Metal-organic frameworks-based nanomedicines to promote cancer immunotherapy: Recent advances and future directions
Chenqian Feng and Xiaoyan Liang contributed equally to this study.
Abstract
Cancer immunotherapy uses the body's immune system to fight tumors by restoring natural antitumor responses. Metal-organic frameworks (MOFs), characterized by their unique crystalline porous structures formed from metal ions linked by organic ligands, offer a promising solution. Recent studies have unveiled the potential of MOFs in cancer immunotherapy. The exceptional porosity and surface area, coupled with their extraordinary thermal and chemical stability, bring significant advantages for efficient drug loading and delivery of immunotherapeutic agents. The adaptability of MOFs further enhances the controlled release of immunotherapeutic drugs within target cells and increases tumor sensitivity to other therapies such as photodynamic, photothermal, and radiotherapy. This multifunctional carrier contributes to modulating the tumor microenvironment and reactivating antitumor immunity, providing a comprehensive strategy for cancer treatment. In this review, we summarize the applications of MOFs in immune checkpoint blockade, immunomodulator delivery, and cancer vaccine delivery, and discuss existing challenges in their use for immunotherapy. This discussion aims to offer insights for developing better treatments and enhancing the efficacy of immunotherapy.
1 INTRODUCTION
The 2020 Global Cancer Statistics Report, issued by the World Health Organization, projected an estimated 19.3 million new cancer cases and 10 million cancer-related deaths by the end of 2020.1 Tumor immunotherapy stands as promising therapies through inhibition of negativeherapeutic avenue aimed at managing and eradicating tumors by reinstating the body's natural antitumor immune response, thus revitalizing the tumor-immune cycle.2 In 2011, a significant milestone was achieved when the FDA greenlit the first immune checkpoint inhibitory (ICI) antibody, ipilimumab, targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).3 This groundbreaking achievement led to a paradigm shift in cancer treatment. Notably, James Allison and Tasuku Honjo were honored with the Nobel Prize in Medicine in 2018 for their pivotal role in the “Discovery of cancer therapies through inhibition of negative immune regulation.” Presently, immunotherapeutic strategies encompass a spectrum of approaches, including immune checkpoint blockade (ICB), chimeric antigen receptor T cell therapy (CAR-T), immunomodulatory agents, and cancer vaccines.4, 5 Several immunotherapies have garnered FDA approval and have shown great potential in clinical trials.
Despite the great clinical success of immunotherapy, it may lead to immune-related adverse events (irAEs), such as autoimmune conditions and inflammatory responses, due to the over-activation of immune system.6 Additionally, researchers are also exploring a variety of combination immunotherapies to address low response rates in immunotherapy patients.7 In this context, the advancement of delivery systems is crucial for ensuring both efficacy and safety in therapy. Metal-organic frameworks (MOFs) are crystalline porous materials composed of metal ions or clusters with organic ligands. The unique structure of MOFs creates void spaces, resulting in a specific surface area of up to 8000 m2·g−1.8 By altering the metal ion centers, organic ligands, or incorporating different functional groups, MOFs can be tailored to enhance their porosity,9 thermal stability,10 and catalytic properties.11, 12 This tunability makes MOFs highly adaptable to various applications.13-15 Notably, they offer tunable structures, high porosity, extensive specific surface areas, and excellent thermal and chemical stability. Common types of MOFs include zeolite imidazole frameworks (ZIFs),15 materials of institute Lavoisier frameworks (MILs),16 and porphyrin-based organic structures with pore-channel frameworks (PCNs).17 MOFs are typically synthesized through the evaporation-solvent method, diffusion method, hydrothermal or solvent-thermal method, as well as ultrasonic and microwave methods.18
In recent years, nanoscale MOFs with excellent biocompatibility and biodegradability have gained traction in biomedical applications.19, 20 The porous nature of MOFs affords them a substantial specific surface area, facilitating higher drug loading compared to nanocarriers like liposomes.21 Notably, the relatively unstable metal–ligand bonds in MOFs enable these nanosystems to undergo controlled degradation within lysosomes or tumor cells, thereby achieving controlled drug release.22 The structural adaptability of MOFs makes them easy to modify for diverse functionalities, making them widely used in drug delivery and tumor therapy.23 Although numerous reviews have comprehensively summarized and discussed the applications and advancements of MOFs in the field of tumor therapy, there remains a relative paucity of literature specifically addressing the roles and applications of MOFs in cancer immunotherapy.24, 25 This area warrants further exploration and research. Through a thorough investigation and in-depth analysis of the latest cutting-edge studies, we have identified significant potential for MOFs in the delivery of immunotherapeutic agents and the induction of immune responses. Moreover, they serve as sensitizers for various therapies such as chemodynamic therapy (CDT), Ferroptosis, photodynamic therapy (PDT), radiation therapy (RT), photothermal therapy (PTT), and Sonodynamic therapy (SDT), thereby modulating the tumor microenvironment (TME) and engaging with the immune system to restore antitumor immunity.26 As shown in Figure 1, this review aims to outline new avenues for cutting-edge research on MOF-based nanosystems in cancer therapy, with a focus on the application in cancer immunotherapy and the accompanying challenges.
2 MULTIFUNCTIONAL MOFs
MOFs, a class of porous carbon-based materials, have garnered considerable attention due to their unique properties. MOFs are formed through the self-assembly of inorganic metal ions or clusters with organic ligands via coordination bonds, resulting in structures with rich diversity and high customizability. The construction of MOFs involves a wide variety of metal ions, including transition metal ions such as Zn2+, Cu2+, Ni2+, and Fe3+; main group metal ions such as Al3+, Ca2+, and Mg2+; and other metal ions such as Zr, Ti, and Cr. Among these, Ca2+, Mg2+, Zn2+, Fe3+, Ti, and Zr are particularly suitable for the development of biocompatible MOFs.27 Ligands play a crucial role in the development of MOF materials, providing a rich and effective array of options for constructing multifunctional MOFs. Commonly used ligands include carboxylates, phosphonates, nitrogen-containing heterocycles, and crown ethers. The selection and design of MOF ligands can be optimized through various strategies, such as adjusting the size and shape of the ligands, introducing functional groups, regulating the rigidity and flexibility of the ligands, and selecting the metal centers. These strategies enable the optimization of MOF properties, such as pore size, surface area, chemical stability, and catalytic activity, thereby expanding the applicability of MOF materials across different fields.
Moreover, the versatility of MOFs extends beyond traditional uses, as their tunable pore sizes and surface functionalities make them ideal candidates for advanced catalytic processes and environmental remediation. In catalysis, MOFs can act as supports for metal nanoparticles, enhancing catalytic efficiency and selectivity in chemical reactions. Their ability to encapsulate enzymes and other biomolecules also opens new avenues in biocatalysis, potentially revolutionizing drug delivery system. Additionally, the integration of MOFs with other materials, such as polymers and nanomaterials, is being explored to create hybrid systems that leverage the strengths of each component, offering unprecedented performance in various domains. The biocompatibility of certain MOFs also paves the way for their use in biomedical applications, where they can serve as carriers for targeted drug delivery, enhancing the efficacy and reducing the side effects of therapeutic agents. The ability of MOFs to host a variety of guest molecules within their porous frameworks allows for controlled release mechanisms, which is particularly advantageous in improving antitumor effect. In conclusion, by using their unique structural properties and integrating advanced technologies, MOFs are expected to contribute to the next-generation cancer immunotherapy.
3 ADVANCEMENTS AND CHALLENGES IN CANCER IMMUNOTHERAPY
Immunotherapy has introduced a novel paradigm in disease management and treatment, leading to numerous clinical breakthroughs. At its core, immunotherapy can fully mobilize the body's own immune system, through a series of complicated and delicate synergistic mechanisms, to eliminate pathological cells while safeguarding healthy tissues. This approach consequently triggers a long-lasting, tissue-specific immune response. Moreover, it offers a more personalized treatment option, which can be tailored to the unique immunological profile of individual patients, thereby enhancing its effectiveness and minimizing adverse effects. One of the most prominent successes of immunotherapy is in the treatment of various cancers, where therapies like ICB, adoptive cell therapy, and cancer vaccines have revolutionized the landscape of oncology. For instance, checkpoint inhibitors work by blocking proteins that prevent T-cells from attacking cancer cells, effectively “releasing the brakes” on the immune system. Adoptive cell therapy, on the other hand, involves genetically modifying a patient's own T-cells to better recognize and attack cancer cells. Cancer vaccines aim to stimulate the immune system to recognize cancer-specific antigens, thereby preventing the development or recurrence of malignancies.
However, despite its significant clinical successes, the application of immunotherapy remains constrained, being effective for only certain diseases and specific patient populations. This limitation primarily arises from various immunosuppressive mechanisms, such as low immunogenicity of the disease itself, insufficient antigen presentation, and restricted T-lymphocyte infiltration, which severely hinder the efficacy of immunotherapy. Additionally, off-target toxicity of immune drugs, instability and unpredictability of therapeutic outcomes, and tissue heterogeneity pose significant challenges that impede further development. Another critical aspect to consider is the TME, which can be highly immunosuppressive, creating physical and biochemical barriers that prevent immune cells from effectively targeting the tumor. Research is ongoing to develop combination therapies that can modify the TME to be more conducive to immune cell activity, thereby enhancing the overall efficacy of immunotherapy.
Moreover, emerging technologies such as next-generation sequencing and advanced bioinformatics tools are enabling the identification of novel biomarkers and therapeutic targets, which could potentially overcome some of the current limitations. Personalized medicine, supported by comprehensive genomic and proteomic profiling, holds promise for tailoring immunotherapeutic strategies to the specific molecular characteristics of an individual's disease, thus improving outcomes and expanding the applicability of these treatments. In conclusion, while immunotherapy has already made a profound impact on the treatment of various diseases, especially cancer, ongoing research and technological advancements are essential to overcome its current limitations. With continuous innovation and a deeper understanding of the immune system's complexities, the future of immunotherapy holds the potential for even more groundbreaking advancements in disease management and treatment.
4 APPLICATION OF MOFS IN CANCER IMMUNOTHERAPY
In the realm of cancer immunotherapy, MOFs, as an innovative biomaterial, can enhance antitumor immune responses on multiple critical levels. First, MOFs can markedly improve drug delivery efficiency and control drug release at tumor sites. Next, their metal ion components can augment immune effects and enhance therapeutic efficacy. Furthermore, MOFs can be utilized in multimodal imaging techniques, providing precise guidance and monitoring for cancer immunotherapy. Accordingly, this chapter will review recent advancements and applications of MOFs in cancer immunotherapy over the past few years, as illustrated in Table 1.
| Classification | Name | MOF | Metal ion | Cancer model | Ref. |
|---|---|---|---|---|---|
| Immune checkpoint inhibitors | nivolumab | ZIF-8 | Zn2+ | 4T1 tumor-bearing mice | [28] |
| BMS1166 | ZIF-8@PEG-FA | Zn2+ | K7M2 tumor-bearing mice | [29] | |
| Dy-TCPP | Dy-TCPP | Dy3+ | HCT 116 tumor-bearing mice | [30] | |
| αPD-L1 | PCN-224 | Zr | CT26 tumor-bearing mice | [31] | |
| siIDO1 | ZIF-8 | Zn2+ | Luci GL261 tumor-bearing mice | [32] | |
| BMS-986205 | Cu-BTC | Cu2+ | 4T1 tumor-bearing mice | [33] | |
| D-1-MT | HA/ZIF-8 | Zn2+ | K7M2 tumor-bearing mice | [34] | |
| NLG919 | UiO-66 | Zr4+ | 4T1 tumor-bearing mice | [35] | |
| Immunomodulator | CCCP | ZIF-8 | Zn2+ | 4T1 tumor-bearing mice | [36] |
| R837, 1 MT | ZIF-8 | Zn2+ | B16F10 tumor-bearing mice | [37] | |
| perforin, granzyme B | ZIF-8@CaCO3 | Zn2+ | 4T1 tumor-bearing mice | [38] | |
| CpG ODN | MIL101-NH2 | Fe3+ | 4T1 tumor-bearing mice | [39] | |
| Fe | M2pep-Fe-TCPP | Fe2+, Fe3+ | H22 tumor-bearing mice | [40] | |
| R837, αCD47, αPD-L1 | Hf-DBP | Hf | CT26 tumor-bearing mice | [41] | |
| CpG ODN, DMXAA, MOF-801 | MOF-801 | Zr | Hepa1-6 tumor-bearing mice | [42] | |
| Zn2+, αPDL1 | Gd-MOF-5 | Gd3+, Zn2+ | 4T1 tumor-bearing mice | [43] | |
| Cancer vaccine | Apt-Cell | ZIF-8 | Zn2+ | B16 tumor-bearing mice | [44] |
| OVA | ZIF-8 | Zn2+, Al3+ | EG7-OVA tumor-bearing mice | [45] | |
| nMOFs, CpG | Hf-DBBF-Ir | Hf, Ir | MC38 | [46] | |
| personalized vaccine | cMOF | Fe2+, Fe3+ | B16F10 tumor-bearing mice | [47] |
- Abbreviations: MOF, metal-organic frameworks; OVA, ovalbumin; PCN, pore-channel frameworks;
4.1 MOFs for regulating the TME
In the field of tumor therapy, MOFs have exhibited tremendous potential in modulating the TME due to their unique structures and tunability. Through meticulous design and functionalization, MOFs can intervene in critical factors within the TME, thereby improving its immunosuppressive state and enhancing the efficacy of immunotherapy. Specifically, MOFs can regulate the TME in various ways. On one hand, they can augment the oxygen supply to tumor tissues, thereby alleviating hypoxia-induced immunosuppression. For instance, a reported study introduced a biomimetic self-supplying oxygen nanocatalytic drug, O2@UiO-66@ICG@RBC, which employs the porous structure of MOFs to load oxygen-generating agents, achieving sustained oxygen supply within tumors.48 This strategy provides a direction for improving hypoxic tumor therapy and facilitates the sensitivity of tumor cells to immunotherapy.
On the other hand, MOFs can deplete glutathione (GSH) within tumor tissues, thereby disrupting their redox balance. GSH is a crucial antioxidant found in high concentrations within the TME, capable of neutralizing reactive oxygen species (ROS) and reducing the efficacy of immunotherapy. In the study conducted by Ma et al., a bimetallic Zn2+/Cu2+ co-doped MOF was reported to deplete GSH and generate ROS, thereby dismantling the antioxidant defense mechanism of tumor cells and creating favorable conditions for immunotherapy.49
Furthermore, MOFs can act as catalysts to continuously enhance the TME through sustained catalytic reactions. The MnFe2O4@MOF nanoplatfrom designed by researchers exemplifies this approach. This material can catalyze the conversion of H2O2 to O2 by a Fenton-like reaction in an acidic TME.50 This catalytic reaction not only mitigates the hypoxic state of tumors but also produces a significant amount of ROS, directly damaging tumor cells and thereby augmenting the antitumor efficacy of PDT. In summary, by modulating the TME through mechanisms such as enhancing oxygen supply, depleting GSH, and sustaining catalytic reactions, MOFs can significantly improve the effectiveness of immunotherapy.
Tumor-infiltrating lymphocytes (TILs) often face resistance, while the presence of immunosuppressive cells, particularly myeloid-derived suppressor cells (MDSCs), poses significant challenges in combating “cold“ tumors.51-53 To overcome this challenge, Chen et al.54 and colleagues devised a method involving the utilization of cationic dendrimer molecules (PDGs) loaded with guicasibine, which were then bound to PCN-224 through electrostatic adsorption. The combination of GEM with PDT facilitated the infiltration of cytotoxic T lymphocytes (CTLs) into the tumor, aiding in the remodeling of the TME.
4.2 MOFs for improved ICB therapy
PD-1 is a crucial immunosuppressive molecule found in high levels on the surface of activated T cells.55 Its primary function is to shield normal tissues from immune attack by engaging with PD-L1, thereby obstructing T cell receptors and suppressing costimulatory signaling. When tumor cells express PD-L1, they can bind to PD-1 on T cells, hindering their activation and proliferation.56 This interaction facilitates immune evasion by tumors. In recent years, ICIs have become more widely used in clinical practice.57 ZIFs offer several advantages, including high porosity, stability, and tunable surface properties, making them promising candidates for targeted drug delivery.58 Recent studies have demonstrated the efficacy of MOFs in delivering ICIs.28-31
In a study by Alsaiari et al.,28 the delivery of nivolumab (NV), a PD-1 inhibitor, was achieved using biomimetic ZIFs. By encapsulating NV in NV-ZIF, the researchers achieved controlled release kinetics, improving the drug's efficacy in treating hematologic malignancies. Additionally, the surface modification of ZIF-8 with cancer cell membranes demonstrates a novel approach to targeted delivery, which could significantly impact the treatment of solid tumors. Ge et al.29 utilized ZIF-8 as a carrier loaded with curcumin and the PD-L1 inhibitor BMS-1166 to augment the immunotherapeutic response of PD-1/PD-L1 blockade by inducing immunogenic cell death (ICD) through autophagic cell death. This highlights ZIF-8's potential in enhancing PD-1/PD-L1 blockade therapies.
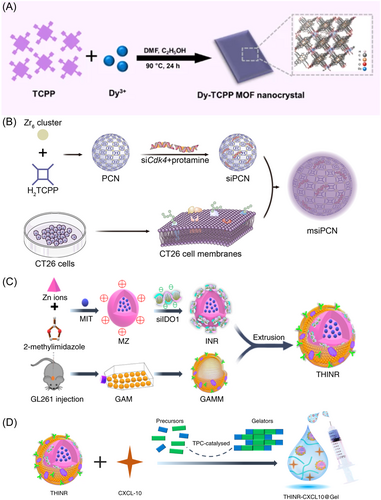
Jiang et al.30 pioneered the synthesis of MOF nanocrystals with distinct two-dimensional morphology, employing the rare-earth element dysprosium (Dy) coordinated with Tetrakis(4-carboxyphenyl) porphyrin (TCPP) as shown in Figure 2A, demonstrated promising results in downregulating PD-L1 expression in tumor cells. Bai et al.31 developed tumor cell membrane-encapsulated PCN-224 for targeted delivery of photosensitizers and small interfering RNAs (siRNAs) to knock down cyclin-dependent kinase 4 (Cdk4) (Figure 2B). The study highlighted a novel strategy for targeted delivery of therapeutic agents to inhibit Cdk4, thereby modulating PD-L1 expression and enhancing antitumor immune responses. IDO-1 inhibitors are another class of ICIs.59 Indoleamine 2,3-dioxygenase (IDO-1) serves as an endogenous immunosuppressive mediator, promoting the accumulation of regulatory T cells (Treg) and dampening T cell activity through tryptophan (Trp) depletion within the microenvironment.60-62 By blocking IDO-1 activity, IDO-1 inhibitors enhance the immune system's ability to attack tumor cells.63 Zhang et al.32 using MOFs like ZIF-8 for dual delivery of chemotherapeutics and siRNA to target ICD and counteract Treg-mediated immunosuppression, enhancing localized drug concentration and reducing systemic toxicity (Figure 2C,D). This dual delivery system not only promises increased localized drug concentration but also precise targeting, thereby reducing systemic toxicity. Similarly, Du et al.'s33 development of a GSH-sensitive MOF for delivering an IDO-1 inhibitor and an NO donor underscores the capability of responsive nanocarriers to leverage the TME for therapeutic gain. The rapid disintegration of these carriers in response to GSH levels ensures timely release of therapeutic agents, which is crucial for maximizing the pharmacological response while minimizing side effects. Furthermore, Fan et al.34 used ZIF-8 for delivering both a chemotherapeutic and IDO-1 inhibitor in osteosarcoma, showcasing the adaptability of nanocarriers across different cancers. These strategies enhance and personalize cancer treatments.
In the field of cancer research, employing nanotechnology for drug delivery systems significantly enhances the efficacy of immunotherapy treatments.64, 65 The integration of nanotechnology in cancer treatment through the development of hybrid nanomedicines exemplifies a significant advancement in targeted cancer therapy.66 The integration of MOF and nanotechnology enables the precise targeted delivery and the controlled release of ICI. By employing UiO-66 as a scaffold, Ding et al.35 utilized UiO-66 to create functionalized AuMOF nanoparticles for controlled loading and release of the IDO inhibitor NLG929. Through π-π stacking and gold-thiol interactions, the nanoparticles ensured drug stability and effective release in response to specific triggers like light and the tumor biochemical environment. This approach addresses challenges in drug delivery, such as nonspecific distribution and premature release.
4.3 MOFs for immunomodulator-based cancer immunotherapy
Studies have shown that MOFs exhibit great potential as multifunctional nanocarriers in tumor immunotherapy.67-70 Firstly, as a multifunctional nanocarrier, MOF is highly tunable and controllable for efficient delivery and release of immunomodulators. pH-responsive nanocarriers have attracted much attention for their ability to respond to the acidic TME for controlled release.71, 72 ZIF-8 nanoparticles are widely utilized as carriers for tumor therapy due to their high porosity, adjustable pore size, ease of preparation, and pH responsiveness.36 However, the inherent therapeutic efficacy and immunogenicity of ZIF-8 remain uncertain. Initial observations by Ding et al.35, 36 found that ZIF-8 NPs cause tumor cell death through necrosis and ICD by releasing inflammatory molecules and DAMPs, enhancing immunogenicity and initiating an immune response. In a related study, Zhao et al.38 used pH-sensitive ZIF-8 NPs loaded with perforin and granzyme B, targeting CD8+ T cells, which showed enhanced cytolytic activity in acidic lysosomal environments. Adding elements like calcium carbonate ensures immunostimulatory agents are released where needed, boosting CTL activation and effectiveness.
Secondly, the structural features of MOF allow it to achieve targeted delivery through various modifications, such as chemical modification and biological modification, thus increasing the enrichment of immunomodulators at the tumor site and reducing the damage to healthy tissues. Zhang et al.37 advanced the use of ZIF-8 in targeted nanoparticle therapies for immunotherapy. By customizing nanoparticles with ligands like hyaluronic acid (HA) and mannose, they target tumor and immune cell markers, enhancing therapeutic delivery and addressing immune evasion in tumors.73-75 In a separate study, Yang et al.44 encapsulated mammalian cells with ZIF-8 NPs using the AS1411 aptamer, inducing ICD in cancer cells. This process activates immune pathways, including tumor necrosis factor signaling, cytokine-cytokine receptor interaction, and toll-like receptor signaling. In particular, MOF carriers enable the induction of ICD via aptamers, thus enhancing the immunotherapeutic effect.76 These findings suggest a potential adjuvant role for ZIF-8 NP coating in tumor therapy.
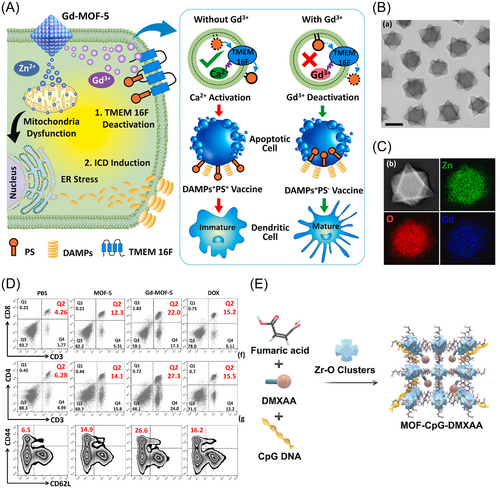
Interestingly, MOF may also have a role as an immune adjuvant. Through its unique composition and recognition properties, MOF not only serves as a carrier for therapeutic agents but also actively engages with the immune system, driving antitumor responses. Dai43 and colleagues designed a MOF containing Gd3+ and Zn2+ as metal nodes, with 1,4-benzyl dicarboxylic acid as the organic ligand. Their aim was to investigate the MOF's impact on cellular signaling in the immune response. They found that intracellular Gd3+ competes with Ca2+ for binding to TMEM 16 F, which results in the inhibition of phosphatidylserine (PS) externalization by suppressing TMEM 16 F activity. This process is depicted in Figure 3A–D. Zn2+ acts as an immunomodulator, suppressing immunosuppressive PS signaling, and simultaneously serves as an ICD inducer, enhancing immunostimulatory signaling. Hepatocellular carcinoma (HCC) is a common primary cancer characterized by high morbidity and mortality.77 Chen et al.42 engineered MOF-801 via the combination of Zr6 clusters and fumaric acid. Acting as a conveyance mechanism, MOF-801 demonstrates the ability to trigger the cGAS-STING-NF-κB signaling cascade by recognizing toll-like receptor 4 (TLR4) at remarkably low concentrations (70 ng/mL). This activation expedites the maturation of DCs. CpG ODNs and the STING agonist DMXAA were incorporated into MOF-801 to counter immunosuppression and provoke potent antitumor immunity in HCC settings (refer to Figure 3E). Theirs study underscores the multifaceted capabilities of MOF-801 in cancer immunotherapy.
The combination of precise tumor targeting, controlled release of immune adjuvants and synergistic photoimmunotherapy significantly improves tumor cytotoxicity, ultimately leading to high cure rates and minimal systemic side effects. The strategic manipulation of tumor-associated macrophages (TAMs) highlights a sophisticated approach in the treatment of cancer.78, 79 TAMs, which infiltrate tumors, exhibit dual behaviors that significantly influence cancer therapy outcomes.80 M1-like TAMs are beneficial for their tumor-destroying capabilities and ability to trigger Th1-type immune responses, while M2-like TAMs promote tumor growth, angiogenesis, metastasis, and suppress T cell-mediated antitumor immunity.81, 82 Wei et al.‘s40 developed iron-based MOFs targeting M2-like TAMs using an M2pep-modified system to reprogram M2 macrophages into M1 macrophages. This strategy boosts the immune system's ability to fight cancer and reduces recurrence risks. Ni et al.41 used Hf-DBP nanoparticles to co-deliver IMD and αCD47, regulating macrophage function and countering tumor-induced immune suppression. This multi-target approach integrates immunomodulatory and phagocytosis-promoting agents, transforming the TME into a more immunostimulatory state. In summary, as a carrier of immunomodulators, MOF has good biocompatibility, targeting and controllability, and is expected to become an effective tool for tumor immunotherapy.
4.4 MOFs for enhanced cancer vaccine strategies
Vaccination, widely recognized as a secure and efficacious preventive measure against various infections.83 However, developing vaccines for cancer remains an immense challenge. MOFs have emerged as an effective tool to enhance cancer immunotherapy through efficient antigen delivery, immune stimulation and induction of ICD. MOFs facilitate the efficient loading and delivery of antigens, such as ovalbumin (OVA), to target cells.84 Zhong et al.45 initiated a synthesis of nanoparticles (termed ZANPs), integrating aluminum, the model antigen OVA, and ZIF-8. They achieved a loading efficiency of 30.6% using ZANPs, demonstrating the effectiveness of MOFs in antigen delivery.
MOFs can enhance antigen immunostimulatory properties by facilitating intracellular antigen release, promoting cross-presentation, and immune activation. Ni et al.‘s development of hafnium-based MOFs for targeted cancer therapy is a significant breakthrough in immunotherapy.46 These MOFs bind and deliver CpG oligodeoxynucleotides, activating the immune system directly within theTME. Exposure to X-rays enables MOFs to generate ROS, releasing tumor antigens and DAMPs, which enhance antigen-presenting cell maturation and boost CTL populations in tumor-draining lymph nodes. Combined with ICIs, this strategy significantly enhances innate and adaptive immune responses, leading to notable tumor regression and sustained immune memory, highlighting MOFs’ potential in in situ cancer vaccines.
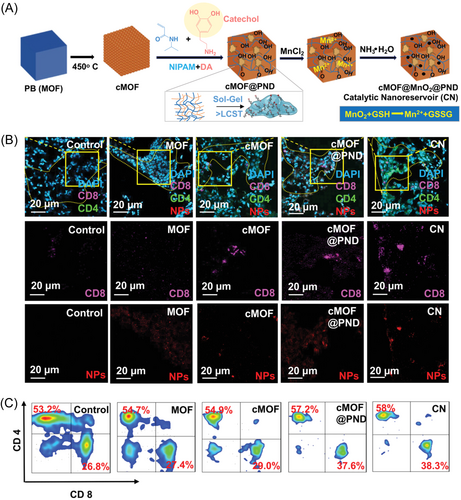
Nanocarriers based on MOFs can also promote the recruitment of antigen-presenting cells.85 MOFs can attract DCs into the TME enhancing T cell-mediated immune responses, which are crucial for inhibiting tumor metastasis. Yalamandala et al.47 introduced a novel in situ catalytic nanoserver (CN), comprising a PNIPAAM/PDA hybrid nanogel, catechol-functionalized MOFs, and MnO2, targeting lung metastasis and enhancing immune responses (Figure 4). This MOF-based nanocarrier catalyzes redox reactions to generate Mn2+, induces apoptosis and releases antigens within tumor metastases. Catechol-functionalized MOFs capture released antigens, forming a reservoir that promotes prolonged immune activation. This process not only triggers ICD but also attracts DCs, enhancing T-cell mediated immune responses and promote prolonged immune activation. These findings collectively stress the potential of MOFs in developing in-situ cancer vaccines, offering promising avenues for enhancing cancer immunotherapy. MOFs serve as multifunctional platforms for cancer immunotherapy through efficient antigen delivery and immune stimulation, promoting immune responses and tumor regression. Further research into MOF-based strategies holds promise for developing more effective in-situ cancer vaccines, further enhancing immune responses, and promoting tumor regression.
4.5 MOFs for immunogenicity-augmented cancer immunotherapy
ICD is a distinct type of apoptosis that allows immune-capable hosts to initiate specific immune responses against antigens from dead cells.86, 87 Surface-exposed calreticulin (CRT) sends “eat me” signals to DCs; released high-mobility group box 1 (HMGB1) enhances DC maturation and antigen presentation to CTLs; and secreted ATP drives CTL infiltration into tumors.54 This process counteracts the tumor's immunosuppressive environment and enhances the effectiveness of immunotherapy. MOFs-based drug delivery systems trigger ICD by integrating chemotherapeutic drugs, photosensitizers, radiosensitizers, and immunomodulators.88-90 These systems specifically target and release drugs at the tumor site, improving biosafety and therapeutic outcomes.91, 92 This section will overview the role of MOFs in inducing ICD for tumor treatment.
4.5.1 Chemotherapy-driven immunogenic activation
Chemotherapy remains one of the most effective strategies for treating cancer.93, 94 Recently, the emergence of chemoimmunotherapy, which combines chemotherapy and immunotherapy, has shown promising synergistic effects in cancer treatment. Chemotherapeutic agents such as paclitaxel, oxaliplatin (Oxa), and doxorubicin (DOX) not only directly kill cancer cells but also activate both innate and adaptive immune responses, thereby inducing ICD.95, 96 To enhance the effects of chemotherapy-induced ICD, Wang et al.97 introduced a novel biomimetic nanosystem utilizing ZIF-8 nanoparticles to enhance chemotherapy-induced ICD and modulate the immunosuppressive TME (iTME), as shown in Figure 5A–C. Biomimetic materials often have the ability to be self-adaptive and self-healing.99 By integrating ZIF-8 nanoparticles that release lactate oxidase (Lox) and Oxa, this system targets the metabolic and immunologic dysregulation within tumors. Within this system, Lox is released to convert lactate, thereby shifting the acidic tumor environment, promoting the polarization of macrophages from M2 to M1, and reducing Tregs. The release of Oxa further induces ICD, facilitating the clearance of cancer cells by CTLs and M1 macrophages. Hu et al.100 formulated PCN-Oxpt/PEG, a multifunctional, biocompatible nanotherapeutic that targets the TME to enhance the efficacy of cancer treatments through chemotherapy, ferroptosis, and immunomodulation. This system uses a chemically modified form of oxaliplatin, Oxpt (IV)-COOH, to deplete GSH in tumor cells, facilitating the generation of hydrogen peroxide and subsequent production of cytotoxic hydroxyl radicals via ferroptosis, thereby promoting ICD. Enhanced by dual-mode imaging capabilities, this approach optimizes drug delivery and effectiveness.
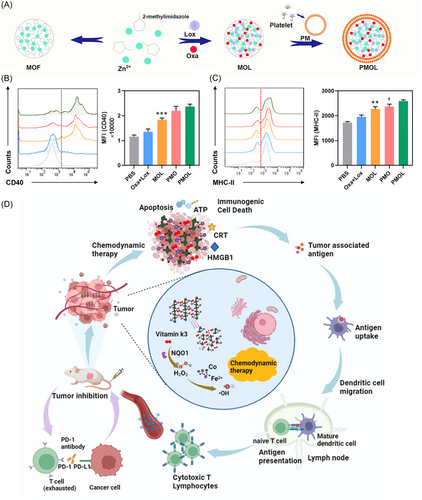
4.5.2 Ferroptosis-driven immunogenic activation
Ferroptosis, a recently identified mode of nonclassical programmed cell death, is triggered by the accumulation of lipid peroxidation products which compromise cellular structures and functions.101-104 This process promotes ICD by emitting “find me” signals.105 Integrating iron into MOFs has proven to be an effective method for inducing ferroptosis in cancer cells.106 For instance, Yang et al.107 synthesized mFe(SS)/DG by reacting FeCl3⋅6H2O with dithiobisethanolic acid at room temperature. Similarly, Wang98 and his team used a combination of ferrocene and cobalt(II) acetate tetrahydrate (Co(OAc)2⋅4H2O) in a hydrothermal reaction to create a cobalt-ferric framework, Co-Fc (Figure 5D). These MOFs have been demonstrated to facilitate the production of Fe2+, thereby inducing ferroptosis in tumor cells.
4.5.3 PDT-driven immunogenic activation
PDT utilizes three inherently safe components: a photosensitizer, light, and oxygen in the target tissue. This combination produces ROS, predominantly singlet oxygen (1O2), which initiate cell death through apoptosis and necrosis.108, 109 PDT also has a strong immunogenic effect and can induce ICD.110, 111 For the photodynamic effect, MOFs frequently incorporate porphyrins as organic ligands to create porous nanocarriers.
MOFs are modified to significantly increase NIR absorption, which can more effectively initiate PDT in deeper tissues.112, 113 In an innovative research, Li et al.114 synthesized PCN-222 nanoparticles using a solvothermal technique with TCPP as a ligand, Zr4+ as the metal center, and dichloroacetic acid as a modifier. These NPs were then transformed into spindle-shaped PCN-SUs with enhanced NIR absorption through a sulfonation reaction. This modification, introducing sulfonic acid anions to the TCPP ligand, led to porphyrin ring distortions that effectively narrowed the energy gap between the HOMO and LOMO, thereby significantly enhancing NIR absorption. The resulting PCN-SU nanoparticles exhibited superior photoactive properties, notably in generating singlet oxygen under NIR light, effectively inducing ICD, and promoting DC maturation more efficiently than their PCN-222 counterparts. This development shows the potential of PCN-SU as a powerful tool in cancer immunotherapy. Wang et al.115 successfully developed PCN-224 nanoparticles using a solvothermal method with TCPP as a ligand, Zr4+ as the metal core, and DMF as the modifier. They incorporated BSO into the nanoparticles, which was targeted to tumor sites with HA. BSO inhibits GSH synthesis and GPX activity while increasing ROS, inducing ferroptosis and effectively combining with PDT to promote ICD in tumor cells. Simultaneously, Cai et al.116 enhanced this design by adding ACF and CpG within an HA coating to inhibit hypoxia-inducible factor 1-alpha (HIF-1α).
Some MOFs are capable of altering the TME and alleviating hypoxia, a common challenge in solid tumors, thereby improving the efficacy of PDT.117, 118 Sun et al.119 engineered another variant of PCN-224, incorporating carbocyanide 3-chlorophenylhydrazine (CCCP), a compound that disrupts mitochondrial function, within a manganese dioxide (MnO2) shell. This innovative shell assists in the catalytic decomposition of hydrogen peroxide (H2O2) within the tumor environment, thereby alleviating hypoxia. CCCP intensifies mitochondrial dysfunction leading to enhanced autophagy. This, in turn, facilitates the release of tumor antigens, boosting the immune system's recognition and destruction of tumor cells, thus promoting autophagy-driven cell death and enhancing the overall immunogenic response. In addition to the commonly used Zr4+, other metal ions such as Al3+, Fe3+, and Mn3+ can also coordinate with porphyrins to create MOFs with varied functionalities. Chen et al.120 developed a new nanomaterial, PCN(Al), using a solvent-thermal method with Al3+ and TCPP, subsequently enhancing it by depositing Cu (II) and Pt nanoparticles on its surface and modifying it with NH2-PEG-FA (Figure 6A). This configuration allowed Cu (II) to target and reduce intracellular GSH, elevating ROS levels, while Pt nanoparticles converted H2O2 to O2, reducing hypoxia and enhancing PDT's effectiveness in triggering ICD and a systemic immune response (Figure 6B–L). Concurrently, Li et al.121 synthesized PCN(Fe), using a similar approach with Fe3+ and TCPP, and enhanced it with Pt nanoparticles and a coating of RBCMs, improving circulation time and tumor targeting, thereby boosting PDT's antitumor efficacy.
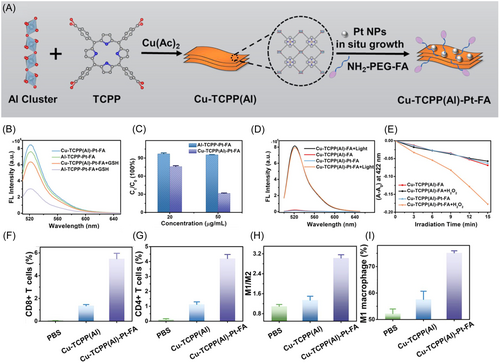
MOFs offer a promising platform for synergistically integrating diverse therapeutic modalities, such as the combination of PDT and CDT, thereby expand the eradication of cancer cells.122-124 The PDT capabilities of Pt nanoparticles are enhanced by their ability to generate 1O2. Additionally, in the presence of GSH, the iron in PCN(Fe) undergoes reduction from Fe3+ to Fe2+, promoting CDT. Both PDT and CDT work synergistically to intensify intracellular oxidative stress and amplify the ICD effect. Yu et al.125 and Zhao et al.126 have advanced nanoparticle-based therapies by integrating photodynamic and chemodynamic mechanisms to enhance tumor-targeted treatments. Yu et al. encapsulated tirapazamine (TPZ) in PCN(Fe) for simultaneous PDT and CDT applications, leveraging the dual mechanism to amplify intracellular oxidative stress and ICD. Zhao et al. developed PCN(Mn) nanoparticles, coordinated with Mn3+ and TCPP, for the delivery of vorinostat, modifying the nanoparticles with polyethyleneimine and AS1411 for targeted cancer therapy. These modifications facilitated the release of therapeutic agents within tumor cells, enhancing histone acetylation and activating the STING pathway, thereby boosting both innate and adaptive immune responses and significantly augmenting the therapeutic efficacy of PDT and CDT.
MOFs are fruitful in enhancing NIR absorption and PDT efficacy.127, 128 Xie et al.129 and Lu et al.130, 131 conducted studies to enhance the efficiency of PDT through the synthesis of novel MOFs and porphyrin modifications. Xie et al. formulated PTP using a palladium-coordinated π-extended porphyrin (Pd-TBP), TCPP, and Zr4+, which exhibited dual NIR emission properties under a single excitation wavelength, useful for oxygen monitoring in tumor environments. Lu et al. modified porphyrin structures to chlorins, significantly boosting singlet oxygen production with the new DBC-UiO and TBC-Hf complexes, showing superior PDT efficacy compared to previous porphyrin-based frameworks.
Collectively, these investigations stress the potential of MOFs in cancer immunotherapy, showing their innovative modifications and applications in PDT-driven ICD. These advancements encompass the engineering of MOFs to enhance NIR absorption, modulate the TME, stimulate immune responses, and incorporate multiple therapeutic strategies. Each study contributes a distinct insight into the customization of MOFs to address the inherent limitations of conventional light-guided therapies, such as inadequate penetration depth, hypoxia, and insufficient tumor specificity. These findings illustrate a coherent progression from fundamental material synthesis and functionalization to sophisticated systems engineered for multimodal therapeutic and targeting mechanisms. Collectively, these initiatives have enhanced the efficacy, safety, and precision of PDT, advancing it towards clinical implementation in cancer treatment.
4.5.4 Radiodynamic therapy-driven immunogenic activation
Conventional visible light-activated MOFs used in PDT systems suffer from limited tissue penetration depth, typically less than 1 cm, which hampers their efficacy in treating deep-seated tissues. In contrast, RT employs deeply penetrating X-rays that directly damage DNA and indirectly generate hydroxyl radicals (-OH) to eradicate tumor cells, albeit with lower immunogenicity compared to PDT.132 Zhao et al.133 introduced a novel approach for PDT by developing a MOF-based system that incorporates lanthanide-doped scintillant nanoparticles (SNPs) on the surface of PCN-224. This innovation aims to enhance deep-tissue radiodynamic therapy (RDT) and bolster antitumor immunity. Their method harnesses soft X-ray irradiation to activate a nanoprobe, facilitating energy transfer from SNPs to PCN-224 and significantly boosting ROS generation, particularly at depths of approximately 3 cm. Additionally, Li et al.134 engineered ultrasmall MOF arrays, termed TZMs, utilizing tantalum (Ta) and zirconium (Zr) as metal nodes and TCPP as the photosensitizer (Figure 7A). These structures effectively attenuate X-ray energy, promoting hydroxyl radical production and facilitating energy transfer to enhance 1O2 generation, thereby augmenting the efficacy of RDT (Figure 7B–F).
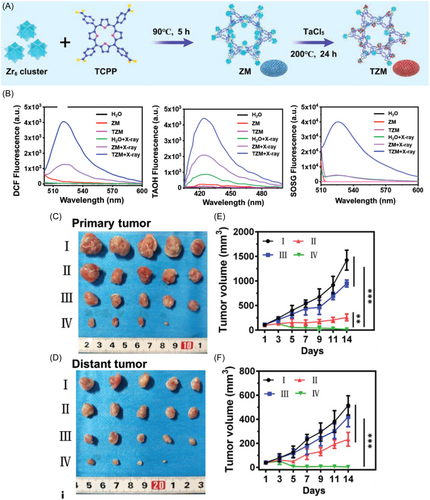
Hafnium (Hf)-based MOFs have been documented to significantly augment the therapeutic effectiveness of ionizing radiation, such as X-rays, and serve as a potent adjunct therapy in synergy with cancer immunotherapy.135 Within the Hf-periodic framework, heavy-element metal clusters efficiently absorb radiation, leading to the generation of -OH radicals. Concurrently, they transfer energy to neighboring photoconductors through inelastic scattering of photoelectrons in the radiation-excited hot spot region, facilitating the production of 1O2. As the X-ray absorption cross-section correlates positively with the atomic number of heavy metals, MOFs comprising heavy metals exhibit enhanced X-ray adsorption, thereby bolstering the radiotherapeutic effect. Ni et al.88 have proposed the utilization of elemental bismuth (Bi), the heaviest naturally occurring nonradioactive element, for constructing MOFs as nano-sensitizers. The augmented kinetic effects of radiotherapy by Bi-MOFs induce stronger local immune activation.
4.5.5 Photothermal therapy (PTT)-driven immunogenic activation
PTT, which induces tumor cell death by generating high temperatures under near-infrared light irradiation, holds significant promise for cancer treatment.136, 137 PTT offers several advantages including high spatial selectivity and specificity, no resistance development, minimally invasive procedures, and minimal side effects.138 By adding imaging agents and immunodrugs at the same time, MOFs can achieve both diagnostic and therapeutic functions. This dual function facilitates real-time monitoring of efficacy and targeted therapies, thereby improving overall treatment outcomes.18 The study by Zhang et al.139 demonstrates the development of multifunctional nanoparticles termed DIMP NPs for potential therapeutic applications. These NPs were fabricated by incorporating ICG and DOX into ZIF-8 nanoparticles, followed by surface modification with polyvinylpyrrolidone (PVP). The inclusion of ICG within the NPs allows for various imaging modalities and therapeutic interventions, such as PTT and chemotherapy, while also enabling real-time monitoring of treatment efficacy.
Modified MOFs can be delivered to targeted tumor cells. This specificity reduces systemic side effects, increases the concentration of therapeutic drugs at the tumor site, and enhances the photothermal and chemotherapy effects. MIL-100, a material containing iron ions, can generate photothermal effects when irradiated with a 671-nm laser. Building on this property, Ni et al.140 incorporated the anticancer drug mitoxantrone into MIL-100 and further modified it with HA to target tumors. Similarly, Liu et al.141 developed nanoparticles by co-loading ICG and Oxa into MIL-100, also utilizing HA for targeted delivery, as illustrated in Figure 8A. Moreover, when these nanoparticles are combined with a PD-L1 antibody, the resulting chemo-PTT not only targets tumors but also activates T-cells (Figure 8B–L). This activation enhances the efficacy of ICB, thereby promoting a systemic antitumor immune response.
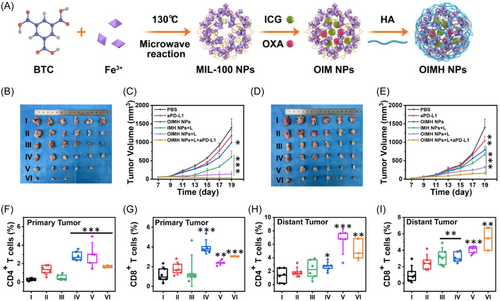
By precisely controlling the release of immunomodulatory agents, MOFs can up-regulate immune response factors such as HSP70 to improve the efficacy of thermal immunotherapy.142, 143 Guo et al.143 developed a novel approach for the programmed upregulation of immune response factors, enhancing the efficacy of thermal immunotherapy. They synthesized ZrMOF-NH2 nanoparticles as carriers, encapsulating CSNO and GGA within nanoparticle channels, followed by sealing with l-menthol. Surface modification with triphenylphosphine yielded the GCZMT nano-amplifier. This nano-amplifier, administered intravenously, accumulates at tumor sites and responds well to microwave-induced heating. It mediates microwave thermotherapy, releasing NO, inducing mitochondrial damage, and promoting apoptosis. The combined effects of microwave heat, NO, and GGA systematically up-regulate HSP70 expression, fostering cytotoxic CD4+ and CD8+ T cell responses for effective antitumor immunotherapy. MOFs act as vectors to directly regulate the immune response, demonstrating an innovative approach to immunotherapy that programs the immune system to enhance antitumor activity by controlling agent release.
4.5.6 SDT-driven immunogenic activation
Ultrasound-triggered SDT represents a noninvasive treatment approach that leverages ultrasound sensitizers, ultrasound sources, and oxygen to produce ROS that kill cancer cells, induce ICD, and activate antitumor immunity.144, 145 Porphyrin-based MOFs can act as ultrasound sensitizers to produce ROS upon ultrasound stimulation and enhancing RDT-induced ICD, thereby enhancing antitumor immune responses.146-148 Zhan et al.149 introduced a novel nanovaccine platform named cMn-MOF@CM, which involves the conjugation of Mn-MOF with the immune adjuvant CpG via TCPP and Mn2+. This platform encapsulates within the membrane of melanoma B16 cells that overexpress OVA. The Mn-MOF component of this nanoplatform plays a crucial role in catalyzing the conversion of H2O2, a substance overly abundant in tumors, into oxygen. Consequently, this action helps alleviate tumor hypoxia and diminish intracellular GSH levels. With the sensitizing effect of porphyrin-based MOFs on ultrasound, the cMn-MOF@CM platform efficiently induced ROS generation upon ultrasound stimulation, effectively promoting cancer cell death. This mechanism is complemented by CpG-induced ICD, which prompts DC maturation and T cell activation, ultimately bolstering the antitumor immune response.
MOFs enable precise delivery of therapeutic agents to tumors and mitochondria, reducing unintended effects while maximizing treatment effectiveness. Luo et al.150 and his team explored the potential of MOFs in SDT. They developed MOFs utilizing a Zr-TCPP base to encapsulate the toll-like receptor agonist TLR7, enhancing SDT. Engineered with cancer cell membrane coatings and triphenylphosphonium (TPP) modifications, these MOFs exhibit dual-targeting capabilities toward tumors and mitochondria, crucial for ATP production and apoptosis induction. This targeted approach aims to synergize SDT with TLR7-mediated DC maturation, potentially offering a potent antitumor effect with reduced side effects. In both studies, MOFs were observed to enhance the efficacy of SDT by fostering ICD and eliciting antitumor immune responses.
4.6 MOFs for imaging and diagnostic promotes immunotherapy
In the field of cancer therapy, the employment of MOFs for imaging and diagnostic purposes has significantly augmented the effectiveness of immunotherapy. Firstly, the integration of diagnosis and treatment enables a more precise localization of tumor characteristics, such as its location, size, and type. This enhanced specificity allows immunotherapy to target specific tumor cells, thereby augmenting therapeutic precision.33, 151 For instance, the HIFU-specific MOF nanosystem (ADMOFs) precisely guides HIFU surgery in MOF-guided imaging by targeting tumors and enhancing photoacoustic/magnetic resonance imaging effects, inhibiting tumor growth and eliminating lung metastasis, suggesting a promising strategy for enhancing cancer theranostics through combining hypoxia-activated chemo-immunotherapy with HIFU.151
Furthermore, the integrated diagnostic-therapeutic technique facilitates real-time guidance during the treatment process, ensuring that immunotherapeutic agents or cells are delivered accurately to the tumor site for optimal efficacy. Concurrently, it also enables the assessment of treatment outcomes, providing crucial insights for subsequent therapeutic planning.152, 153 In a study, Ma et al. developed a PD-L1-based electrochemical aptamer sensor that harnesses the synergistic effects of Fe3O4@UiO-66 nanocomposites and DNA nanocages.152 This innovation achieved real-time, high-sensitivity, and high-specificity guidance and prognostic evaluation for tumor immunotherapy. Concurrently, Feng et al. introduced EV-ANCHOR, a fluorescence aptamer sensor based on MOFs and cholesterol-triggered signal amplification, which enabled the efficient isolation and sensitive detection of PD-L1-positive extracellular vesicles in plasma.153 This advancement significantly enhanced the efficacy of cancer diagnosis and the real-time assessment of immunotherapy.
Moreover, given the significant variations among cancer patients, standardized treatment protocols may not yield the optimal outcomes. The integrated diagnostic-therapeutic technology allows for the formulation of personalized immunotherapy regimens based on specific patient characteristics, such as disease severity, overall health status, and tumor type. This tailored approach ensures that immunotherapy is aligned with the patient's unique needs.155, 156 Fan et al.39 explore the use of GSH-sensitive MIL101-amino MOFs in the ICG-CpG@MOF nanocarrier for targeted cancer therapy. This system delivers indocyanine green (ICG) and cytosine-phosphate-guanine (CpG) to tumors, leveraging the EPR effect for passive targeting and enabling advanced imaging (fluorescence, photoacoustic, photothermal, MRI). The GSH-responsive MOF releases immune adjuvants in the tumor environment, turning “cold” tumors “hot” by increasing immune activity. The study shows that ICG-CpG@MOF achieves tumor cell death and immune enhancement through photodynamic and PTT under 808-nm laser irradiation, leading to significant tumor cytotoxicity, high cure rates, and minimal side effects. Liu et al. construct Er3+-doped NaLnF4@MOF core@shell nanoparticles that integrate NIR-driven PDT and NIR-II imaging, enhancing the accuracy of therapy location tracking and significantly improving tumor inhibition when combined with α-PD-L1 immunotherapy.154 In conclusion, the integration of diagnostic and therapeutic techniques in cancer therapy has ushered in more precise, efficient, and personalized immunotherapy strategies, ultimately contributing to improved patient outcomes and quality of life.
5 CHALLENGES AND PERSPECTIVES
The application of MOFs in cancer immunotherapy presents both promising opportunities and notable challenges. Despite the significant attention that immunomodulatory MOFs have garnered, several hurdles need to be addressed. Firstly, the range of immunomodulators that can be effectively loaded into MOFs is currently limited to chemical drugs and antigens. There is a scarcity of research focused on the delivery of biological macromolecules like nucleotide drugs and cytokines, which also possess immunomodulatory functions. This limitation necessitates further exploration to optimize synthesis protocols, enhance loading efficiency, and improve the stability of MOFs while maintaining the biological activity of the loaded agents.
Moreover, the development of stimulus-responsive functionalized MOFs is essential. These intelligent biomaterials could potentially improve delivery efficiency, reduce systemic toxicity, and minimize drug waste. Introducing compounds responsive to various internal and external stimuli could pave the way for more effective cancer therapies. Additionally, creating MOFs that can mimic complex physiological processes through multilayer structures, core-shell designs, and sequential release strategies could significantly advance staged dosing and treatment efficacy. There remains an untapped potential in combining immunomodulatory MOFs with imaging and tracing capabilities, which could revolutionize the diagnosis and treatment of diseases.
While MOF platforms offer numerous benefits such as ease of functionalization, adjustable pore size, large surface area, and porous structure, their current synthesis methods are complex and multifaceted. This complexity could hinder their clinical application due to potential issues with long-term safety and biocompatibility. Improving biocompatibility through the use of endogenous biomolecules and less toxic metal ions, enhancing stability using water-containing solvents and high-valent metal ions, and achieving ideal nanosizes through advanced synthesis techniques are critical areas for future research. Focusing on green synthesis methods, such as surface modifications using biocompatible polymers like polyethylene glycol, chitosan, and silica could also improve the biocompatibility and stability of MOFs. Additionally, more reliable long-term toxicity data and systemic evaluations of metabolism and degradation pathways are crucial. The stability, aggregation, and premature clearance of MOFs during blood circulation present significant challenges that need to be addressed through careful evaluation of particle size, surface functionalization, and other factors.
In conclusion, while MOFs hold great promise for cancer immunotherapy, addressing the challenges related to synthesis, stability, biocompatibility, and targeted delivery is essential for their successful clinical application. Further research and innovation in these areas will be pivotal in unlocking the full potential of MOFs in the fight against cancer.
6 CONCLUSION
Immunotherapy has profoundly transformed the pathology and clinical management of cancer. However, many malignant tumors remain resistant to immunotherapy, often due to inadequate immunogenicity. It is thus rational to enhance the immunogenicity of tumors to achieve better therapeutic outcomes. MOFs, known for their excellent biocompatibility and biodegradability, hold promise for improving immunogenicity and amplifying the efficacy of immunotherapy, presenting significant advantages over other nanomaterials. In this article, therefore, the application of MOF in immunotherapy is reviewed, mainly covering the role of MOF in the regulation of TME, delivery of immunotherapeutic agents and enhancement of immunogenicity. MOF carriers can enable targeted delivery and release of immunotherapeutic agents in a variety of ways, including helping to induce ICD via aptamers, modulating the TME to reshape the immune response, as well as playing a role in multimodal imaging and therapeutic combination applications. Importantly, due to its unique composition and recognition profile, MOF also actively engages with the immune system to facilitate antitumor responses. These studies have not only expanded the application of MOF in immunotherapy, but also provided new ideas and strategies for tumor immunotherapy.
Despite these advancements, MOF-based immunotherapy is still in its nascent stages. Numerous scientific and technological challenges related to the engineering of MOFs for immunotherapy still need to be addressed. Strategies for combination therapy using MOFs require careful selection of therapeutic modalities to ensure efficacy and safety. The potential for over-immunization in vivo demands cautious consideration, necessitating the rational design of synergistic therapeutic systems that are grounded in a thorough understanding of the mechanisms underlying various treatments. Additionally, there is an urgent need for an improved toxicity evaluation system for MOFs and for further investigation into the mechanisms of immunotherapy. Therefore, future work should be devoted to further improving the design and preparation of MOF vectors for more precise and efficient tumor immunotherapy.
AUTHOR CONTRIBUTIONS
Chenqian Feng and Xiaoyan Liang drafted the initial manuscript and prepared the figures. Rangrang Fan and Min Mu revised the figures for the entire manuscript. Liangxue Zhou and Gang Guo edited the manuscript, guided, and administered the work. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was financially supported by Science and Technology Planning Project of Shihezi University (2023AB047), National Natural Sciences Foundation of China (31971308, 82102767, and U1903211), National S&T Major Project (2019ZX09301-147), and Sichuan Science and Technology Program (2022YFS0007).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




