Phospholipids with two polyunsaturated fatty acyl tails: an important driver of ferroptosis
In a recently published study in Cell,1 a group led by Brent R. Stockwell performed a detailed investigation of the relationship between Phospholipids harboring PUFA tails at both the sn1 and sn2 positions (PL-PUFA2s) and ferroptosis. They found that dietary PL-PUFA2s could induce the initiation and propagation of lipid peroxidation that contributed to ferroptosis (Figure 1).
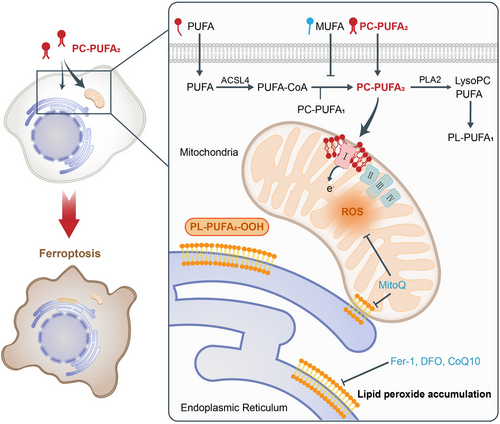
Ferroptosis is an iron-dependent non-apoptotic form of cell death and excess iron has been reported to promote phospholipid peroxidation and mitochondrial dysfunction.2 PLs containing one PUFA tail (PL-PUFA1s) are characterized by high abundance within mammalian cells and the presence of di-acyl allyl groups within the PUFA tails renders them susceptible to oxidation. Therefore, PL-PUFA1s are believed to actively participate in lipid peroxidation, leading to ferroptosis. However, a recent discovery revealed that protecting PL-PUFA2s, rather than PL-PUFA1s, from oxidative degradation could confer ferroptosis resistance to cells.3 This interesting finding caught the attention of Qiu et al. and they subsequently conducted substantial experiments to explore the function of PL-PUFA2s in cell death.
Initially, Qiu et al. proved there existed a strong correlation between PL-PUFA2s and ferroptosis. They observed that although many types of PLs were capable of causing cell death, phosphatidylcholines (PCs), especially PC-PUFA₂s, exhibited the most specific ability to induce ferroptosis among them. Previous studies showed that accumulation of oxidized PE-PUFA precipitated ferroptosis. However, in this study, the supplementation of PE-PUFA was less effective than PC-PUFA in inducing ferroptosis. The authors suggested that this may stem from the difficult uptake of exogenous PE-PUFA, coupled with its heightened cytotoxicity. The elevation of ferroptosis biomarkers, accrual of lipid peroxides, and observed synergistic effects with established ferroptosis inducers further corroborated the ferroptosis-inducing potential of PL-PUFA2s. Moreover, liquid chromatography (LC)-mass spectrometry (MS)-based targeted lipidomics substantiated the correlation between the abundance of PC-PUFA2s and cellular susceptibility to ferroptosis. In aging and degenerative diseases, iron accumulation contributes to vulnerability to lipid oxidation and consequential ferroptosis. The authors found that PL-PUFA2 was the main substrate for lipid oxidation in the hippocampus of senescent mice and the brain tissue of patients with Huntington's disease, indicating that the apparent depletion of PL-PUFA2s might serve as a marker of ferroptosis.
Then, Qiu et al. explored the role of exogenous PL-PUFA2s in cellular systems. By extracting and analyzing lipids from the cells treated with PL-PUFA2s, they found that in contrast to PL-PUFA1s, exogenous PL-PUFA2s exhibited enhanced stability within the cellular environment, potentially explaining the higher ferroptosis inducibility of exogenous PL-PUFA2s. Furthermore, by employing isotope labeling of the fatty acyl tails of exogenous PC-PUFA2s, researchers observed their successful incorporation into cellular membranes, instigating lipid remodeling processes.
Previous research revealed that free PUFAs promoted ferroptosis while free monounsaturated fatty acids (MUFAs) inhibited ferroptosis, but the mechanism was unclear. In this study, Qiu et al. provided one proper explanation by conducting experiments. The co-treatment with free PUFAs had a larger synergistic effect with PC-PUFA1s than PC-PUFA2s. Based on this phenomenon, they postulated that the interaction between PC-PUFA1s and free PUFAs may result in the formation of PC-PUFA2s, thereby exacerbating cell death. This hypothesis was partially substantiated by a marked increase in the content of PC-PUFA2s in cells treated with free PUFAs. Another finding that free MUFAs decreased the levels of PC-PUFA2s demonstrated that MUFAs hampered the synthesis of PC-PUFA2s. Additionally, the authors proved that PC-PUFA2s were proximal mediators of ferroptosis.
The above results prompted Qiu et al. to explore the mechanism underlying PC-PUFA2s-induced ferroptosis. Employing the affinity pull-down assay with MS, they observed that PL-PUFA2s exhibited a specific binding affinity towards mitochondrial electron transport chain (ETC) complex I, in alignment with outcomes derived from gene set enrichment analysis (GSEA). Numerous studies have indicated mitochondrial phospholipid composition, particularly cardiolipin (CL) and Ether phospholipids, had a significant impact on the function of ETC complex I. However, investigations into the interaction of PLs with specificity in the tail with complex I are still scant. It is conceivable that PL-PUFA2s possess the capability to bind to specific sites within the structural domains of complex I, akin to CL, and cause protein conformational changes, ultimately resulting in increased reactive oxygen species (ROS) production. Subsequently, the co-staining experiment and LC-MS analysis revealed a heightened accumulation of exogenous lipids within both the mitochondria and the endoplasmic reticulum (ER). This observation may result from the prevalence of endogenous PL-PUFA2 trafficking mechanisms within mitochondria, a phenomenon hitherto found in yeast but not yet identified in mammalian cells. These investigations collectively underscored the pivotal role of mitochondria as a significant site for the accumulation of PC-PUFA2s, further emphasizing their involvement in the induction of ferroptosis.
Therefore, the authors assessed the potential impact of PC-PUFA2s accumulation on mitochondrial physiology. The presence of PC-PUFA2s resulted in elevated levels of mitochondrial ROS (mtROS), accompanied by a decline in mitochondrial membrane potential and dampening of mitochondrial respiration. This finding underscored the ability of PC-PUFA2s to induce mitochondrial oxidative stress. However, the addition of other ETC complex inhibitors caused mitochondrial oxidative stress but failed to induce ferroptosis, and a similar phenomenon appeared in one prior research.4 They collectively suggest the significant role of phospholipids and in this study PL-PUFA2 might induce mtROS production, concurrently serving as the substrate for mtROS oxidation. Consequently, Qiu et al. evaluated the role of mtROS in PC-PUFA2-induced ferroptosis. The utilization of mitochondria-targeted ROS scavengers effectively mitigated lipid peroxidation and ferroptosis, demonstrating the significance of mtROS. Moreover, the potency of PC-PUFA2s in inducing ferroptosis was diminished in cells with reduced mitochondrial content. However, in these cells, spare accumulation of PC-PUFA2s within organelles such as the ER might serve as the primary initiator of the lethal effect.
Co-staining of cells with fluorescent probes targeting lipid peroxides along with organelle markers revealed a notable increase in lipid peroxide levels after treatment with PC-PUFA2s, particularly in ER. This demonstrated that ER was the principal site of lipid peroxidation. However, further experiments did not find any evidence of ER stress following treatment with PC-PUFA2s.
Qiu et al. pioneered the elucidation of the significant ability of dietary PL-PUFA2s to induce ferroptosis. Their seminal work revealed the mechanism through which dietary PL-PUFA2s, especially PC-PUFA2s, promote ferroptosis (Figure 1). Numerous PC-PUFA2s are absorbed by cells and subsequently undergo lipid remodeling. Intriguingly, these PC-PUFA2s predominantly accumulate in mitochondria and interact with ETC complex I, thereby elevating mtROS levels. Consequent mitochondrial peroxide production precipitates the accumulation of lipid peroxidation within the ER through mitochondria-ER contact sites, ultimately inducing ferroptosis. In addition, the authors found that PL-PUFA1 could bind to peroxisome-related proteins. Considering PUFA has been proven to interact and activate the peroxisome proliferator-activated receptors (PPARs),5 we speculate that a similar mechanism may exist for PL-PUFA1, which needs to be further explored.
Although Qiu et al. have outlined a foundational framework for understanding the ontogeny of ferroptosis induced by dietary lipids, particularly PL-PUFA2s, there exist several challenges that must be addressed by future studies. These include the specific pathways through which dietary MUFAs modulate the synthesis of PL-PUFA2s in vivo, the exact binding site of PC-PUFA2s within ETC complex I, and so on.
In summary, this latest research has significantly advanced our understanding of the intricate interplay between ferroptosis and lipid metabolism. Additionally, PL-PUFA2 may be established as the discerning marker for evaluating cellular vulnerability to ferroptosis.
AUTHOR CONTRIBUTIONS
C.L. wrote the manuscript and prepared the figure. X.Z. provided valuable discussion. L.Z. approved the final version of the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by Chinese National Natural Science Funds (31925013 and U20A20393), and a special program from the Ministry of Science and Technology of China (2021YFA101000).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
Not applicable.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




