Physiological and genomic features of Paraoceanicella profunda gen. nov., sp. nov., a novel piezophile isolated from deep seawater of the Mariana Trench
Graphical Abstract
A novel piezophilic alphaproteobacterium, strain D4M1T, was isolated from deep seawater of the Mariana Trench. The combined evidence shows that it represents a novel species of a novel genus in the family Rhodobacteraceae, for which the name Paraoceanicella profunda gen. nov., sp. nov. is proposed. The complete genome contained 5,468,583-bp with a G + C content of 70.2 mol% and contained 4,855 protein-coding genes and 78 RNA genes. Genomic analysis revealed abundant clues on bacterial high-pressure adaptation and piezophilic lifestyle.
Abstract
A novel piezophilic alphaproteobacterium, strain D4M1T, was isolated from deep seawater of the Mariana Trench. 16S rRNA gene analysis showed that strain D4M1T was most closely related to Oceanicella actignis PRQ-67T (94.2%), Oceanibium sediminis O448T (94.2%), and Thioclava electrotropha ElOx9T (94.1%). Phylogenetic analyses based on both 16S rRNA gene and genome sequences showed that strain D4M1T formed an independent monophyletic branch paralleled with the genus Oceanicella in the family Rhodobacteraceae. Cells were Gram-stain-negative, aerobic short rods, and grew optimally at 37°C, pH 6.5, and 3.0% (w/v) NaCl. Strain D4M1T was piezophilic with the optimum pressure of 10 MPa. The principal fatty acids were C18:1 ω7c/C18:1 ω6c and C16:0, major respiratory quinone was ubiquinone-10, and predominant polar lipids were phosphatidylglycerol, phosphatidylethanolamine, and an unidentified aminophospholipid. The complete genome contained 5,468,583-bp with a G + C content of 70.2 mol% and contained 4,855 protein-coding genes and 78 RNA genes. Genomic analysis revealed abundant clues on bacterial high-pressure adaptation and piezophilic lifestyle. The combined evidence shows that strain D4M1T represents a novel species of a novel genus in the family Rhodobacteraceae, for which the name Paraoceanicella profunda gen. nov., sp. nov. is proposed (type strain D4M1T = MCCC 1K03820T = KCTC 72285T).
1 INTRODUCTION
The deep sea, accounting for approximately 75% of the total ocean volume and hosting 62% of the global biosphere (Fang, Zhang, & Bazylinski, 2010), is a reservoir of remarkably diverse archaea and bacteria. The extreme physical–chemical factors (high salinity, high pressure, and low temperature) in the deep sea may have considerable influences on microbial life. For example, high pressure, the most unique physical parameter in the deep sea, decreases membrane permeability and stability, impedes energy metabolism, and inactivates proteins (Jebbar, Franzetti, Girard, & Oger, 2015; Picard & Daniel, 2013). Thus, piezophiles must evolve physiological and genomic adaptations to grow under high-pressure conditions. Microorganisms use different strategies to thrive in high-pressure conditions, such as synthesizing piezolytes, improving permeability and stability of cell membrane, regulating gene expression, and modifying genome features (Oger & Jebbar, 2010; Simonato et al., 2006). Despite the fact that greater than 88% of the ocean's biosphere is above 10 MPa (water depths of 1,000 m or more), a limited number of piezophiles have been isolated and characterized (Picard & Daniel, 2013; Zhang, Wu, & Zhang, 2018).
During our recent campaign of investigating the diversity of culturable microbes in the deep ocean, we isolated a novel piezophilic bacterium D4M1T, which was closely related to the species in the family Rhodobacteraceae within the class Alphaproteobacteria. The family Rhodobacteraceae (type genus, Rhodobacter) contains more than 130 genera (www.bacterio.net/), many members of which have been isolated from the marine environment (Albuquerque, Rainey, Nobre, & da Costa, 2012; Chang et al., 2018; Chang, Meng, Du, & Du, 2019). Additionally, some members have been isolated from deep-sea environment, such as members belonging to the genera Acidimangrovimonas (Jiang, Xu, Shao, & Long, 2014), Brevirhabdus (Wu et al., 2015), Celeribacter (Lai, Cao, Yuan, Li, & Shao, 2014), Citreicella (Lai et al., 2011), Marinibacterium (Li, Lai, et al., 2015), Meridianimarinicoccus (Ren et al., 2019), Pararhodobacter (Lai, Liu, Yuan, Xie, & Shao, 2019), Profundibacterium (Lai et al., 2013), and Thiobacimonas (Li, Tang, Liu, & Jiao, 2015). In this study, the marine bacterial strain D4M1T was characterized using a polyphasic approach, along with the genome sequence analysis and high-pressure adaptation.
2 MATERIALS AND METHODS
2.1 Strains and culture conditions
A deep seawater sample was collected at a depth of 10,890 m from the Mariana Trench (142.4°E, 11.4°N; site MT) in December 2016. The sample (1 ml) was serially diluted with 10 ml sterilized natural seawater and spread onto a selective D4 agar medium (1.0 L seawater, 0.2 g yeast extract, 3.0 g HEPES, 2.0 g xylose, 17.0 g agar, and pH 7.0) under atmospheric pressure. Subsequently, a white-colored strain D4M1T was isolated by restreaking single colonies onto D4 agar plates at 10°C. The strains grew well on marine agar 2216 (MA; BD Difco) or in marine broth 2216 (MB; BD Difco) medium and were routinely cultivated on MA or MB in this study, unless noted otherwise. Stock cultures were stored at −80°C with 20% (v/v) glycerol. The phylogenetically related type strains, Oceanicella actignis DSM 22673T (=PRQ-67T), Thioclava electrotropha DSM 103712T (=ElOx9T), and Oceanibium sediminis MCCC 1H00233T (=O448T), were obtained from the Leibniz Institute DSMZ–German Collection of Microorganisms and Cell Cultures (DSMZ) and Marine Culture Collection of China (MCCC), respectively.
2.2 DNA extraction, genomic, and phylogenetic analyses
Genomic DNA was extracted from liquid cultures of strain D4M1T after being cultivated in MB for 36 hr using the ChargeSwitch® gDNA Mini Bacteria Kit (Life Technologies) according to the manufacturer's instructions. The 16S rRNA gene of strain D4M1T was amplified and sequenced by using conserved primers Bac8F (5′-AGAGTTTGATCATGGCTCAG-3′) and U1492R (5′-GGTTACCTTGTTACGACTT-3′), as reported previously (Cao et al., 2016). The 16S rRNA gene sequence was identified using global alignment algorithm implemented at the EzBioCloud server (https://www.ezbiocloud.net/; (Yoon et al., 2017)). Phylogenetic analysis of 16S rRNA gene was conducted with MEGA 5.0 package (Tamura et al., 2011), using the Kimura two-parameters model with the neighbor-joining (Saitou & Nei, 1987) and maximum-likelihood (Felsenstein, 1981) algorithms, respectively. The tree topology was calculated by bootstrap analysis based on 1,000 bootstraps.
Purified genomic DNA was quantified by TBS-380 fluorometer (Turner BioSystems Inc.). The complete genome was sequenced using a combination of Pacific Biosciences (PacBio) RS and Illumina sequencing platforms (Shanghai Majorbio Bio-pharm Technology Co., Ltd.). For PacBio sequencing, 8–10 k insert whole-genome shotgun libraries were generated and sequenced on a PacBio RS instrument using standard methods. For Illumina sequencing, 500 bp paired-end library were generated and sequenced using Illumina Hiseq Xten. The genome was assembled using Velvet assembler (v1.2.09) with a kmer length of 17 and “PacBioToCA with Celera Assembler” pipeline (Chin et al., 2013; Koren et al., 2012) with both the PacBio reads and Illumina reads. The genome sequences of Thioclava electrotropha Elox9T (NBXF00000000), Thioclava pacifica DSM 10166T (AUND00000000), Rhodobacter megalophilus DSM 18937T (FZOV00000000), Rhodobacter johrii JA192T (PZZW00000000), Paenirhodobacter enshiensis DW2-9T (JFZB00000000), Oceanicella actignis CGMCC 1.10808 (FRDL00000000), and Oceanibium sediminis O448T (QGNX00000000) were obtained from the NCBI website. The Oceanicella actignis DSM 22673T genome sequence (IMG Genome ID: 2593339287) was downloaded from the Genome portal of the Joint Genome Institute (JGI) (http://genome.jgi.doe.gov/). The genomic DNA G + C content was estimated from the genome sequence. A whole-genome-based phylogenetic tree was reconstructed based on the whole-genome protein sequences using CVTree3 (http://tlife.fudan.edu.cn/cvtree/cvtree/) with K-value = 6 (Zuo & Hao, 2015). The genomic analyses were performed as described previously (Cao, Lai, Yuan, & Shao, 2015) using the tools available on the Integrated Microbial Genomes (IMG) server (https://img.jgi.doe.gov) (Chen et al., 2019).
2.3 Phenotypic, physiologic, and biochemical analyses
Images of cells of strain D4M1T were obtained with a transmission electron microscopy (JEM-1230; Jeol) after glutaraldehyde prefixation and uranyl acetate staining of cells grown on MA at 37°C for 30 hr. Growth characteristics were determined by the measurement of optical density at 600 nm (OD600) using a NanoDrop 2000c spectrophotometer (Thermo Scientific). The growth temperature was evaluated at 4, 10, 20, 25, 30, 37, 40, 45, and 50°C in duplicates in 10 days. The salinity range (0, 0.5, and 1%–10% (intervals of 1%) of NaCl, w/v) and pH range (pH 4.0–11.0 (intervals of 1 unit), added with 20 μmol/L HOMOPIPES, MES, PIPES, HEPES and CAPS buffers, respectively) were investigated as previously described in duplicates (Lai et al., 2014). Gram-staining, oxidase, and catalase activity were carried out according to the test procedures described by Dong and Cai (2001). Growth under anaerobic condition was tested in LB liquid medium (for fermentation) and in LB supplemented with Na2SO4 or NaNO3 (10 mmol/L, for anaerobic respiration) with oxygen-free N2 gas phase (200 kPa) in sealed sterile vials at 37°C for 7 days. Poly-β-hydroxybutyrate (PHB) production was determined by using Nile blue A staining and an upright fluorescence microscope (ECLIPSE Ni-U; Nikon) according to a previous study (Ostle & Holt, 1982). Determination of the hydrostatic pressure range for growth was carried out in hydrostatic pressure vessels under a pressure range of 0.1–80 MPa (intervals of 10 MPa) at the optimal temperature (37°C), with oxygen-saturated Fluorinert (FC-40, 3M Company. 25% of total volume) added to supply oxygen (Kato, Sato, & Horikoshi, 1995). Other biochemical tests were carried out using API 20NE, API ZYM strips (bioMérieux) and GEN III microplates by Biolog system (Biolog Microstation™) according to the manufacturer's instructions. Some tests in API strips, such as reduction of nitrate, fermentation of D-glucose, hydrolysis of aesculin, and utilization of citrate, were also re-examined by conventional biochemical identification as described by Dong and Cai (2001).
2.4 Chemotaxonomic analysis
The fatty acid and polar lipid profiles of strain D4M1T were analyzed on exponential growth phase of cultures grown in MB at 37°C for 48 hr. Fatty acids in whole cells were saponified, extracted, and methylated using the standard protocol of Microbial IDentification Inc. (MIDI, Sherlock Microbial Identification System, version 6.0B). The fatty acids were analyzed by gas chromatography (GC, Agilent Technologies 6850) and identified by using the TSBA 6.0 database of the Microbial Identification System (Sasser, 1990). Polar lipids were extracted from 100 mg of freeze-dried cells using a chloroform/methanol system, separated by two-dimensional thin-layer chromatography (TLC) on silica gel 60 F254 plates (Merck), and then identified with molybdophosphoric acid as the spray reagent according to a previously described method (Tindall, Sikorski, Smibert, & Krieg, 2007). The fatty acid and polar lipid profiles of reference strains Oceanicella actignis DSM 22673T and Thioclava electrotropha DSM 103712T were performed in parallel with strain D4M1T under the same condition. The respiratory quinone was extracted from freeze-dried cells with chloroform/methanol (2:1, v/v) and evaporated to dryness at 35°C. The extracts were resuspended in chloroform/methanol (2:1, v/v) and subsequently purified by TLC on GF254 silica gel plates (Branch of Qingdao Haiyang Chemical Co. Ltd.) with n-hexane/ether (17:3, v/v). The respiratory quinones were measured by HPLC-MS system (Agilent) (Wu et al., 2015).
3 RESULTS AND DISCUSSION
3.1 Phylogenetic and phylogenomic analyses
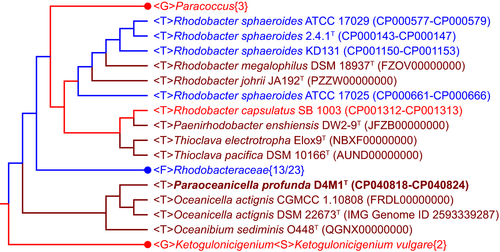
16S rRNA gene sequence analysis showed that strain D4M1T had the highest 16S rRNA gene sequence similarity of 94.2% with Oceanicella actignis PRQ-67T and Oceanibium sediminis O448T, followed by Thioclava electrotropha ElOx9T (94.1%). Genera are generally described as agglomerates of nodal species and internodal strains (Gillis, Vandamme, De Vos, Swings, & Kersters, 2001), for which similarity values around 94.5%–95% are commonly used for genus differentiation (Ludwig et al., 1998; Yarza et al., 2014). Based on these criteria, strain D4M1T likely represent a novel genus in the family Rhodobacteraceae. Phylogenetic analysis based on 16S rRNA gene sequence showed that strain D4M1T formed an independent monophyletic branch paralleled with the genus Oceanicella within the family Rhodobacteraceae, suggesting that it may represent a novel genus within the family Rhodobacteraceae (Figure 1 and Figure A1).
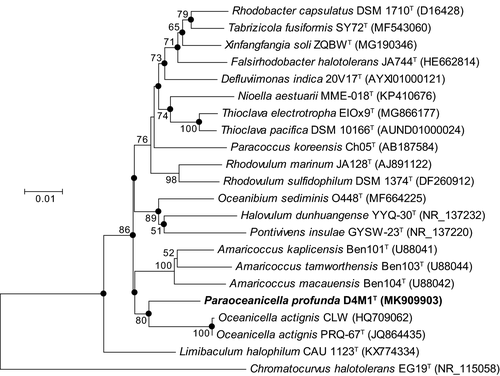
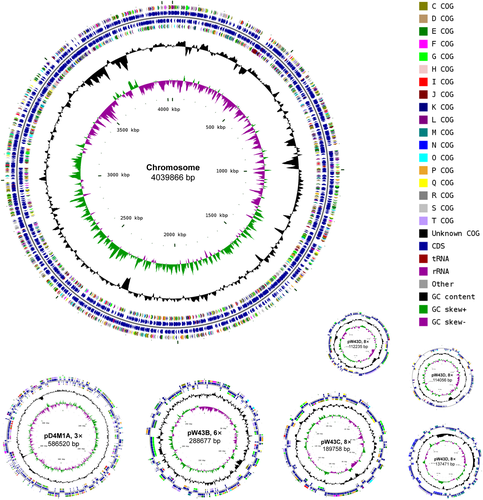
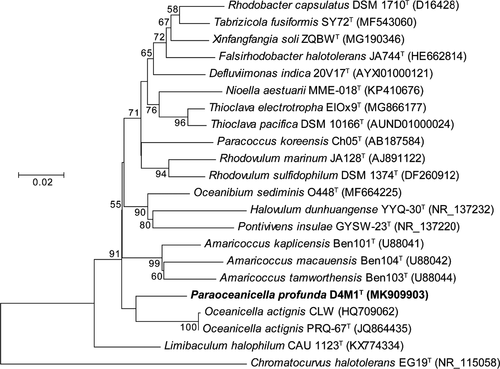
Phylogenomic analysis, previously suggested to provide a better taxonomic framework at the genus and higher levels (Chun et al., 2018), was further carried out to provide a better taxonomic characterization. A total of 2.36 Gb of clean data were generated from the genome sequencing of D4M1T. The final assembly has 431-fold coverage for the complete genome, which contains 5,468,583-bp with a G + C content of 70.2 mol%. The complete genome consists of a circular chromosome of 4,417,125 bp and six plasmids ranging from 112,235 bp to 586,520 bp in length (Table 1 and Figure 2). The assembled and annotated genome of D4M1T has been deposited in GenBank (accession numbers: CP040818–CP040824) and JGI portal (GOLD ID: Gp0432545; IMG Taxon ID: 2828513066). A whole-genome-based phylogenomic tree (Figure 3) showed that strain D4M1T formed an independent monophyletic branch within the family Rhodobacteraceae. This result supports that strain D4M1T represents a genus-level taxon in agreement with the result of 16S rRNA gene phylogeny.
| Content | Chromosome | Plasmids | |||||
|---|---|---|---|---|---|---|---|
| pD4M1A | pD4M1B | pD4M1C | pD4M1D | pD4M1E | pD4M1F | ||
| Size (bp) | 4039866 | 586520 | 288677 | 189758 | 137471 | 114056 | 112235 |
| G + C content (mol%) | 70.0 | 71.0 | 71.5 | 71.7 | 71.1 | 61.2 | 71.4 |
| Protein-coding genes | 3588 | 525 | 241 | 171 | 111 | 124 | 95 |
| Average gene size (bp) | 940 | 967 | 1037 | 1001 | 1028 | 809 | 1011 |
| Coding density (%) | 83.5% | 86.6% | 86.6% | 90.2% | 83.0% | 88.0% | 85.6% |
| Gene assigned to COG | 2996 | 462 | 211 | 154 | 74 | 84 | 76 |
| tRNA | 54 | 3 | 0 | 0 | 0 | 0 | 0 |
| rRNA operon (23S, 16S and 5S) | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| ncRNA | 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| GenBank accession | CP040818 | CP040819 | CP040820 | CP040821 | CP040822 | CP040823 | CP040824 |
3.2 Morphology and physiology properties
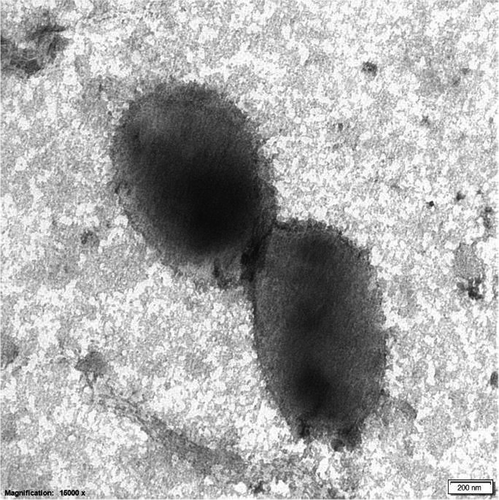
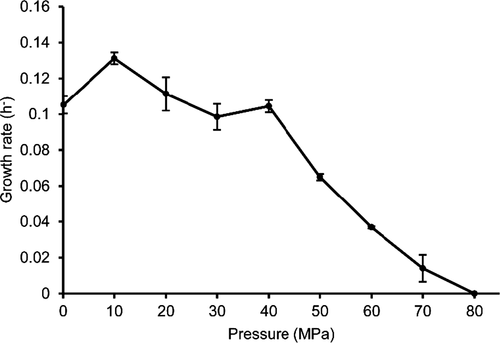
Cells of strain D4M1T are Gram-stain-negative, oxidase- and catalase-positive, aerobic, short rods (1.0–1.5 × 0.6–0.8 μm, Figure A2). Growth of the novel strain occurs between pH 5.0–8.0 (optimum 6.5), 10–45°C (optimum 37°C), and in the presence of 0.5%–8.0% (w/v) NaCl (optimum 3.0%). The novel strain contains poly-β-hydroxybutyrate (PHB) inside the cells. Strain D4M1T is piezophilic, with the optimum growth pressure of 10 MPa and tolerance up to 70 MPa (Figure A3). Anaerobic growth was not observed in LB medium nor in LB medium supplemented with 10 mmol/L of Na2SO4 or NaNO3. Results of carbon utilization (Biolog GEN III), API ZYM and 20NE tests are given in Table 2 and the species description below. Strain D4M1T is distinguishable from their closest relatives in physiological characteristics as shown in Table 2.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Growth at 10°C | + | − | + | − |
| Optimum temperature | 37 | 50 | 35 | 37 |
| Growth in 8% NaCl | + | + | + | − |
| Growth at pH 5 | + | − | − | − |
| G + C content (mol %) | 70.2 | 72.3 | 63.8 | 65.8 |
| Enzyme activity | ||||
| Lipase (C14) | w | + | − | + |
| α-Chymotrypsin | + | − | − | − |
| α-Galactosidase | − | − | + | + |
| β-Galactosidase | − | − | + | + |
| β-Glucuronidase | − | − | + | + |
| α-Glucosidase | w | − | + | + |
| β-Glucosidase | − | − | + | + |
| Utilization of: | ||||
| D-Glucose | w | − | − | w |
| L-Arabinose | w | − | + | + |
| D-Mannose | − | − | w | + |
| D-Mannitol | + | − | + | + |
| D-Maltose | − | − | + | + |
| Potassium gluconate | + | − | + | + |
| Malic acid | w | − | + | + |
| Trisodium citrate | − | − | + | + |
| D-Galactose | + | − | + | + |
| 3-Methyl glucose | − | + | w | − |
| L-Rhamnose | − | + | − | − |
| D-Sorbitol | + | − | + | + |
| D-Aspartic acid | − | + | − | + |
| Glycyl-L-proline | − | + | + | − |
| L-Arginine | − | + | − | + |
| L-Aspartic acid | − | + | + | + |
| L-Pyroglutamic acid | − | + | − | − |
| p-Hydroxy-phenylacetic acid | + | − | + | − |
| D-Lactic acid methyl ester | − | + | − | − |
| L-Lactic acid | + | + | − | + |
| Bromo-succinic acid | + | − | + | − |
| Tween 40 | − | + | − | − |
| α-Hydroxy-butyric acid | − | + | + | − |
| Sensitive to: | ||||
| Lincomycin | − | − | − | + |
| Guanidine HCl | + | + | − | + |
Note
- Strains: 1, strain D4M1T; 2, Oceanicella actignis DSM 22673T; 3, Thioclava electrotropha DSM 103712T; 4, Oceanibium sediminis MCCC 1H00233T. All data were experimentally determined in this study under the same conditions. Characteristics are scored as: +, positive; -, negative; w, weakly positive.
3.3 Fatty acids, polar lipids, and quinone composition
The predominant fatty acid of strain D4M1T was summed feature 8 (41.7%, C18:1 ω7c/C18:1 ω6c) and C16:0 (36.9%) (Table A1). There were obvious differences in fatty acid profile between strain D4M1T and reference strains DSM 22673T and DSM 103712T. C18:1 ω7c/C18:1 ω6c were present in a much higher amount in reference strains DSM 22673T and DSM 103712T than in strain D4M1T, but the amount of C16:0 was much lower in the reference strains than in strain D4M1T.
The major isoprenoid quinone of strain D4M1T was ubiquinone 10 (Q-10), which was the same as its related taxa in the family Rhodobacteraceae (Albuquerque et al., 2012; Y. Q. Chang, Meng, Du, & Du, 2019; Lai et al., 2014). The polar lipids of strain D4M1T consisted of phosphatidylglycerol (PG), phosphatidylethanolamine (PE), an unidentified aminophospholipid (PN), an unidentified glycolipid (GL), and several unidentified phospholipids (PL) as shown in Figure A4, which were similar to those of reference strains DSM 22673T and DSM 103712T, except some minor differences in unidentified phospholipids.
3.4 Genome annotation and analysis
The genome was shown to encode 4,942 predicted genes including 4,855 protein-coding genes, 12 rRNAs (four 5S rRNA, four 16S rRNA, and four 23S rRNA), 57 tRNAs, and 9 ncRNAs. Complete genome analysis revealed that the 4,855 protein-coding genes constituted 98.2% of the total genes in the genome, but only 79.3% were predicted with functions. Furthermore, there were 4,057 genes (82.1%) assigned to 24 different clusters of orthologous groups (COGs, Table A2), 1,489 genes (30.1%) connected to KEGG pathways, and 1,106 genes (22.4%) connected to MetaCyc pathways.
Analysis of the complete genome indicated the presence of different genes that are most likely linked to life at high pressure. Microbes are thought to preserve membrane fluidization and functionality at high pressure and low temperature in the deep sea by increasing the proportion of unsaturated fatty acids in their membrane lipids (Cao et al., 2015; Simonato et al., 2006). Strain D4M1T contains high proportions of monounsaturated fatty acids, summed feature 8 (41.7%, C18:1 ω7c/C18:1 ω6c), probably for improving membrane piezo-adaptation. Genomic analysis showed the presence of thirty-seven genes involved in biosynthesis of unsaturated fatty acids, including four fatty acid desaturase genes (Table A3). Pressure-induced chaperones proposed to help in maintaining protein folding (Oger & Jebbar, 2010) were also encoded adjacent to the unsaturated fatty acids biosynthesis genes in D4M1T genome, including the OmpH which was thought to function as a nutrient transporter in nutrient-limited deep sea (Table A3).
It is well known that many piezophiles change their respiratory chains in order to adapt to pressure (Oger & Jebbar, 2010). The genome was found to contain genes encoding cytochrome bd-type quinol oxidase and cytochrome cbb protein complex (Table A3), which were involved in specific piezo-adaptations in respiratory chain (Chikuma, Kasahara, Kato, & Tamegai, 2007; Qureshi, Kato, & Horikoshi, 1998). F1F0 ATP-synthase was shown to facilitate energy-yielding processes in high-pressure adaptation (Souza, Creczynski-Pasa, Scofano, Graber, & Mignaco, 2004). It was remarkable that two sets of the F1F0 ATP-synthase genes were identified in the genome of strain D4M1T (Table A3).
Deep-sea bacteria were also found to accumulate protein-stabilizing solutes at high pressure, such as piezolytes β-hydroxybutyrate (β-HB) and oligomers of β-HB (Martin, Bartlett, & Roberts, 2002). PHB was detected in the cells of strain D4M1T in this study, and genes that encoded the enzymes required for β-HB and PHB synthesis were present in the genome, including 1 β-HB dehydrogenase and 3 polyhydroxyalkanoate synthase genes (Table A3). The PHB inside the cells could also serve as intracellular carbon and energy reserves, which have been linked to pressure adaptation (Martin et al., 2002; Methe et al., 2005). Genes involved in biosynthesis and transport of compatible solutes, such as glycine betaine, were also identified in the genome, including genes encoding choline dehydrogenase and transcriptional repressor BetI (Table A3). It was suggested that trehalose protects proteins and cellular membranes from inactivation or denaturation caused by a variety of stress conditions, including high hydrostatic pressure (Simonato et al., 2006). Nineteen genes in the genome were predicted to encode trehalose biosynthesis and trehalose-specific transporters (Table A3), which were probably involved in pressure adaptation.
Additionally, the genome of D4M1T has six copies of glnA, including the counterpart of the pressure-upregulated glnA (IMG Gene OID: 2828515862) in piezophile Shewanella violacea DSS12 (Ikegami, Nakasone, Kato, Nakamura, et al., 2000). Furthermore, the pressure-regulated regulator ntrBC in S. violacea DSS12 was also identified in the genome of D4M1T (Table A3), which was predicted to play a role in activation of transcription of pressure-regulated promoters (Ikegami, Nakasone, Kato, Usami, & Horikoshi, 2000).
The increasing number of rRNA operons in a bacterial genome was previously proposed to represent a strategy for adapting to specific selective pressures from the environment (Klappenbach, Dunbar, & Schmidt, 2000). The genome of the strain was found to contain four rRNA operons (Table 1), which may correlate with the adaptation to the deep-sea environment. Pressure is thermodynamically coupled to temperature. One “universal” response to environmental pressures is the biosynthesis of stress proteins (Kültz, 2005). The genome encoded 6 heat shock protein genes and 4 cold shock protein genes (Table A3), which were previously reported to be induced when exposed to high pressure (Simonato et al., 2006). Our results suggest that hydrostatic pressure is an important environmental stress that drives the adaptation of heat shock protein genes and cold shock protein genes in deep-sea microorganisms.
The genome analysis revealed insights into the piezophilic lifestyle of the novel isolate and provided a reference for further phylogenomic, comparative genomic, and functional studies of the relative species in the deep ocean. However, further specific experiments need to be addressed in the future to find out the precise function of the genes involved in high-pressure adaptation and the molecular adaptation mechanisms.
4 CONCLUSION
Strain D4M1T exhibits the typical characteristics of the family Rhodobacteraceae, but it is also distinguishable from its closest relatives in the phylogenetic analysis of 16S rRNA gene sequence, the phylogenomic analysis based on whole-genome protein sequences, the fatty acids profiles, the enzyme activities, the carbon utilization, the G + C contents, and the low 16S rRNA gene sequence similarity (≤95.8%) to the type species of the closely related genera of the family Rhodobacteraceae. Therefore, from the polyphasic evidence, strain D4M1T represents a novel species of a novel genus for which the name Paraoceanicella profunda gen. nov., sp. nov. is proposed.
4.1 Description of Paraoceanicella gen. nov
Paraoceanicella (Pa.ra.o.ce.a.ni.cel'la. Gr. prep. para, beside, alongside of; N. L. fem. n. Oceanicella, a bacterial generic name; N. L. fem. n. Paraoceanicella, a genus adjacent to Oceanicella).
Cells are aerobic, Gram-stain-negative, oxidase- and catalase-positive, short rods (1.0–1.5 × 0.6–0.8 μm). The G + C content of the genomic DNA of the type strain of the type species is 70.2 mol%. The predominant fatty acids are summed feature 8 (C18:1 ω7c/C18:1 ω6c), and C16:0. PG, PE, and an unidentified PN are the predominant polar lipids. Q-10 is the major isoprenoid quinone.
The type species is Paraoceanicella profunda.
4.2 Description of Paraoceanicella profunda sp. nov
Paraoceanicella profunda (pro.fun'da. L. adj. profunda from the deep).
Cells are aerobic, Gram-stain-negative, oxidase- and catalase-positive, short rods (1.0–1.5 × 0.6–0.8 μm). Growth occurs at salinities from 0.5% to 8.0% (optimum 3.0%), from pH 5.0 to 8.0 (optimum 6.5), and at temperatures between 10 and 45°C (optimum 37°C). Anaerobic growth does not occur in LB medium nor in LB medium supplemented with 10 mM of Na2SO4 or NaNO3. The optimum pressure for growth was 10 MPa with tolerance up to 70 MPa. Positive for nitrate reduction, alkaline phosphatase, esterase(C4), esterase lipase (C8), lipase (C14), leucine arylamidase, valine arylamidase, cystine arylamidase, α-chymotrypsin, acid phosphatase, naphthol-AS-Bl-phosphohydrolase, α-glucosidase, arginine dihydrolase, gelatin hydrolysis and urease activities; negative for trypsin, α-galactosidase, β-galactosidase, β-glucuronidase, β-glucosidase, beta-glucosidase (aesculin hydrolysis), N-acetyl-β-glucosaminidase, α-mannosidase, α-fucosidase, indole production, or D-glucose fermentation. Utilizes the following carbon sources: D-glucose, L-arabinose, D-sorbitol, D-mannitol, D-arabitol, malic acid, potassium gluconate, D-fructose, D-fructose-6-PO4, D-galactose, D-fucose, L-fucose, glycerol, L-alanine, L-glutamic acid, D-galacturonic acid, L-galactonic acid lactone, D-gluconic acid, D-glucuronic acid, glucuronamide, p-hydroxy-phenylacetic acid, methyl pyruvate, α-keto-glutaric acid, bromo-succinic acid, γ-amino-butyric acid, β-hydroxy-D,L-butyric acid, L-serine, glucuronamide, quinic acid, D-saccharic acid, L-lactic acid, acetoacetic acid, propionic acid, and acetic acid. The predominant fatty acid is summed feature 8 (C18:1 ω7c/C18:1 ω6c) and C16:0. Q-10 is the major isoprenoid quinone. The predominant polar lipids consist of PG, PE, and an unidentified PN. The G + C content of the genomic DNA is 70.2 mol%.
The type strain D4M1T (=MCCC 1K03820T = KCTC 72285T) was cultured from a deep-water sample obtained at a depth of 10,890 m of the Mariana Trench (142.4°E, 11.4°N; site MT). The 16S rRNA and genome sequences are submitted to GenBank under accession numbers MK909903 and CP040818–CP040824, respectively.
ETHICS STATEMENT
None required.
ACKNOWLEDGMENTS
The authors acknowledge all crews and the on-board colleagues of M/V Zhangjian for collecting the seawater samples of the Mariana Trench in December 2016. This study was funded by the National Key R&D Program of China (grant No. 2018YFC0310600) and the National Natural Science Foundation of China (41706146).
CONFLICT OF INTERESTS
None declared.
AUTHOR CONTRIBUTIONS
JC supervised the project. PL, WD, and QL carried out the experiments. LP and JC analyzed the data. PL, JC, and JF wrote the manuscript with support from RL, YW, LW, and ZX.
APPENDIX 1

| Fatty acids | 1 | 2 | 3 |
|---|---|---|---|
| C10:0 3-OH | — | — | 1.9 |
| iso-C11:0 3-OH | 1.6 | — | — |
| iso-C12:0 | 1.3 | 0.2 | 0.2 |
| C12:0 | — | — | 0.2 |
| Summed feature 2 | 3.7 | 2.5 | — |
| Summed feature 3 | 1.7 | 0.2 | 2.0 |
| C16:0 | 36.9 | 4.0 | 2.4 |
| C17:1 ω7c | 0.8 | — | — |
| C17:1 ω8c | — | 1.0 | 0.2 |
| C17:0 | — | 2.5 | 1.4 |
| Summed feature 8 | 41.7 | 65.1 | 82.7 |
| C18:0 | 7.5 | 10.0 | 6.0 |
| C18:1 ω7c 11-methyl | 3.5 | 10.2 | 0.6 |
| C18:0 3-OH | — | 2.9 | 0.4 |
Note
- Taxa: 1, strain D4M1T; 2, Oceanicella actignis LMG 25334T; 3, Thioclava electrotropha DSM 103712T; data of all strains were from this study under the same condition. Values are percentages of total fatty acids; —, not detected. *Summed features represent groups of two or three fatty acids which could not be separated by GLC with the MIDI system. Summed feature 2, C14:0 3-OH/iso-C16:1 I; summed feature 3, C16:1 ω7c/ω6c; summed feature 8, C18:1 ω7c/ω6c
| COG categories | Code | Gene count | Percent (%) |
|---|---|---|---|
| Amino acid transport and metabolism | E | 646 | 13.36 |
| Carbohydrate transport and metabolism | G | 334 | 6.91 |
| Cell cycle control, cell division, chromosome partitioning | D | 44 | 0.91 |
| Cell motility | N | 65 | 1.34 |
| Cell wall/membrane/envelope biogenesis | M | 219 | 4.53 |
| Chromatin structure and dynamics | B | 5 | 0.10 |
| Coenzyme transport and metabolism | H | 213 | 4.40 |
| Cytoskeleton | Z | 1 | 0.02 |
| Defense mechanisms | V | 93 | 1.92 |
| Energy production and conversion | C | 279 | 5.77 |
| Extracellular structures | W | 5 | 0.10 |
| Function unknown | S | 261 | 5.40 |
| General function prediction only | R | 542 | 11.21 |
| Inorganic ion transport and metabolism | P | 306 | 6.33 |
| Intracellular trafficking, secretion, and vesicular transport | U | 58 | 1.20 |
| Lipid transport and metabolism | I | 254 | 5.25 |
| Mobilome: prophages, transposons | X | 52 | 1.08 |
| Nucleotide transport and metabolism | F | 106 | 2.19 |
| Posttranslational modification, protein turnover, chaperones | O | 175 | 3.62 |
| Replication, recombination, and repair | L | 134 | 2.77 |
| Secondary metabolites biosynthesis, transport, and catabolism | Q | 225 | 4.65 |
| Signal transduction mechanisms | T | 193 | 3.99 |
| Transcription | K | 407 | 8.42 |
| Translation, ribosomal structure, and biogenesis | J | 219 | 4.53 |
| IMG gene OID | Locus tag | Protein |
|---|---|---|
| Biosynthesis of unsaturated fatty acids | ||
| 2828513318 | Ga0392526_253 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828513436 | Ga0392526_371 | Glycerol-3-phosphate acyltransferase PlsY |
| 2828513500 | Ga0392526_436 | 1-acyl-sn-glycerol-3-phosphate acyltransferase |
| 2828513541 | Ga0392526_477 | acyl-CoA thioesterase-1 |
| 2828513884 | Ga0392526_820 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828514031 | Ga0392526_967 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828514243 | Ga0392526_1179 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828514304 | Ga0392526_1240 | fatty acid desaturase |
| 2828514475 | Ga0392526_1411 | acyl transferase domain-containing protein/NADPH:quinone reductase-like Zn-dependent oxidoreductase/acyl carrier protein |
| 2828514527 | Ga0392526_1463 | enoyl-[acyl-carrier protein] reductase I |
| 2828514528 | Ga0392526_1464 | 3-oxoacyl-[acyl-carrier-protein] synthase-1 |
| 2828514529 | Ga0392526_1465 | 3-hydroxyacyl-[acyl-carrier protein] dehydratase/trans-2-decenoyl-[acyl-carrier protein] isomerase |
| 2828514544 | Ga0392526_1480 | acyl-CoA thioesterase YciA |
| 2828514651 | Ga0392526_1587 | fatty acid desaturase |
| 2828514809 | Ga0392526_1745 | fatty acid desaturase |
| 2828515056 | Ga0392526_1992 | enoyl-CoA hydratase |
| 2828515178 | Ga0392526_2114 | 1-acyl-sn-glycerol-3-phosphate acyltransferase |
| 2828515283 | Ga0392526_2219 | 3-hydroxyacyl-CoA dehydrogenase/enoyl-CoA hydratase/3-hydroxybutyryl-CoA epimerase |
| 2828515303 | Ga0392526_2239 | 1-acyl-sn-glycerol-3-phosphate acyltransferase |
| 2828515340 | Ga0392526_2276 | long-chain acyl-CoA synthetase |
| 2828515835 | Ga0392526_2771 | 3-hydroxyacyl-[acyl-carrier-protein] dehydratase |
| 2828515979 | Ga0392526_2915 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828516231 | Ga0392526_3167 | 3-oxoacyl-[acyl-carrier-protein] synthase II |
| 2828516233 | Ga0392526_3169 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828516304 | Ga0392526_3240 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828516395 | Ga0392526_3331 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828516464 | Ga0392526_3400 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828516468 | Ga0392526_3404 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828516565 | Ga0392526_3501 | Glycerol-3-phosphate acyltransferase PlsX |
| 2828516609 | Ga0392526_3545 | enoyl-[acyl-carrier protein] reductase I |
| 2828516730 | Ga0392526_3666 | enoyl-CoA hydratase |
| 2828516791 | Ga0392526_3727 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828516807 | Ga0392526_3743 | 3-oxoacyl-[acyl-carrier protein] reductase |
| 2828516926 | Ga0392526_3862 | enoyl-CoA hydratase |
| 2828517203 | Ga0392526_4139 | enoyl-[acyl-carrier protein] reductase I |
| 2828517211 | Ga0392526_4147 | fatty acid desaturase |
| 2828517574 | Ga0392526_4510 | 3-oxoacyl-[acyl-carrier protein] reductase |
| Chaperone | ||
| 2828513714 | Ga0392526_650 | molecular chaperone DnaK |
| 2828513716 | Ga0392526_652 | molecular chaperone DnaJ |
| 2828515281 | Ga0392526_2217 | Zn-dependent protease with chaperone function |
| 2828515832 | Ga0392526_2768 | regulator of sigma E protease |
| 2828515833 | Ga0392526_2769 | outer membrane protein insertion porin family |
| 2828515834 | Ga0392526_2770 | Skp family chaperone for outer membrane proteins, OmpH |
| 2828516688 | Ga0392526_3624 | HSP20 family molecular chaperone IbpA |
| 2828516690 | Ga0392526_3626 | molecular chaperone Hsp33 |
| Respiratory chain | ||
| 2828513683 | Ga0392526_619 | cytochrome d ubiquinol oxidase subunit I |
| 2828513684 | Ga0392526_620 | cytochrome d ubiquinol oxidase subunit II |
| 2828515783 | Ga0392526_2719 | cbb3-type cytochrome oxidase maturation protein |
| 2828515787 | Ga0392526_2723 | cytochrome c oxidase accessory protein FixG |
| 2828515788 | Ga0392526_2724 | uncharacterized membrane protein |
| 2828515789 | Ga0392526_2725 | cytochrome c oxidase cbb3-type subunit 3 |
| 2828515790 | Ga0392526_2726 | cytochrome c oxidase cbb3-type subunit 4 |
| 2828515791 | Ga0392526_2727 | cytochrome c oxidase cbb3-type subunit 2 |
| 2828515792 | Ga0392526_2728 | cytochrome c oxidase cbb3-type subunit 1 |
| F1F0 ATP-synthase | ||
| 2828514969 | Ga0392526_1905 | F1F0 ATP-synthase subunit delta |
| 2828514970 | Ga0392526_1906 | F1F0 ATP-synthase subunit alpha |
| 2828514971 | Ga0392526_1907 | F1F0 ATP-synthase subunit gamma |
| 2828514972 | Ga0392526_1908 | F1F0 ATP-synthase subunit beta |
| 2828514973 | Ga0392526_1909 | F1F0 ATP-synthase subunit epsilon |
| 2828515088 | Ga0392526_2024 | F1F0 ATP-synthase, membrane subunit b |
| 2828515089 | Ga0392526_2025 | F1F0 ATP-synthase, membrane subunit b |
| 2828515090 | Ga0392526_2026 | F1F0 ATP-synthase, membrane subunit c |
| 2828515091 | Ga0392526_2027 | F1F0 ATP-synthase, membrane subunit a |
| 2828515092 | Ga0392526_2028 | F1F0 ATP-synthaseprotein I |
| 2828516954 | Ga0392526_3890 | F1F0 ATP-synthase subunit beta |
| 2828516955 | Ga0392526_3891 | F1F0 ATP-synthase subunit epsilon |
| 2828516956 | Ga0392526_3892 | F1F0 ATP-synthaseprotein I |
| 2828516957 | Ga0392526_3893 | F1F0 ATP-synthase subunit 2 |
| 2828516958 | Ga0392526_3894 | F1F0 ATP-synthase subunit a |
| 2828516959 | Ga0392526_3895 | F1F0 ATP-synthase subunit c |
| 2828516960 | Ga0392526_3896 | F1F0 ATP-synthase subunit b |
| 2828516961 | Ga0392526_3897 | F1F0 ATP-synthase subunit alpha |
| 2828516962 | Ga0392526_3898 | F1F0 ATP-synthase subunit gamma |
| PHA/PHB synthesis | ||
| 2828513971 | Ga0392526_907 | 3-hydroxybutyrate dehydrogenase |
| 2828514023 | Ga0392526_959 | putative acetyltransferase |
| 2828514219 | Ga0392526_1155 | hydroxymethylglutaryl-CoA lyase |
| 2828514509 | Ga0392526_1445 | apolipoprotein N-acyltransferase |
| 2828514635 | Ga0392526_1571 | polyhydroxyalkanoate synthase |
| 2828514700 | Ga0392526_1636 | polyhydroxybutyrate depolymerase |
| 2828514800 | Ga0392526_1736 | poly(3-hydroxybutyrate) depolymerase |
| 2828514849 | Ga0392526_1785 | polyhydroxyalkanoate depolymerase |
| 2828514923 | Ga0392526_1859 | 3-hydroxyacyl-CoA dehydrogenase |
| 2828515161 | Ga0392526_2097 | acetoacetyl-CoA reductase |
| 2828515162 | Ga0392526_2098 | acetyl-CoA C-acetyltransferase |
| 2828515163 | Ga0392526_2099 | polyhydroxyalkanoate synthase |
| 2828515164 | Ga0392526_2100 | polyhydroxyalkanoate synthesis repressor PhaR |
| 2828515217 | Ga0392526_2153 | 3-hydroxybutyryl-CoA dehydrogenase |
| 2828515283 | Ga0392526_2219 | 3-hydroxyacyl-CoA dehydrogenase/enoyl-CoA hydratase/3-hydroxybutyryl-CoA epimerase |
| 2828515284 | Ga0392526_2220 | acetyl-CoA C-acetyltransferase |
| 2828515305 | Ga0392526_2241 | 3-hydroxybutyryl-CoA dehydratase |
| 2828515724 | Ga0392526_2660 | 3-hydroxybutyryl-CoA dehydrogenase |
| 2828516037 | Ga0392526_2973 | 3-oxoacyl-(acyl-carrier-protein) synthase/nodulation protein E |
| 2828516295 | Ga0392526_3231 | hydroxymethylglutaryl-CoA lyase |
| 2828516711 | Ga0392526_3647 | putative acetyltransferase |
| 2828516927 | Ga0392526_3863 | 3-hydroxybutyryl-CoA dehydrogenase |
| 2828517202 | Ga0392526_4138 | polyhydroxyalkanoate synthase |
| 2828517335 | Ga0392526_4271 | acetyl-CoA C-acetyltransferase |
| 2828517488 | Ga0392526_4424 | hydroxymethylglutaryl-CoA lyase |
| 2828517899 | Ga0392526_4835 | apolipoprotein N-acyltransferase |
| 2828517938 | Ga0392526_4874 | acetyl-CoA C-acetyltransferase |
| Glutamine synthesis and regulation | ||
| 2828513735 | Ga0392526_671 | glutamine synthetase |
| 2828514055 | Ga0392526_991 | glutamine synthetase |
| 2828514780 | Ga0392526_1716 | glutamine synthetase |
| 2828514889 | Ga0392526_1825 | glutamine synthetase |
| 2828515862 | Ga0392526_2798 | glutamine synthetase |
| 2828516007 | Ga0392526_2943 | ntrX two-component system, NtrC family, nitrogen regulation response regulator NtrX |
| 2828516008 | Ga0392526_2944 | ntrY two-component system, NtrC family, nitrogen regulation sensor histidine kinase NtrY |
| 2828516009 | Ga0392526_2945 | ntrC two-component system, NtrC family, nitrogen regulation response regulator GlnG |
| 2828516010 | Ga0392526_2946 | ntrB two-component system, NtrC family, nitrogen regulation sensor histidine kinase GlnL |
| 2828516194 | Ga0392526_3130 | glutamine synthetase |
| Betaine and trehalose biosynthesis and transport | ||
| 2828513193 | Ga0392526_128 | choline dehydrogenase |
| 2828513583 | Ga0392526_519 | choline dehydrogenase |
| 2828513584 | Ga0392526_520 | choline-sulfatase |
| 2828513585 | Ga0392526_521 | TetR/AcrR family transcriptional repressor of bet genes |
| 2828513586 | Ga0392526_522 | glycine betaine/proline transport system substrate-binding protein |
| 2828513587 | Ga0392526_523 | glycine betaine/proline transport system permease protein |
| 2828513588 | Ga0392526_524 | glycine betaine/proline transport system ATP-binding protein |
| 2828513835 | Ga0392526_771 | glycine betaine/proline transport system substrate-binding protein |
| 2828513836 | Ga0392526_772 | drug/metabolite transporter (DMT)-like permease |
| 2828513837 | Ga0392526_773 | DNA-binding Lrp family transcriptional regulator |
| 2828513838 | Ga0392526_774 | DNA-binding HxlR family transcriptional regulator |
| 2828514400 | Ga0392526_1336 | choline monooxygenase |
| 2828515533 | Ga0392526_2469 | BCCT family betaine/carnitine transporter |
| 2828516142 | Ga0392526_3078 | glycine betaine/proline transport system substrate-binding protein |
| 2828516143 | Ga0392526_3079 | glycine betaine/proline transport system permease protein |
| 2828516144 | Ga0392526_3080 | glycine betaine/proline transport system ATP-binding protein |
| 2828516539 | Ga0392526_3475 | choline dehydrogenase |
| 2828514833 | Ga0392526_1769 | Acetyltransferase (isoleucine patch superfamily) |
| 2828515886 | Ga0392526_2822 | glucosylglycerol-phosphate synthase |
| 2828516592 | Ga0392526_3528 | trehalose/maltose transport system substrate-binding protein |
| 2828516593 | Ga0392526_3529 | trehalose/maltose transport system permease protein |
| 2828516594 | Ga0392526_3530 | trehalose/maltose transport system permease protein |
| 2828516595 | Ga0392526_3531 | ABC-type sugar transport system ATPase subunit |
| 2828517438 | Ga0392526_4374 | trehalose 6-phosphate synthase |
| 2828517439 | Ga0392526_4375 | trehalose 6-phosphate phosphatase |
| 2828517440 | Ga0392526_4376 | (1->4)-alpha-D-glucan 1-alpha-D-glucosylmutase |
| 2828517441 | Ga0392526_4377 | 4-alpha-glucanotransferase |
| 2828517442 | Ga0392526_4378 | maltooligosyltrehalose trehalohydrolase |
| 2828517443 | Ga0392526_4379 | glycogen operon protein |
| 2828517444 | Ga0392526_4380 | 1,4-alpha-glucan branching enzyme |
| 2828517445 | Ga0392526_4381 | maltose alpha-D-glucosyltransferase/alpha-amylase |
| 2828517644 | Ga0392526_4580 | multiple sugar transport system ATP-binding protein |
| 2828517645 | Ga0392526_4581 | multiple sugar transport system permease protein |
| 2828517646 | Ga0392526_4582 | multiple sugar transport system permease protein |
| 2828517647 | Ga0392526_4583 | multiple sugar transport system substrate-binding protein |
| 2828517648 | Ga0392526_4584 | LacI family transcriptional regulator |
| Cold and heat shock proteins | ||
| 2828513475 | Ga0392526_411 | CspA family cold shock protein |
| 2828513528 | Ga0392526_464 | ribosome-associated heat shock protein Hsp15 |
| 2828513634 | Ga0392526_570 | heat shock protein HspQ |
| 2828514209 | Ga0392526_1145 | heat shock gene repressor HrcA |
| 2828514210 | Ga0392526_1146 | molecular chaperone GrpE (heat shock protein) |
| 2828514746 | Ga0392526_1682 | heat shock protein HtpX |
| 2828514758 | Ga0392526_1694 | CspA family cold shock protein |
| 2828515042 | Ga0392526_1978 | CspA family cold shock protein |
| 2828515213 | Ga0392526_2149 | heat shock protein HslJ |
| 2828516605 | Ga0392526_3541 | cold shock CspA family protein |
Open Research
DATA AVAILABILITY STATEMENT
The GenBank [/EMBL/DDBJ] accession numbers for the 16S rRNA gene sequence of strain D4M1T are MK909903: https://www.ncbi.nlm.nih.gov/nuccore/MK909903. The assembled and annotated genome of D4M1T described in this paper has been deposited in GenBank (accession number: CP040818-CP040824: https://www.ncbi.nlm.nih.gov/assembly/GCA_005887635.2) and JGI portal (GOLD ID: Gp0432545, https://gold.jgi.doe.gov/project?xml:id=Gp0432545; IMG Taxon ID: 2828513066, https://img.jgi.doe.gov/cgi-bin/m/main.cgi?section=TaxonDetail&page=taxonDetail&taxon_oxml:id=2828513066).