Standardization of 3D printing parameters to control the size and shape of pores in Polylactic acid scaffolds
Abstract
The challenge of three-dimensional (3D) printing by polymeric extrusion in tissue bioengineering is to control with precision the microarchitecture and porous interconnectivity of scaffolds, as well as search for models that allow and facilitate the development of personalized constructs that meet optimal characteristics for the regeneration of significant bone defects. In this study, anatomically accurate scaffolds were designed and printed to a critical size defect from a microtomography image of the rat calvaria. Different software is used to design a scaffold with exact anatomy. With Ultimaker Cura software, distinct printing parameters were standardized, permitting the printing of different types of pores and graded porosity scaffolds, with exact adaptation to the bone defect, utilizing a commercial 3D printer with a fused deposition modeling technique and compensating for the limitations of the method. The scaffolds were characterized by evaluating their mechanical properties and surface characteristics (pore size and porosity), employing scanning electron microscopy images, verifying that the size and shape of the pores were controlled, and evaluating cell viability and cell distribution on the 3D printed scaffold. Therefore, this work proves that by standardizing the printing parameters, it was possible to print a unique customized scaffold, controlling the shape and size of pores.
1 INTRODUCTION
Bone is formed by mineralized, heterogeneous, and hierarchical connective tissue, with an organic and inorganic matrix in constant change, where different biochemical and cellular processes intervene; the primary function of bone is to form the skeletal system, which protects and supports the organs of the human body and allows their mobility and stores calcium, phosphate, and other minerals, as well as containing the bone marrow.1-3 Currently, bone tissue regeneration is a challenge for tissue engineering (TE), its main objective being to regenerate, maintain or improve bone tissue lost or injured by various etiological factors, such as syndromes, traumatic injuries, and infections that require bone debridement and resection of bone tumors.4, 5 The main treatments for bone regeneration in the clinical setting are based on the use of grafts (autografts, allografts, and alloplastics)6, 7; however, none of them are adapted to the shape and size of the defect, which is a significant challenge in extensive anatomical regions, as is the case with critical bone defects.5
Current treatments are not entirely predictive of these defects, so TE has developed scaffolds as a primary strategy for bone regeneration. Scaffolds are artificial three-dimensional (3D) structures that provide a suitable environment for cell adhesion, migration, proliferation, and differentiation, mimicking the extracellular matrix's (ECM) function. At the structural level, they support the mechanical loads applied while bone regeneration occurs. Scaffolds must be biocompatible, bioresorbable, and biodegradable to be accepted by the body and eventually replaced by healthy tissue.7-9 Structural features include a biomimetic design, which can be customized for each patient.
Among the various methods of 3D scaffold fabrication are particle leaching, gas foaming, phase separation, and electrospinning. However, their main disadvantage is that their fabrication properties are unpredictable, resulting in limited control of characteristics such as pore size, porosity, and scaffold geometry.6, 8 To overcome these challenges, the fused deposition modeling (FDM)-based 3D printing technique has emerged as an ideal approach that allows control over such properties and can customize scaffolds with precise geometries for bone defects through medical imaging.9-11 One of the advantages of using the FDM 3D printing technique is that it allows the use of different thermoplastic polymers approved by the Food and Drug Administration such as polycaprolactone and Polylactic acid (PLA).12 In this work, PLA polymer was used due to its biocompatibility, biodegradability, and good mechanical properties for TE.
Although the use of extrusion-based 3D printing technique has potential in bone TE, there are still certain drawbacks related to obtaining complex structures, such as graded scaffold porosity and pore sizes (in microns), as well as the anatomy of the bone defect. Thus, modification of impression parameters has been reported to improve dimensional accuracy, part quality, and mechanical properties.13-15 In addition, pore sizes and shapes in scaffolds are indispensable as they function as topological signals for cells that can promote adhesion, proliferation, and influence osteogenic differentiation. The range of pore size that has been reported is (100–400 µm), while pores considered favorable for vascularization are within the range of 500–1000 µm.16-18 The shape or type of pore (blind, closed, and open pore) also plays an essential role in bone regeneration, as it influences the response at the cellular (adhesion and proliferation) and tissue (vascularization) levels.12, 19 Therefore, in the present work, scaffolds with graded porosity, that is, scaffolds with different pore sizes and three types of pores, were designed and printed so that the performance of the scaffold would not be limited.
In recent years, 3D printed scaffolds with stem cells have been studied due to their self-renewal, differentiation, and immunomodulation characteristics, which are widely described in the literature.20-22 In this project, we worked with dental pulp-derived mesenchymal stem cells (DPSCs) because their use in bone regeneration has acquired an important role, as several studies have shown that DPSCs can produce a high volume of mineralized matrix, present high cell proliferation, and are easily induced to the bone lineage.23, 24 Therefore, the main objective of this work was to standardize the printing parameters with Ultimaker Cura software to print a scaffold with unique characteristics (graded porosity and three pore types) that had a favorable cellular response for future applications in bone TE.
2 RESULTS
2.1 Scaffold design with graded porosity, including the three pore types
To print a customized scaffold, with graded porosity and three types of pores, a critical size bone defect was made, and a microtomographic study was taken to obtain a 3D image and from this image, design the scaffold with the characteristics mentioned above, using specialized software. Figure 1 shows the different steps for the design of the scaffold.
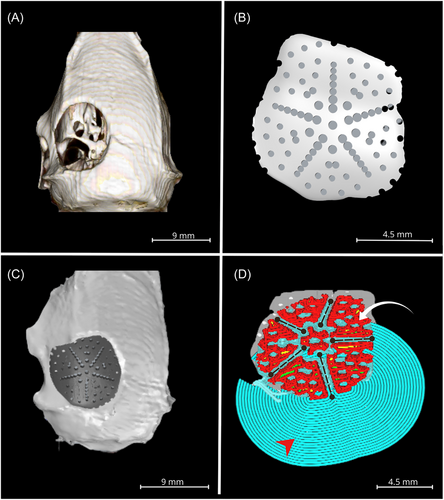
First, a 3D surface was created from a microtomographic images with InVesalius software of a Wistar rat calvaria with a critical-size defect 9 mm in diameter (Figure 1A).
From the image of the defect, a scaffold was designed with three types of pores (blind, closed, and open) and graduated porosity with Rhinoceros 3D software (Figure 1) that, when verified in the image, precisely sealed the defect, considering its size and anatomy (Figure 1C).
The scaffold design was transformed into a G-Code format to be printed using Ultimaker Cura lamination software. Figure 1D depicts the thin layers of the object-to-print, which possess actual printing material, support, and adhesion.
2.2 Standardization of printing parameters
To achieve the impression of a scaffold with graduated porosity and the three types of pores, respecting the concavity of the bone defect, more than 50 printing parameters were standardized in Ultimaker Cura software. Table 1 presents each parameter, including temperature, speed, infill, shell, and others.
| Variable | Parameter |
|---|---|
| Infill | |
| Infill density (%) | 20 |
| Infill line distance (mm) | 2 |
| Infill pattern | Zigzag |
| Infill line multiplier | 1 |
| Infill overlap percentage (%) | 10 |
| Infill layer thickness (mm) | 0.18 |
| Gradual infill steps | 0 |
| Print speed | |
| Print speed (mm/s) | 10 |
| Infill speed (mm/s) | 10 |
| Wall speed (mm/s) | 5 |
| Outer wall speed (mm/s) | 10 |
| Inner wall speed (mm/s) | 10 |
| Top/bottom speed (mm/s) | 6 |
| Travel speed (mm/s) | 80 |
| Initial layer speed (mm/s) | 5 |
| Skirt/brim speed (mm/s) | 5 |
| Enable acceleration control | n/a |
| Enable jerk control | n/a |
| Material | |
| Printing temperature (°C) | 200 |
| Printing temperature initial layer (°C) | 205 |
| Initial printing temperature (°C) | 190 |
| Final printing temperature (°C) | 185 |
| Build plate temperature (°C) | 50 |
| Build plate temperature initial layer (°C) | 50 |
| Enable retraction | Yes |
| Retract at layer change | Yes |
| Retraction distance (mm) | 1.5 |
| Retraction speed | 40 |
| Shell | |
| Wall thickness (mm) | 1.05 |
| Wall line count | 10 |
| Top/bottom thickness (mm) | 0.72 |
| Top thickness (mm) | 0.72 |
| Top layer | 0 |
| Bottom thickness (mm) | 0.72 |
| Bottom layers | 2 |
| Fill gaps between walls | Nowhere |
| Horizontal expansion (mm) | −0.11 |
| Quality | |
| Layer height (mm) | 0.18 |
| Initial layer height (mm) | 0.18 |
| Line width (mm) | 0.2 |
| Wall line width (mm) | 0.2 |
| Outer wall line width (mm) | 0.2 |
| Inner wall (s) line width (mm) | 0.2 |
| Top/bottom line width (mm) | 0.2 |
| Infill layer line width (mm) | 100 |
| Travel | |
| Combing mode | Not in skin |
| Avoid printed parts when traveling | Yes |
| Avoid supports when traveling | n/a |
| Travel avoid distance (mm) | 0.625 |
| Z hop when retracted | n/a |
| Experimental | |
| Minimum polygon circumference (mm) | 0.2 |
| Maximum resolution (mm) | 0.5 |
| Maximum travel resolution (mm) | 0.4 |
| Build plate adhesion | |
| Build plate adhesion type | Brim |
| Brim width (mm) | 5 |
| Brim line count | 15 |
| Brim only outside | Yes |
- Note: Describes the standardization of the printing parameters for a scaffold with heterogenous porosity and different pore types, using the software Ultimaker Cura in the advanced setting.
It is essential to mention that the 200-micron extrusion nozzle was continuously changed because the diameter of the exit orifice was deformed due to the high pressure exerted on it, causing changes in the resolution of the scaffold.
2.3 Printing of the scaffolds with graduated porosity and three types of pores
Once the printing parameters were standardized, images of the 3D printed scaffold were taken to verify that it complied with the characteristics described in the design, and that it maintained the shape and geometry of the bone defect, and when the support and adhesion material was removed, the structure and porosity were maintained. Figure 2 shows the printed scaffold that includes the three types of pores: closed, open, and blind, respecting the graduated porosity. Figure 2A reveals the scaffold printed with support and adhesion material, essential parameters for printing; in this image, the concavity and convexity of the scaffold are evident. Figure 2B is a front-view image of the scaffold, where it can be observed; since its diameter is 9 mm, it corresponds to the diameter of the bone defect that served as a model for its design. Figure 2C corresponds to a concave front view of the scaffold without support and adhesion material; the porosity is observed on the entire surface.
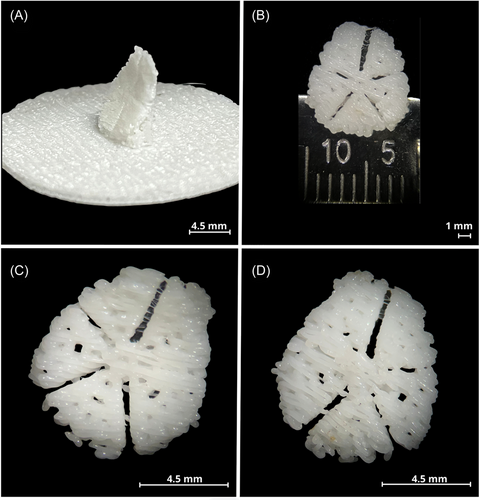
Finally, Figure 2D has a convex front view without support and adhesion material.
Therefore, by standardizing the printing parameters, it was possible to have a customized 3D printed scaffold with graded porosity and three types of pores.
2.4 Superficial characterization of the scaffold by scanning electron microscopy (SEM)
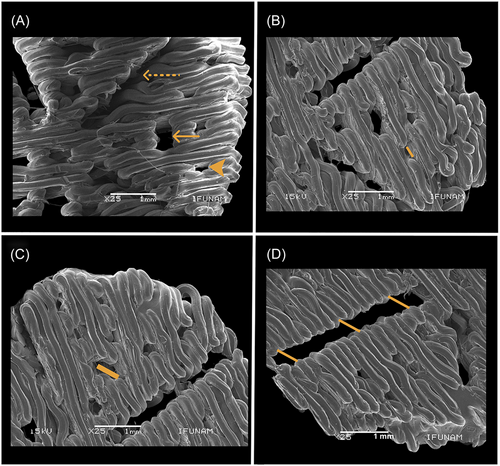
To analyze the surface characteristics of the scaffold, SEM images were taken to obtain the pore sizes, using ImageJ software to evaluate the surface of the scaffolds in greater detail and to observe the three types of pores. Figure 3A shows a panoramic image of the three types of pores indicated with the arrows, the dotted line corresponds to the open pore, the solid line corresponds to the blind pore and the arrow without a line corresponds to the closed pore. Figure 3B is a representative image. of one of the areas of the scaffold, the yellow line shows the diameter of a closed pore of 326.80 µm, Figure 3C shows the diameter of a blind pore of 639.96 µm, Figure 3D shows an open pore where the yellow line near the center area of the scaffold has a diameter of 474.667 µm, the middle area measures 412.04 µm and the edge has a diameter of 234.38 µm. SEM images allowed us to measure and observe in detail the surface of 3D printed scaffolds with graded porosity and three types of pores and verify that the pores were without printing material inside them.
2.5 Graded porosity
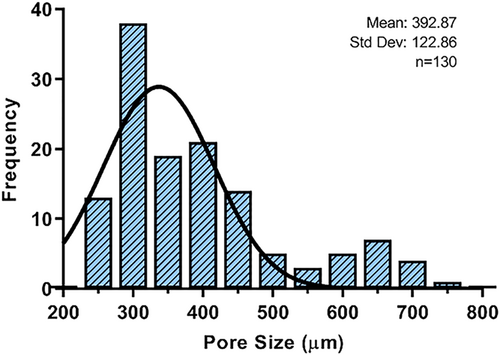
Measurements made on SEM images verify the graded porosity, with the largest pore sizes concentrated in the center of the scaffold and decreasing at the edges. Figure 4 depicts a graph with the pore size ranging from 200 µm minimum to 700 µm maximum, with the average pore size being 392.87 µm and the most frequent pore size, 300 µm. The distribution of pore sizes observed in the histogram confirms the graduated porosity (Figure 4), where the most significant number of pores with smaller diameters are found at the lower limit, and these decrease in frequency and increase in diameter as they approach the upper limit. The histogram shows the distribution of the pores within the scaffold, where the most prominent pores are in the central part to favor vascularization, remembering that the central part of the bone defect is a critical area for regeneration due to the lack of blood circulation, and the smallest pores are at the edge of the scaffold to favor cell adhesion and migration of the cells close to the native tissue. The results confirm that the distribution of the pores in the printed scaffolds is in accordance with the design. Finally, the scaffolds have an average porosity percentage of 68.14%.
2.6 Comparison of printed and designed pore sizes
In the SEM images, it can be observed that some printed pores are slightly smaller than those in the design due to the thermal expansion and subsequent cooling of the PLA during the 3D printing process. Therefore, it was necessary to conduct a statistical analysis, assuming there was no significant difference between the sizes of the printed pores and those of the design.
A Kolmogorov–Smirnov normality test was performed to verify this, where the significance was less than 0.05, indicating that the data do not follow a normal distribution. Therefore, a Mann–Whitney U test was conducted, where the significance was 0.913, and the data were considered statistically significant at less than p = 0.05. Therefore, it is confirmed that it was possible to standardize the printing parameters that controlled the pores' sizes and shapes. SPSS software was employed to analyze all the data.
2.7 Mechanical properties
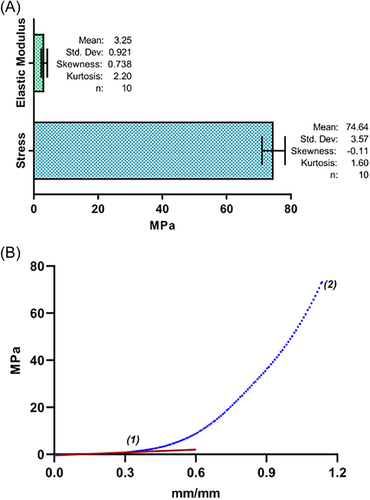
A compression test was performed using a Universal Machine to evaluate the maximum compression and the elastic modulus, with the objective of comparing the data obtained with the mechanical properties of cortical bone described in the literature, which is the bone tissue that makes up the cranial bones. Figure 5 depicts a typical stress-strain curve of the 3D PLA scaffolds obtained, where point 1 represents the coordinates of elastic deformation and elastic limit and point 2 represents the deformation coordinates at the beginning of the fracture of the material and the maximum stress. The maximal average compression obtained was 74.642 MPa, while the elastic modulus obtained was 3.35 MPa.
2.8 Cell viability assay and cell distribution and morphology in 3D printed scaffold
After evaluating the surface characteristics and mechanical properties of the scaffolds, DPSC cells were seeded on the 3D printed scaffolds with graded porosity and three types of pores to evaluate cell viability; in addition, SEM images were taken to observe cell adhesion and distribution. Figure 6 shows the cell viability, there is no statistical difference between Day 1 and Day 7, while there is a difference with Day 14. However, these results corresponded to expectations because the peak of cell growth occurs in the first days; subsequently, it usually decreases because the cells begin to secrete ECM.
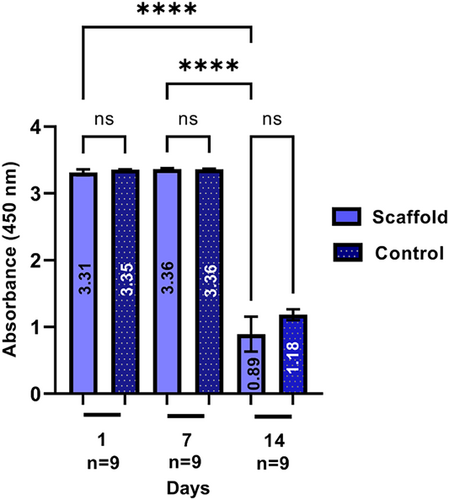
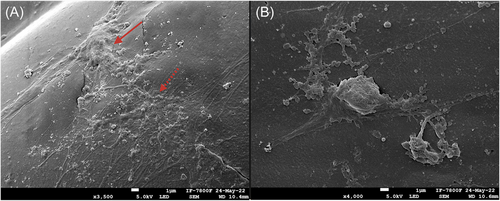
Figure 7 shows the distribution and adhesion of DPSC cells on the 3D printed scaffolds. Observing cell conglomeration and intercellular contact with another set of cells and maintaining the cellular morphology of the DPSCs confirms the good cell viability of the previous assay.
3 DISCUSSION
3D printed objects in the medical field have been used primarily as anatomical models (for education and training purposes), surgical planning (approach, surgical strategy, and choice of device), and the fabrication of implants, prostheses, and surgical guides.25 However, in bone-TE, there are few clinical cases in which medical images are implemented for the design of scaffolds and, later, their printing for bone regeneration.26-29 This is mainly because bone is a complex tissue; therefore, it is necessary to improve the scaffolds’ physical and chemical characteristics to be able to utilize them in the clinical setting.
For the 3D printing of scaffolds, there are different additive manufacturing techniques; in the present study, the FDM 3D printing technology was employed, which is an economical, convenient, and accessible method compared to the others. Printing technologies quickly lead to using scaffolds and implants for bone regeneration in a personalized manner.30 FDM is the methodology with the lowest resolution compared to the remaining methods,31 which implies a challenge in obtaining complex structures, such as the graduated porosity of the scaffolds and the pore sizes (in microns) presented in this work. On including the anatomical characteristics of the bone defect, the main objective of this study, therefore, was to standardize the printing parameters employing Ultimaker Cura software to achieve 3D printing of scaffolds with FDM technology with graduated porosity and pore sizes in microns, which are necessary for bone regeneration.
The scaffolds' personalized design is feasible through medical images obtained from microtomography, as shown in Figure 1. The steps that were carried out to obtain the 3D scaffold for this study agree with what was described by Lal et al.,32 that is, a 3D model creation that relies on data from the 3D Digital Imaging and Communications in Medicine (DICOM) format, which must be converted into a file format that the 3D printer can recognize.32
On the other hand, there is a large amount of literature that reports the printing of 3D scaffolds aimed at modeling bone defects in vivo, considering that what has been reported indicates a variety of flat scaffolds that, despite their possessing the size and thickness, none of these adapts to the anatomical shape of the bone. However, this work shows that by standardizing the printing parameters, it is possible to obtain scaffolds printed precisely in terms of the defect and that the printing is also effective since the results of this study reveal that once the scaffold is printed, it complies with the size and shape of the pores established from its design33-37 (Figures 2 and 3).
To achieve good dimensional accuracy in FDM printed parts, the optimal setting of process parameters varies depending on the material, the complexity of the part's geometry, the type of material, and the chemical composition.10 In this project, the PLA polymer was used, which, in comparison with other materials utilized with the FDM technique, presents more difficulty in the control of its dimensional precision due to its volume change and the residual stress caused by the high melting temperatures and printing; thus, the configuration of critical parameters can reduce these limitations.38
According to other works, the process parameters can be divided into three main sets. The first data set includes process-related parameters, the second set comprises machine-specific parameters, and the third set is related to part geometry, the majority of the essential parameters reported in the work of Abas et al.39 (layer, height, perimeters, infill density, print speed, nozzle temperature, and bed temperature), which coincide with the parameters modified and reported in this work, except for a fill angle of 90° and a print orientation of 90°. However, in this work, other complementary parameters were recorded (see Table 1). The importance of standardizing these is that they directly influence the quality of the piece, its dimensional precision, and its mechanical properties,40 in this work, these were essential to achieve printing the sizes and shapes of the pores in microns, in addition to maintaining its dimensional precision concerning the bone model.
Among the results obtained in this work was the sintering of scaffolds with a heterogeneous surface and graduated porosity; in Figure 3, we can observe the three types of pores (closed, blind, and open) on five different areas of the scaffold surface. According to Chung et al.,41 the closed pore that is isolated within the structure favors cell adhesion and the migration of nearby native cells; for this reason, these pores were concentrated at the edges of the defect, as may be seen in Figure 3B. The blind pore, similar to the closed pore but possessing a larger diameter, as shown in Figure 3C, was printed in the center area of the scaffold. Finally, we find the open pore or microchannel, which connects the internal with the external structure, as observed in Figure 3C. In Figure 3D, the scaffold surface is divided by microchannels to allow the flow of liquids or gases, and the latter is essential for vascularization.42
There is diverse literature reporting that the pore sizes that promote osteogenesis range from 100 to 1000 µm.41, 43 Although there is no consensus on the ideal pore size in bone regeneration, some studies reveal that the pore size that favors the proliferation of adhesion and even influences osteogenic differentiation is 100–400 µm30, 44 Lutzweiler et al.45 points out that porous 3D environments provide several signals that can affect cells. Based on mechanotransduction, external forces are transmitted through actin filaments and are translated into biological signals directed toward the nucleus, triggering various signaling pathways. Consequently, the material's mechanical properties and the curvature provided by the pore walls act as physical cues, thus playing a role in cell fate. The pore size that favors vascularization, therefore the diffusion of oxygen and nutrients, is between 500 and 1000 µm.17, 45, 46
In this project, it was possible to print exact scaffolds for the bone defect, with pore sizes ranging from 200 to 700 µm (Figure 4). The most prominent pores were designed in the center of the scaffold. In contrast, the pores with larger diameters, the small ones, were located at the edges of the scaffold, with the aim of not limiting the functioning of the scaffold by considering both cellular response and internal vascular growth, both necessary in any regeneration process.
Another critical parameter is porosity, the percentage of empty spaces in a solid. The scaffolds with the three types of pores presented an average of 68% porosity, compared to cortical bone, which has 3%–15% porosity, while the trabecular bone possesses one of 50%–90% porosity.17 This percentage coincides with the porosity of the trabecular bone and is greater than that of the cortical bone of rat calvaria (site of the model defect for the design). However, porosity is an indispensable measure of the permeability of nutrients and the oxygen exchange necessary for any regeneration process. Therefore, although the scaffolds possess greater porosity than cortical bone, this can benefit the regeneration process, as demonstrated by findings in the literature.47-49 High porosity also affects the scaffolds' mechanical properties, as noted by Karageorgiou et al.17
Concerning the mechanical properties, the results revealed in Figure 5 represent a stress-strain curve of the 3D printed scaffolds, where the average maximal compression obtained was 74.642 MPa, while the elastic modulus was 3.35 MPa, compared to cortical bone tissue, which has a maximal compression of 131 MPa and an elastic modulus of 10,100 MPa,50, 51 it becomes evident that the mechanical properties of these printed scaffolds are not similar to those of the cortical bone, the native tissue to which the use of these scaffolds is directed. However, it is noteworthy that molecular weight, filament diameter, and extrusion temperature influence the mechanical properties of 3D printed scaffolds, and it has also been reported that increasing porosity leads to a decrease in elastic properties.42
However, it is essential to recall that the cortical tissue of the skull does not receive significant mechanical forces, as would the cortical bone of the femur or other anatomical sites.
In addition, their cellular response was verified with viability assays that demonstrate that the stem cells derived from the dental pulp (DPSC) remained viable on Days 1 and 7, having a decrease on Day 14, which coincides with a study previous, where the same cells will be used at different evaluation times (1, 3, 7, 14, and 21 days), decreasing their cell viability from Day 14, the growth peak occurs in the first days, subsequently, it usually decreases because the cells begin to secrete ECM.52 On the other hand, the SEM micrographs show the efficient cell adhesion on the 3D scaffolds, as well as the cell distribution and morphology, which agrees with that presented by Jain et al.53 Where the cells were aggregated in large groups and the intercellular contact of the cells is observed, through the cytoplasmic extensions, confirming the excellent response of cell viability in the 3D printed scaffold (Figures 6 and 7).
In this research work, it was possible to design and print a 3D scaffold in the shape of the defect, with graduated and heterogeneous porosity, with mechanical properties appropriate to the anatomical site to which it is directed, using the FDM printing technique and standardizing the printing parameters. With future perspectives in mind, using these parameters to control the size and geometry of the pores in personalized scaffold designs will be aimed at clinical bone regeneration.
4 CONCLUSIONS
A personalized scaffold with unique characteristics was designed and printed using a commercial 3D printer with a FDM technique, managing to standardize more than 50 printing parameters not previously described in the literature. These parameters allow for controlling the size and shapes of the pores, closed, open, and blind, compensating for the limitations of the technique. On the other hand, by seeding dental pulp cells, the proposed parameters and the scaffold manufactured showed an excellent biological response of DPSC, as confirmed by the biocompatibility assay based on cell adhesion, cell interaction over the scaffold, and cell viability, making it a promising alternative for TE and future analysis at in vivo defect models.
5 MATERIALS AND METHODS
5.1 PLA scaffolding design
The scaffold design with graduated porosity and three types of pores was made from a microtomographic medical image, which had a critical-size bone defect of 9 mm in diameter in a Wistar rat calvaria.
As mentioned, the image format was in the DICOM format, which was modified to Standard Tessellation Language with InVesalius software.
The programs used 3D Builder, Autodesk Meshmixer, and Rhinoceros 3D to carry out the exact design regarding the defect.
Finally, with Ultimaker Cura software, the G-code format was changed, where more than 50 printing parameters were modified to print an ideal scaffold with the exact anatomical characteristics of the defect, graduated porosity, and three types of pores.
5.2 Scaffolding printing
The scaffolds were printed by the fused deposition technique (FDM) with layer-by-layer extrusion using a 3D printer (Monoprice Select—Mini 3D printer) with manual calibration, with an extrusion tip of 200 microns in diameter, raising the temperature to 205°C to melt the PLA filament.
PLA white with a filament 1.75 mm in diameter and a molecular weight average of 182,000 g/mol.
5.3 Evaluation of the surface structure of the scaffold
Three samples of 3D-printed scaffolds were imaged using backscattered secondary electrons utilizing a JEOL 5600LV SEM at 25 kV operating voltage and ×25 magnification.
The samples were previously prepared with a gold film coating by plasma-assisted sputtering to favor conductivity (IFUNAM Central Microscopy Laboratory). With these images, the surface characteristics of the scaffolds were evaluated, and 130 pore sizes were measured using ImageJ software.
The results made it possible to compare the pore size before and after printing.
5.4 Evaluation of the porosity of the 3D-printed scaffold
5.5 Mechanical tests
A uniaxial compression test was performed on 10 samples of 3D printed scaffolds using a Universal Mechanical Testing Machine (INSTRON 5567) at a speed of 1 mm/min; a 4.5-kN load cell was utilized at a temperature of 23°C and a humidity of 50%, and elastic modulus and maximal stress were calculated.
5.6 Cell culture
Mesenchymal stem cells derived from dental pulp (DPSC) characterized at the Medical Research Unit in Oncological Diseases, Oncology Hospital, Centro Médico Nacional Siglo XXI, IMSS Mexico (74), were cultured and expanded in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, a solution of antibiotics, (penicillin [100 IU/mL], streptomycin [100 μg/mL], and fungizone [0.3 μg/mL]). The cultures were maintained at a temperature of 37°C and in an atmosphere of 95% air and 5% CO2 in an environment with 100% humidity. The 3D printed scaffolds were sterilized by ethylene oxide gas, and the cells were seeded in triplicate at a density of 2 × 104 cells/ml in 46-well plates, and the control group was the DPSC cells seeded in the wells.
5.7 Cell viability assay
To evaluate the viability of DPSC cells on the 3D-printed scaffolds, 20 μL of WST-1 reagent (Roche) was used per 200 μL of culture medium and incubated for 4 h. Subsequently, the solution was transferred to a 96-well plate to perform the reading at a wavelength of 450 nm in an ELISA reader (Chromate, Awareness). The assay was performed in triplicates (n = 9) at 1, 7, and 14 days.
5.8 Cell distribution and morphology in 3D printed scaffold
The cells' integration, distribution, and morphology in the 3D printed scaffold were observed by secondary electrons utilizing a JSM 7800F SEM at 25 kV operating voltage and ×3500 and ×4000.
Samples were washed with phosphate buffer saline, fixed with 4% paraformaldehyde, and subjected to graded ethanol dehydration.
The samples were previously prepared with a gold film coating by plasma-assisted sputtering to favor conductivity (IFUNAM Central Microscopy Laboratory) after 7 days of cultivation of the DPSC.
5.9 Statistical analysis
A Kolmogorov–Smirnov normality test was performed, where the significance was less than 0.05, indicating that the data did not follow a normal distribution. Therefore, a Mann–Whitney U test was conducted, where the significance was 0.913, and the data was considered statistically significant at less than p = 0.05. This test was for comparison of printed and designed pore sizes. One-way analysis of variance with a post-hoc Tukey test was performed, where the significance was less than 0.0001 (****). This test was for the viability assay.
AUTHOR CONTRIBUTIONS
Lucía Pérez-Sánchez: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); writing—original draft (equal); writing—review and editing (equal). Misael A. Ortiz de la O: Methodology (equal); software (equal); validation (equal); writing—original draft (equal). Marco A. Álvarez-Pérez: Investigation (equal); methodology (equal); resources (equal); supervision (equal); validation (equal); writing—original draft (equal); writing—review and editing (equal). Monserrat Llaguno-Munive: Investigation (equal); resources (equal); validation (equal); writing—original draft (equal). Osmar A. Chanes-Cuevas: Methodology (equal). Janeth Serrano-Bello: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); methodology (equal); project administration (equal); resources (equal); supervision (lead); writing—original draft (equal); writing—review and editing (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
Lucía Pérez-Sánchez thanks CONACYT scholarship support (No. 752129 with CVU: 853492) for her support in her Ph.D. studies in the Programa de Maestría y Doctorado en Ciencias Médicas, Odontológicas y de la Salud (PMDCMOS), UNAM. The authors want to thank the supported program projects by DGAPA-UNAM-PAPIIT-IN218223, IN112022, and IN202924.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting this study's findings are available from the corresponding author upon reasonable request.




