Platelets: Novel Biomaterials for Cancer Diagnosis and Therapeutic Delivery
ABSTRACT
Platelets play a pivotal role in cancer detection and metastasis, serving both as novel liquid biopsy biomarkers and as versatile carriers in nanomedicine. Tumor-educated platelets (TEPs) undergo molecular alterations influenced by the tumor microenvironment, with their RNA profiles—including mRNA, circular RNA, and long noncoding RNA—offering potential for early cancer detection, prognosis, and treatment monitoring. Additionally, platelet-derived extracellular vesicles (PEVs) and activation markers (e.g., P-selectin, CD40L) further enhance their diagnostic utility. However, standardization of platelet biomarker analysis remains a challenge for clinical implementation. Concurrently, nanotechnology is leveraging the natural biocompatibility and targeting properties of platelets to develop platelet-based drug delivery systems and bioinspired nanomaterials, improving therapeutic precision and efficacy. Moreover, artificial intelligence (AI)-driven biomarker analysis is refining TEP and PEV profiling, accelerating advances in precision oncology. Future research should focus on establishing standardized protocols, optimizing platelet-based nanomedicine, and integrating AI to enhance diagnostic accuracy and therapeutic efficacy. By bridging biological insights with clinical applications, platelets hold significant promise as transformative tools in precision oncology.
1 Introduction
The timely identification of primary tumors and early metastatic spread is essential for the effective initiation of remission-inducing therapies [1]. In light of the often limited success of current cancer treatments and the anticipated rise in the global incidence of cancer, there is an urgent need for novel diagnostic approaches to detect cancer at its earliest stages, whether in primary or metastatic forms [2]. While tissue biopsies remain an important complement to imaging techniques, providing valuable insights into the molecular characteristics of tumors—such as specific mutational signatures associated with resistance to certain therapies—they come with significant drawbacks [3-5]. Tissue sampling is an invasive procedure that can be uncomfortable for patients and carries inherent risks. Moreover, certain tumor sites, such as those located in the brain, are not easily accessible for biopsy.
The growing demand for such innovations highlights the critical necessity of developing techniques capable of identifying these diseases at their inception. Specifically, the advancement of noninvasive imaging modalities that can target a universal molecular marker shared by all tumors holds great promise as a transformative diagnostic tool. A growing area of interest in cancer diagnostics is the analysis of biomarkers released by tumors into the bloodstream and other bodily fluids [6]. Liquid biopsies, which include the sampling of these biofluids, offer a noninvasive alternative to tissue biopsies, with the distinct advantage of being repeatable throughout a patient's treatment course. Analyzing the molecular composition of these biological fluids provides valuable insights into both the primary tumor and distant metastatic sites. Liquid biopsy techniques encompass the detection of circulating tumor cells (CTCs) [7-9], extracellular vesicles (EVs) [10, 11], cell-free DNA and tumor-educated platelets (TEPs) [12], each serving as valuable sources of cancer-associated biomarkers. While these liquid biopsy methods provide unique molecular data individually, their combined use enhances diagnostic accuracy, enabling the generation of highly detailed and comprehensive cancer profiles.
An emerging biosource with considerable promise in the context of liquid biopsies is blood platelets, which are gaining attention for their potential to provide valuable insights into cancer biology and metastasis [13-15]. Platelets are small, anucleate cell fragments (2-4 µm) that arise from larger progenitor cells known as megakaryocytes [16]. Once released into the bloodstream, platelets become the second most abundant cell type in peripheral blood, surpassed only by red blood cells. The normal platelet counts in human blood ranges from 150,000 to 450,000 per microliter. Despite their abundance, platelets have a relatively short lifespan, typically ranging from 8 to 11 days, after which they are cleared from circulation and degraded primarily in the spleen [17]. Although platelets are anucleate cell fragments, they contain a range of organelles and granules in their resting state, including mitochondria, lysosomes, α-granules, and dense granules. On average, resting platelets house approximately 5–8 mitochondria, with the quantity of α-granules correlating linearly to platelet size [18, 19]. Energy production in platelets occurs via glycolysis and mitochondrial respiration. Notably, recent studies have demonstrated that platelet mitochondria undergo fission upon activation, a process that relies on dynamin-related protein 1 (Drp1) [19]. Moreover, MutT Homolog 1 (MTH1) has been identified as a key factor in protecting mitochondria from oxidative stress, with platelets lacking MTH1 showing diminished aggregation and impaired thrombin-induced calcium mobilization [20].
Since their discovery in 1881, platelets have been the subject of extensive research, revealing their multifaceted roles in various physiological processes, including hemostasis, thrombosis, immune responses, inflammation, and notably, cancer metastasis [21]. These small yet highly bioactive cells carry an array of molecules, which play critical roles in cancer progression. Research has shown that platelets play a crucial role in establishing early metastatic niches, which support the dissemination of cancer cells to distant organs. Interestingly, certain signaling factors driving this process may not solely originate from tumor cells but instead be derived from platelets themselves. This platelet-driven recruitment of immune cells may be a crucial early step in the establishment of metastatic niches, underscoring the importance of platelets in the metastatic cascade.
The interplay between platelets and tumor cells is intricate and reciprocal. Studies indicate that CTCs adhere to platelets as a strategy to evade immune detection, while platelets, in turn, actively participate in multiple stages of metastasis [22, 23]. They facilitate the adhesion of CTCs to the vascular endothelium, a crucial step in the extravasation process, and may also contribute to the epithelial-to-mesenchymal transition (EMT) of tumor cells [24, 25]. Furthermore, preclinical studies have shown that both direct and indirect interactions between platelets and tumor cells play a significant role in enhancing tumor proliferation and promoting metastatic dissemination [26, 27]. Platelets possess a sophisticated endocytosis mechanism, which allows them to internalize and store tumor-derived proteins, RNA, and other small molecules. This uptake alters the transcriptome and proteome of platelets, equipping them to influence tumor progression more effectively [28]. In turn, the interaction with tumor cells stimulates platelets to secrete soluble activating factors, further promoting tumor metastasis and disease progression [29-31]. These findings underscore the central role of platelets as active participants in the tumor microenvironment, making them an intriguing target for therapeutic intervention. Thus, The diagnostic potential of TEPs has recently attracted considerable attention. Platelets have the ability to capture and internalize RNA, proteins, and other molecules from tumor cells, thereby altering their own molecular profile. These modifications can be identified and analyzed for diagnostic applications. The RNA signature of TEPs and platelet-derived extracellular vesicles (PEVs) holds promise as a biomarker for minimally invasive blood tests. This makes TEPs an invaluable tool for early cancer detection and accurate tumor localization, offering a noninvasive and efficient alternative to conventional biopsy procedures. However, this limited lifespan has critical implications for the consistency and reliability of platelet RNA profiles in liquid biopsy applications. Platelets are not merely passive carriers of RNA; rather, they can selectively uptake, splice, and modify their RNA cargo in response to external stimuli, including tumor-derived signals [25, 32]. This dynamic nature of platelet RNA processing suggests that platelet lifespan plays a crucial role in determining the temporal stability and consistency of RNA biomarkers for cancer diagnosis.
The primary objective of this review is to investigate the emerging potential of platelets as a valuable source of biomarkers for cancer diagnosis and their feasibility as a liquid biopsy tool. In this review, we examine a range of studies that highlight the promising use of platelet-derived proteins and RNA as biomarkers in tumor diagnostics and metastasis. We also address the current limitations and challenges that must be addressed before these biomarkers can be effectively incorporated into clinical practice. While substantial evidence supports the use of platelets in cancer diagnostics, we explore the technical, biological, and regulatory obstacles that persist and highlight the further research required to facilitate their translation into routine clinical applications.
2 The Internaction Between Platelet and Tumor Cells
The interactions between platelets and cancers are fundamental to the metastatic process, with both physical and biochemical factors shaping this critical relationship. While the connection between platelets and cancer cells was first observed in the late 19th century, the full scope of platelet involvement in metastasis remains incompletely understood. As CTCs navigate through the bloodstream toward distant organs, platelets are among the first cells they encounter [33]. Notably, only about 0.1% of individual CTCs persist beyond 24 h in circulation, with an estimated half-life of approximately 1 h, significantly limiting their metastatic potential [34, 35]. However, the presence of platelets, which often surround CTCs upon their initial entry into the circulatory system, provides them with a physical shield against the harmful fluid shear stresses (FSS) encountered in the bloodstream [36]. This platelet-derived “cloak” not only safeguards CTCs from mechanical injury but may also confer a metabolic advantage, further supporting their survival and dissemination (Figure 1). Under these conditions, platelets may preferentially divert the metabolic pathways of CTCs toward glycolysis, a process known to support cell survival under stressful conditions [37-39]. Furthermore, the disruption of platelet-cancer cell interactions could potentially expose CTCs to increased shear stress, which might lead to the destruction of these CTCs. This disruption could impair the metabolic advantage that platelets confer to CTCs, potentially reducing their ability to survive in the bloodstream and limiting their metastatic potential.
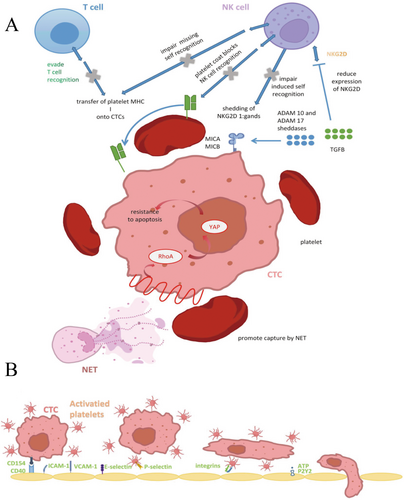
2.1 The Direct Interaction Between Tumors and Platelets
For stable adhesion between platelets and tumor cells, tumor cells employ various mechanisms to activate platelets, triggering their aggregation and creating a protective microenvironment around CTCs. Extensive research has demonstrated that tumor-induced platelet activation and aggregation play a crucial role in enhancing tumor cell survival and metastatic potential. Once activated, platelets become highly adhesive, forming clusters around CTCs to shield them from fluid shear stress and immune surveillance, thereby facilitating their passage through the circulatory system (Figure 1).
Tumor cells can directly induce platelet activation in several ways (Figure 2). One mechanism is the release of soluble platelet agonists into the circulation, which activate platelets upon contact. Another pathway involves direct interaction between tumor cells and platelets, a process known as tumor cell-induced platelet aggregation (TCIPA) [40]. Various molecular signals are involved in TCIPA, including high-morbidity group box 1 (HMGB-1)-Toll-like receptor 4 (TLR4), CD40L–CD40 interactions [41], TXA2–TXR, and ADP–P2Y12 [42-46]. The alarmin protein HMGB1, which can be released by dying tumor cells circulating in the bloodstream, serves as a natural ligand for TLR4 on platelet surfaces [47]. This interaction activates the myeloid differentiation factor 88 (MyD88)-dependent cGMP–PKG signaling pathway [48]. Furthermore, during the EMT process, CTCs of breast cancer deposit extracellular matrix (ECM) proteins, such as type I collagen, around themselves, further influencing their microenvironment and metastatic potential [49]. This collagen forms an essential scaffold for platelet interaction, facilitating successful colonization of metastatic sites. Podoplanin, a surface ligand expressed on tumor cells, binds to C-type lectin-like receptor 2 (CLEC2) on platelets, playing a crucial role in platelet activation, which is upregulated in various tumor types [50, 51] and contributing to venous thromboembolism (VTE) in cancer patients [52]. This interaction signals through immunoreceptor tyrosine-based activation motifs (ITAM) can activate downstream signaling molecules protein kinase C and integrin-αIIbβ3 as well as the subsequent granule release. In vivo experiments in mice have shown that inhibiting this interaction can effectively suppress platelet aggregation and tumor metastasis [52]. Several studies have demonstrated that platelet activation in response to tumors is largely mediated by ITAMs, such as glycoprotein VI (GPVI), CLEC2, and FcγRIIa, which are expressed on platelets [53-56]. Beyond these well-established soluble factors, some cancer cell types express G protein-coupled receptors (GPCRs), such as CD97, whose extracellular domain interacts with platelets, initiating their activation [57] (Figure 2).
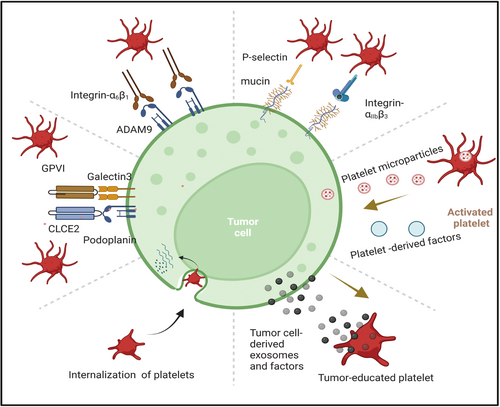
Tumor cell-induced platelet activation plays a crucial role in supporting tumor survival, proliferation, and metastasis. Notably, platelets contribute to the metastatic progression of solid tumors by promoting EMT through TGF-β signaling. Both platelet-derived TGF-β and direct platelet-tumor cell interactions have been shown to trigger key signaling pathways, including TGF-β/SMAD and NF-κB. This activation drives the transition to a more invasive mesenchymal phenotype, thereby enhancing metastatic potential [24]. This transition includes the upregulation of EMT-associated genes like Snail1 and Vim [58]. Additionally, CD40 on platelets may activate NF-κB signaling in tumor cells, further promoting their invasive behavior [59]. Beyond TGF-β, PDGF has also been implicated in promoting EMT in cancer cells through several signaling pathways, including Notch-1, NF-κB, STAT-1, mTOR, Bcl-2, and p38/MAPK [60-62]. Inhibition of either TGF-β from platelets or NF-κB signaling in cancer cells has been shown to prevent metastasis in vitro, suggesting that targeting these pathways could limit tumor spread. The platelet cloak surrounding CTCs thus provides them with a source of TGF-β1, promoting a more invasive, mesenchymal-like phenotype and enhancing their ability to extravasate and colonize distant tissues. Platelets also contribute to endothelial arrest by bridging the gap between CTCs and the endothelial cells through interactions between the GPIb-IX-V receptors on platelets and von Willebrand factor (vWF) on the endothelial surface [63, 64]. Alternatively, CTCs may engulf platelets to escape immune surveillance, incorporating platelet-derived proteins such as regulator of G protein signaling 18 (RGS18), which upregulates human leukocyte antigen E (HLA-E) expression on the tumor cell surface. HLA-E can then bind to NKG2A receptors on natural killer (NK) cells, inhibiting NK cell activity and thereby facilitating metastasis [65]. These integrins are critical for the platelet-cancer cell interaction, and inhibiting ITGB3 disrupts platelet-tumor cell adhesion, which could serve as a potential therapeutic strategy to reduce metastasis. This interaction is crucial for tumor cells to arrest at metastatic sites and initiate extravasation.
2.2 The Indirect Interaction Between Tumors and Platelets
Beyond direct activation, tumor cells can indirectly stimulate platelets by activating procoagulant pathways. For instance, tumor cells can induce the generation of thrombin through the activation of coagulation factors, including cancer procoagulant (CP), which acts as a factor X activator [43]. This procoagulant activity leads to platelet activation through the extrinsic coagulation pathway. Tumor cells also secrete soluble procoagulant factors, such as thrombin and tissue factor (TF)-bearing extracellular vesicles [66], which mimic vessel injury and trigger platelet aggregation by activating the extrinsic coagulation pathway (Figure 2). Pancreatic cancer cells, for example, have been shown to directly generate thrombin, further stimulating platelet activity [42].
Additionally, tumor cells can induce platelet activation indirectly by engaging innate immune cells in circulation. For example, carcinoma mucins can simultaneously bind to P-selectins on platelets and L-selectins on neutrophils, fostering crosstalk between these two cell types. This interaction, along with reciprocal signaling between platelets and neutrophils via P-selectin and its ligand, P-selectin glycoprotein ligand-1 (PSGL-1), leads to the activation of both cell populations [67] (Figure 2). In addition, neutrophil extracellular traps (NETs)—which consist of DNA and proteases released by neutrophils—have been shown to possess the ability to activate platelets and induce thrombosis. There is significant evidence supporting tumor-induced NET formation, establishing it as a key mechanism for tumor-mediated platelet activation [68].
Platelets store a variety of growth factors within their α-granules, including PDGF, vascular endothelial growth factor (VEGF), and TGF-β, which are released upon platelet activation [69] (Figure 2). These factors can be exploited by CTCs to enhance proliferation and evade apoptosis, particularly in response to chemotherapy [70, 71]. Additionally, platelets play a crucial role in establishing early metastatic niches. By releasing chemokines such as CXCL5 and CXCL7, they can recruit granulocytes to the tumor site, even in the absence of direct tumor signaling [72]. Experimental studies have demonstrated that blocking CXCL5/7 receptors (CXCR2) or depleting platelets and granulocytes prevents early metastasis formation in animal models [24]. The CXCL5 axis has been particularly implicated in facilitating breast cancer metastasis to bone [73]. Furthermore, RNA sequencing of single CTCs and CTC clusters has identified the overexpression of platelet-associated gene markers, such as ITGA2B (integrin alpha-IIb; CD41) and ITGB3 (integrin alpha-V beta-3; CD61), within CTC data sets [74-76]. Platelet-derived autotaxin (ATX), an enzyme responsible for generating the lipid signaling molecule lysophosphatidic acid (LPA), interacts with tumor-expressed integrin αVβ3, thereby promoting breast cancer metastasis to bone [77]. Additionally, ATX enhances CTC survival by preventing anoikis, a form of cell death triggered by detachment from the extracellular matrix, through activation of the RhoA-Gα12/13-YAP-1 signaling pathway [57]. Moreover, platelet-derived TGF-β1 and matrix metalloproteinase-1 (MMP-1) are instrumental in facilitating bone metastases, while platelet uptake of tumor-derived proteins further supports distant metastatic progression [37]. Beyond their role in metastasis, platelets also contribute to tumor angiogenesis by secreting a range of angiogenic regulators, including VEGF, to sustain tumor growth and vascularization [78, 79].
In addition, platelets also release a variety of pro-survival, proangiogenic, and immune-modulating factors in a contact-independent manner. These factors can help construct and maintain both primary and metastatic tumor microenvironments. Platelets can synthesize prostaglandin H2 (PGH2), which is converted into several bioactive isomers, including prostaglandin E2 (PGE2) and thromboxane A2 (TXA2), both of which exert pro-tumoral effects such as promoting angiogenesis and inducing stromal cell proliferation [80]. PEVs, including microparticles and exosomes, are rich in microRNAs and can interact with tumor cells through functional membrane proteins such as GPIbα, integrin αIIbβ3, and P-selectin [81-84]. These interactions actively support tumor cell proliferation and facilitate metastatic progression. Collectively, these findings indicate that tumor cells may engage in a form of ‘platelet mimicry,’ acquiring and leveraging platelet-derived lipids, nucleic acids, and proteins to evade immune detection while simultaneously enhancing their growth and metastatic potential.
3 Overview of Platelet Markers
Platelet markers are critical indicators of platelet function, reflecting their involvement in cancer metastasis, thrombosis, and inflammation. These markers include platelet count, activation markers (such as P-selectin, GPIIb/IIIa, and soluble CD40L), PDEVs, transcriptomic profiles of TEPs, and platelet-derived cytokines like platelet factor 4 (PF4) and TGF-β. These markers not only signal platelet activation and aggregation but also influence tumor progression, angiogenesis, and metastasis. As such, they hold significant potential for cancer diagnostics, prognosis, and therapeutic monitoring, providing insights into the molecular mechanisms underpinning tumor cell interactions with platelets (Figure 3).
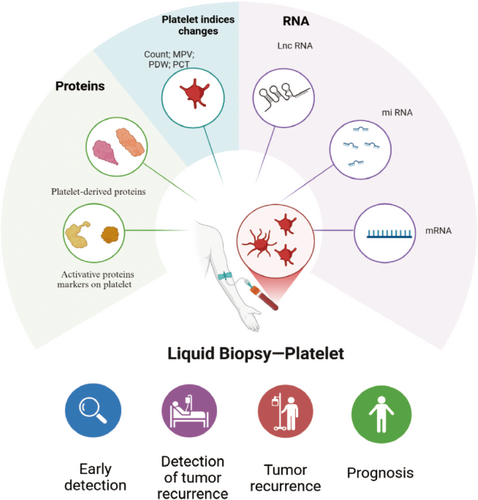
3.1 Platelet Indices Changes
Clinical studies have indicated that an elevated platelet count may serve as a predictive marker for certain cancers and a potential tool for monitoring tumor progression. Research on thrombocytosis has shown that nearly one-third of patients diagnosed with lung or colorectal cancer within a 2-year timeframe presented with no apparent malignancy-related symptoms aside from an increased platelet count [85]. This finding highlights the potential of thrombocytosis as a subtle yet significant indicator of underlying cancer, even in the absence of other clinical signs. Moreover, in cancers such as ovarian, lung, and gastric cancers, thrombocytosis is often associated with hematogenous metastasis and disease recurrence [86-88].
The production of platelets is regulated by thrombopoietin (TPO), a cytokine primarily synthesized in the liver. TPO binds to the thrombopoietin receptor, c-Mpl, located on megakaryocytes, thereby promoting their growth and maturation within the bone marrow [89]. Once mature, megakaryocytes extend cytoplasmic projections known as proplatelets, which eventually fragment to form platelets. Research suggested that IL-6 plays a critical role in the induction of thrombocytosis and may influence tumor cell survival and chemotherapy efficacy [89]. In clinical settings, particularly among breast cancer patients, the association between thrombocytosis and poorer survival outcomes is more pronounced in inflammatory tumor subtypes [90]. These subtypes exhibit heightened activity in inflammation-related and immune-modulatory signaling pathways, suggesting that inflammation may intensify the link between elevated platelet counts and adverse prognosis. While IL-6 plays a significant role, additional factors such as granulocyte–macrophage colony-stimulating factor (GM-CSF), CCL5, and PF4 are also secreted by tumor and stromal cells within the tumor microenvironment [91, 92]. These factors drive platelet production by promoting megakaryocyte proliferation. Notably, studies in mouse models of breast cancer have demonstrated that inhibiting these factors suppresses tumor-associated megakaryocyte proliferation and the subsequent rise in platelet production, highlighting potential therapeutic strategies for mitigating thrombocytosis in cancer [93].
In addition to alterations in platelet count, studies have also highlighted significant differences in other platelet indices, such as mean platelet volume (MPV), platelet crit (PCT), and platelet distribution width (PDW), among cancer patients [94, 95]. Elevated MPV values have been observed in various types of cancer, particularly in more advanced stages, such as gastric cancer [96], esophagus cancer [97], and lung cancer [98]. A meta-analysis conducted by Xia et al. reviewed 11 studies and found a strong correlation between high PDW levels and poor overall survival in cancer patients, particularly in those with breast cancer and pharyngo-laryngeal cancer. Additionally, high PDW was notably associated with lymph node metastasis, suggesting that this platelet index could serve as a potential prognostic marker for metastatic spread [99]. In the study by Karateke et al., the authors confirmed the prognostic value of platelet indices in gynecological conditions [100]. The highest MPV, PDW, and PCT values were observed in patients with endometrial cancer identified by histopathological examination, compared to the control group. The authors concluded that these platelet indices could potentially aid in identifying patients at risk for malignancies, even before histopathological examinations are conducted. This finding underscores the utility of platelet indices as noninvasive biomarkers, which could assist in the early detection and diagnosis of cancer, offering a cost-effective and accessible method for monitoring patients at risk.
3.2 Tumor-Educated Platelets
Platelets have emerged as significant carriers of various RNA biomarkers, including messenger RNA (mRNA), long noncoding RNA (lncRNA), circular RNA (circRNA), and mitochondrial RNA. Despite lacking a nucleus, they possess essential molecular machinery for RNA processing, including a functional spliceosome and a protein translation system [32]. This enables them to carry out a full range of RNA-related processes, such as pre-mRNA splicing, pre-microRNA maturation, and mRNA translation. Upon interaction with tumor cells or tumor-associated biomolecules, platelets undergo significant alterations in their RNA profiles. This transformation gives rise to TEPs, which mirror the molecular modifications induced by the tumor microenvironment [101-103].
Although the majority of platelets' RNA content originates from their precursor cells, megakaryocytes, they are also capable of absorbing tumor-derived RNAs from both the bloodstream and the tumor microenvironment. Platelets can directly uptake circulating mRNA from the peripheral blood, a process that enhances their RNA content with tumor-specific signatures [102]. External signals or activators can also trigger platelet activation, which in turn induces specific splice variants of pre-mRNAs. As a result, these changes generate unique mRNA profiles that could have valuable implications for cancer diagnostics, potentially offering a noninvasive means of detecting and monitoring tumor-related molecular alterations [32, 104-106]. This capacity of platelets to store and process tumor-derived RNA opens up avenues for developing liquid biopsy-based diagnostic tools, providing crucial insights into the molecular landscape of cancer. Thus, specific changes in platelet RNA signatures may have been linked to the presence of early-stage tumors, metastatic spread, and poor prognosis in the future.
3.3 Platelet-Derived Vesicles
Platelets communicate with tumor cells either through direct interactions or indirectly via EVs released from tumor or platelet cells. EVs are small membrane-bound particles secreted by various cell types, including erythrocytes, megakaryocytes, platelets, leukocytes, and tumor cells, and they can be detected circulating in the bloodstream [107]. These vesicles represent a promising avenue for liquid biopsy applications, providing valuable molecular information for cancer diagnostics. Notably, platelets have the ability to capture tumor-specific EVs, which may carry tumor-associated RNAs, thereby enhancing their functional role in molecular crosstalk within the tumor microenvironment [102, 108].
PEVs are diverse in nature and, based on their biogenesis and size, can be categorized into various subtypes. Platelet microparticles (PMPs), the most prevalent form of PEVs, represent approximately 70%–90% of all EVs present in the bloodstream [109, 110]. The discovery of these vesicles can be traced back to 1967, scientists identified them as small procoagulant structures originating from activated platelets [111]. The vesiculation process involves the budding of the platelet's cytoplasm and membrane, resulting in the formation of PEVs. These vesicles carry a diverse array of bioactive molecules, including cytokines, functional enzymes, mRNA, and noncoding RNA, all originating from the platelet's internal environment [112].
Interestingly, platelets contain an average of four mitochondria, and during the formation of PEVs, some of these may be incorporated into larger ones [113]. A proteomic analysis has confirmed that larger microparticles tend to harbor mitochondrial proteins, while smaller ones do not [113]. Despite the growing interest in PEVs, there is still no standardized nomenclature for the various populations of EVs originating from platelets or megakaryocytes. Future research is needed to refine the characterization of these vesicular populations, which could help to standardize their classification and improve their diagnostic utility.
While further studies are required to fully elucidate the complex interactions between tumor cells, platelets, and their vesicles, the examination of PEVs and their associated cargo holds promise in identifying potential cancer biomarkers. When combined with the analysis of tumor-derived EVs and TEPs, PEVs could significantly enhance diagnostic approaches, providing more robust and noninvasive tools for cancer detection and monitoring.
3.4 Activation Markers
Platelet activation markers, such as P-selectin, GPIIb/IIIa, and soluble CD40L (sCD40L), play crucial roles in the process of tumor-induced platelet activation. The expression of sialyl-Lewisx and sialyl-Lewisa, both of which are recognized by selectins, is elevated, suggesting that selectin-mediated interactions may contribute to tumor metastasis in many cancers [114, 115]. Among the selectins, P-selectin is specific to platelets and plays a unique role in metastatic progression. Its expression is upregulated by various platelet surface receptors, particularly those linked to metastasis. Key ligands for P-selectin include P-selectin glycoprotein ligand-1 (PSGL-1) and CD44 [115]. Upon platelet activation, P-selectin rapidly translocates from α-granules to the platelet membrane, where it binds to its ligands, triggering a cascade of downstream effects [116]. Notably, P-selectin promotes the expression of matrix metalloproteinases (MMPs) such as MMP-2 and MMP-9, as well as integrin β3 in CTCs, enhancing their invasive potential and facilitating metastasis [117]. In addition, P-selectin stimulates the secretion of cytokines like IL-10 and IL-4, which further support tumor cell proliferation, survival, and metastatic spread [118]. Furthermore, P-selectin mediates interactions between platelets and neutrophils, leading to the formation of NETs, which play a pivotal role in immune evasion and CTC extravasation [119]. By recruiting platelets, P-selectin helps establish a pro-metastatic microenvironment that supports tumor spread [120]. Moreover, P-selectin can recruit various bone marrow-derived cells to the site of metastasis, further enhancing the metastatic process [121].
Another crucial integrin in platelet-mediated tumor metastasis is αIIbβ3 (also known as GPIIb/IIIa or the fibrinogen receptor) [49, 122]. This integrin is abundant on platelets and plays a central role in platelet deformation, adhesion, and thrombus formation [123, 124]. αIIbβ3 is a key mediator of TCIPA, and its activation can be triggered by various agonists, such as TXA2, thrombin, and ADP [125, 126]. CTCs express αvβ3 integrins, and the crosslinking of αIIbβ3 on platelets with αvβ3 on CTCs facilitates platelet adhesion to the tumor cells. Beyond adhesion, αIIbβ3 also activates intracellular signaling pathways, such as the PI3K-Akt pathway, which promotes the release of VEGF from platelets [125, 127]. In turn, increases VEGF expression in CTCs, enhancing their metastatic potential.
Moreover, platelets express CD40 ligand (CD154), which is critical for angiogenesis [128]. Studies in CD40-deficient mice have demonstrated impaired tumor growth and reduced tumor vasculature, particularly in mammary gland tumors, highlighting the role of CD40 in tumor progression [129]. Platelet-derived CD40L directly interacts with CD40 receptors on tumor cells, promoting neovascularization within the tumor microenvironment and facilitating the establishment of secondary metastatic niches. As such, understanding the precise mechanisms of tumor-induced activation could offer novel platelet markers in diagnosis or recurrence.
4 Platelet Biomakers in Liquid Biopsy
Platelets offer several advantages as a biosource for liquid biopsy over rarer and more challenging-to-obtain cancer cells. Given their abundance in circulation, platelets can be easily isolated in clinical laboratories, and their RNA content remains of high quality. Moreover, platelet RNA profiles are stable for up to 48 h at room temperature, making them more accessible for clinical analysis without significant degradation [130, 131]. In contrast to nucleated cells, platelets are largely unaffected by isolation procedures, preserving the integrity of their RNA profiles. These features position platelets as a robust and reliable source for monitoring cancer progression and potentially identifying new biomarkers for early detection and treatment efficacy.
4.1 mRNA, circRNA, and lncRNA in Platelets
Platelets undergo notable alterations in their RNA profile upon interacting with tumor cells or tumor-associated proteins [102, 103], enabling them to act as valuable reservoirs for potential RNA biomarkers [132]. Recent studies have leveraged sequencing technologies to analyze platelet mRNA profiles, with the goal of detecting and monitoring cancer in patients [15, 133]. circRNAs, a distinct class of RNA highly enriched in platelets, originate primarily from the exons of protein-coding genes through a process called back-splicing. Selective isolation of linear transcripts has led to the identification of several unique circRNAs within platelets [134, 135]. Furthermore, the profiling of lncRNAs and microRNAs in blood has demonstrated significant potential in enhancing cancer diagnostics [136-139].
4.2 Proteins in Platelets
The proteome of platelets is diverse, consisting of proteins derived from megakaryocytes, proteins endocytosed from other sources, and proteins synthesized within platelets themselves. Identifying and distinguishing tumor-derived proteins from those that may fluctuate due to tumor-independent processes is critical for using platelets as a source for cancer biomarkers. Platelets contain secretory granules that release their contents upon activation, and they also harbor a wide array of membrane surface receptors that play key roles in platelet activation and adhesion [21, 140]. To analyze platelet proteins, the first step involves platelet isolation from whole blood, followed by platelet lysis and enzymatic or chemical protein digestion [141]. Recent advancements in mass spectrometry have significantly enhanced our ability to explore the platelet proteome, revealing numerous proteins that may serve as potential biomarkers for cancer diagnosis [141-144]. The PlateletWeb platform, a comprehensive database for human platelet proteomics, allows for the functional analysis of over 5000 proteins, providing valuable insights into platelet biology in the context of cancer and other diseases [145].
4.3 Platelet-Derived Microvesicles (PMVs)
PMVs represent another promising class of tumor biomarkers. PMVs are small vesicles shed by activated platelets, and while their precise roles in cancer progression are still under investigation, the increased platelet activity associated with tumor development results in elevated levels of PMVs in circulation. These PMVs including the expression of platelet membrane surface markers such as cluster of differentiation CD31, CD41, CD42, CD61, CD62, and CD63, as well as phosphatidylserine (PS) [146]. These features facilitate their interaction with the surrounding cellular environment. The concentration of PMVs has been shown to correlate with tumor stage, making them potential indicators of disease progression [147]. PMVs carry a variety of bioactive molecules, including proteins, growth factors, and genetic material such as RNA, mRNA, and microRNAs [148, 149]. Notably, studies have demonstrated that PMVs in cancer patients exhibit a distinct profile, which may be leveraged as a complex biomarker for cancer detection and monitoring [150]. This unique cargo could provide insights into tumor behavior and metastasis, offering a valuable tool for liquid biopsy applications.
5 Clinical Applications and Challenges
5.1 Transcriptomics to Identify TEP Biomarkers
A growing body of research underscores the utility of TEPs as a rich source of cancer biomarkers. Studies have leveraged a variety of techniques to identify and monitor cancer types using the RNA profile of TEPs. Table 1 summarizes key studies highlighting the potential of TEPs as biomarkers for tumor diagnosis and predicting therapeutic response [15, 101, 102, 133, 151-164].
| Performance | ||||||
|---|---|---|---|---|---|---|
| Author | Biomarkers | Tumor types | Patients number | Sensitivity | Specificity | AUC |
| RNA for tumor detection | ||||||
| Xing et al. [151] | ITGA2B | NSCLC | 243 | 0.912 | 0.565 | 0.888 |
| Liu et al. [152] | MAX, MTURN, HLA-B | LC | 225 | — | — | 0.734 |
| Yao et al. [153] | TPM3 | BC | 549 | — | — | 0.971 |
| Yang L et al. [154] | TIMP1 | CRC | 286 | — | — | 0.958 |
| Nilsson et al. [155] | EML4-ALK | NSCLC | 77 | EML4–ALK in platelets correlated with shorter PFS in crizotinib-treated patients (HR: 3.5) | ||
| Xu et al. [156] | Panel | CRC | 132 | 0.885 | 0.868 | 0.920 |
| In ‘t Veld et al. [15] | Panel | Solid | 1628 | 0.640 | 0.990 | 0.910 |
| Gao et al. [157] | Panel | Ovarian | 465 | — | — | 0.918 |
| Best et al. [101] | Panel | Solid | 228 | 0.710 | — | — |
| Best et al. [133] | Panel | NSCLC | 624 | 0.890 | ||
| Li et al. [158] | lncRNA linc GTF2H2-1, RP3-466P17.2, lnc ST8SIA4-12 | LC | 329 | — | — | 0.895 |
| Dong et al. [159] | SNORD55 | NSCLC | 290 | 0.793 | 0.683 | 0.803 |
| Dong et al. [160] | snRNA U1, U2, U5 | LC | 405 | — | — | 0.840 |
| Sol et al. [161] | Panel of RNAs | GBM | 240 | — | — | 0.810 |
| Bingqi et al. [162] | LNCAROD, SNHG20, LINC00534, and TSPOAP-AS1 | CRC | 54 | — | — | 0.780 |
| Yuanji et al. [163] | Panel of mRNA and lncRNA | NPC | 33 | — | — | — |
| Statoshi et al. [164] | IL-1 β | MM | 16 | — | — | — |
| Proteins for tumor detection | ||||||
| Peterson et al. [165] | VEGF | CRC | 35 | 0.825 | ||
| PF4 | 0.869 | |||||
| PDGF | 0.818 | |||||
| Strohkamp et al. [166] | Clusterin | CRC | 30 | 0.867 | 0.933 | |
| GSH-S | 0.933 | 0.867 | ||||
| cofilin-1 | 1.000 | 0.800 | ||||
| Hinterleitner et al. [167] | PDL1 | NSCLC | 128 | High platelet PD-L1 predicted a PFS benefit in patient treated with immune checkpoint inhibitor (HR 4.74) | ||
| Marta et al. [166] | Panel | Ovarian | 114 | 0.960 | 0.880 | 0.831 |
- Abbreviations: AUC, area under the curve; BC, breast cancer; CRC, colorectal cancer; GBM, glioblastoma GBM, glioblastoma; LC, lung cancer; NPC, nasopharyngeal carcinoma; NSCLC, non-small cell lung carcinoma.
A notable study employed microarray analysis on isolated platelets to identify an RNA signature capable of differentiating glioblastoma patients from healthy individuals. This analysis revealed several altered transcripts. Among these, four genes (WFDC1, FKBP5, IL1R2, and TPCN1) exhibited the most significant differential expression [102]. In 2022, Ye et al. discovered four specific lncRNA markers of platelets associated with colorectal cancer (CRC). These markers include LNCAROD, SNHG20, LINC00534, and TSPOAP-AS1. The expression levels of these lncRNAs were markedly increased in both platelets and serum samples from individuals diagnosed with colorectal cancer [162]. In subsequent studies, researchers further refined algorithms to enhance the accuracy of differential gene identification in platelets. One such study used the thromboSeq pipeline to analyze mRNA from 283 samples, achieving a 96% accuracy in differentiating lung cancer patients from healthy controls [168]. One study applied RNA sequencing using the thromboSeq pipeline, combined with particle-swarm optimization (PSO)-enhanced algorithms and ANOVA statistics, to detect sarcoma. The diagnostic accuracy was 87% for the validation set (n = 53 samples). This approach allows for the selection of gene panels most suitable for diagnosing cancer in a specific cohort, demonstrating the efficacy of PSO in tailoring diagnostic tools for different cancer types [133].
Another algorithm successfully predicted the presence of MET amplification, EGFR mutations, and KRAS mutations in tumors [101]. The capability of detecting tumor-driving mutations through platelet RNA surrogate signatures underscores the potential of TEPs as a promising tool for predicting cancer treatment responses. Furthermore, a Venn diagram comparison between sarcoma signatures and those obtained from non-small cell lung cancer (NSCLC) and low-grade glioma (LGG) suggested that each cancer type has a unique signature derived from TEPs' RNA profiles [133, 169]. Additionally, the development of the digital SWARM algorithm has provided new insights into monitoring GBM progression. The algorithm demonstrated that the TEP score could differentiate between false-positive progression and true progression, with an AUC of 0.86. When applied to RNA-seq data from NSCLC patients and healthy controls, a 48-biomarker panel was identified, achieving 92.5% sensitivity, 82.7% specificity [170]. Emerging technologies are also being explored to complement the RNA biomarker panel approach. One such study employed NanoString hybridization techniques to analyze CTCs and plasma cfRNA from patients with metastatic lung cancer. NanoString analysis revealed potential interactions between PF4 and several proteins, including CCL5, TGFB1, SRGN, and SPARC [171].
Altogether, these studies suggest that the advanced data analysis techniques, such as PSO-enhanced algorithms and thromboSeq, are crucial for optimizing the diagnostic utility of TEPs. These approaches not only enhance the accuracy of TEP-based cancer diagnostics but also hold promise for other liquid biopsy sources. Despite promising results from proof-of-concept studies, future research should incorporate larger sample sizes to improve the robustness of prediction algorithms, especially in distinguishing between true and false-positive progression in cancer monitoring [161].
5.2 Proteomics to Identify TEP Biomarkers
Recent proteomic analyses of platelets in patients with lung and pancreatic cancers have revealed notable differences in their platelet protein profiles compared to those of age- and sex-matched healthy controls. This differential platelet proteome suggests that platelets may serve as a reliable biomarker for early cancer detection. Interestingly, after the surgical resection of the tumor, the platelet proteome showed signs of normalization, further supporting the idea that platelet proteins can not only detect the presence of cancer but also monitor disease progression and response to treatment [172].
Recently, a specific group of platelet protein biomarkers has been identified that can distinguish between ovarian cancer patients and those with benign tumors in ovarian cancer. Using partial least squares discriminant analysis (PLS-DA) of platelet protein, researchers found significant differences in the protein expression patterns between ovarian cancer (stages III or IV) and benign lesions. The model exhibited remarkable diagnostic accuracy (96% in sensitity and 88% in specificity). Even tested on an independent sample set, the model maintained strong performance [173]. In a separate study on colon cancer, enzyme-linked immunosorbent assays (ELISAs) were used to analyze platelet and plasma samples between colon cancer patients and healthy controls. Significant differences were observed in the median levels of VEGF, PF4, and PDGF. Collectively, these biomarkers demonstrated strong discriminatory power, with an AUC of 0.893 and a P-value of < 0.0001, highlighting their potential as effective diagnostic markers for colon cancer [165].
Antitumor therapies can significantly influence the platelet proteome, further highlighting the potential of platelet protein content as a valuable biomarker for cancer screening and monitoring therapeutic response [174]. The alterations in platelet proteome induced by treatment may provide real-time insights into the effects of therapies on tumor progression and treatment efficacy. One promising marker in this context is PD-L1 expression on platelets. Beyond its expression on tumor cells, recent studies have demonstrated that tumor cells can transfer PD-L1 to platelets, effectively “educating” them with tumor-associated molecules [167]. This interaction between tumor cells and platelets has led to the development of a novel algorithm designed to quantify the PD-L1 payload on circulating platelets. This algorithm has proven to be highly effective in predicting treatment responses to immune checkpoint inhibitors, outperforming traditional histological methods of PD-L1 quantification.
These studies underscore the growing evidence that platelet-derived proteins and their profiles can serve as powerful biomarkers for the detection and monitoring of various cancers, including lung, pancreatic, ovarian, and colon cancers. The ability of platelet proteomics to distinguish between cancerous and noncancerous conditions, along with its potential to track tumor progression and response to treatment, positions it as a promising tool for noninvasive liquid biopsy applications in oncology.
6 Platelet-Based Nanomedicine
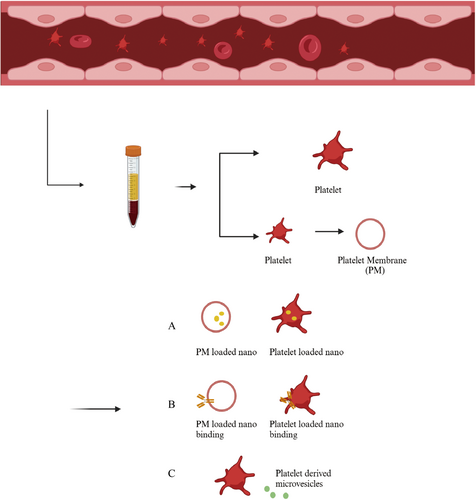
Due to their unique interactions with tumor cells and the presence of specific biomarkers, platelets have become an innovative focus in nanotechnology-based cancer therapies. Recent advances in nanomaterials have leveraged platelet properties to enhance drug delivery, improve imaging, and inhibit tumor progression (Table 2) [175-180, 182-184, 206]. The schematic diagram of the preparation of platelet-based nanomaterials is shown in Figure 4.
| Nanoparticles | Nanoplatform | Content | Response way | Author |
|---|---|---|---|---|
| Tumor-based platelet-targeted nanoparticles | ||||
| BDCCN | FeIII -doped C3N4 | CA | PH | Yue et al. [175] |
| BSA-IRLA@RVs | Scaffold of bovine serum albumin | NOS with l-Arginine/IR783 | Laser | Ma et al. [176] |
| CyBA/PFM | Amphiphilic phenyl boronated fucoidan | Fucoidan segment | H2O2 | Ying et al. [177] |
| DPC@ICD-Gd-Tic | Liposome | Ticagrelor/IR780 | GSH | Xu et al. [178] |
| TM33-GON/TNA | Gelatin/oleic acid | Tanshinone IIA | MMP2 | Jin et al. [179] |
| Fu-Uk/ICG@SiAu | Mesoporous silica-coated gold | Indocyanine green/urokinase-type plasminogen activator | Laser | Lee-Hsin et al. [180] |
| PLP-D-R | Copolymer poly(etherimide)-poly(lactic-coglycolic acid)2 | Dox/R300 | MMP2 | Suping et al. [181] |
| IGBN | Copper-based MOF | Pt-Arg prodrug | PH | Sijie et al. [182] |
| Ptx@AlbSNO | AlbSNO | Paclitaxel/NO | GSH | Yan et al. [183] |
| CREKA-Lipo | Liposome | Ticagrelor | PH | Yinlong et al. [184] |
| Platelet membrane-coated nanoparticles | ||||
| PM-NP/PTX | Dextran | Paclitaxel | Thrombin | Yinxin et al. [185] |
| PSCI | MSNs | Cinnamaldehyde/IR780 | US | Chunyu et al. [186] |
| PLTM-DOX@MPDA | MPNs | Dox | PTT | Dandan et al. [187] |
| PLTM-CS-pPLGA/FBu | (PLGA) copolymer | Bufalin | pH | Haijun et al. [188] |
| PMS | Mesoporous Fe single-atom | — | PTT | Pengyuan et al. [189] |
| PG@HGNs | Hollow gold | pH | Jiahui et al. [190] | |
| PCLP-CUR | Chitosan-modified liposome | Curcumin | pH | Shengli et al. [191] |
| PMBNs | Biomimetic mesoporous organosilicon | TBP-2 | GSH | Shiping et al. [192] |
| PCDD | The third-generation poly-l-lysine dendrimer | Ce6/docetaxel | ROS | Huixian et al. [193] |
| PMNPs | PLGA | Dox | PTT | Lin et al. [194] |
| PINPs@PM | poly(d,l-lactide-co-glycolide) | Indocyanine green | PTT | Yin et al. [195] |
| H-L-D-Z@PM | ZIF8 | Dox | pH | Xingyu et al. [196] |
| HMMD@PG | Hollow mesoporous copper sulfide | MnO2/Dox | PTT | Ying et al. [197] |
| IR780@PLGA/DOX | PLGA | IR780/dobiroxacin | PTT | Weijing et al. [198] |
| Living platelets-based nanoparticles | ||||
| PDNGs | Nanogels | Dox | tumor | Qi et al. [199] |
| IO/PG@DOX-Ce6 | Iron oxide (IO)/polyglycerol | Ce6 | Laser | Qirui et al. [200] |
| αMM-PEG@PLTs | MnO2-coated porphyrinic | α-MT | US | Liqiang et al. [201] |
| PLT@PDA-DOX | Polydopamine | Dobiroxacin | PTT | Ting et al. [202] |
| CAR-T-P aPDL1@gel | Hyaluronic acid hydrogel | CAR-T cells/P–aPDL1 | Thrombin | Quanyin et al. [203] |
| P-P-IO | aPDL1/IONPs | Endothelium | Yu Gao et al. [204] | |
| HVJ-E@P | Inactivated sendai virus (HVJ-E) complex | — | pH | Tomoyuki et al. [205] |
| PMVs-based nanoparticles | ||||
| RGD@MNPs/DOX | — | Melanin nanoparticles/doxorubicin | PTT | D'Ambrosi et al. [206] |
- Abbreviations: CA, chlorogenic acid; Dox, doxorubicin; PTT, photothermal therapy.
6.1 Tumor-Targeted Nanomaterials Utilizing Platelets
Platelets are frequently involved in tumor progression through interactions between their membrane receptors and specific biomarkers on tumor cells. Platelet-targeted therapies, designed to engage these receptors, have demonstrated both promising imaging capabilities and antitumor effects. Given their critical role in cancer progression, platelets represent a compelling target for the development of innovative therapeutic strategies. Researchers have developed living platelet-targeted nanoparticles designed to exploit these interactions, yielding promising imaging and antitumor effects.
One innovative approach has involved the use of detergent-treated living platelets, which lack adhesion and aggregation properties, to act as “platelet decoys.” These decoys have demonstrated the ability to inhibit platelet attachment to matrix proteins in vitro, significantly reducing tumor metastasis in murine models [207]. To counteract platelet-mediated tumor protection, Zhou and colleagues developed albumin-based nanoparticles that coat the surface of living platelets with ADP, GPVI agonist, and thrombin activation sites. This nanoparticle system enhances tumor blood vessel permeability and facilitates T-cell infiltration, thereby boosting the effectiveness of antitumor immunotherapy [208]. A challenge associated with systemic administration of antiplatelet drugs—such as aspirin and clopidogrel—is their nonspecific targeting, which can lead to unwanted platelet depletion and functional disruption. To address this issue, Wang et al. designed a hydrogen peroxide (H₂O₂)-responsive near-infrared (NIR) probe-triggered nanosystem, termed CyBA/PFM. This self-assembling nanoplatform actively binds thrombi by targeting highly expressed P-selectin and facilitates the delivery of therapeutic polymers and imaging probes to platelet-rich arterial thrombi [177]. A more aggressive antiplatelet approach was developed by Li et al., who engineered a nanocomposite system composed of a block copolymer core (PEI-(PLGA)₂) loaded with both the monoclonal antiplatelet antibody R300 (targets GPIbα, a key subunit of the platelet vascular hematopoietic factor receptor, leading to platelet depletion via microaggregation and doxorubicin (Dox). This dual-action nanocomposite exhibits a potent antitumor effect by simultaneously inhibiting living platelet-mediated tumor protection and delivering cytotoxic therapy directly to cancer cells [181]. Another promising development comes from Ma et al., who created a BSA-IRLA@RVs complex that selectively targets platelets using a red blood cell-membrane-encapsulated protein nanoparticle system. This construct incorporates a cRGD immune membrane structure, allowing it to actively target both platelets and tumor cells by exploiting the overexpression of integrins αIIbβ3 and αvβ3. The system enhances tumor selectivity while maintaining biocompatibility and immune evasion properties, making it a promising candidate for future clinical applications [176].
6.2 Platelet Membrane (PM)-Based Nanomaterials
One of the most widely explored applications of PM-coated nanomaterials is their ability to enhance drug delivery and facilitate targeted therapy at tumor sites. These systems utilize either natural or genetically engineered platelet membranes as coatings for nanoparticles, improving biocompatibility and enabling tumor-specific drug transport. Since Zhang's group first introduced polymeric nanoparticles enveloped in platelet plasma membranes in 2015, PM-based biomimetic delivery systems have gained significant interest as a promising strategy for cancer therapy [209]. King and colleagues developed PM-coated silica nanoparticles conjugated with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to selectively target CTCs. These biomimetic nanoparticles successfully localized to metastasizing tumor cells within the TME and induced apoptosis in both prostate and breast cancer cells [210, 211]. This approach offers a potential strategy for preventing metastasis by directly eliminating CTCs before they colonize secondary sites. Another innovative strategy was developed by Zhuang et al., who encapsulated small interfering RNA (siRNA) within acid-responsive porous metal-organic framework (MOF) nanoparticles coated with platelet membranes. This P-MOF–siRNA complex demonstrated specificity in targeting breast cancer cells, leveraging platelet membrane surface proteins for tumor recognition [212]. The use of MOFs as siRNA carriers enhances stability, cellular uptake, and controlled release, allowing precise gene silencing for therapeutic intervention.
PM-coated nanomaterials represent a rapidly advancing frontier in biomimetic drug delivery and theranostics. These systems provide tumor-targeting capabilities, improved drug stability, and reduced immune clearance, enhancing therapeutic efficacy while minimizing off-target effects.
6.3 Living Platelets-Based Nanomaterials
In addition to PM-coated nanomaterials, living platelets have also been explored as drug delivery carriers for cancer treatment. Researchers have leveraged platelets' natural affinity for tumor cells to transport therapeutic agents directly to tumor sites. One widely used approach involves loading drugs, such as DOX, into platelets through passive osmosis, allowing the platelets to carry and release the drug upon reaching the tumor microenvironment [213].
Several laser-activated platelet-based drug delivery systems have demonstrated promising antitumor effects. For instance, BNPD-Ce6@Plt, developed by Xu et al., and the Au-Hb@PLT complex, engineered by Xia et al., exhibited significant therapeutic efficacy in glioblastoma mouse model [214, 215]. These platelet-based nanosystems enhance tumor targeting and retention, leveraging photoactivation to precisely release therapeutic agents upon laser irradiation, thereby minimizing systemic toxicity. Beyond glioblastoma, similar engineered platelet-based systems have been tested across multiple tumor models, showing positive therapeutic outcomes. For example, Nishikawa et al. developed platelets incorporating inactivated Sendai virus (hemagglutinating virus of Japan; HVJ) envelope (HVJ-E) and injected them into B16F10 melanoma-bearing mice. This approach successfully reduced tumor size, highlighting the potential of platelets as a Trojan horse for delivering viral particles in cancer therapy [205]. Another innovative strategy involves engineered platelets equipped with POI ligand-tethered HSP90 (DePLTs), generated through ligand-directed covalent labeling. These platelets selectively target postsurgical tumor sites, recognizing hemorrhagic regions and undergoing activation accordingly. Once activated, DePLTs can transfer labeled HSP90 to cancer cells via PMPs, inducing proteasomal degradation of intracellular POIs and release free HSP90 to redirect extracellular POIs to the lysosome for proteolysis, ultimately suppressing cancer recurrence and metastasis [216].
Despite the promising therapeutic potential of living platelet-based nanomaterials, several challenges remain in their large-scale application. First, Ethical and regulatory concerns surrounding the use of human-derived platelets in experimental therapies. Then, donor-specific variability, as platelets are highly dynamic cells that communicate with their environment (e.g., TEPs). This raises concerns regarding whether engineered platelets derived from non-autologous donors could have unintended effects on tumor progression [217].
6.4 PMVs-Based Nanoparticles
PMVs, as the most abundant form of extracellular vesicles, play a significant role in tumor invasion and metastasis. Derived from platelets, PMVs retain certain platelet characteristics, including surface markers such as CD41, CD42, P-selectin, and functional molecules like platelet-activating factor, angiogenic factors, and various chemokines [218]. It has been established that PMVs contribute to tumor progression in a manner similar to platelets. As such, they represent a promising candidate for the development of anticancer therapeutics [219].
Some native p-EVs were shown to inhibit tumor growth by transferring miRNA-24 (miR-24) [220]. Several studies have demonstrated that PMVs, as a novel nano-scale delivery platform, can effectively carry therapeutic agents for the treatment of cancer and related conditions. In one study by Jing et al., a nanomedicine composed of RGD peptide-modified nanoscale platelet vesicles (RGD-NPVs) encapsulating melanin nanoparticles (MNPs) and DOX was developed. This RGD-NPVs@MNPs/DOX formulation successfully evaded immune clearance, targeted tumor cells, and, upon laser irradiation, activated and released the drug [206]. The findings revealed that this nanoparticle-based approach enhanced radiotherapy efficacy and significantly reduced tumor cell viability. Despite these promising advances, research on the use of PMV-based carriers for anticancer drug delivery remains underexplored, and further investigation into the potential of PMVs in cancer treatment is warranted.
6.5 Side Effects and Safety
While platelet-based nanomaterials have demonstrated great promise in targeted drug delivery, tumor imaging, and immunotherapy, several safety concerns and potential side effects must be addressed before clinical translation. First, Platelets possess numerous surface glycoproteins and immune-modulating molecules, making them susceptible to immune recognition and clearance by the host immune system. In autologous applications, where a patient's own platelets are used, the risk of immune rejection is minimal. However, in allogeneic or bioengineered platelet-based nanomaterials, there is a potential risk of immune activation, leading to platelet clearance, hypersensitivity reactions, or inflammatory responses. Additionally, engineered platelet-derived carriers may influence immune cell activation, potentially leading to unwanted immunosuppression or hyperactivation of the immune system, which could interfere with antitumor immunity [221]. In addition, A major concern in using platelet-based systems for drug delivery is the risk of unintended thrombosis. Platelets naturally adhere to endothelial cells, aggregate, and participate in clot formation, which raises concerns about their potential to induce thrombotic events when used as therapeutic carriers. Several studies suggest that platelet-derived nanoparticles or platelet membrane-coated drug carriers could lead to increased coagulation factor activation, raising the risk of deep vein thrombosis (DVT), pulmonary embolism, or even stroke in susceptible patients [221-223]. Strategies such as surface modification of platelet membranes or controlled-release formulations may help mitigate excessive clotting risks. Finally, off-target accumulation of platelet-derived carriers in noncancerous inflamed tissues or healthy vasculature remains a concern. For example, platelet membrane-coated nanoparticles could inadvertently accumulate in the spleen, liver, or lungs, leading to potential off-target toxicity and altered pharmacokinetics [213]. By systematically addressing these toxicity and safety concerns, platelet-based nanomedicine can be safely integrated into clinical oncology, paving the way for more effective, personalized cancer therapies.
6.6 Challenges and Limitations
Despite the promising potential of TEPs as molecular markers for cancer detection, several challenges and limitations remain in their implementation for liquid biopsy applications. These limitations span pre-analytical procedures, biomarker specificity, tumor heterogeneity, and the influence of external factors on platelet marker profiles. Addressing these challenges is essential to enhance the accuracy and reliability of TEP-based cancer diagnostics.
One major challenge in using TEPs as biomarkers is the pre-analytical stage, which requires strict standardization of blood collection and processing. Several key issues include: (1) Sample handling and time sensitivity. The delay between blood sampling and analysis must be minimized to prevent alterations in platelet RNA profiles; (2) Consistent testing conditions. To ensure reproducibility and comparability, all patient samples should be collected and processed under standardized conditions. (3) Use of buffers for platelet preservation: Proper buffering solutions must be employed during sample preparation to maintain the stability of target biomarkers and prevent degradation. The second is to detect cancer-specific biomarkers. Although encouraging results have been achieved in detecting cancer-specific biomarkers in TEPs, pre-analytical processing and analytical methodologies remain nonstandardized. The lack of standardized protocols hinders the widespread adoption of TEP-based liquid biopsies, particularly for early-stage cancer diagnosis. Establishing consistent processing workflows and quality control measures is essential to improve the clinical applicability of TEP analysis. Third, TEPs are predominantly influenced by tumor type and by tumor progression and metastases. Additionally, different molecular tumor subtypes (e.g., HER2-amplified vs. wild-type breast cancer) elicit distinct changes in platelet RNA profiles. This is likely due to differential “education” stimuli exerted by specific tumor subtypes, leading to variable biomarker expression in TEPs [224]. Fourth, heterogeneity in platelet RNA content is another critical limitation. The RNA composition of platelets is influenced by multiple factors, including: (1) The transcriptional activity of bone marrow-derived megakaryocytes, which produce platelets. (2) RNA sequestration and splicing events during platelet circulation, leading to patient-specific variations [225, 226]. (3) Differences in platelet lifespan and turnover rates, which may contribute to variability in RNA profiles among individuals. As a result, large-scale validation studies with diverse patient cohorts are necessary to account for inter-patient variability and refine biomarker selection. Then, Platelet marker profiles can also be affected by comorbidities and lifestyle factors, which introduce additional variability. These include, medications (e.g., anticoagulants, chemotherapy, or immunotherapy), physical activity and dietary habits, psychological stress and immune status, and a recent cancer diagnosis and ongoing treatments. To minimize these confounding variables, prospective clinical trials must carefully control for external influences and establish standardized blood collection protocols. Finally, Platelets stored at room temperature are prone to bacterial contamination and spontaneous activation and degranulation. On the other hand, platelets stored at lower temperatures exhibit Functional impairments, or reduced viability and altered biological activity. Future research should focus on developing standardized protocols, large-scale validation studies, and improved preservation techniques to fully harness the potential of platelets in cancer detection and treatment monitoring.
6.7 Clinical Translation and AI-Driven Advancements
Advancements in technology are poised to significantly enhance our ability to detect and monitor cancer using platelet-based biomarkers. In particular, the integration of machine learning (ML) and artificial intelligence (AI) algorithms in biomarker pattern recognition and recurrence prediction is expected to revolutionize the field. These computational approaches can improve feature selection, optimize diagnostic performance, and increase the sensitivity and specificity of cancer detection in liquid biopsy applications. Various ML techniques can be employed to extract meaningful features from liquid biopsy analytes, ensuring high detection accuracy. For instance, Wong et al. applied deep learning, decision trees, Naïve Bayes, and averaged one-dependence estimators, achieving a 77% sensitivity for detecting stage I cancers—a significant improvement over the 48% sensitivity reported in the original data set [227]. Andreas Halner et al. developed DEcancer, an ML-based model that improved stage I cancer detection sensitivity from 48% to 90% across eight cancer types and 812 cancer-free individuals [228]. AncerSEEK, a multi-analyte test first reported by Cohen et al. in 2018, demonstrated the ability to detect multiple cancers through blood samples by analyzing circulating tumor DNA (ctDNA) mutations and protein biomarkers [229].
Recent studies have explored gradient boosting and deep learning-based models to enhance cancer detection accuracy. The Light Gradient Boosting Machine (LGBM) model, optimized through a random search for hyperparameters, has been employed for cancer classification. By using tenfold cross-validation, LGBM models are ensemble-weighted to obtain highly accurate final predictions [230]. Researchers have also leveraged convolutional neural networks (CNNs) and boosting techniques to evaluate and refine classifier performance for liquid biopsy data [231]. While AI-driven approaches show great promise, their clinical application faces several challenges. First, large-Scale Data Collection. Reliable ML models require extensive patient data to reduce bias and improve generalizability. Second, standardization of sample collection protocols. Variability in sample processing can introduce bias, necessitating rigorous standardization. Third, interpretability of AI models. Developing transparent AI frameworks that allow clinicians to understand and trust the model's decision-making process is crucial. Given the success of AI-driven cancer diagnostics, a dedicated platelet biomarker database will be essential for improving TEP-based liquid biopsy applications. Future efforts should focus on Building a centralized repository of platelet-related biomarkers, integrating AI-driven analytics to classify cancer subtypes based on platelet RNA/protein signatures and Enhancing interpretability to facilitate clinical adoption and regulatory approval.
7 Conclusion
In conclusion, the use of platelets as a tool for cancer diagnostics and therapy is an emerging field with immense potential. While liquid biopsy technologies and platelet-based nanomedicine continue to evolve, overcoming standardization and validation challenges is crucial for their clinical adoption. With further advancements in AI-driven biomarker discovery, nanomaterials, and engineered platelets, these innovative approaches could revolutionize precision oncology, offering more effective, noninvasive cancer detection and treatment strategies.
Author Contributions
Xin Wang: conceptualization (equal), data curation (equal), writing–original draft (lead). Jie Chen: conceptualization (equal), methodology (equal), supervision (equal), validation (equal), visualization (equal), writing–review and editing (equal). Hubing Shi: conceptualization (equal), data curation (equal), supervision (equal), validation (equal), writing–review and editing (lead).
Acknowledgments
Figures 2, 3 and 4 in this review were created using BioRender.com (https://biorender.com). This work was supported by (1) the National Key Research and Development Program of China (2022YFA1207300 [2022YFA1207303]); (2) National Natural Science Foundation of China (No. 82172634); (3) Key Program of the Science and Technology Bureau of Sichuan (No. 2021YFSY0007); and (4) 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYYC20013).
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report. All data and information in this review can be found in the reference list.




