer tissues, acting as a critical regulator for cancer cell proliferation. Three hundred eighty-three thousand three hundred eighty-four rRNA mutants are very common in colorectal cancer,421 so ψ modification of reduced ribosomes may affect translation in cancer cells. Ψ can regulate the misreading of mRNA, and might have a unique application in the development of RNA drugs.
6.7 N4-acetylcysteine
ac4C is currently the only type of acetylation modification in eukaryotic RNAs, mainly occurring on tRNAs and rRNAs and, to a lesser extent, mRNAs.422 ac4C in tRNA helps to maintain translational stability and plays a role in thermophilic bacteria for their heat resistance.423, 424 ac4C is vital in the diagnosis and treatment of cancer. Studies have found that cancer patients have a more pronounced increase in ac4C than standard nucleosides and that ac4C levels are significantly lower after tumor resection.425 In addition to cancer diagnostics, ac4C has a regulatory role in bladder, gastric, and colorectal cancers.426, 427 N-acetyltransferase 10 (NAT10) exhibits acetyltransferase and RNA binding activity and can catalyze ac4C modification. NAT10-mediated acetylation improves mRNA stability and translational efficiency.428 High expression of NAT10 is necessary for the tumorigenic properties of bladder cancer. Regulation of ac4C via NAT10 involves proliferation, migration, and stem cell properties of bladder cancer cells.429, 430 In gastric cancer, ac4C acts as the inducer of hypoxia tolerance through glycolytic mechanisms. NAT10 receives regulation of a critical transcription factor HIF-1α in the hypoxia response, which triggers the activation of the HIF-1 pathway and regulates ac4C to enhance hypoxia tolerance in gastric cancer cells.431 NAT10 mediates an increase in ac4C modification and promotes the binding of polysaccharides, which enhances translational efficiency and induces central sensitization for neuropathic pain.432 Knockdown of NAT10 significantly reduced macrophage inflammatory responses, whereas overexpression resulted in the opposite result. It has been demonstrated that NAT10 regulates macrophage inflammatory responses through the NOX2–ROS–NF-κB pathway.433 The abundance of ac4C modifications in lncRNA significantly differed in AD as obtained by highly repetitive sequence analysis.434 These studies highlight the critical role of ac4C in cancer development by controlling various pathological events, such as glucose metabolism reprogramming and cancer hypoxia.
6.8 2ʹ-O-methylation
2ʹ-O-methylation is the main methylation of the 2′-OH portion of the ribose and is widely present in tRNA, rRNA and mRNA in mammalian cells.435 Absence of 2′-O-methylation in vertebrate development leads to severe morphological defects and embryonic lethality.422, 436 Fibrillarin is a 2′-O-RNA methyltransferase located in the dense proto-fiber component of the nucleolus accumbens.437 Fibrillarin is an essential nucleolin protein involved in pre-rRNA processing and regulates rRNA transcription, and it is overexpressed in different cancers.438 2ʹ-O-methylated modification in ncRNAs is involved in colorectal carcinogenesis.439 Among these ncRNAs, SNORD11B functions in cancer proliferation and invasion and increases the processing and maturation of 18S rRNA by introducing 2ʹ-O-methylation on 18S rRNA G509 site. lncRNA ZFAS1 is significantly overexpressed in several human malignant tumors, including colorectal, hepatocellular, and gastric cancers, and knockdown of ZFAS1 inhibits cancer cell proliferation and migration and increases apoptosis.440 Overexpression of NOP58 reverses this inhibition of ZFAS1 and promotes 2ʹ-O-methylation.441 Another small nucleolar RNA, SNORD104, can be knocked down by antisense oligonucleotide in Ishikawa cells to reduce proliferation and migration in endometrial cancer.442 In this study, SNORD104 is found to bind to the 2′-O-methyltransferase fibrillarin and increases PARP1 2′-O-methylation.442 In malignant melanoma and acute myeloid leukemia, 2ʹ-O-methylation has also been recognized as a new target for treatment.443, 444 Loss of function of FTSJ1, a characteristic 2′-O-methyltransferase targeting tRNA, has been identified as the cause of nonsyndromic X-linked intellectual disability.445, 446 Another 2′-O-methyluridine methyltransferase, TRMT44, is associated with idiopathic epilepsy.447 It still demands more studies to understand the role of 2ʹ-O-methylation in preventing and treating diseases.
6.9 LLPS in RNA modifying enzymes
Many RNA modifications have their own “reader,” “writer,” and “eraser” proteins that play an important role in the relationship between previously mentioned RNA modifications and diseases. For example, YTHDC1 undergoes LLPS requiring m6A, and the nuclear YTHDC1–m6A condensates maintain cell survival and the undifferentiated state of acute myeloid leukemia cells.324 Possibly, m6A acts as a bridge between LLPS and RNA to fine-tune the subcellular localization of RNA to implement or even modify its functions in controlling cancer cells' occurrence, proliferation, apoptosis, and metastasis.178 Proteins with disordered structure are likely to undergo LLPS or have some involvement in forming membraneless organelles. As the m1A “writer” protein TRMT6/61A is significantly enriched in SG-chelated mRNAs, it was shown that TRMT6/61A methyltransferase localizes to SGs under stress and protects mRNAs during heat shock.448 ALKBH5 specifically binds to proteins in the paraspeckles and forms phase-separated droplets that bind to the paraspeckles through its C-terminal IDR.317 In a hypoxic environment, proteins in the paraspeckles before the upregulation of ALKBH5 expression are induced with its rapid condensation to promote the formation of paraspeckles, reflecting an essential role in hypoxia induction.448 ADAR1 is localized to SGs formed in response to arsenite-induced oxidative stress or long dsRNA transfection.449 METTL3 has a large number of disordered structures that are not only capable of LLPs independently but also of stabilizing METTL14 to bind constitutively to each other and form heterodimers. In addition, certain LLPS behaviors of METTL3 cancer mutants have now been observed.306 DNMT2 can relocate from the nucleus to SGs and participate in the protein processing of RNA.450 In sum, many RNA-modifying enzymes harbor IDRs that must be explored in their association with LLPS.
6.10 Relationship between RNA modifications and LLPS
The phase boundaries of condensates can be controlled by changing RNA length, sequence, structure, modifications, and RNA–RNA and RNA–protein interactions.255 The use of RNA–RNA and RNA–protein interactions to influence RNA-driven LLPS realizes that it is possible to use RNA to control condensates, such as the LLPS regulation of m6A.451 The RNA modifications can alter LLPS behaviors by refining RNA–RNA and RNA–protein interactions.
As mentioned earlier, membrane-free fluidic regions play a role in many cellular functions; they are often enriched in RNA and influence the transcriptome. For example, nuclear speckles, which are significant regulators of gene expression and contain proteins and RNAs involved in gene expression, play a role in some cancers, and speckles, working together with p53, can directly enhance gene activity. Speckle-associated p53 target genes are more likely to be involved in tumor suppressor functions, such as stopping cell growth and triggering cell suicide, than other p53 target genes.452, 453 Among them, nuclear speckles or splicing speckles, also called interchromatin granule clusters, are meaningful RNA-containing membraneless chambers that function as sites for RNA splicing and RNA m6A methylation. LLPS of these compartments selects biomolecules to become concentrated, presumably to implement their functions.454, 455 Phase-separated multivalent interactions are mediated by scaffold proteins with tandem repeat binding sites with other partners or proteins with IDRs (or low-complexity structural domains), often in conjunction with nucleic acids.253 The m6A can be recognized by the YTH structural domain for LLPS in the IDR, and the LLPS potential can be further facilitated by multiple m6A modifications to the RNA.261
The YTH family of binding proteins of m6A consists of a m6A-binding YTH domain of approximately 15 kD and a low complexity domain of approximately 40 kD.298 Under the action of LLPS, some low-complexity structures (e.g., YTHDF family) form protofibrils, hydrogels or droplets.149 The heated sample of YTHDF proteins becomes turbid when warmed to 37°C, indicating that the warming causes lower critical solution temperature LLPS. Adding as low as 10% glycerol and reducing the salt concentration decreases the YTHDF2 concentration required for the LLPS transition to 1−8 µM.261 These results demonstrate that YTHDF2 LLPS is enhanced by increasing protein concentration but weakened by salt. Furthermore, studies reveal that polymethylated m6A-RNA provides a scaffold to juxtapose multiple YTHDF proteins, causing these proteins to undergo LLPS through interactions between their low-complexity domains.261 It possibly demonstrates that m6A-RNA binding to the YTH domain regulates LLPS.261
YTHDF proteins localize to neuronal RNA granules, P-bodies, and SGs, each of which is considered to be a phase-separated compartment in the cytosol raising the possibility that LLPS may govern the localization of these proteins and potentially m6A-mRNA. It is well known that nearly all m6A formation in mRNA is catalyzed by the METTL3–METTL14 heterodimeric methyltransferase.456 METTL14 knockout has been conducted to examine the effect of m6A-mRNA on YTHDF2 protein localization to SGs and P-bodies. Results reflected that METTL14 knockout did not alter the formation of SGs, but there was less YTHDF2 relocalization in SGs.261 In addition, the YTHDF2 mutant that lacks m6A binding exhibited less relocalization to SGs in wild-type cells, indicating that binding to m6A mRNA is necessary for YTHDF2 to partition into SGs efficiently. YTHDF2 does not enrich the P-body in the cells with METTL14 knockdown, suggesting that YTHDF2 being included in P-bodies to form complexes is accompanied by m6A-mRNA. In SGs, m6A-mRNA occupies a higher percentage than in total cellular mRNA, further confirming that polymethylated m6A RNAs play an important role in LLPS.
Additionally, it is found that, unlike other forms of RNA-scaffolded LLPS, m6A regulates the fate of cytoplasmic m6A through the scaffold YTHDF protein, leading to the formation of phase-separated YTHDF-m6A-mRNA complexes that then partition into phase-separated structures in cells.261 This series of experiments by Rise et al. demonstrated that m6A regulates the fate of mRNA by scaffolding YTHDF proteins to form phase-separated YTHDF–m6A–mRNA complexes. The presence of m6A enhances RNA–RNA and RNA–protein interactions, targeting different intracellular condensates. Furthermore, O-GlcNAcylation modification can occur on YTHDF1 and YTHDF3 to modulate the stability, assembly and disassembly of SGs.457 The regulatory mechanism of O-GlcNAcylation in RNA m6A methylation highlights additional complexity to the posttranscriptional regulation function of LLPS.
Investigation shows that the LLPS of YTHDC1 and 3′ end processing factor FIP1L1 can be enhanced by binding to the m6A sites, probably playing a crucial role in their interaction and APA regulation.458 LLPS of YTHDC1 also undergoes liquid-like condensates in highly active enhancers marked by m6A-enhancer RNAs.451 YTHDC1/m6A-enhancer RNAs condensates can further facilitate BRD4 coactivator condensates formation to implement enhancer activation and gene transcriptional control.451 Considering these essential findings, m6A might provide a regulatory LLPS mechanism based on m6A multivalency, potentially a new strategy for cancer treatment.
RNA is a crucial component of phase-separated cellular compartments, and large amounts of protein-free mRNA can be released from multimers into the cytoplasm under stress conditions.459 Free RNA is susceptible to aberrant interactions with misfolded proteins and undergoes protein accumulation, inducing the formation of irreversible RNA–protein coaggregation. Participation of m1A in mRNA granulation and protection during stress via TRMT6/61A methyltransferase.14 Strong binding of TDP-43 to m1A stimulates cytoplasmic mislocalization and formation of TDP-43 gel-like aggregates.460 TDP43 promotes stemness in breast cancer stem cells, and deletion of TDP43 inhibits the progression of triple-negative breast cancer.461, 462 m7G is predominantly present in mRNA processing and protein synthesis. Within mRNAs, m7G can be selectively recognized by quiver proteins (QKIs). m7G regulates mRNA stability and translation under stress conditions, and the bound complex QKI7 interacts with SGs core proteins (via the C-terminus) and shuttles internally. Through extensive experimental validation, Zhao et al.15 found that QKIs can regulate target mRNA metabolism and cellular drug resistance by binding m7G. Also, A-to-I editing has been shown to be associated with alterations in the interaction sites of proteins involved in LLPS.16 However, there are still plenty of unknowns in investigating the relationship between RNA modifications and LLPS. The phenomenon and underlying mechanism of many RNA modifications in LLPS have not yet been discovered.
6.11 RNA modifications regulate LLPS to affect disease development
As mentioned above, m6A enhances the LLPS potential of mRNA, which in turn regulates various metabolic processes in organisms.261 Considering that both LLPS and m6A might participate in multiple pathological progression, it would be reasonable to hypothesize that LLPS can be regulated by m6A to control disease development.
Human papillomavirus (HPV), a diverse family of small double-stranded DNA viruses, is the major risk factor for various types of cancers, including cervical cancer, head and neck cancer, and nonmelanoma skin cancer. The E7 protein encoded by high-risk HPVs contributes to HPV-associated carcinogenesis through multiple signaling pathways. Viral E7 mRNA contains modified m6A that IGF2BP1 stabilizes in HPV-infected cells.463 Heat treatment has been reported to reverse HPV-associated tumorigenesis both in vitro and in vivo, which enhances IGF2BP1 aggregation depending on the presence of m6A-modified E7 mRNA to generate obvious m6A E7 mRNA–IGF2BP1 granules,463 indicating that controlling LLPS is a possible cancer therapeutic strategy in the m6A context. RNA driving IGF2BP1 LLPS has also been documented in several research studies.464, 465 IGF2BP1 is an important m6A modulatory gene that has always increased in many malignant tissues. RBM15 contains IDR and potentially forms “liquid-like” condensates in vivo and in vitro.466 RBM15 condensates are prone to contact or partially overlap with nuclear speckles rather than other membraneless chambers.466 Most importantly, RBM15 condensates promote the m6A modification of the transcripts of highly expressing cancer-related genes and are partially colocalized with m6A-modified transcripts in the nucleus.466 In myeloid leukemia, m6A is found to be required for YTHDC1 to undergo LLPS, generating nuclear YTHDC1–m6A condensates to maintain cell survival and myeloid leukemic undifferentiated state.324 METTL3 can also undergo LLPS, and its self-interacting ability provides the necessary multivalency for LLPS of mRNA m6A methyltransferase complex.306 Researchers also investigated the LLPS behavior of METTL3 cancer mutants, including R508H, R415C, and E516K, finding that R415C and E516K mutants fail to undergo LLPS separation.306 Because the METTL3 has been documented to function in many types of cancers,329, 334 modulating its LLPS behavior may be a potentially critical facet for inhibiting cancer progression.
Many cancer-related genes can undergo LLPS and be regulated by m6A modification. Low-complex domain RNA-binding protein FUS can mediate LLPS.467 FUS is overexpressed in prostate adenocarcinoma, and patients with FUS overexpression show a low survival rate. Increased abundance of m6A modification promotes FUS expression to enhance P53 and Gpx4 expression to promote the proliferation of prostate adenocarcinoma.468 m6A-modified RNAs have been shown to prevent cytoplasmic TLS/FUS aggregation induced by hyperosmotic stress.469 SGs in U2OS cells induced by NaAsO2 treatment are enriched of m6A modified mRNA, and m6A binding of YTHDF proteins enhances SG formation.17 YTH domain family proteins, including YTHDF and YTHDC subtypes, have been reported to function in several types of cancers.470, 471 YTHDF2 has an essential regulatory role in various cancers (Figure 6),472-474 and acts as either an oncogene or tumor suppressor gene in different cancers depending on context.475 Generally, YTH domain family proteins play an essential role in tumor proliferation, invasion, migration, metabolism, and apoptosis.
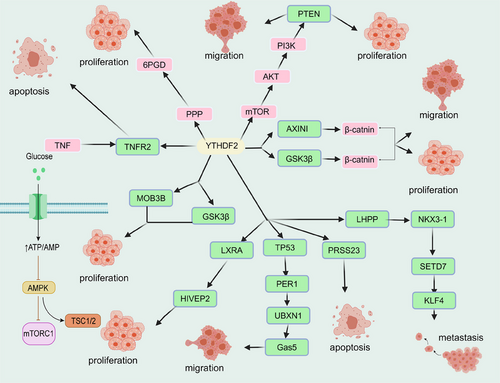
In addition, it has been noted that SG formation can be beneficial for the survival of stressed cells, and persistent induction of SGs can cause cell death. SG assembly can be attenuated by circRNA–CREIT to activate the RACK1/MTK1 apoptosis signaling pathway in doxorubicin-resistant triple-negative breast cancer and combinatorial treatment of SG inhibitor ISRIB and doxorubicin would synergistically suppress the growth of doxorubicin-resistant triple-negative breast cancer.476 In another report, chemotherapy-driven SGs have been shown to disassemble by glucocorticoids,477 suggesting that controlling SGs assembly can increase cancer cell chemosensitivity. However, a study has argued that m6A modification in mRNAs plays a minimal role, if any, in mRNA recruitment to SGs.478 Generally, m6A modification might modulate LLPS to regulate the development of cancer-representative diseases under certain circumstances. However, more LLPS phenomena and their precise mechanism should be further exploited to benefit disease therapy.
7 IMPLICATIONS FOR DISEASE TREATMENT
7.1 Phase-separated polymers can be used for diagnostics
Errors at any step of cell division might lead to aberrance in chromosome replication. Meanwhile, rearrangements and aneuploidy of the genome might occur in steps, such as chromosome condensation, breakdown of sister chromatids, and individualization of chromosomes as spatial segregates. Therefore, it is reasonable to apply phase-separated polymers for disease diagnostics.
The expression of human Ki-67 protein strictly correlates with cell proliferation and can be used as a value-added marker of tumor cells.479 Only antigens can be localized in the nucleus during inter-LLPS cell division, and proteins would be precisely localized on chromosomes during mitosis. The Ki-67 protein presents during all active cell cycle phases (G1, S, G2, and mitosis). It is absent from resting cells (G0), making it an excellent marker for determining the so-called growth fraction of a given cell population.480 Ki-67 acts as a space, electrostatic charge barrier, or surfactant by phase-separating individual mitotic chromosome arms.481 Generally, modulation by LLPS has the potential to enhance cancer diagnosis. To utilize LLPS behaviors for tumor diagnostics, real-time tracking and mapping of the numerous endogenous proteins at once in living cells is critical. Recently, TransitID has been applied to unbiasedly map endogenous proteome trafficking among cytosol and different organelles in living cells, including nucleolus and SGs.482 Such a powerful approach for distinguishing protein populations even under the occurrence of LLPS can be conducive to developing precision medicine.
7.2 SGs can be targets for preventing disease progression and therapeutic resistance
Untranslated mRNPs bind over weak interactions and form cores or droplets that aggregate into SGs.483, 484 Normally, SGs are in dynamic equilibrium with polyribosomes, whereas pharmacological intervention or stress disrupts this equilibrium and blocks translation by inhibiting translation initiation.484, 485 It has been demonstrated that SGs regulate the body's behavior in various cancers, including lung, breast, pancreatic, and gastric cancer (Figure 7).486-493 Particularly, SGs show strong antiapoptotic effects in pancreatic cancer.494 Breast cancer cell lines containing SGs exhibit lower sensitivity and less apoptosis when exposed to drugs.495 Generally, the formation of SGs would help the cancer cell to cope with the encountered stress to survive. Several studies have shown that some mRNA molecules contained in SGs can undergo a complete translation cycle and participate in and regulate various cellular biological processes.496 SGs-related components are upregulated in multiple tumor cells, including G3BP1 and G3BP2. In lung cancer, G3BP1 has been shown to negatively regulate the p53 tumor suppressor gene by interacting with lncRNA to suppress apoptosis.497, 498 In non-small cell lung cancer, a member of the TRIM protein family named MG53 has been revealed to modulate G3BP2 activity to inhibit SGs formation and suppress cancer progression.499 In addition, SGs may also be triggered in the high-pressure tumor microenvironment, where SGs coordinate the cellular response to the harsh cellular environment. Targeting SGs by interfering with SGs formation or assembly and altering stress conditions can affect tumor progression and sensitize cancer cells to chemotherapeutic agents.500 Sodium arsenite induces production-dependent SGs, participates in the regulation of apoptosis, protects cells in response to stress, inhibits apoptosis, and promotes cell survival.499, 501 Silvestrol-induced production of nondependent SGs impairs cellular resistance to stress, reduces cell survival, and promotes apoptosis.502 Formation of SGs can contribute to the establishment of doxorubicin resistance, and circRNA–CREIT attenuates doxorubicin-induced SGs formation via the PKR/eIF2α axis in triple-negative breast cancer.476 In fact, many widely used chemotherapeutics have been found to induce SGs formation to drive cancer cells to obtain chemoresistance. SGs regulate tumor proliferation, apoptosis, invasion and drug resistance by participating in various tumor-related signaling pathways.503, 504 mTOR is a critical signaling pathway that regulates cellular biological behavior, and studies have found that upregulation of mTOR promotes SGs formation in cancer cells.501, 505 Mutations in the RAS genes (K-RAS, N-RAS, and H-RAS), the most frequently mutated genes in tumor cells, regulate the biosynthesis and catabolism of lipid signaling molecules, and contribute to the formation of SGs.499, 506 Therefore, engineering these pathways to destroy the aberrant formation of SGs and impaired SGs disassembly can contribute to eliminate the pathological phenomena in cancer.
As one of the most representative membraneless chambers, SGs also have an essential role in the pathophysiology of neurodegenerative diseases.507 SGs in neurons appear to be different from SGs that are generated rapidly after stress, possibly due to the presence of cytoplasmic factors that inhibit LLPS, such as posttranslational modifications, protein chaperones, specific cytoskeletal proteins, and so on.485 Neurodegenerative diseases with their slower pressure changes may also make the body's regulatory mechanisms more adaptable.147 Mutations in genes encoding pathologic aggregation proteins such as TDP43 and FUS lead to excessive accumulation of SGs in cells exposed to stress.508, 509 The adaptive response and accumulation of SGs to transient stress is fundamentally susceptible to aggregation-based disease, and this high local concentration of increased amyloidogenic interactions would lead to the formation of persistent pathological oligomers. Targeted SGs could be used as a new option for treating neurodegenerative diseases. Studies have demonstrated the therapeutic benefit of inhibiting SGs-associated RNA-binding proteins. Knockdown of Atxn2 in an animal model of ALS overexpressing TDP43 increased the median lifespan of the animals by 80%,510 and inhibition of T1A1 prolonged survival of tau mice by 26%.511 In addition, targeting the neural pathways involved in SGs, including the mTOR pathway and the eIF2α pathway, is also considered as a potential therapeutic means.
7.3 RNA enzymes act as potential anticancer drug targets
RNA methylation is catalyzed by nucleoside methyltransferases, which act as critical proteins in a variety of RNA modifications (writers, erasers, and readers). Recently, developing anticancer epigenetic drugs has become a hot topic in cancer therapy research.512, 513 METTL3 is a well-known m6A methyltransferase and is upregulated in multiple tumors and metastatic tissues. Elvitegravir, an anti-HIV drug, directly targets METTL3 to prevent metastasis of esophageal squamous cell carcinoma.514 Actually, small-molecule STM2457 has been designed to inhibit METTL3 and show antitumor ability against acute myeloid leukemia.515 Etrazepam, a thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia and aplastic anemia, has been shown to act as a potential METTL3 inhibitor.516 In addition, renal inflammation and programmed cell death associated with the administration of cisplatin were attenuated in the presence of METTL3 silencing.517 METTL14 displays extremely high methyltransferase activity and is a crucial RNA-binding scaffold that is essential in recognizing substrate RNAs. The SOCS family of proteins is involved in the negative feedback regulation of cytokine signaling, which disrupts cytokine downstream signaling and leads to the development of inflammatory and autoimmune diseases and malignant tumors.518 METTL14 can inhibit the proliferation and metastasis of colorectal cancer by targeting oncogenic lncRNA XIST.519 METTL14 is both a promoter and a tumor suppressor in cancer, and activators and inhibitors targeting METTL14 have recently been reported. METTL14 affects many biological processes and plays multiple roles in cancer,520 making it a potential drug target. ALKBH5, a demethylase of m6A, is involved in the induction of cancer immune escape and adaptive immune responses.521 Since ALKBH5 has both cancer-inhibitory and cancer-promoting roles, methods to induce and inhibit ALKBH5 are a viable therapeutic option.522 In addition, ALKBH5 demonstrated a regulatory role in renal impairment. ALKBH5 knockout mice exhibit less pathologic injury and better renal function, and the imposition of the ALKBH5 inhibitor IOX1 promotes Ccl28 modification to inhibit inflammatory cells.523 ALKBH5 has a high potential targeting capacity, and compounds, including small molecule modulators and protein hydrolysis-targeted chimeras function at targeting the ALKBH5 regulatory mechanism.524, 525 Fibrillarin in 2ʹ-O-methylation has been reported to be a favorable target against tumors because it is an autoantibody for various cancers.446 Fibrillarin as a crucial constitutive protein affecting ribosome abundance and composition, offers significant possibilities for targeting key ribosome biogenesis components to reduce genotoxic activity in cancer cells.526 Investigating inhibitors targeting RNA modifications for the application of diseases is promising, but it is necessary to consider the role of LLPS during treatment as discussed above.
7.4 Targeting LLPS in drug design and delivery
Transcriptional control affects nearly all biological processes and events, including cancer initiation, proliferation, metastasis, recurrence, and drug resistance. LLPS has been observed to contribute to the maintenance of cellular homeostasis. Therefore, regulating the LLPS in forming SQSTM1/p62, NRF2, or FIP200 condensates would be conducive to recovering cellular homeostasis and preventing cancer progression. Furthermore, LLPS is also connected to cancer chemotherapeutic resistance, especially for involving with the formation of SGs. For example, cisplatin has been shown to suppress the formation of SGs.527 Meanwhile, future drug delivery system designs should focus on utilizing LLPS to enhance drug concentration and precision on targets. Based on some of the current applications of LLPS in drug delivery, precise delivery of drugs by RNA phase change to form polymers is possible.
Traditional drug development typically involves drugs that bind to and inhibit pathogenic targets, targeting proteins, and RNA via small molecule or RNA Phase modulators based on their propensity to phase separate.255, 528 Phase modulators can directly target RNA or proteins in IDR.529 In general, IDR are generally considered nondruggable therapeutic targets because they do not display stable, well-defined binding sites. Depending on the screening of inhibitors of protein–protein interactions and phenotypic screening, a small molecule targeting the inherently disordered region of p53 interaction with MDM-2 has been developed.530 YTHDF1 can bind to ribosomal proteins to facilitate translation of the mRNAs it targets,531 providing a direction for this protein to be used in targeted drug development.
Numerous anomalous phase-separated drug screening and development platforms have been established to screen phase-separated targeted drugs. In 2023, Wang et al.532 established DropScan, which could perform extensive screening and multidimensional analysis, identifying LY2835219, a condensate that solubilizes Ewing sarcoma fusions. Through this recognition of phase separation and the application of deep learning techniques, this platform could, in the future, screen suitable drugs in neurodegenerative, cardiovascular diseases, and so on.
Currently, there are also several applications of LLPS in drug delivery.533, 534 Regarding drug protection and degradability, Feng et al.535 used LLPS coupled with sodium alginate beads to enable drug release downstream in the gastrointestinal tract. The use of LLPS to deliver drugs, forming LLPS droplets to insulate their internal drug from physicochemical properties such as temperature, can lead to the design of LLPS systems capable of transporting active protein cascades to specific locations in human patients.536 In 2020, research validates the drug delivery potential of LLPS condensates by successfully delivering myoglobin to human mesenchymal stem cells using characteristics such as the noncytotoxic nature of the condensates and their spontaneous interaction with cell membranes.537 Nevertheless, LLPS that might impair or even reverse the destination is one of the prospects for designing and delivering cancer drugs.
8 CONCLUSION AND OUTLOOK
LLPS drives the assembly of multiple membraneless chambers of concentrated protein and RNA molecules through multivalent interactions. Multiple combinations between multivalent interactions driving LLPS transitions can lead to abnormal agglutination and amyloid formation and subsequently contribute to neurodegenerative disease, carcinogenesis, cancer progression, and therapeutic resistance.
Although the mechanism of LLPS in neurodegenerative diseases is not yet apparent, the link between abnormal phase separation and the pathology of several proteins has been well documented. LLPS concerning gene fusions and mutations can also affect cancer through many pathways, such as SPOP–DAXX bodies, PML vesicles and FET fusion proteins. Aberrant chromosome condensation generated by LLPS can induce tumorigenesis, and controlling the integrity of the nuclear microenvironment via LLPS can be tailored for cancer therapy. However, it should be noted that LLPS is just a biophysical phenomenon, and its functions in disease-promoting or inhibiting are background dependent.
In addition to regulating nuclear structure, LLPS has a regulatory role in the transcription and translation. RNAs are a major constituent of several types of phase-separated condensation, especially in RNA granules. Many condensates described to date contain mRNA, ncRNA, small molecules, and enzymes that can be used to modulate pathologic condensates or hard-to-administer targets. A variety of RNA modifications are either up- or downregulated in cancer and are involved in regulating the development of cancer cell fate. RNA modification is also involved in various diseases including cardiovascular disease, neurodevelopment and reproduction. Some of these modifications are observed to phase-segregate in the formation of SGs. Regulatory RNA metabolizing enzymes with a large number of IDR regions are now available as oncogenic modulators and as new therapeutic targets. In recent years, the development of anticancer RNA therapeutics, including in vitro transcribed mRNA drugs, RNA vaccines, ASOs, and saRNA, has rapidly evolved.538-540
By discussing the close connection between LLPS, RNA modification, and diseases, it is believed that modulating RNA biology with LLPS as an alternative strategy for cancer treatment has a great outlook. However, the current research on LLPS mainly focus on proteins. Due to the scarcity of studies on LLPS with RNA modifications, it is impossible to draw arbitrary conclusions. More endeavors should be paid to enrich the understanding of the role of RNA, especially RNA modifications, in LLPS to contribute to disease treatment.
AUTHOR CONTRIBUTIONS
Xinyue Zhang, Lin Yuan, Wanlu Zhang, Yi Zhang, Chunting Li, Qun Wu, and Yongye Huang participated in different parts of writing. Lin Yuan, Yongye Huang, and Min Wu composed and edited the manuscript. Xinyue Zhang and Wanlu Zhang illustrated the figure and artwork in consultation with coauthors. The article has received approval from all authors.
ACKNOWLEDGMENTS
We thank members in our laboratory for helpful discussion. This study was funded by the National Natural Science Foundation of China (Nos. 82201594, 81502582). Funding was also provided by the Fundamental Research Funds for the Central Universities (N182004002, N2220002), Natural Science Foundation of Liaoning Province (2021-MS-104, 2022-YGJC-39, 2022-MS-228), Fundamental Scientific Research Fund of Liaoning Provincial Education Department (LJKQZ2021002), Key Laboratory of Bioresource Research and Development of Liaoning Province (2022JH13/10200026), National Key Research Project of MOST (2023YFA0915000), Fund of the Affiliated Wenzhou Hospital of Zhejiang Traditional Chinese Medical and Pharmaceutic University, and Wenzhou Institute University of Chinese Academy of Sciences.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




