Bacterial Outer Membrane Vesicles: From Physics to Clinical
Jun Zhou, Shuang Zou, and Derong Dai should be regarded as Joint first authors.
ABSTRACT
Bacterial outer membrane vesicles (OMVs) are nanoscale vesicular structures naturally produced by Gram-negative bacteria during growth. These vesicles encapsulate a diverse array of bioactive molecules, including proteins, nucleic acids, and lipopolysaccharide, contributing to a range of bacterial processes such as toxin delivery, horizontal gene transfer, and biofilm formation. OMVs play crucial roles in bacterial pathogenesis and host immune modulation, and their presence is implicated in a variety of clinical conditions affecting the respiratory, gastrointestinal, immune, cardiovascular, and urinary systems. The unique properties of OMVs offer promising avenues for clinical translation, including their use as vaccines (against bacterial, viral, parasitic, and tumor-associated), diagnostic tools (for bioimaging and molecular diagnostics), drug delivery vehicles (for antibiotics, anti-cancer therapeutics, and nucleic acids) and regenerative medicine. However, several challenges hinder the widespread clinical adoption of OMVs, including heterogeneity in composition depending on growth conditions, incompletely understood mechanisms of cargo loading and release, inherent immunogenicity and potential toxicity, and limitations in scalable production. This review aims to provide a comprehensive overview of OMVs biogenesis, composition, function, and association with human disease, while also exploring current challenges and future development directions for clinical application.
1 Introduction
Cells produce and release a variety of membrane-bound materials, which are spherical nanostructures derived from the lipid bilayer of the cell surface. These structures are collectively referred to as membrane vesicles, encompassing a range of entities such as microvesicles, exosomes, lysosome, and virus-like particles [1, 2]. These vesicles, originating from different cellular sources, play essential roles in intercellular communication, molecular transport, and immune modulation, reflecting their functional diversity and biological significance. Among these, vesicles released by bacteria, particularly Gram-negative species, have garnered increasing attention due to their unique structural and functional attributes. The primary bacterial vesicles include outer membrane vesicles (OMVs), bacterial extracellular vesicles (BEVs), and cytoplasmic membrane vesicles, each contributing to distinct physiological and pathological processes.
OMVs, in particular, were first observed in the 1960s through electron microscopy studies of bacterial structures [3]. These vesicles were initially described in an auxotrophic Escherichia coli strain under lysine-limited growth conditions [4], marking the beginning of a new era in bacterial research. Subsequent studies expanded the understanding of OMVs, demonstrating their presence in patients with meningococcal infections, which suggested their active involvement in bacterial pathogenesis [5]. Over time, researchers have isolated OMVs from a wide range of Gram-negative bacteria, including Veronigrobacterium spp [6]., Vibrio cholerae [7], Pseudomonas aeruginosa [8], Helicobacter pylori [9], and Salmonella spp [10]., using advanced techniques like centrifugation, filtration, and size exclusion chromatography. These findings have underscored the ubiquity of OMVs across bacterial species and their potential relevance in human health and disease.
OMVs are not merely byproducts of bacterial physiology; they serve as active mediators of various critical processes. These include the delivery of virulence factors, horizontal gene transfer (HGT), defense mechanisms against bacteriophages and antibiotics, detoxification, and intercellular communication [11]. Notably, OMVs contribute significantly to bacterial competition within ecological niches, enabling bacteria to adapt to dynamic environments while modulating their interactions with host organisms [3, 12]. This dual role of OMVs—facilitating bacterial survival and influencing host-pathogen dynamics—has positioned them as central players in microbial ecology and infectious diseases.
Over the past five decades, extensive research has shed light on the formation mechanisms and biological functions of OMVs. For instance, Lee et al. [13] provided a comprehensive overview of the biochemical, biological, and proteomic aspects of OMVs, emphasizing their complex composition and multifaceted roles. Schwechheimer et al. [14] proposed a general mechanism for OMVs biogenesis, highlighting the interplay between bacterial envelope components. Meanwhile, Kulp and Kuehn [11] detailed the unique biological processes mediated by OMVs, distinguishing them from other secretion mechanisms. Further, Kaparakis-Liaskos and Ferrero [15] explored how OMVs induce host pathology or immune tolerance, revealing their dual role in host-pathogen interactions. Intriguingly, Koukoulis et al. [16] suggested that OMVs might traverse the intestinal lumen to cause systemic and neuroinflammation, potentially linking bacterial infections to diseases like Parkinson's. These studies collectively underscore the diverse and intricate roles of OMVs in bacterial physiology and pathogenesis.
Recent research has also shifted focus toward the translational potential of OMVs. LiM et al. discussed the concept of OMVs-based vaccine platforms, exploring design considerations such as antigen localization, adjuvant functionalities, and immune response optimization [17]. Similarly, Li et al. [3] analyzed the structural and compositional aspects of OMVs that contribute to their biomedical applications, such as drug delivery and diagnostics. Such findings highlight the versatility of OMVs as tools for addressing pressing medical challenges, ranging from infectious diseases to cancer therapy.
Despite these advances, the field of OMVs research remains dynamic and evolving, necessitating periodic updates and comprehensive reviews of the latest findings. This review aims to provide a holistic overview of the biological characteristics, functions, clinical relevance and application of OMVs. By synthesizing current knowledge, it seeks to elucidate the mechanisms underlying OMVs biogenesis, their diverse roles in human disease, and the challenges and opportunities associated with their clinical translation. Ultimately, this study aspires to bridge the gap between basic research and clinical application, paving the way for innovative therapies and diagnostic tools based on OMVs.
2 Structural Characterization and Biogenesis Mechanisms of Bacterial OMVs
The Gram-negative bacterial envelope is a uniquely complex structure, comprising two distinct lipid bilayers: the inner membrane (IM) and the outer membrane (OM). These membranes are separated by the periplasmic space, which houses a peptidoglycan (PG) layer [18, 19]. This layered architecture provides structural integrity and functional specialization, enabling Gram-negative bacteria to survive and adapt to diverse environmental pressures. OMVs are primarily composed of lipopolysaccharide (LPS) on their outer leaflet, while phospholipids dominate both leaflets of the IM [12]. This composition reflects the intricate lipid and protein dynamics within the bacterial envelope, which are critical for the biogenesis and functionality of OMVs.
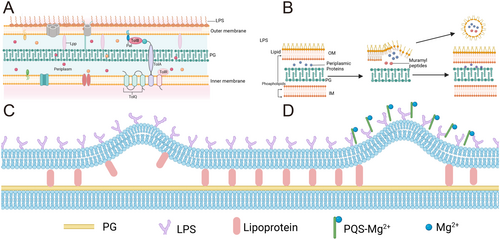
The periplasmic space, occupying approximately 7%–40% of the total cell volume, spans about 13 nm and serves as a pivotal region for the coordination of cellular processes [20]. The thin and rigid PG layer within the periplasm is anchored to the OM and IM by structural proteins such as Braun lipoprotein (Lpp) and outer membrane protein A (OmpA) [20]. These proteins play essential roles in maintaining envelope stability by forming molecular linkages between the PG and the OM. Additionally, the PG is non-covalently bound to the PG-associated Lpp (Tol-Pal) complex, which extends across the periplasm to connect the OM and IM [21]. This structural arrangement ensures the mechanical stability of the bacterial envelope while facilitating dynamic processes such as vesicle formation and secretion (Figure 1A and Table 1).
| Substances that affect/regulate OMVs | |
|---|---|
| Omp | |
| Lpp | Act as molecular backbone linking OM to PG layer |
| OmpA | Binds to potential PG layers in a non-covalent manner |
| Tol-Pal complex | |
| TolA, TolQ, and TolR | Form a complex in the IM that affects division |
| TolB | Periplasmic protein, interacts with LPP, OmpA and Pal |
| Pal | Promotes membrane stability |
| Membrane protein that regulates phospholipid transport | |
| VacJ/Yrb | Environmental stressors such as antibiotic pressure indirectly affect OMVs release |
2.1 Current Paradigms in OMVs Biogenesis
The biogenesis of OMVs is a highly regulated and multifaceted process, influenced by the structural and biochemical properties of the bacterial envelope. Early studies by Burdett and Murray [22] revealed that reduced cross-linking between the PG and OM triggers OMVs formation. This finding highlighted the role of envelope integrity in vesicle production, suggesting that localized disruptions in the PG-OM linkage could initiate vesicle budding. Lpp, serving as a molecular staple connecting the OM to the PG layer, is a critical regulator of OMVs production. Its inactivation has been shown to increase vesicle formation in P. aeruginosa, E. coli, and S. Typhimurium [23]. Similarly, OmpA, which binds non-covalently to the PG, is another key component of OMVs. The loss or dysregulation of OmpA often leads to excessive vesicle formation, underscoring its role in maintaining envelope homeostasis [23, 24]. The Tol-Pal complex, comprising TolA, TolB, TolQ, TolR, and Pal, is another critical player in OMVs biogenesis [25]. TolA, TolQ, and TolR form a complex at the IM, while TolB is a periplasmic protein that interacts with Lpp, OmpA, and Pal [26]. Pal, localized to the inner leaflet of the OM, directly interacts with the PG layer to promote membrane stability [25, 26]. Inactivation of these proteins disrupts cell division and compromises envelope integrity, leading to increased OMVs production [25, 26]. This highlights the interconnectedness of bacterial envelope stability, cell division, and vesicle formation.
Beyond structural proteins, phospholipid dynamics also play a crucial role in OMVs biogenesis. Gram-negative bacteria release OMVs through mechanisms involving phospholipid accumulation and membrane proteins that regulate phospholipid transport, such as VacJ/Yrb [27]. Environmental stress factors, including antibiotic pressure, can further modulate OMVs release. Regulatory molecules like VacJ/Yrb and quorum sensing signals such as Pseudomonas quinolone signal (PQS) have been identified as pivotal factors in this process, particularly during biofilm formation [28]. These findings suggest that OMVs biogenesis is not merely a passive process but rather an adaptive response to environmental and cellular cues.
2.2 Secretory Dynamics and Extracellular Release of OMVs
The extracellular release of OMVs is a complex and dynamic process that remains incompletely understood. Maintaining cellular viability during vesicle formation adds an additional layer of complexity to this phenomenon [29]. Biochemical and genetic studies have provided evidence that OMVs shedding is a regulated process influenced by envelope cross-linking, accumulation of envelope components, and specific lipid insertion into the OM. Several models have been proposed to explain the mechanism of OMVs generation, each highlighting distinct aspects of bacterial envelope dynamics [30]: 1. The Lpp-PG Model: This model posits that the lack of Lpp linkage leads to localized detachment of the PG from the OM, resulting in bulging and subsequent release of OMVs [31, 32] (Figure 1B). The detachment creates regions of membrane instability, allowing the OM to protrude outward and form vesicles. This mechanism underscores the importance of molecular linkages in maintaining envelope integrity and regulating vesicle formation; 2. The PG Turnover Model: This model suggests that when the products of normal PG turnover cannot be effectively internalized, they accumulate in the periplasmic space, exerting turgor pressure on the OM. This pressure causes the OM to swell and eventually shear off, releasing OMVs [33, 34] (Figure 1C). This model highlights the role of metabolic byproducts in vesicle biogenesis, suggesting that disruptions in PG metabolism can directly influence OMVs production; 3. The LPS Charge Model: This model relates OMVs formation to the charge properties of LPS [35, 36]. P. aeruginosa produces both negatively charged and neutrally charged LPS, with OMVs released under oxidative stress predominantly comprising negatively charged LPS. Researchers propose that ionic interactions between PQS and Mg²⁺ in the OM enhance anionic repulsion between LPS molecules, leading to membrane blebbing. Consequently, an increase in negatively charged LPS may facilitate OMVs release [28] (Figure 1D). This model underscores the importance of membrane composition and electrostatic interactions in vesicle formation.
Each of these models provides valuable insights into the mechanisms underlying OMVs biogenesis. However, it is likely that multiple pathways operate simultaneously, reflecting the complexity and adaptability of bacterial envelope dynamics. Furthermore, the interplay between these mechanisms may vary depending on bacterial species, environmental conditions, and growth phases. For example, stress conditions such as nutrient limitation, oxidative stress, or antibiotic exposure may preferentially activate specific biogenesis pathways, thereby altering the composition and functionality of OMVs [37]. These findings emphasize the need for further studies to integrate these models into a unified framework, elucidating the molecular and environmental factors that govern OMVs production.
In summary, the biogenesis and release of OMVs represent a finely tuned process that is critical for bacterial survival, adaptation, and interaction with their environment. Understanding the structural and biochemical underpinnings of OMVs formation not only sheds light on bacterial physiology but also opens new avenues for leveraging these vesicles in therapeutic and diagnostic applications.
3 Functional Diversity and Mechanistic Insights Into OMVs-Mediated Bacterial Processes
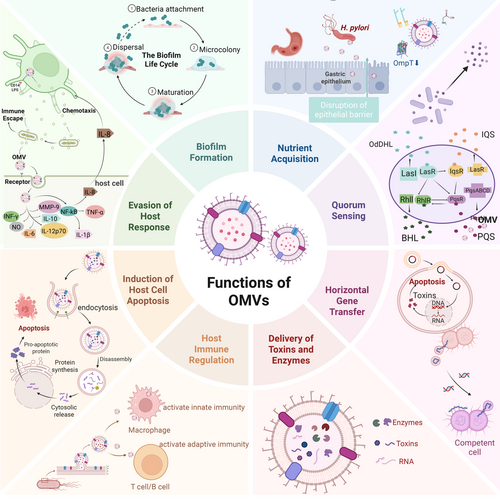
The functional versatility of OMVs is a direct consequence of their dynamic composition, which is influenced by bacterial species, growth conditions, and environmental stressors. This adaptability allows OMVs to serve as vehicles for delivering specific cargo, such as enzymes, signaling molecules, and genetic material, to target cells. Such targeted delivery not only enhances bacterial competitiveness but also contributes to the complexity of microbial communities. Furthermore, OMVs provide a mechanism for bacteria to rapidly respond to environmental changes, including shifts in nutrient availability, exposure to antibiotics, and host immune pressures.
In addition to their role in bacterial physiology, OMVs have emerged as key players in pathogenesis, immune modulation, and biofilm dynamics. Their ability to carry and deliver virulence factors, toxins, and immune-modulatory molecules highlights their importance in mediating host-pathogen interactions. This section explores the diverse functions of OMVs, with a focus on their roles in communication, adaptation, and pathogenicity, while also examining the underlying mechanisms that enable these processes.
3.1 OMVs-Mediated-Facilitated Intercellular Communication and Bacterial Adaptation
OMVs significantly enhance bacterial intercellular communication, enabling the exchange of information and resources within bacterial populations and across species. This capability is essential for coordinating group behaviors, such as quorum sensing and biofilm formation, which are critical for bacterial survival in complex and competitive environments. By acting as carriers of signaling molecules and genetic material, OMVs facilitate interactions that extend beyond the immediate vicinity of individual cells [38, 39].
One of the most striking features of OMVs is their ability to enhance nutrient acquisition, particularly under conditions of nutrient limitation. By packaging degradative enzymes, OMVs enable bacteria to access resources that would otherwise be unavailable. These enzymes breakdown macromolecules into absorbable forms, providing a competitive advantage in resource-limited environments. For example, OMVs from P. aeruginosa have been shown to release amino acids from environmental proteins, supporting bacterial growth and proliferation [8, 40, 41]. This mechanism not only sustains bacterial metabolism but also highlights the ecological significance of OMVs as tools for resource exploitation.
In host-associated environments, OMVs production is often upregulated to counteract host defenses and adapt to hostile conditions. For instance, V. cholerae modifies its OM composition via OMVs to resist antimicrobial peptides and bile salts [42]. This adaptive response involves the selective enrichment or depletion of specific membrane components, demonstrating the ability of OMVs to mediate rapid phenotypic changes in response to environmental pressures. Such dynamic adaptations underscore the role of OMVs in facilitating bacterial survival and colonization in challenging environments.
Beyond nutrient acquisition, OMVs are instrumental in mediating quorum sensing, a process that enables bacteria to coordinate collective behaviors through the secretion and detection of signaling molecules. By encapsulating hydrophobic quorum sensing molecules, such as PQS, OMVs protect these signals from degradation and facilitate their transport over long distances [38, 39, 43]. This stabilization and delivery mechanism enhances the efficiency of quorum sensing, allowing bacterial populations to regulate gene expression in a synchronized manner. As a result, OMVs contribute to the coordination of behaviors such as virulence factor production, biofilm development, and antibiotic resistance.
Another critical function of OMVs is their role in HGT, which enhances genetic diversity and accelerates bacterial evolution. By encapsulating DNA, plasmids, and other genetic elements, OMVs provide a protected environment for the transfer of genetic material between cells [44, 45]. This mechanism is particularly significant in the dissemination of antibiotic resistance genes and virulence factors, as demonstrated in hypervirulent Klebsiella pneumoniae, where OMVs transfer resistance and virulence genes to classical K. pneumoniae [45]. The ability of OMVs to facilitate HGT highlights their importance in shaping bacterial populations and driving evolutionary processes.
Biofilm formation represents another area where OMVs play a crucial role. Biofilms are complex, multicellular structures that provide bacteria with protection against environmental stressors, including antibiotics and immune attacks. OMVs contribute to biofilm development by integrating into the biofilm matrix and promoting bacterial aggregation [46]. In addition to their structural role, OMVs enhance biofilm stability by carrying multivalent adhesins and other matrix components [47, 48]. This dual functionality not only strengthens the biofilm but also facilitates its dynamic remodeling in response to environmental changes.
Interestingly, OMVs can also act as nucleation sites within biofilms, promoting the initial stages of biofilm formation and maintaining community cohesion [49]. This function is particularly important in environments where bacterial populations are exposed to fluctuating conditions, as OMVs help stabilize the biofilm and ensure its resilience. Moreover, OMVs contribute to the hydrophobicity of bacterial surfaces, further enhancing biofilm formation capacity [50]. This property allows bacteria to adhere to a wide range of surfaces, including host tissues and medical devices, thereby increasing their ecological versatility.
While OMVs are essential for biofilm formation, they can also play a role in biofilm disruption. For example, OMVs carrying natural antimicrobial compounds have been shown to inhibit biofilm formation in competing bacterial species [51]. Additionally, OMVs can enhance the efficacy of antibiotics against biofilms by delivering enzymes that degrade the biofilm matrix or by facilitating the penetration of antibiotics into the biofilm [52]. This dual role of OMVs in biofilm dynamics underscores their versatility as both facilitators and disruptors of bacterial communities.
The interplay between OMVs and bacterial adaptation mechanisms highlights their central role in bacterial ecology. By mediating communication, resource acquisition, and biofilm dynamics, OMVs enable bacteria to thrive in diverse environments and respond dynamically to environmental challenges. This adaptability not only underscores the biological significance of OMVs but also presents opportunities for targeting OMVs in the development of novel antimicrobial strategies (Figure 2).
3.2 OMVs-Mediated Bacterial Pathogenicity
OMVs serves as highly efficient delivery systems for transporting virulence factors, toxins, and other bioactive molecules to host cells. This ability to selectively package and deliver pathogenic cargo allows bacteria to manipulate host cellular processes, evade immune responses, and establish infections. The structural and biochemical versatility of OMVs makes them particularly effective in mediating pathogenic interactions, as they can traverse physical barriers and deliver their contents directly to target cells [53, 54]. By acting as carriers of molecular effectors, OMVs contribute to the complexity and severity of bacterial infections, highlighting their importance in host-pathogen dynamics.
A key feature of OMVs is their role in the targeted delivery of virulence factors, which are critical for bacterial survival and colonization within host environments. For instance, H. pylori OMVs carry a diverse array of exotoxins, enzymes, and small non-coding RNAs (sncRNAs) that modulate host immune responses and promote bacterial persistence in the gastric mucosa [15, 55]. The selective enrichment of specific cargo within OMVs reflects the highly regulated nature of vesicle biogenesis, which enables bacteria to tailor their pathogenic strategies to specific host environments. This targeted delivery mechanism not only enhances the efficiency of bacterial virulence but also minimizes the metabolic cost associated with producing and deploying these factors.
In addition to delivering virulence factors, OMVs facilitate the dissemination of toxins within host tissues. For example, OMVs from Aggregatibacter actinomycetemcomitans transport leukotoxin (LtxA), a potent cytotoxin that targets host immune cells. Interestingly, larger OMVs (diameter > 300 nm) are preferentially associated with LtxA, suggesting that vesicle size may influence cargo sorting and delivery efficiency [56]. This observation underscores the complexity of OMVs-mediated toxin transport, as vesicle size, composition, and surface properties can all impact their interactions with host cells. Similarly, OMVs from enterohemorrhagic E. coli (EHEC) (O157) carry Shiga toxin, which induces host cell apoptosis and inflammatory responses [57]. The ability of OMVs to deliver such potent toxins highlights their role as key mediators of bacterial pathogenicity.
OMVs also contribute to bacterial resistance against host immune defenses. For instance, P. aeruginosa utilizes OMVs to confer resistance to polymyxin B, an antimicrobial peptide that targets bacterial membranes [58]. This resistance is achieved through the incorporation of specific membrane components into OMVs, which act as decoys to neutralize the antimicrobial peptide. Such strategies not only protect bacterial populations from host defenses but also enhance their ability to establish and maintain infections. Furthermore, the mobile colistin resistance enzyme MCR-3, carried by OMVs, facilitates bacterial evasion of host phagocytosis, highlighting the dual role of OMVs in resistance and pathogenicity [59]. Indeed, OMVs-mediated pathogenicity is further enhanced by their role in biofilm dynamics. During biofilm development, OMVs production is often upregulated in response to environmental cues. For example, P. aeruginosa produces OMVs enriched with matrix-degrading enzymes during biofilm dispersion, facilitating the breakdown of the biofilm matrix and enabling bacterial escape [60]. This process not only promotes the dissemination of bacteria but also enhances their ability to colonize new environments. The dual role of OMVs in biofilm formation and dispersion underscores their importance in bacterial survival and adaptability.
Another critical aspect of OMVs-mediated pathogenicity is their ability to modulate host cell signaling pathways, often leading to apoptosis or other forms of programmed cell death. For example, the OmpA protein in OMVs has been shown to promote pro-inflammatory responses in macrophages and induce apoptosis in lung epithelial cells [61]. This dual role of OMVs in inflammation and apoptosis underscores their importance in disrupting host cellular homeostasis. Similarly, OMVs from avian pathogenic E. coli (APEC) induce necroptosis in chicken macrophages while activating the nuclear factor-kappa B (NF-κB) signaling pathway, further elucidating their role in the pathogenic mechanisms of APEC infection [62]. The ability of OMVs to trigger such diverse cellular responses highlights their versatility as pathogenic tools.
In some cases, OMVs-mediated apoptosis involves highly specific interactions with host cellular components. For instance, OMVs from K. pneumoniae activate death signaling pathways in human bronchial epithelial cells, leading to cell death and tissue damage [63]. Notably, OMVs from EHEC (O157) are internalized by host cells via endocytosis, where the EHEC hemolysin escapes from lysosomes to target mitochondria, ultimately inducing apoptosis [64]. These findings demonstrate the sophisticated mechanisms by which OMVs manipulate host cell biology to promote bacterial survival and pathogenesis.
In addition to inducing apoptosis, OMVs can modulate host gene expression to create a more favorable environment for bacterial colonization. For example, OMVs from Porphyromonas gingivalis deliver sRNA45033, which targets the host CBX5 gene, leading to DNA methylation changes in the p53 gene [65]. This epigenetic modification not only promotes apoptosis but also triggers the release of inflammatory factors, contributing to the development of periodontitis. Such interactions between OMVs and host epigenetic machinery highlight the intricate ways in which bacteria exploit OMVs to influence host cellular processes.
Overall, OMVs serve as versatile and highly effective tools for bacterial pathogenicity, enabling bacteria to deliver virulence factors, evade immune defenses, and manipulate host cellular processes. Their ability to mediate such diverse functions highlights their central role in host-pathogen interactions and underscores the complexity of bacterial pathogenesis. Understanding the mechanisms underlying OMVs-mediated pathogenicity provides valuable insights into bacterial biology and offers potential targets for therapeutic intervention (Figure 3).
3.3 The Role of OMVs in Immune Regulation
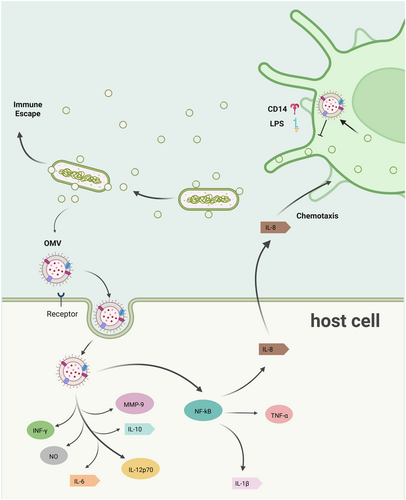
OMVs is also important for the interaction between bacteria and the host immune system, exerting both stimulatory and modulatory effects on immune responses. These vesicles serve as potent immunomodulators due to their ability to carry a diverse array of microbial components, including LPS, outer membrane proteins (OMPs), and nucleic acids, which interact with host immune receptors. Through these interactions, OMVs can either activate immune responses or facilitate immune evasion, depending on the context and the specific bacterial species involved [2, 66, 67]. This dual role underscores the complexity of OMVs-mediated immune regulation and highlights their significance in shaping host-pathogen dynamics.
OMVs are well-known for their ability to activate innate immune responses by interacting with pattern recognition receptors such as Toll-like receptors (TLRs) and Nod-like receptors (NLRs). For instance, OMVs from P. gingivalis have been shown to induce selective tumor necrosis factor (TNF) tolerance through TLR4 and mTOR-dependent pathways [66]. This interaction highlights the capacity of OMVs to fine-tune the host's inflammatory response, potentially contributing to immune evasion while maintaining a level of inflammation that facilitates bacterial survival. Similarly, OMVs from Acinetobacter baumannii enhance immune evasion by modulating PRR signaling pathways, thereby dampening the host's ability to mount an effective immune response [67].
One of the key mechanisms by which OMVs activate innate immunity is through the delivery of LPS, a potent activator of TLR4. The structural and compositional diversity of LPS in OMVs can influence the magnitude and type of immune response elicited. For example, OMVs containing penta-acylated LPS typically induce a robust pro-inflammatory response, while those with tetra-acylated LPS may trigger a more subdued response [68]. This variability allows bacteria to modulate host immune responses in a context-dependent manner, thereby enhancing their adaptability and survival within host environments.
In addition to LPS, OMVs carry other immunostimulatory molecules, such as lipoproteins and PGs, which can activate NLRs and other cytosolic immune sensors. For example, OMVs from Actinobacillus pleuropneumoniae have been shown to induce the release of pro-inflammatory cytokines, including interleukin (IL) and TNF [69]. This activation not only amplifies the host's inflammatory response but also underscores the role of OMVs in bridging extracellular and intracellular immune signaling pathways. As well, OMVs play a significant role in shaping adaptive immune responses. By delivering antigens to antigen-presenting cells (APCs) such as dendritic cells (DCs) and macrophages, OMVs facilitate the activation of T cells and the subsequent development of adaptive immunity. For instance, OMVs from H. pylori have been shown to modulate both innate and adaptive immune responses, enabling the bacterium to establish persistent infections in the gastric mucosa [70]. This modulation involves the selective activation of regulatory T cells (Tregs) and the suppression of effector T cell responses, thereby creating an immunosuppressive environment that favors bacterial survival.
Interestingly, the immunomodulatory effects of OMVs are not limited to antigen presentation. OMVs can also influence the polarization of T helper (Th) cell subsets, thereby shaping the overall immune response. For example, OMVs from P. gingivalis have been shown to promote a Th17-dominated response, which is characterized by the production of IL-17 and other pro-inflammatory cytokines [66]. This response is particularly relevant in chronic infections, where Th17 cells contribute to tissue inflammation and pathology.
Beyond activating and modulating immune responses, OMVs also play a critical role in immune evasion. One of the primary strategies employed by OMVs is the sequestration of immune effectors, such as antimicrobial peptides and antibodies. For instance, OMVs from P. aeruginosa act as decoys for antimicrobial peptides, effectively reducing their concentration at the bacterial cell surface and enhancing bacterial survival [58].
Another immune evasion strategy involves the suppression of host immune responses through the delivery of immunosuppressive molecules. For example, OMVs from H. pylori carry vacuolating cytotoxin A, which induces apoptosis in T cells and suppresses the activation of B cells [71, 72]. This suppression not only impairs the host's ability to mount an effective immune response but also facilitates the establishment of chronic infections. Additionally, OMVs from P. gingivalis deliver small RNAs that modulate host gene expression, further contributing to immune evasion [65].
In certain contexts, OMVs can promote immune tolerance, thereby preventing excessive inflammation and tissue damage. This is particularly relevant in commensal bacteria, where OMVs contribute to the maintenance of host-microbiota homeostasis. For example, OMVs from Bacteroides fragilis have been shown to deliver polysaccharide A to DCs, promoting the induction of Tregs and the suppression of inflammatory responses [73]. This mechanism not only prevents immune-mediated damage to the gut epithelium but also highlights the potential of OMVs as therapeutic agents for modulating immune responses in inflammatory diseases.
The ability of OMVs to modulate immune responses has significant implications for their use in therapeutic applications. For instance, OMVs can be engineered to serve as vaccine platforms, delivering antigens and adjuvants to elicit protective immunity against bacterial, viral, and even tumor-associated antigens [17]. Additionally, the immunomodulatory properties of OMVs can be harnessed for the treatment of autoimmune and inflammatory diseases, where excessive immune activation plays a central role in disease pathology. However, the inherent immunogenicity and potential toxicity of OMVs pose significant challenges to their clinical application, necessitating further research to optimize their safety and efficacy profiles.
In summary, OMVs play a multifaceted role in immune regulation, encompassing the activation of innate and adaptive immunity, the modulation of immune responses, and the facilitation of immune evasion. Their ability to interact with a wide range of immune receptors and pathways underscores their versatility as immunomodulatory agents. Understanding the mechanisms underlying OMVs-mediated immune regulation not only provides valuable insights into host-pathogen interactions but also opens new avenues for the development of OMVs-based therapeutics and vaccines. As research in this field continues to advance, OMVs hold significant promise as tools for modulating immune responses in both infectious and non-infectious diseases (Figure 3).
4 Clinical Relevance and Disease Associations of OMVs
The clinical implications of OMVs are vast and diverse, ranging from their involvement in infectious diseases to their emerging roles in inflammatory disorders, neurodegeneration, and even oncogenesis. Their ability to cross biological barriers, such as the blood-brain barrier (BBB), and modulate host immune responses underscores their significance in disease progression. Importantly, the structural and compositional heterogeneity of OMVs, influenced by bacterial species, growth conditions, and environmental stressors, adds complexity to their roles in clinical contexts. This variability not only affects their pathogenic potential but also presents challenges for their therapeutic and diagnostic applications.
In this section, we delve into the specific roles of OMVs in the pathogenesis and progression of infectious diseases, as well as their associations with inflammatory disorders, neurodegenerative diseases, and cancer. By exploring these aspects, we aim to provide a comprehensive understanding of the clinical significance of OMVs and their potential implications for disease management and therapeutic intervention.
4.1 OMVs in the Pathogenesis and Progression of Infectious Diseases
OMVs are key mediators of bacterial pathogenicity, contributing to the initiation, progression, and severity of infectious diseases. Their ability to encapsulate and deliver virulence factors, toxins, and other bioactive molecules allows them to interact with host cells in ways that enhance bacterial colonization, immune evasion, and tissue damage. This section delves into the diverse mechanisms through which OMVs influence the pathogenesis of respiratory, urinary, and central nervous system (CNS) infections, as well as their broader implications in systemic and localized diseases.
In respiratory infections, OMVs play a pivotal role in facilitating bacterial colonization and persistence within the host. For example, OMVs from P. aeruginosa are enriched with virulence factors, including proteases, elastases, and adhesins, which disrupt host epithelial barriers and promote bacterial adhesion [12, 33, 74]. These vesicles degrade tight junction proteins, such as occludin and claudins, thereby increasing epithelial permeability and facilitating bacterial invasion. The ability of OMVs to modulate host cell signaling pathways further amplifies their pathogenic potential. In chronic respiratory conditions like cystic fibrosis, the contribution of OMVs to disease progression is particularly pronounced. OMVs from P. aeruginosa induce epigenetic modifications in lung macrophages, impairing their ability to clear bacterial infections and promoting a pro-inflammatory environment [75, 76]. This dual role of OMVs in immune modulation and tissue damage underscores their significance in the pathogenesis of respiratory infections. Oral pathogens, such as P. gingivalis, also produce OMVs that contribute to respiratory conditions like aspiration pneumonia. These vesicles reduce the viability of lung epithelial cells, disrupt tight junctions, and induce apoptosis, linking oral infections to respiratory diseases [77]. The ability of OMVs to act as carriers of virulence factors and modulators of host immune responses highlights their central role in respiratory infections.
OMVs are equally significant in the context of urinary tract infections (UTIs), particularly those caused by uropathogenic E. coli. These vesicles enhance bacterial colonization by delivering adhesion factors and biofilm-associated proteins, which facilitate the formation of biofilms on the urinary epithelium [78]. Biofilms not only provide a protective niche for bacteria but also increase their resistance to antibiotics and host immune responses, complicating the treatment of UTIs. In addition to promoting colonization, OMVs play a crucial role in the dissemination of antibiotic resistance genes. For instance, OMVs from A. baumannii have been shown to carry the blaNDM-1 gene, which confers resistance to carbapenems [79]. This HGT mechanism significantly exacerbates the challenge of treating UTIs caused by multidrug-resistant bacteria. The dual role of OMVs in biofilm formation and the spread of resistance genes underscores their importance in the pathogenesis and progression of UTIs.
The ability of OMVs to cross the BBB and induce neuroinflammation is a critical factor in CNS infections. OMVs from Neisseria meningitidis facilitate bacterial penetration of the BBB by degrading tight junction proteins, such as occludin and ZO-1 [80-83]. Once in the CNS, these vesicles activate microglial and astroglial cells, triggering inflammatory responses that contribute to the development of bacterial meningitis. OMVs from P. gingivalis have also been implicated in neuroinflammatory processes. These vesicles activate the NLRP3 inflammasome in microglia, leading to the production of pro-inflammatory cytokines and the phosphorylation of tau proteins in neurons [84]. This mechanism not only exacerbates CNS infections but also establishes a potential link between bacterial infections and neurodegenerative diseases, such as Alzheimer's disease. The ability of OMVs to modulate host immune responses and disrupt neuronal homeostasis highlights their dual role in CNS infections and neurodegeneration.
4.2 OMVs-Associated Inflammatory Disorders
OMVs have been shown to play a pivotal role in exacerbating inflammatory bowel disease (IBD), a chronic and relapsing inflammatory condition of the gastrointestinal tract. OMVs released by adherent-invasive E. coli, a pathobiont frequently associated with Crohn's disease, significantly contribute to intestinal inflammation by upregulating TLR-2 and TLR-4 expression in intestinal epithelial cells [84]. This upregulation enhances the host's sensitivity to bacterial products, triggering pro-inflammatory signaling cascades that lead to the secretion of cytokines such as IL-6 and TNF-α. These cytokines, in turn, recruit immune cells to the site of inflammation, perpetuating a cycle of immune activation and tissue damage. In addition to promoting inflammation, OMVs disrupt the intestinal epithelial barrier, a key feature of IBD pathogenesis. By altering the expression and localization of tight junction and adhesion junction proteins, such as occludin and E-cadherin, OMVs weaken the physical barrier that separates the intestinal lumen from underlying tissues [84]. This barrier disruption facilitates the translocation of bacteria and their products into the lamina propria, further amplifying local inflammation and contributing to systemic immune activation. This process not only exacerbates intestinal inflammation but also increases the risk of extraintestinal complications, highlighting the systemic impact of OMVs in IBD. Interestingly, OMVs also influence metabolic pathways that affect gut homeostasis. For example, OMVs produced by commensal bacteria like Serratia spp. have been shown to promote stable colonization and enhance the host's ability to resist pathogenic infections [85]. These beneficial effects contrast with the pro-inflammatory actions of OMVs from pathogenic bacteria, underscoring the context-dependent roles of OMVs in gut health and disease.
OMVs play a dual role in shaping the composition and function of the gut microbiota, influencing both commensal and pathogenic bacterial populations. On one hand, OMVs from commensal bacteria contribute to the maintenance of microbial balance by promoting biofilm formation and facilitating bacterial colonization [86]. These vesicles carry enzymes, adhesins, and other factors that support the aggregation and stability of beneficial bacterial communities on the intestinal epithelium. By enhancing biofilm integrity, OMVs help create a protective barrier against pathogenic bacteria, thereby supporting gut homeostasis. On the other hand, OMVs from pathogenic bacteria, such as certain strains of E. coli, disrupt microbial balance by promoting the growth and colonization of harmful bacteria [87, 88]. These vesicles deliver virulence factors and biofilm-associated proteins that enhance the competitive fitness of pathogens, often at the expense of commensal populations. This disruption of microbial balance, or dysbiosis, is a hallmark of many inflammatory disorders, including IBD. Dysbiosis not only exacerbates local inflammation but also impairs the host's ability to regulate immune responses, creating a feedback loop that perpetuates disease progression. Moreover, OMVs facilitate inter-bacterial communication through the delivery of quorum sensing molecules, which regulate collective bacterial behaviors such as biofilm formation and virulence factor production [38, 39]. By modulating these interactions, OMVs influence the ecological dynamics of the gut microbiota, with significant implications for both health and disease. These interactions highlight the complexity of OMVs-mediated effects on the gut microbiota and their role in shaping the inflammatory landscape of the gastrointestinal tract.
Periodontal disease, a chronic inflammatory condition affecting the supporting structures of the teeth, provides another example of the pathogenic potential of OMVs. OMVs from P. gingivalis, a keystone pathogen in periodontitis, are instrumental in promoting dysbiosis of the oral microbiota and driving chronic inflammation [89]. These vesicles carry a wide range of virulence factors, including proteases, lipids, and small RNAs, which contribute to tissue destruction, immune modulation, and microbial imbalance. A key mechanism by which OMVs exacerbate periodontal disease is through the degradation of the oral mucosal barrier. Proteases carried by OMVs degrade extracellular matrix (ECM) components and tight junction proteins, facilitating the invasion of P. gingivalis into gingival tissues. This invasion triggers a robust immune response, characterized by the recruitment of neutrophils and macrophages to the site of infection. However, rather than resolving the infection, the immune response is often dysregulated, leading to the release of matrix metalloproteinases (MMPs) and other tissue-damaging enzymes. These processes drive alveolar bone resorption and tooth loss, hallmark features of advanced periodontitis [90].
In addition to promoting tissue destruction, OMVs modulate host immune responses in ways that favor bacterial survival and persistence. For example, OMVs induce the production of pro-inflammatory cytokines, while simultaneously suppressing the activity of antimicrobial peptides and other immune effectors [89]. This dual role in immune activation and evasion enables P. gingivalis to establish chronic infections, further highlighting the pathogenic potential of OMVs in periodontal disease.
4.3 Emerging Evidence for OMVs Involvement in Oncogenesis and Tumor Progression
In recent years, accumulating evidence has highlighted the multifaceted roles of OMVs not only in pathogenic bacterial infections but also in tumor development, progression, and metastasis. These vesicles, through their interactions with host cells, have emerged as critical mediators in the tumor microenvironment. One prominent mechanism by which OMVs contribute to tumor progression is by transporting immunosuppressive molecules, such as PD-L1, along with other regulatory factors. These components effectively impair the host's immune surveillance by reducing the antigen-presenting capacity of DCs and macrophages. This dampens T cell activation, weakens the host's anti-tumor immune response, and consequently enables tumor cells to evade immune detection and destruction [15]. The immunosuppressive properties of OMVs are particularly significant in the context of tumors, where immune evasion is often a prerequisite for sustained tumor growth, survival, and metastasis. By creating an immunosuppressive microenvironment, OMVs not only shield tumor cells from immune attack but also facilitate their proliferation and dissemination.
Chronic inflammation, a hallmark of tumorigenesis, is another critical factor influenced by OMVs. These vesicles are known to activate inflammatory pathways in host cells, thereby fostering a pro-carcinogenic environment that supports tumor initiation and progression [91]. The persistent activation of inflammatory signaling cascades by OMVs contributes to the recruitment of immune cells, the release of pro-inflammatory cytokines, and the generation of reactive oxygen species (ROS), all of which can induce DNA damage and promote genetic instability. Such processes are central to the early stages of tumorigenesis. Moreover, certain microRNAs carried by OMVs may play a pivotal role in tumor development by downregulating tumor suppressor genes, thereby disrupting cellular homeostasis and facilitating uncontrolled cell growth [92]. These microRNAs act as potent regulators of gene expression, and their delivery via OMVs underscores the sophisticated mechanisms through which bacteria can influence host cell biology to their advantage.
As tumors progress, angiogenesis becomes a crucial process for supplying oxygen and nutrients to the rapidly growing tumor mass. OMVs have been shown to actively participate in this process by releasing vascular endothelial growth factor (VEGF) and other pro-angiogenic factors [91]. These factors stimulate the proliferation and migration of endothelial cells, leading to the formation of new blood vessels that sustain tumor growth and provide pathways for metastatic dissemination. Additionally, OMVs enhance the invasiveness of tumor cells by regulating the expression and activity of MMPs. These enzymes degrade components of the ECM, thereby weakening the structural barriers that confine tumor cells. The proteins and nucleic acids carried by OMVs further influence tumor cell-ECM interactions, promoting ECM remodeling and increasing its degradation [93]. This enables tumor cells to detach from the primary site, invade surrounding tissues, and enter the bloodstream or lymphatic system, ultimately facilitating distant metastasis.
The role of OMVs in enhancing tumor cell invasiveness and promoting metastasis is particularly concerning, as metastasis is the leading cause of cancer-related mortality. By modulating the tumor microenvironment, OMVs not only increase the metastatic potential of tumor cells but also contribute to the establishment of pre-metastatic niches in distant organs. These niches provide a favorable environment for circulating tumor cells to colonize and thrive, further underscoring the significance of OMVs in cancer progression.
In addition to their roles in tumor growth and metastasis, OMVs have been implicated in the development of chemoresistance, a major obstacle in effective cancer treatment. By carrying resistance-related genes or proteins, OMVs enable tumor cells to acquire mechanisms that reduce the efficacy of chemotherapy. This reduces the intracellular concentrations of these drugs, thereby minimizing their cytotoxic effects and allowing tumor cells to survive and proliferate despite treatment [94-96]. The contribution of OMVs to chemoresistance is further compounded by their ability to modulate the tumor microenvironment in ways that protect tumor cells from the effects of chemotherapy. For instance, OMVs can deliver anti-apoptotic proteins and other survival factors that counteract the pro-apoptotic signals induced by chemotherapy.
Overall, the involvement of OMVs in oncogenesis and tumor progression highlights their dual role as facilitators of both tumor development and therapeutic resistance. Their ability to influence key processes such as immune evasion, inflammation, angiogenesis, metastasis, and chemoresistance underscores the complexity of their interactions with host cells and the tumor microenvironment. As research into OMVs continues to expand, understanding their molecular mechanisms and functional diversity will be critical for developing novel therapeutic strategies aimed at targeting these vesicles to combat cancer effectively (Table 2).
| Disease types | The roles and impacts of OMVs | Reference |
|---|---|---|
| Sepsis | “OMVs activate the immune system through the LPS-induced activation of the TLR4 signaling pathway, triggering Systemic Inflammatory Response Syndrome (SIRS), exacerbating sepsis, and leading to multiple organ dysfunction.” | [11] |
| Pulmonary infection | OMVs carry virulence factors that activate the TLR signaling pathways in lung cells, triggering a strong inflammatory response and promoting pathogen colonization and invasion. | [12, 33, 74] |
| UTI | OMVs enhance bacterial colonization by delivering virulence factors and antibiotic resistance genes, promoting the inflammatory response of urothelial cells and exacerbating UTIs. | [78] |
| Neurological infection | OMVs cross the BBB, activating inflammatory pathways in the CNS, inducing neuroinflammation and neuronal damage, potentially exacerbating CNS infections such as meningitis. | [80, 83] |
| IBD | OMVs upregulate TLR receptors in intestinal epithelial cells, activating inflammatory responses, disrupting the intestinal barrier, affecting the gut microbiota, and triggering intestinal inflammation. | [97, 98] |
| Periodontal disease | OMVs carry virulence factors (such as BspA, proteases, etc.), activating immune responses, exacerbating inflammation and destruction of periodontal tissues, and contributing to the progression of periodontal disease. | [89, 90] |
| Cardiovascular disease | OMVs induce inflammatory responses in endothelial cells, promote atherosclerotic plaque formation, increase vascular permeability, and exacerbate the progression of cardiovascular diseases. | [99, 100] |
| Tumor | OMVs influence the tumor microenvironment, immune evasion, angiogenesis, and cell metastasis, promoting tumor development and progression, while also showing potential as an anti-tumor therapeutic strategy. | [15, 101] |
5 Translational Applications and Biotechnological Advancements in OMVs Research
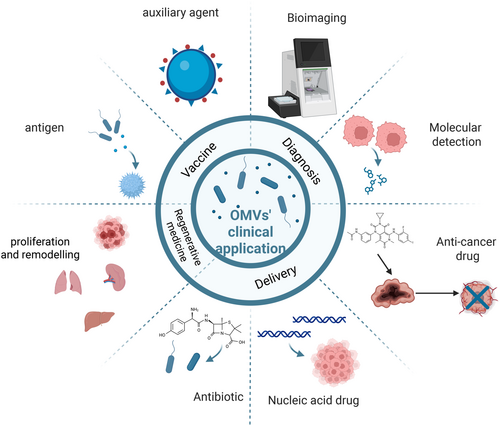
The burgeoning interest in OMVs stems not only from their intrinsic biological roles but also from their remarkable adaptability as platforms for biomedical innovation. Their unique combination of natural immunogenicity, structural stability, and cargo-carrying capacity positions them at the forefront of next-generation therapeutic and diagnostic strategies. Recent advances in nanotechnology, synthetic biology, and bioengineering have further expanded the horizons of OMVs research, enabling precise modifications to enhance functionality, specificity, and safety. These developments are paving the way for OMVs to transcend their traditional roles in bacterial physiology and emerge as versatile tools for addressing unmet clinical needs.
A critical driver of OMVs’ translational potential lies in their ability to interface seamlessly with both microbial and mammalian systems. Their nanoscale dimensions facilitate efficient tissue penetration and cellular uptake, while their lipid bilayer structure ensures protection of encapsulated cargo against enzymatic degradation. Moreover, the inherent diversity of OMVs’ molecular repertoire—spanning antigens, nucleic acids, and signaling molecules—provides a foundation for engineering multifunctional systems. By leveraging these attributes, researchers are now developing OMVs-based solutions that integrate targeted delivery, immune modulation, and real-time diagnostics into unified platforms. Such innovations highlight the transformative potential of OMVs in bridging the gap between microbial biology and clinical innovation.
5.1 Versatile Therapeutic Potential of OMVs
While OMVs have long been recognized for their roles in bacterial pathogenesis, breakthroughs in bioengineering and synthetic biology have unveiled their remarkable versatility as therapeutic platforms [2, 15, 102, 103]. Their unique structural architecture—comprising a lipid bilayer embedded with immunogenic OMPs and LPS—confers exceptional biocompatibility, stability, and low cytotoxicity. These properties, combined with their nanoscale dimensions (typically 20–300 nm), enable OMVs to efficiently traverse biological barriers and facilitate long-range molecular transport [101, 104-108]. Crucially, the inherent immunogenicity of OMVs, driven by LPS and OMPs, positions them as self-adjuvanting vaccine candidates capable of eliciting robust humoral and cellular immune responses [2, 105]. The clinical validation of this approach is exemplified by the FDA-approved meningococcal serogroup B (MenB) vaccine, which utilizes OMVs as a core component to protect infants against N. meningitidis infections [109]. This milestone underscores the translational feasibility of OMVs-based strategies.
Recent advances in genetic engineering have further expanded OMVs’ therapeutic scope. Through modular modifications—such as surface display of targeting ligands or encapsulation of heterologous cargo—OMVs can be engineered for precision-targeted delivery [104]. For instance, antigen-presenting scaffolds like ClyA fusion proteins enable plug-and-play display of tumor-specific epitopes, while chemical conjugation strategies allow precise anchoring of fluorophores or monoclonal antibodies for diagnostic applications [110, 111]. Such adaptability extends to drug delivery, where OMVs demonstrate unparalleled versatility in transporting diverse payloads, including small-molecule antibiotics (e.g., gentamicin [112]), chemotherapeutic agents (e.g., doxorubicin [113]), and nucleic acids. The vesicles’ ability to protect labile cargos from enzymatic degradation while maintaining controlled release kinetics enhances therapeutic efficacy across disease models. Emerging applications in theranostics highlight OMVs’ dual functionality as imaging probes and therapeutic carriers. Fluorescently labeled OMVs, engineered with luciferase or near-infrared dyes, enable real-time tracking of tumor targeting and biodistribution in vivo [110, 111]. Innovations such as melanin-loaded OMVs (OMV-Mels) leverage endogenous pigment synthesis pathways to create photoacoustic contrast agents for high-resolution tumor imaging [114]. Concurrently, OMVs’ diagnostic potential is being harnessed to detect disease-specific biomarkers in biofluids, offering a minimally invasive alternative to conventional assays [111].
The convergence of these attributes—modular design, multifunctionality, and scalable production—positions OMVs at the forefront of precision medicine (Figure 4). Ongoing efforts to optimize cargo loading efficiency, reduce endotoxicity through LPS modification, and standardize manufacturing protocols will accelerate their transition to clinical use. As the field evolves, OMVs-based platforms are poised to revolutionize therapeutic paradigms in oncology, infectious diseases, and regenerative medicine, bridging the gap between microbial biology and cutting-edge biomedical innovation.
5.2 OMVs in Vaccine Development
Traditional vaccines stimulate the immune system by engaging both innate and adaptive immune responses, typically relying on appropriately sized antigenic components and pathogen-specific epitopes to ensure efficacy [115-117]. OMVs represent a paradigm shift in vaccine design, offering distinct structural and functional advantages over whole-cell bacterial vaccines. Their nanoscale dimensions not only facilitate passive drainage into lymphatic vessels but also enhance active uptake by APCs, such as DCs and macrophages, through receptor-mediated endocytosis. This dual mechanism significantly improves antigen delivery efficiency and ensures robust activation of both humoral and cellular immunity [75]. Furthermore, OMVs inherently possess pathogen-associated molecular patterns like LPS and OMPs, which act as natural adjuvants to amplify immune priming without requiring exogenous additives. This intrinsic immunogenicity, combined with their ability to preserve native antigen conformation, enables OMVs to elicit durable immune memory—a critical feature for long-term protection against recurrent infections.
The versatility of OMVs as vaccine platforms is exemplified by their successful application across diverse bacterial pathogens. For instance, OMVs derived from Bordetella pertussis have demonstrated potent protection in murine models by inducing neutralizing antibodies against pertactin and filamentous hemagglutinin, key virulence factors in whooping cough pathogenesis [118]. Similarly, H. pylori OMVs loaded with urease antigens triggered mucosal IgA responses in immunized mice, reducing gastric colonization and inflammation [119]. In P. aeruginosa infections, a bivalent OMVs formulation incorporating diphtheria toxoid and alum adjuvant synergistically enhanced IgG2a and IgG1 titers in burn wound models, achieving > 80% reduction in bacterial load and mitigating tissue necrosis [120]. Innovative multivalent approaches, such as tetravalent Shigella OMVs vaccines targeting serotypes 2a, 3a, 6, and flexneri 2a, have shown cross-protective efficacy in challenge studies, while bivalent formulations against P. gingivalis and A. actinomycetemcomitans significantly reduced periodontal inflammation and alveolar bone loss [121, 122]. Beyond pathogen-specific applications, OMVs from probiotic E. coli Nissle 1917 exhibit dual immunomodulatory and antimicrobial properties, enhancing macrophage phagocytosis while disrupting P. aeruginosa biofilm matrices through delivery of biofilm-degrading enzymes [123, 124]. These findings underscore the adaptability of OMVs in addressing both infectious and dysbiosis-related conditions (Table 3).
| OMVs source | Animal model | Immunization modalities | Protective effect | Reference |
|---|---|---|---|---|
| A. baumannii | ICR | Intramuscular | 73.3% survival rate (7.14% for controls) | [125] |
| Borderella bronchiseptica | BALB/c | Hypodermic | Significant increase in lgG content | [126] |
| Boredetella pertussis | BALB/c | Intraperitoneal | Reduced bacterial burden in lung | [127] |
| Borrelia Burgdorferi | New Zealand White Rabbits | Intramuscular | Reduced bacterial burden in skin | [128] |
| Brucella | BALB/c | Intraperitoneal | Reduced bacterial burden in spleen | [129] |
| Burkholderia mallei | C57BL/6 | Nasal | 100% survival rate (0% for controls) | [130] |
| Burkholderia pseudomallei | BALB/c | Hypodermic | 50% survival rate (0% for controls) | [131] |
| E. coli | C57BL/6 BALB/c | Intraperitoneal | 80%–100% survival rate (20% for controls) | [132] |
| Haemophilus influenzae | BALB/c | Nasal | Reduced bacterial burden in nasopharynx | [133] |
| H. pylori | C57BL/6 | Oral | Reduced bacterial burden in gastric | [134] |
| K. pneumoniae | C57BL/6 | Intraperitoneal | 80%–100% survival rate (0% for controls) | [135] |
| N. meningitidis | Swiss | Intraperitoneal | 80%–90% survival rate (0%–10% for controls) | [136] |
| P. gingivalis, A. actinomycetemcomitans | BALB/c | Nasal | Reduced bacterial burden in oral | [122] |
| P. aeruginosa | BALB/c | Hypodermic | 100% survival rate (0% for controls) | [120] |
| Salmonella enterica serotype Choleraesuis | BALB/c | Nasal/Intraperitoneal | 16.7% survival rate (0% for controls), but longer survival time | [137] |
| Salmonella Enteritidis | BALB/c | Nasal/Intraperitoneal | 92% survival rate (0% for controls) | [138] |
| Salmonella Typhi, Salmonella Paratyphi A | BALB/c | Oral | 90% survival rate (0% for controls) | [139] |
| S. Typhimurium | C3H/HeJ C3H/HeN | Intraperitoneal | Reduced bacterial burden in spleen, liver and lymphatic node | [140] |
| Shigella Castellani | BALB/c | Oral | 83.4% survival rate (0% for controls) | [121] |
| V. cholerae | New Zealand White rabbits | Oral | 100% survival rate (0% for controls) | [141] |
The clinical translation of OMVs-based vaccines reached a milestone with the development of MenB vaccines. Conventional polysaccharide-based strategies faced insurmountable challenges due to structural mimicry between MenB capsular polysaccharides and human neural cell adhesion molecules, raising risks of autoimmune reactions [142]. This impasse was overcome in the 1980s with the Norwegian OMVs vaccine, which utilized detergent-extracted OMVs from N. meningitidis serogroup B. Clinical trials in adolescents demonstrated 57% protective efficacy, attributed to antibodies targeting PorA porins and other surface-exposed antigens [143]. Building on this foundation, the MeNZB vaccine developed during New Zealand's 1990s MenB epidemic achieved 77% reduction in disease incidence through targeted immunization of high-risk populations [144]. These successes catalyzed the creation of the first quadrivalent MenB OMVs vaccine, which integrates recombinant antigens (fHbp, NHBA, NadA) with detergent-treated OMVs to broaden strain coverage [145]. Phase III trials in infants revealed that two doses elicited protective serum bactericidal activity (SBA ≥ 4) in > 90% of recipients, with antibody titers against fHbp, NHBA, and NadA increasing 12–20-fold [109, 146, 147]. Real-world surveillance following Bexsero's inclusion in the UK's National Immunization Program (2015) documented 75% decline in MenB cases among vaccinated infants, validating its public health impact [148]. The favorable safety profile—transient injection-site pain (60%), fever (30%), and fatigue (20%) without severe adverse events—further supports OMVs platforms’ suitability for pediatric use [109]. This trajectory highlights OMVs’ transformative potential in combating antigenically complex pathogens through rational antigen selection and delivery optimization.
The application spectrum of OMVs-based vaccines has been dramatically broadened through innovations in genetic engineering and nanomaterial sciences. For instance, recombinant E. coli engineered to express heterologous antigens from Groups A and B Streptococcus (GAS/GBS) demonstrated remarkable cross-protection in murine models, achieving > 90% survival rates against lethal GAS infections through synergistic activation of both Th1 and Th17 immune pathways [149]. Beyond their role as antigen carriers, bioengineered OMVs exhibit intrinsic adjuvant properties. Flagellum-deficient Salmonella OMVs, for example, bypass TLR5-mediated tolerance while retaining TLR4/MyD88 signaling capacity, thereby stimulating balanced humoral (IgG1/IgG2a) and mucosal (sIgA) immune responses against enteric pathogens [150]. Such dual functionality positions OMVs as self-adjuvanting systems that overcome limitations of traditional alum-based formulations.
To address biosafety concerns in live-attenuated vaccine platforms, researchers have developed LPS-deficient OMVs from Brucella abortus through msbB gene deletion. These modified OMVs eliminate endotoxin-related pyrogenicity while preserving immunodominant OMPs like Omp25, enabling safe intranasal administration with sustained mucosal residency (> 72 h) when encapsulated in thermoresponsive Pluronic F-127 hydrogels [151, 152] (Figure 5A). Nanomaterial conjugation strategies further enhance vaccine performance: Bovine serum albumin (BSA)-coated OMVs improve colloidal stability and antigen presentation efficiency (Figure 5B) [153], while dendritic mesoporous organosilica nanoparticles (DMONs) conjugated to OMVs enable pH-controlled antigen release in lysosomal compartments (Figure 5C) [154]. These innovations have collectively elevated serum antibody titers by 3-5-fold compared to native OMVs in vaccination models [155].
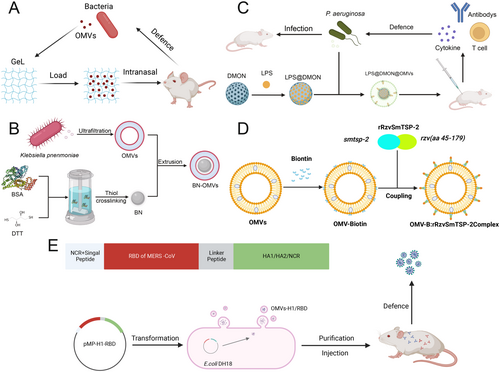
The versatility of OMVs platforms extends beyond bacterial targets. Shehata's pioneering work demonstrated that OMVs decorated with H1N1 hemagglutinin and MERS-CoV spike proteins via SpyTag/SpyCatcher technology induced neutralizing antibodies against both viruses, achieving cross-protective efficacy in challenge studies (Figure 5D) [156]. Similarly, OMVs presenting Schistosoma mansoni SmTSP-2 antigens elicited Th2-polarized immune responses that reduced hepatic egg burdens by 78% in vaccinated mice (Figure 5E) [157], while Trypanosoma cruzi Tc24 antigen-displaying OMVs conferred sterile immunity against lethal parasitemia through CD8+ T cell activation [158]. These successes underscore OMVs’ capacity to bridge prokaryotic and eukaryotic antigen presentation systems.
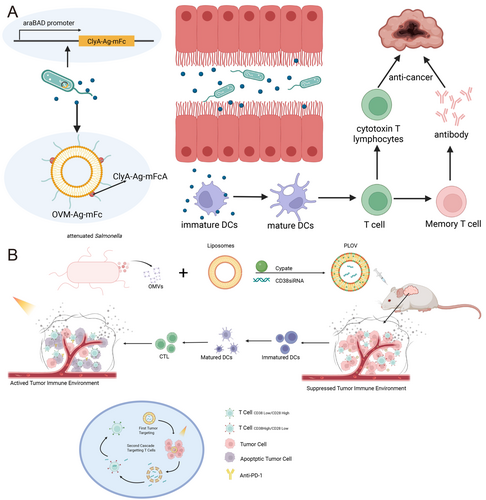
In oncological applications, OMVs are emerging as potent cancer vaccine platforms. Wang et al. [159] engineered OMVs to display HPV16 E7 oncoprotein antigens fused to ClyA scaffold proteins, which promoted DCs maturation and cytotoxic T lymphocyte responses that suppressed tumor growth by 85% in TC-1 mouse models. Multi-antigen co-display strategies, such as co-expressing HER2 and MUC1 tumor-associated antigens on OMVs, have shown synergistic effects in breaking immune tolerance against heterogeneous tumors [160]. Fusing tumor antigens to ClyA allows for simplified antigen display via plug-and-play systems, demonstrating significant anti-tumor [105]. Remarkably, tumor-targeted OMVs secreted by engineered E. coli accumulate preferentially in hypoxic tumor cores via enhanced permeability and retention (EPR) effects, directly delivering therapeutic payloads while activating antitumor macrophages [101] (Figure 6A). Emerging evidence suggests OMVs possess intrinsic oncolytic properties. E. coli-derived OMVs were shown to downregulate cancer stem cell markers (CD44/CD133) by 60%–75% through Wnt/β-catenin pathway inhibition while inducing DNA damage responses via ROS generation [161]. In orthotopic glioblastoma models, OMVs modified with BBB-traversing peptides achieved tumor-selective apoptosis through caspase-3/7 activation and necroptosis pathway induction (Figure 6B) [162]. These multimodal mechanisms—combining immunomodulation, direct cytotoxicity, and microenvironment remodeling—position OMVs as next-generation theranostic agents in precision oncology.
5.3 OMVs-Based Molecular Diagnostics and Imaging
Bioimaging plays a pivotal role in advancing life sciences and medicine by enabling real-time, high-resolution monitoring of physiological and pathological processes. Leveraging their inherent modifiability, biosafety, and capacity for long-range transport, OMVs have emerged as versatile tools for molecular diagnostics and imaging, offering unprecedented opportunities for early disease detection and dynamic monitoring.
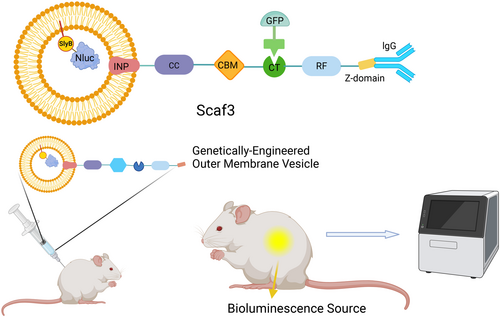
Fluorescence imaging remains one of the most widely utilized techniques due to its sensitivity and specificity. In Chen's pioneering study, OMVs were engineered as multifunctional platforms integrating antibody recognition and biosensing capabilities through dual structural modifications [163]. Internally, the luminescent enzyme Nanoluciferase (Nluc) was encapsulated within OMVs via the SlyB lipoprotein (Lpp) anchor, ensuring stable luminescence signals while preserving vesicle integrity. Externally, a Z-domain scaffold derived from Protein A was surface-anchored using ice-nucleating proteins (INPs), enabling antibody conjugation for targeted imaging. To further enhance versatility, a trifunctional scaffolding system (Scaf3) was incorporated, allowing orthogonal decoration with Dockerin-tagged fluorescent proteins like GFP [163] (Figure 7). This modular design not only improved imaging resolution but also enabled multiplexed detection of cancer-specific biomarkers. Huang et al. [164] expanded this platform by co-administering OMVs with the substrate furimazine, achieving robust bioluminescence imaging in murine models while circumventing limitations such as photobleaching and autofluorescence inherent to traditional fluorophores. The system demonstrated exceptional spatiotemporal resolution, capturing real-time tumor dynamics with minimal background noise.
Beyond fluorescence, OMVs have been harnessed for absorption-based imaging modalities. Gujrati et al. developed OMV-Mels by genetically engineering E. coli to overexpress tyrosinase while deleting the msbB gene to attenuate virulence [114]. This strategic modification enabled endogenous melanin synthesis within OMVs, capitalizing on its broad-spectrum absorption of visible and near-infrared light. When administered intravenously, OMV-Mels generated strong multispectral optoacoustic tomography signals, enabling precise tumor delineation in vivo. Notably, melanin's high photothermal conversion efficiency also positions OMV-Mels as promising theranostic agents for combined imaging and photothermal therapy. Complementary approaches exploit the intrinsic anionic charge of OMVs’ LPS-rich membranes. By leveraging electrostatic interactions with cationic dyes such as indocyanine green, OMVs can accumulate selectively in tumors, facilitating sensitive photoacoustic imaging with enhanced contrast-to-noise ratios [165]. This charge-driven targeting mechanism bypasses the need for complex ligand conjugation, streamlining clinical translation.
The diagnostic utility of OMVs extends beyond imaging to non-invasive biomarker detection in biofluids. Compared to traditional histologic analyses or imaging tests, OMVs isolated from saliva, serum, or urine offer a minimally invasive alternative for monitoring organ function and disease progression. Recent studies highlight the potential of BEVs as prognostic markers, particularly in inflammatory and infectious diseases [166]. Han's seminal work identified elevated levels of LPS-positive OMVs in the saliva of periodontitis patients, achieving superior diagnostic discrimination compared to healthy controls [111]. This finding underscores the potential of OMVs as liquid biopsy targets, with implications for early diagnosis and therapeutic monitoring. The stability of OMVs in biofluids, coupled with their pathogen-specific molecular signatures, positions them as ideal candidates for point-of-care diagnostics in resource-limited settings.
Advances in microfluidics and high-throughput sequencing have further amplified the diagnostic potential of OMVs. For instance, microfluidic chips functionalized with OMVs’ surface receptors enable rapid enrichment and profiling of vesicle-associated biomarkers from minute sample volumes. Meanwhile, OMVs’ ability to cross biological barriers, including the BBB, opens avenues for diagnosing neurological disorders through cerebrospinal fluid analysis. As the field progresses, integrating OMVs-based diagnostics with artificial intelligence-driven data analysis could revolutionize personalized medicine, enabling real-time disease stratification and treatment optimization.
5.4 OMVs in Drug Delivery
In addition to their established roles in vaccine development and clinical testing, OMVs have emerged as highly versatile functional carriers for advanced drug delivery systems. Their unique attributes, including exceptional drug loading capacity, intrinsic targeting capabilities, and remarkable thermal stability, position them as superior candidates for therapeutic applications. Notably, OMVs demonstrate cross-species transferability between bacterial populations, a property that has been strategically exploited for antibiotic delivery. The pioneering work by Kadurugamuwa established this paradigm through innovative exposure of P. aeruginosa to gentamicin, generating antibiotic-loaded OMVs (g-MVs) that exhibited 3–5-fold enhanced bacteriostatic effects compared to free gentamicin [167]. This enhanced efficacy was attributed not only to the vesicular protection of gentamicin from enzymatic degradation but also to the synergistic action of autofuscin constituents within OMVs that potentiate membrane penetration. Subsequent studies revealed that g-MVs achieve targeted antibiotic accumulation at infection sites through membrane fusion mechanisms, effectively overcoming the critical limitation of poor cellular penetration characteristic of free gentamicin molecules [168]. Building on this foundation, Wu et al. [112] engineered a sophisticated Rif@MSN@OMV hybrid system that combines E. coli-derived OMVs with mesoporous silica nanoparticles (MSNs) pre-loaded with rifampicin. This nanoarchitectured platform demonstrated enhanced bacterial uptake efficiency through both passive diffusion and active transport mechanisms, as illustrated in Figure 8A. Recent advancements by Schulz et al. [169] further validated the therapeutic potential of OMVs through deployment of Cystobacter velatus-derived vesicles, which achieved > 90% reduction in bacterial load through synergistic antibiotic delivery and immunomodulation.
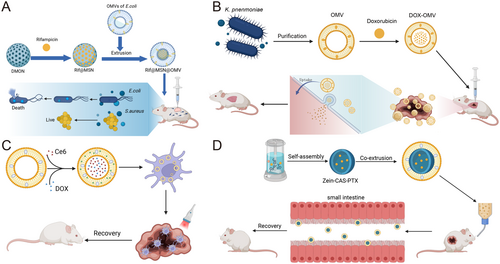
The application spectrum of OMVs has expanded significantly into oncology, particularly for delivering chemotherapeutic agents with suboptimal pharmacokinetic profiles. Doxorubicin (DOX), while effective against various malignancies, suffers from dose-limiting cardiotoxicity and poor tumor selectivity. Kuerban et al. [170] addressed these challenges through innovative loading of DOX into attenuated Klebsiella oxytoca OMVs. The resulting DOX-OMV complexes demonstrated dual therapeutic mechanisms: direct tumor cell cytotoxicity via drug release, coupled with immunogenic activation of tumor-associated macrophages that remodeled the immunosuppressive microenvironment (Figure 8B). This approach reduced tumor volume by 85% while maintaining systemic toxicity within clinically acceptable thresholds. Li et al. [113] extended this concept through co-encapsulation of the photosensitizer chlorin e6 (Ce6) with DOX, creating a theranostic platform that combined photodynamic therapy with chemotherapy. Upon near-infrared irradiation, the Ce6 component generated cytotoxic ROS while simultaneously triggering heat-responsive DOX release, achieving complete tumor regression in triple-negative breast cancer models (Figure 8C). Parallel developments in phytochemical delivery demonstrated that baicalin-loaded OMVs could overcome multidrug resistance in colorectal cancer through P-glycoprotein inhibition and apoptosis induction [171].
Strategic surface modifications have significantly enhanced the tumor-targeting precision of OMVs. The incorporation of RGD peptides (6-Mal-Arg-Gly-Asp) onto OMVs membranes created high-affinity binding to αvβ3 integrins overexpressed on tumor vasculature and metastatic cells, improving tumor accumulation than unmodified vesicles [172]. Nanomaterial conjugation strategies have further optimized drug delivery kinetics—encapsulation of 5-fluorouracil-loaded MSNs within E. coli OMVs extended plasma half-life from 2.3 to 8.7 h while maintaining sustained drug release profiles [173]. Sodium caseinate-coated OMVs demonstrated enhanced colloidal stability and pH-responsive drug release through charge-shielding effects [174] (Figure 8D). To address temporal control challenges, thermosensitive Pluronic F-127 hydrogels have been employed to encapsulate OMVs, creating depot systems that provide localized drug release over 14 days while preventing systemic dispersion [175]. These engineering innovations are systematically compared in Table 4, highlighting progressive improvements in loading efficiency and therapeutic.
| Disease | Specific types | Bacterial strain | Drug | Loading methods | Therapeutic effect | Reference |
|---|---|---|---|---|---|---|
| Infections | PAO1/DH5α | PAO1 | Gentamicin | Antibiotic co-culture | MIC reduced to | [167] |
| E. coli ATCC25922 | E. coli ATCC25922 | Rifampicin-MSNs | Vortex mix | Complete inhibition at 4 µg mL−1 | [112] | |
| enterotoxigenic E. coli | A. baumannii | Levofloxacin | Antibiotic co-culture | Bacterial loads reduced 23.9–28.5-fold. | [94] | |
| Buttiauxella agrestis | B. agrestis | Gentamicin | Antibiotic co-culture | Higher antibacterial effect than free antibiotics | [176] | |
| S. flexneri M90T | S. flexneri M90T | Gentamicin | Antibiotic co-culture | Remove bacteria in cells | [168] | |
| Sorangiineae suborder SBSr073 | S. suborder SBSr073 | Cystobactamid | Bacterial self-contained | Similar inhibitory effect to gentamicin | [169] | |
| Diabetes | Intracranial malignancies | E. coli K1 | NPs | Mixed and extruded | Efficient Brain Accumulation of dOMV@NPs | [177] |
| Lymphoma | E. coli K-12 W3110 | siRNA | Electroporation | % Tumor growth inhibition(TGI) 66.34% | [178] | |
| Triple-negative breast cancer | E. coli BL21 (ΔmsbB) | Paclitaxel+ Redd1-siRNA | Incubation | Tumour reduction and inhibition of metastasis | [179] | |
| Melanoma | Attenuated Salmonella typhimurium | Tegafur+F127 | Mixed and extruded | TGI 70% | [180] | |
| Melanoma | E. coli MG1655 | UNC2025 | Electroporation | TGI 82.05% | [108] | |
| Breast cancer | E. coli MG1655 | Ce6 | Mixed and extruded | TGI 74.44% | [181] | |
| 4T1 breast cancer | E. coli BL21(DE3) ΔmsbB | miR-34a+miR-124+miR-126 | Intracellular expression | Tumour growth restriction | [182] | |
| Non-small cell lung cancer | K. pneumonia ACCC 60095 | Doxorubicin | Incubation | Disappearance of tumour | [170] | |
| Colon carcinomas | E. coli Rosetta | Lysosome-targeting chimera | Intracellular expression | Complete inhibition of tumour growth | [183] | |
| 4T1 breast cancer | E. coli K-12 W3110 | IR780, Cy7, Cy7.5 | incubation | Disappearance of tumour | [165] | |
| Lung cancer | E. Coli@P2O | Adenovirus | Intracellular expression | Inhibition of tumor growth, recurrence metastasis | [184] | |
| Triple-negative breast tumors | E. coli DH5α | Doxorubicin+Ce6 | Incubation | Tumor eradication without recurrence | [113] | |
| Colorectal cancer | E. coli Nissle 1917 | Baicalin | Incubation | Inhibition of vitality and proliferation of HT-29 cells | [171] | |
| Oral squamous cancer | E. coli | MSN-5-FU | Mixed and extruded | Inhibition of tumor growth, metastasis | [173] |
5.5 OMVs in Regenerative Medicine
OMVs have emerged as transformative tools in regenerative medicine, leveraging their unique structural stability, bioactive cargo diversity, and inherent biocompatibility to address multifaceted challenges in tissue repair. Regenerative processes—classically divided into inflammatory regulation, proliferative activation, and remodeling phases [185]—are profoundly influenced by OMVs’ capacity to modulate cellular responses across these critical stages. In anti-inflammatory applications, OMVs derived from Proteus mirabilis demonstrate remarkable therapeutic precision, suppressing RANKL-induced osteoclastogenesis through NF-κB pathway inhibition while preserving osteoblast viability [186]. Preclinical arthritis models reveal a 62% reduction in trabecular bone loss following intra-articular OMVs administration, outperforming conventional bisphosphonate therapies in maintaining joint architecture. This dual action on bone metabolism highlights OMVs’ potential as next-generation biologics for osteoimmunological disorders.
The engineering of advanced nanoprobiotic systems exemplifies OMVs’ adaptability in gastrointestinal regeneration. Hybrid constructs combining E. coli Nissle 1917-derived OMVs with manganese dioxide and metformin exhibit pH-responsive drug release kinetics, achieving 89% remission rates in dextran sulfate sodium-induced colitis models compared to 67% with anti-TNFα monoclonal antibodies [187]. Mechanistic studies attribute this efficacy to synergistic modulation of gut microbiota composition and M2 macrophage polarization, with nanoprobiotics restoring Firmicutes/Bacteroidetes ratios to physiological levels while elevating IL-10 production 3.2-fold versus controls.
While OMVs demonstrate measurable wound closure acceleration (42% epithelialization at Day 7 vs. 28% in saline controls), their regenerative performance in full-thickness skin defects remains secondary to eukaryotic extracellular vesicles [188]. This disparity stems from differential miRNA profiles—OMVs contain 18-fold lower levels of miR-21-5p, a key regulator of fibroblast migration and angiogenesis. However, OMVs-functionalized hydrogels demonstrate superior bacterial clearance (4-log CFU reduction) in infected wounds through sustained β-lactamase inhibitor delivery, suggesting combinational therapeutic advantages.
The antimicrobial-osteogenic synergy of engineered OMVs systems addresses critical needs in orthopedic reconstruction. Tinidazole-loaded OMVs conjugated with Cu-TCPP metal-organic frameworks achieve 96% Staphylococcus epidermidis biofilm eradication while stimulating MC3T3-E1 pre-osteoblast differentiation (ALP activity +217% vs. untreated controls) [189]. Micro-CT analysis reveals these nanocomposites enhance trabecular bone volume fraction by 38% in rat femoral defect models, outperforming commercial bone cement formulations.
Advanced functionalization strategies further expand OMVs’ regenerative scope. Surface immobilization of osteogenic growth peptide transforms OMVs into smart immunomodulators, inducing 83% macrophage polarization toward tissue-reparative M2 phenotypes within 24 h [190]. This engineered platform demonstrates dual-stage therapeutic action: initial anti-inflammatory cytokine secretion (IL-1ra +15.7 pg/mL) followed by sustained VEGF release (321 pg/mL at Day 14) to orchestrate vascularized osseointegration. Such multimodal functionality positions bioengineered OMVs at the forefront of precision regenerative therapies, bridging microbial-derived nanotechnology with clinical translation challenges.
6 Conclusion
Bacterial OMVs, characterized by their nanoscale dimensions and structurally resilient bilayered architecture, have emerged as transformative entities in biomedical research and therapeutic innovation. Their intrinsic biomolecular composition, which faithfully mirrors the parent bacterium's OM and periplasmic constituents, has positioned OMVs as versatile platforms for vaccine development. This is exemplified by the landmark approval of the first OMVs-based vaccine targeting N. meningitidis serogroup B, which has demonstrated clinical efficacy in preventing invasive meningococcal disease [191]. Engineered OMVs, in particular, have unlocked unprecedented potential through advancements in genetic engineering and physicochemical modification strategies. These innovations have enabled precise modulation of OMVs’ immunogenic profiles, targeting specificity, and controlled cargo release kinetics, thereby pioneering novel therapeutic avenues in oncology, antiviral therapies, and antiparasitic interventions, as well as in advanced drug delivery systems and diagnostic assays.
Despite these remarkable advancements, critical challenges persist in translating OMVs-based technologies into routine clinical practice. First, the biogenesis mechanisms of OMVs remain incompletely elucidated [32]. Variations in vesicle size, membrane composition, and cargo loading across bacterial species and growth conditions [37] introduce substantial heterogeneity, complicating large-scale manufacturing processes essential for industrial standardization. Second, the pharmacokinetic behavior of OMVs—including their biodistribution patterns, cellular uptake efficiency, and cargo release dynamics—requires systematic characterization to optimize therapeutic dosing regimens and minimize off-target effects. Third, while OMVs’ inherent immunogenicity drives their adjuvant-like properties in vaccines, components such as LPS and OMPs risk triggering excessive inflammatory cascades, potentially exacerbating tissue damage in sensitive populations. Current strategies to attenuate toxicity, such as LPS truncation via lipid A modification or OMPs depletion, often compromise vesicle stability or immunostimulatory capacity, underscoring the need for innovative engineering approaches. Fourth, the absence of unified regulatory frameworks for OMVs production, quality control, and clinical evaluation hampers cross-institutional reproducibility and delays regulatory approvals [192].
To overcome these barriers, future research should prioritize three interconnected directions: (i) Mechanistic insights into vesiculogenesis: Comparative studies exploring parallels between prokaryotic OMVs and eukaryotic extracellular vesicle biogenesis could illuminate conserved molecular pathways, enabling genetic or pharmacological interventions to standardize OMVs yield and composition (Figure 1A and Table 1). (ii) Functional quantification and clinical translation: High-resolution profiling of OMVs-host interactions—using advanced imaging modalities like cryo-electron tomography and single-vesicle tracking—will establish quantitative structure-activity relationships. Concurrently, expanding Phase II/III clinical trials across diverse pathologies (e.g., solid tumors, chronic infections) will validate OMVs’ therapeutic index in human populations. (iii) Interdisciplinary standardization: Collaborative efforts integrating microbiologists, nanotechnologists, and regulatory bodies must establish Good Manufacturing Practice guidelines for OMVs isolation, characterization, and storage. This includes defining critical quality attributes such as endotoxin units/mg, protein-to-lipid ratios, and nucleic acid contamination thresholds to ensure batch-to-batch consistency.
By addressing these challenges through multidisciplinary innovation, OMVs are poised to transcend their current niche applications, evolving into mainstream tools for precision medicine. Their unique capacity to interface with both microbial and mammalian systems—coupled with engineerable functionalities—heralds a new era in which OMVs will not only augment existing therapeutic paradigms but also redefine diagnostic and regenerative strategies across global healthcare landscapes.
Author Contributions
Jun Zhou: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), resources (equal), software (equal), visualization (equal), writing – original draft (equal), writing – review and editing (equal). Shuang Zou: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), project administration (equal), resources (equal), software (equal), visualization (equal), writing – original draft (equal), writing – review and editing (equal). Derong Dai: conceptualization (equal), data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), project administration (equal), resources (equal), software (equal), visualization (equal), writing – original draft (equal), writing – review and editing (equal). Liqing He: visualization (equal). Xingyu Mou: funding acquisition (equal). Ninglin Zhao: funding acquisition (equal). Hong Li: visualization (equal). Rui Bao: conceptualization (equal), methodology (equal), software (equal), writing – review and editing (supporting), project administration, funding acquisition (equal). All authors have read and approved the final manuscript.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant Number 32370187 to Rui Bao). This study was financially supported by the Postdoctoral Science Foundation of China (Grant Number GZC20241162 to Xingyu Mou, and GZC20241164 to Ninglin Zhao). All figures within this manuscript are drawn and exported in Biorender.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
The authors have nothing to report.




