Recent advances of nanobody applications in diagnosis and detection
Abstract
Nanobodies (Nbs) are the variable domain of heavy-chain antibodies derived from the blood of camelids or sharks. Nanobodies are the smallest antibody fragment with intact antigen-binding ability. Compared to conventional antibodies, nanobodies have unique properties such as small size, excellent stability and solubility, low immunogenicity, ability to recognize hidden epitopes, high tissue penetration, and industrialized production. More excitingly, the camelid-specific amino acid sequences in the framework are mutated to their human heavy-chain variable domain equivalent, which is humanized, to a wide range of applications. These superior characteristics make nanobody an ideal alternative to conventional antibody, showing excellent prospects for various applications in structural biology, molecular imaging, disease diagnosis and therapy, agricultural products, and environmental chemicals detection. With the continuous updating of theories and the rapid development of technology, the screening and expression methods of nanobodies are increasingly mature. Consequently, several technologies to identify and express nanobodies have been established, and various use cases have been described. In this review, we summarize recent advances in the discovery and production of novel nanobodies, and their use in detection and diagnosis platforms.
1 INTRODUCTION
Immunoglobulins (Igs), also referred to as antibodies (Abs), are large proteins produced by B lymphocytes in response to antigens.1 Antibodies consist of two identical light (L) chains and two identical heavy (H) chains, linked by disulfide bonds and noncovalent interactions, resembling a Y-shape (Figure 1A).2, 3 The region near the C-terminus of the light and heavy chains called the constant (C) region has a relatively constant amino acid sequence. Residues near the N-terminal, within the variable (V) region, are sequence diverse, and their composition defines antigen recognition. The V domains of both L and H chains (VL and VH) have significant sequence variability. Each V domain has three short regions with hypervariable amino acid composition and sequence, named hypervariable regions (HVRs). The HVRs form a spatial conformation, the paratope, complementary to the antigenic epitope, also known as the complementary determining regions (CDRs). The conserved stretches of the V domain surrounding the CDRs referred to as the framework regions (FRs). The arms of the Y-shaped antibody are the critical site for antigen recognition, referred to as the fragment of antigen binding (Fab), comprising VL, CL, VH, and CH1. The antibody backbone consists of two constant domains (CH2 and CH3) of each H chain, known as fragment crystallizable (Fc), which regulates the activity of immune cells to facilitate antigen clearance. Intact functional antibodies as biopharmaceutical products or analytical tools are widely used in basic research, environmental and food detection, clinical disease prevention, diagnosis, and treatment.1 The applications of antibodies are limited by their relatively large size (such as IgG with a molecular weight of 150 kDa), the high cost of production and purification, and poor diffusion in tissues and solid tumors.3, 4 Antibody fragments and engineered variants, such as single chain variable fragments (scFv),5 Fab fragments,6 and single domain antibody (sdAb),7 have emerged as practical reagents for detection, due to their small size, low production cost, and the ability to express large quantities of these fragments in bacterial culture.

In 1993, heavy-chain-only antibodies (HCAbs) were discovered in the serum of camelids.8 These naturally occurring HCAbs lack light chains, as well as the CH1 domain of Fc found in human IgG. Thus, HCAbs consist of two identical H chains, each comprising CH2, CH3, a hinge region, and a variable domain responsible for antigen recognition (Figure 1A).9 The variable domain of heavy chain of HCAbs (VHH), at the N-terminal end, has a strong affinity for its cognate antigen, so it is considered the smallest intact antigen-binding fragment.7, 9 The petite size of prolate VHH in the low nanometer size range (dimensions of 4 nm × 2.5 nm × 3 nm and molecular weight of 15 kDa) inspired the name nanobodies (Nbs).9 Similar to the VH of human antibodies, nanobodies have four FRs and three CDRs (Figure 1B), and also, regularly, CDR3 is the main contributor to antigen recognition and specificity. The CDR3 loop of VHH is, on average longer than that of the human VH domain, offering the possibility of multiple studies. The flexible CDR3 loop of VHHs could form a finger-like elongation or a convex complementary site to bind small cavities or concave epitope architectures on the antigen surface such as catalytic site enzymes.9 The longer CDR3 and extended CDR1 loops of VHHs are regularly connected through an additional interloop disulfide bond, which might further contribute favorably to the conformational stability of the VHH. Furthermore, the FR of VHH shares more than 80% sequence homology with human VH of family III (VH3), but four highly conserved hydrophobic amino acids (V37, G44, L45, and W47) in FR2 of the human VH domain are substituted in VHH by more hydrophilic amino acids (F37, E44, R45, and G47) (Figure 1B).10 These mutations increase the solubility and stability of autonomous VHH lacking the partner VL domain.8
Two years after discovering camelid HCAbs, Greenberg et al.11 identified similar heavy chain-only antibodies (Immunoglobulin new antigen receptor, IgNAR) in cartilaginous fish. Each heavy chain of IgNAR consists of five constant domains and an N-terminal variable domain (V-NAR).12 Unlike the variable domains of other antibodies, the V-NAR lacks the CDR2 region but has two additional mutation-prone loops, termed hypervariable loop 2 (HV2) and hypervariable loop 4 (HV4), that may be involved in antigen recognition.13 These autonomously variable domains (VHH and V-NAR) share some general features, such as small size, high stability and solubility, and longer CDR3 loops on average.9 Regardless of their species origin, nanobodies offer substantial advantages over conventional full-length antibodies.8 While recombinant full-length antibodies are costly to produce, nanobodies are easily expressed and amplified in microbial systems such as bacteria, yeast, and fungi owing to their monomeric structure, thus enabling mass production at a lower cost.9 In addition, superior tissue penetration, body distribution, and blood clearance of nanobodies significantly improve the sensitivity and specificity of tumor diagnosis, making them more suitable as noninvasive molecular imaging reagents and diagnostic tools.14 With their strict monomeric behavior and ease of modification by genetic engineering techniques, nanobodies are optimal building blocks for constructing multivalent and multispecific therapeutic and diagnostic molecules, such as combining nanobodies with functional peptides, enzyme, serum albumin, and radionuclides, with improved functionality and potency.15
While the evolutionary driving force for the emergence of functional HCAbs and IgNARs remains unclear, researchers immediately recognized the importance and broad applicability of developing and engineering nanobody, a single domain containing the antigen-binding site, rather than the paired VH-VL domains in conventional antibodies.8, 11 Since camel-derived HCAbs are now more intensively studied, we will focus on summarizing and discussing camel-derived nanobodies. The adaptation of tools for library construction, in conjunction with different screening techniques and expression systems, facilitated the recognition and production of target-specific VHHs from camelid HCAbs.9 To date, VHHs have proven suitable for common diagnostic assay platforms for conventional antibodies, such as enzyme-linked immunosorbent assay (ELISA), electrochemical assay, lateral flow devices, and noninvasive imaging. This review begins with three types of nanobody libraries, followed by a description of the advances of nanobody screening techniques and expression systems, and then summarizes their applications in disease diagnosis and small-molecule detection.11
2 PREPARATION OF NANOBODIES
2.1 Construction of nanobody libraries
The selection of nanobody begins with the construction of a library, a diverse combinatorial repertoire of available VHH genes. Nanobody libraries can be distinguished by the source of VHH genes used for display, ranging from the immune library, naïve library, and semisynthetic/synthetic library.16
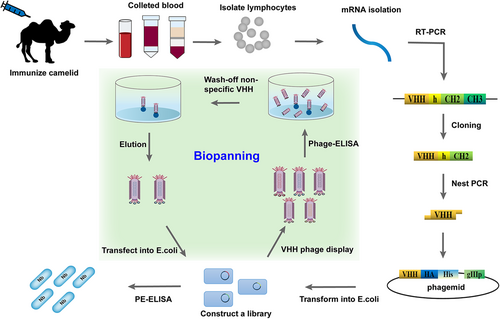
In vivo immunization is the most commonly used strategy for nanobody preparation.17 According to standard immunization protocols, individuals of camelids are first immunized subcutaneously with the target antigen at regular intervals. Typically, each animal is given 4–8 injections of 0.1–1 mg purified antigen for a period of 2 months through which antigen-specific HCAbs undergo affinity maturation.17 Nevertheless, small molecules (molecular weights below 1000 Da), named haptens, should be conjugated to carrier proteins, which provide a scaffold to present it to the immune system to induce an antibody response, or it will be ignored by the body.18 At the peak of the adaptive immune response, peripheral blood was subsequently collected, and mRNA from B lymphocytes was further isolated to synthesize cDNA by reverse-transcribed. The VHH gene sequences (~360 bp) were easily amplified by nested polymerase chain reaction (PCR) and ligated into the cloning vectors representing the immune VHH repertoire of B cells. The recombinant vectors containing the VHH genes are then transformed into host cells, such as Escherichia coli, for library construction (Figure 2).17 The specific nanobodies are generally readily obtained through phage display or other display technologies. The high affinity, specificity, and stability of nanobodies retrieved from immune libraries are ensured due to undergoing somatic maturation in vivo.17 However, the limited size and diversity of the immune libraries, which can produce nanobodies against only a specific antigen or a class of similar antigens used in the immunization step, are key concerns affecting the efficiency of antibody screening.9 The immunological tolerance and bloodstream stability of animals, the possible pathogenicity and toxicity of some antigens, and the overall practical feasibility of scale-up are major constraints naturally present in host animals.17 Therefore, the construction of immune libraries is relatively cumbersome and costly.
By contrast, naïve libraries theoretically allow a wide diversity of nanobodies to recognize any potential antigen.6 Due to the lack of in vivo stimulation of the somatic maturation by immunization, high specificity and affinity of binders can be retrieved only when selecting from a large and diverse naïve VHH library (109–1010 individual clones of which >80% should encode a VHH).17 In addition, molecular evolution after the selection procedure can be employed to increase the diversity of CDRs as well as the affinity and specificity of nanobodies. A naïve library may require at least 1 L of blood samples to be consumed to obtain 109 individual clones, whereas semisynthetic/synthetic libraries without immunization or collection of large amounts of blood have a similar diversity.17
Given the limitations of naïve libraries, the construction of highly diverse and utility semisynthetic/synthetic VHH libraries is probably more worthwhile.19 All steps involved in screening antibodies from semisynthetic/synthetic libraries are performed in vitro, allowing for tightly controlled experimental conditions and ease of operation.17 To generate sufficiently diverse libraries capable of producing nanobodies against any specific antigen, the semisynthetic/synthetic libraries need to randomize the CDR sequence to some degree, that is, mimic somatic hypermutation in camel, precisely tuning the CDRs to recognize any given antigen.9 However, the screened nanobodies may have low affinity because of the lack of somatic maturation stimulated in vivo by immunization, yet affinity enhancement can be achieved by in vitro maturation techniques.19 Unfortunately, full randomization of CDR sequences may generate >1030 different VHH sequences, and this huge diversity causes stability problems because the CDR loop affects antigen binding and folding of the structural domain.14 Moreover, since semisynthetic/synthetic libraries are not selected in vivo for expression as properly folded proteins, they are usually limited by the foldability of the VHH protein.17
2.2 Nanobody screening technologies
Several nanobody screening technologies are developed for selecting nanobodies with particularly desirable properties such as high affinity and stability.9 The phage display was initially developed by Smith in 1985.20 Currently, the phage display is the most widely used technique for specific nanobody screening.9 The technology is a powerful and practical tool that involves the fusion of foreign genes into the genome of phages to be expressed on the phage surface as fusions of foreign proteins/peptides and the pIII coat proteins. In this method, the gene fragment encoding the VHH protein is ligated into a phage cloning vector and subsequently transformed into E. coli to construct the VHH library. To display VHHs, helper phages were added to infect the library when the cells grow to the mid-logarithmic phase. Phages are prepared by polyethylene glycol (PEG) precipitation. Typically, positive clones were enriched after 3–5 rounds of bio-panning.17 Individual clones can be screened by ELISA to produce antigen-specific nanobodies. Positive clones are sequenced to deduce the amino acid of the nanobodies (Figure 2).17 However, a good assay may not be available without the selection of well-designed haptens for screening, or without an adequate library of competitive ligands or “coating antigens” for assay development.16 Another issue to consider, which may also occur with conventional antibodies, is the possibility of eliciting VHHs with a strong binding affinity for the coating haptens, but with weak or no recognition for the free analyte.10 In any case, phage display technology represents a robust, efficient, and inexpensive approach that is particularly suitable for screening large-scale nanobodies. As the field of phage display continues to advance, the limitations of this technology in terms of stability, capacity, and diversity will be improved.21
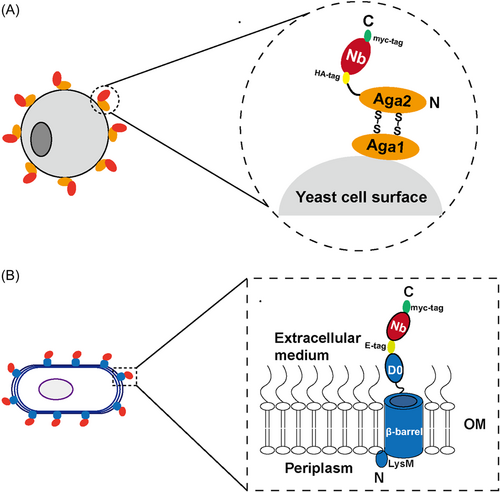
The cell surface display is carried out by fusing an anchoring motif to the N- or C-terminus of the target protein attached to the cell membrane or cell wall components, with the protein being simultaneously produced and directly immobilized on the surface of the microbial cells, including yeast, bacteria, and mammalian cells (Figure 3).17, 22 General benefits of cell surface display versus phage display are reduced background and culture manipulation, allowing for direct selection, screening, and characterization of binders using flow cytometry-based methods with a higher level of display.23 The most common approach for tethering nanobodies or other small proteins to the surface of Saccharomyces cerevisiae and other yeast species is to fuse the protein of interest to the α-agglutinin adhesion receptor (Aga1 and Aga2) on the yeast cell wall.23, 24 Instead, McMahon et al.24 constructed a simplified yeast surface display-based nanobody screening platform in which the nanobodies are directly connected to the yeast cell wall via a single tether to replace the Aga2p-Aga1p linker protein, allowing straightforward, rapid, and low-cost isolation of nanobodies from soluble protein binders to conformationally selective G-protein-coupled receptor stabilizers, and provided a blueprint for identifying nanobodies. Schoof et al.25 used this platform to screen for nanobodies that neutralize SARS-CoV-2. They labeled antigens with biotin or with fluorescent dyes and selected nanobody-displaying yeast over multiple rounds, first by magnetic-activated cell sorting (MACS) and then by fluorescence-activated cell sorting (FACS). The platform allows for direct, rapid, and low-cost isolation of nanobodies and shows promise as an adjunct to molecular structure studies. Bacteria have been developed for display of nanobodies, including both Gram-positive and Gram-negative species.23 Among them, E. coli is the most commonly used bacterial host. As a Gram-negative bacterium, E. coli has two types of biofilms in the cell envelope: the cytoplasmic inner membrane (IM) and the outer membrane (OM). The presence of the OM is a major obstacle to the effective display of antibody fragments in the E. coli display system. To be displayed on the surface of E. coli, proteins of interest produced in the cytoplasm must be translocated through the IM and inserted into the OM, exposing specific proteins to the extracellular milieu. A number of chaperone proteins subsequently assist in the proper folding or disulfide bond formation of the protein domains. Al-ramahi et al.26 integrated the VHH gene as a single copy in bacterial chromosomes and fused it to the intimin OM anchoring domain for E. coli display, merging with DIvERGE technology (the cyclic and DNA-segment directed mutagenesis method) in combination with MACS-based cyclic selection, directly screened and characterized evolved nanobodies binding to a new antigen. Ectopic expression of nanobodies on the envelope of Pseudomonas putida cells has also been reported.27
The yeast two-hybrid (Y2H) system is a well-established method for identifying potential protein–protein interactions.28 The assay is based on the occurrence of gene expression when the DNA-binding domain (BD) and the activation domain (AD), two functionally separable domains organized from eukaryotic transcription factors, are simply nearby. In the two-hybrid system, the cDNA encoding a bait protein (target antigen) is cloned into a vector that allows the expression of bait protein fused to a transcription factor BD, while the cDNA encoding prey protein (VHH protein) is ligated into a vector for the expression of the prey protein fused to a transcription factor AD. If the nanobodies interact with the antigen in the nucleus of the yeast, BD and AD are brought in close proximity and thus become able to activate the transcription of reporter genes.29 Unfortunately, this method is unsuitable for considerably large and diverse libraries and also presents the problem of re-cloning the enriched library to the yeast vectors resulting in low transformation efficiency. In addition, the yeast two-hybrid system is limited by the slow growth of yeast cells. Fortunately, these disadvantages can be remediated by hosting the two-hybrid system in fast-growing bacteria (B2H) rather than yeast.17
The ribosome display technology is a cell-free system with the concept of physical association of phenotype (protein) and genotype (its encoded nucleic acid). The method is based on linking mRNA to its encoded proteins via ribosome to produce mRNA–ribosome–protein ternary complexes, followed by affinity screening using the corresponding ligand (antigen) at the bio-panning step (Figure 4A).30 This technique overcomes the limitation of transformation efficiency; thus, a massive encoded library can be established rapidly, which is assembled for high-throughput screening of ribosome display libraries for rapid nanobody generation. Chen et al.31 developed CeVICA, a cell-free nanobody engineering platform that uses ribosome display for in vitro selection of nanobodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor binding domain (RBD) from a library of 1011 randomized sequences. They validated the binding and virus neutralization activity of 38 predicted VHH families and screened out 30 true binders and 11 neutralizers, and generated a neutralizing agent with an IC50 of 329 picomolar (pM). CeVICA is amenable to automation and generating nanobodies in a rapid, reliable, and scalable manner, providing a technology framework for the incorporation of future refinements, making it a valuable addition to in vitro antibody engineering technologies. The ribosome display technology is a powerful method for accelerating the selection and discovery of novel and active nanobodies. On the other hand, noted limitations in ribosome display are RNase contamination, the stability of protein–ribosome–mRNA ternary complexes, and the functional levels of the ribosome, which depend on the library size.30
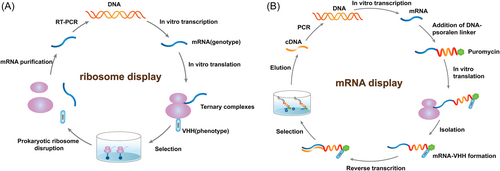
The mRNA/cDNA display technology employs a covalent bond to link mRNA/cDNA and displayed protein, which is more stable than ribosome display. (Figure 4B).17, 21 Odegrip et al.32 proposed the concept of using trans-acting factors to mediate the specific ligation of proteins to encode DNA and established the CIS display technology. Fenderico et al.33 used this technique to screen and identify nanobodies targeting low-density lipoprotein receptor-related protein 6 (LRP6) with sub-nM affinity to LRP6. The in vitro molecular display technology allows precise control of the protein expression environment and dramatically increases the library size. Moreover, the in vitro display system can be easily combined with mutation technologies for directed evolution and better representation of library diversity.16
The above display methods require a physical linkage between phenotype and genotype, possibly leading to screening bias. In 2019, Egloff et al.34 developed NestLink, an innovative technology for binding protein selection and identification. A VHH library is ligated to genetically encoded barcoding peptides, named library nesting. The nested library is sequenced by next-generation sequencing (NGS) and detected via mass spectrometry (MS). By combining library nesting, NGS, and liquid chromatography-tandem mass spectrometry/MS (LC-MS/MS), NestLink establishes a silico genotype–phenotype linkage, working in the absence of physical genotype–phenotype linkage and opens up avenues for the discovery of binding proteins with unique biological properties.
2.3 Nanobody expression systems
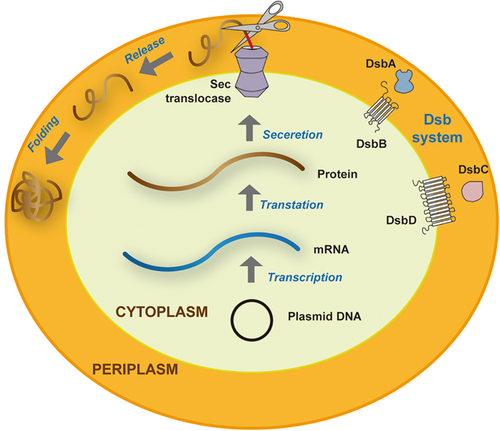
To successfully express and secrete functional and biologically active nanobodies, a variety of expression systems have been established, including prokaryotic, eukaryotic, and plant expression systems.35 In the past few decades, the prokaryotic expression system has been one of the most critical platforms for recombinant protein expression in laboratory environments.14 The most notable bacterial host is E. coli for its simple structure, rapid growth rate with short doubling time, low cost in cultivation medium, and ease of being transformed and maintained.17 Indeed, E. coli is associated with two significant drawbacks, including the expression of endotoxins and the presence of incomplete chaperone machinery.36 The structure of nanobody lacks the Fc domain as well as N-terminal oligosaccharides compared to conventional antibodies. Therefore, periplasm expression, in which exogenous proteins are secreted and accumulated, is the most popular method for expressing nanobodies.35 Nanobodies are first assembled in the cytoplasm of E. coli and then secreted into the periplasmic space via the Sec pathway with the help of the N-terminal leader sequences. The periplasmic space is a compartment with an oxidizing environment, and the presence of chaperones and isomerases, such as the Dsb system could facilitate the correct disulfide bond formation and aid in protein folding (Figure 5).37 Following expression, the osmotic shock method is used for nanobody extraction. Nonetheless, as the insufficiency of chaperones and the limited volume of the compartment, nanobodies are usually expressed at low levels.35 Furthermore, the aggregation and translocation of nanobodies in the cytoplasm are also bottlenecks of high yields. Compared to periplasmic expression, cytoplasmic expression, in which recombinant proteins are translated and accumulated, is 10–100 times more productive. However, most of the purified nanobodies are nonfunctional aggregations existing as inclusion bodies, and the activity needs to be recovered through complete denaturation and refolding.35
To solve the problem of an insoluble form of recombinant protein in the prokaryotic system, other expression systems can be employed instead, such as eukaryotic systems. Yeast expression systems are the common alternative that provides higher yields, sharing some of the features of both prokaryotic and eukaryotic cells.38 The crucial advantages of yeast include the ability to provide proper posttranslational modification processes, fast growth, inexpensive medium, and safe pathogen-free production. S. cerevisiae was the first yeast strain used for the expression of VHH (with yields greater than 100 mg/mL).38 However, the disadvantages of S. cerevisiae, such as plasmid instability and protein hyperglycosylated, have limited the number of commercial products, which has simultaneously promoted the development of methylotrophic yeast expression systems. Pichia pastoris is currently the most valuable and distinguished host for large-scale production and fermentation of nanobodies, possessing excellent secretion capacity to express correctly folded heterologous proteins.9 The industrial interest in this host is also attributed to high cell densities, potent and tightly regulated alcohol oxidase gene-1 (AOX1) promoter as well as the capability of posttranslational modification.35 Gai et al.39 demonstrated the production of Nb11-59 against SARS-CoV-2 on a large scale in P. pastoris, with 20 g/L titers and 99.36% purity, which means that nanobodies produced by yeast expression systems could be rapidly and widely used as biologics.
Mammalian expression systems remain the workhorse for full-length antibody production until today due to the occurrence of functionally and structurally essential glycosylations.35 Nanobodies might require their expression in mammalian cells when produced as Fc-fusions to restore the antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) effector functions or to prolong the half-life in vivo.36 Another fusion, usually difficult to express in bacteria, is nanobody-horseradish peroxidase fusion for colorimetric or electrochemical diagnostics. Thus, mammalian cells are effective platforms for promoting correct complex posttranslational modifications to generate proteins with characteristics similar to proteins present in vivo. However, more expensive production and higher demand system are barriers to their commercialization.36
Insect cell-baculovirus expression systems are based on insect cell lines infected with recombinant baculoviruses to express the exogenous proteins.40 Insect cells require only sterile hoods for suspension culture without expensive equipment or further modifications, which is superior to mammalian cell cultures that require special incubators cultivated at 5% CO2 and 75% humidity.40 Moreover, baculovirus is biosafety and does not pose any threats to vertebrates. Shokrollahi et al.41 used baculoviruses to infect insect cells to express vascular endothelial growth factor (VEGF) receptor-2 specific nanobody (3VGR19). The yield of recombinant 3VGR19 obtained in the baculovirus expression system was comparable to the bacterial expression systems, suggesting that transient infection of insect cells with baculovirus is a promising technology for expressing nanobodies. Using insect cells as a host for protein production platforms also has the advantage that the cytoplasmic environment of insect cells allows for proper protein folding and most posttranslational modifications, which are crucial for the full function of the target protein. However, recombinant protein expression with the insect cell-baculovirus expression system is very time-consuming due to the titration and pretesting of recombinant baculovirus strains before infection, which may otherwise lead to the lysis of insect cells or degradation of the target protein. Also, glycoproteins produced by insect cells have significantly different from those produced by mammalian cells.40
Plant expression systems appear to be an alternative to bacteria and mammalian expression systems owing to their easy transformation and scale-up features.42 In general, recombinant proteins can be obtained in different plant organs, such as leaves, seeds, and roots, and also accumulated in different subcellular compartments, such as cytoplasm, chloroplasts, and endoplasmic reticulum (ER). Plant cells perform the same posttranslational modifications as mammalian cells to express complex proteins and reduce safety issues since their evolution reduces the chance of contamination by toxins and viruses. However, differences between plant and mammalian systems in the N-glycan modification could result in dysfunctional retention of the VHH-Fc fusion protein in the ER of plants. Specific plant glycan residues, such as β (1,2)-xylose and ɑ (1,3)-fucose, may be immunogenic and induce allergic reactions in humans. This limitation can be addressed to retain the glycoprotein in the ER by adding KDEL (ER retention sequence) to the C-terminus of the recombinant protein.43 Park et al.44 transferred a gene expression cassette containing an anti-human epidermal growth factor receptor 2 (HER2) camelid single-domain antibody VHH fused to a human IgG Fc region with a KDEL (ER retention sequence) (VHH-FcK) into tobacco plants via the Agrobacterium-mediated transformation. A series of experimental results showed that plant-derived VHH-FcK could be correctly expressed and assembled with anticancer activity. Plants are ideal candidates as protein production platforms with versatility, affordability, and safety.
3 APPLICATIONS OF NANOBODIES IN DIAGNOSIS AND DETECTION
Antibodies are the primary tool used as biorecognition elements to detect food and environmental contaminants and biomarkers.9 An outstanding feature of these fascinating antibodies is their exceptional ability to identify and bind specific targets in complex environments, and their exquisite specificity has paved the way for their universal application in immunosensors and immunoassays. Consequently, there is a continuous demand for new antigen binders with better durability in research and development. Recent studies have shown that nanobodies are particularly attractive for analytical applications, such as biosensing devices and diagnostic kits, as well as in vivo diagnostics, as they have the versatility and beneficial properties of small size, ideal biodistribution, flexibility, and stability in harsh conditions.9 Several nanobody-based methods, such as ELISA, lateral flow immunochromatography assay (LFIA), flow cytometry analysis, immunoblotting (western-blotting), and positron emission tomography-computed tomography (PET-CT) imaging, have been developed and proved to be feasible.9
3.1 Nanobody as a reagent in clinical diagnosis
3.1.1 Nanobody as a reagent in cancer diagnosis
Biomarkers are defined as indicators to evaluate normal biological processes, pathogenic processes, and responses to exposures or interventions. In recent years, nanobodies have been widely used for detection biomarkers, including targets such as carbohydrate antigen 125 (CA125),45 prostate-specific antigen (PSA),46 and alpha-fetoprotein (AFP),47 which have evolved into a powerful tool in cancer research and have significant implications for early screening, diagnosis and monitoring of disease progression in oncological diseases. Rao et al.48 developed a novel microcantilever immunosensor using nanobodies as the receptor molecules to detect carcinoembryonic antigen (CEA) with a low limit of detection (LOD) as 0.03 ng/mL, achieving three orders of magnitude higher sensitivity. Zhang et al.49 reported the development and validation of a rapid, sensitive, and cost-effective nanobody-based immunoassay for the detection of fibrinogen-like protein 1 (FGL1), a major ligand of lymphocyte-activating gene 3 (LAG-3). This assay could evaluate the expression of FGL1 for patient stratification and for predicting the therapeutic efficacy of targeting the LAG3/FGL1 axis in a more straightforward way than existing methods. The diagnostic potential of nanobody as a probe in immunodetection systems offers possibilities for improving the diagnosis of cancers.
Given their small size, nanobodies can rapidly extravasate and diffuse in tissues to trace and combine with target antigens. In contrast, excess nanobodies are rapidly cleared via the kidney, thereby rapidly decreasing background labels. The extracellular matrix (ECM) forms a major component of the tumor microenvironment. Jailkhani et al.50 demonstrated the specificity of one anti-ECM nanobody (NJB2) using noninvasive in vivo immuno-PET/CT imaging and showed that it could detect multiple models of cancers (including breast cancer, melanoma, and pancreatic ductal adenocarcinoma). 64Cu-labeled NJB2 PET/CT imaging resulted in excellent clarity and signal-to-noise ratios for primary tumors and metastatic lesions seeded from orthotopic tumors that outperformed conventional 18F-2-fluorodeoxyglucose PET/CT imaging. Qin et al.51 developed a 68GA-labeled single-domain antibody tracer (68Ga-NOTA-Nb109) as a PET probe to detect the expression of programmed death receptor ligand 1 (PD-L1) and monitor changes in the expression in MC38 tumor-bearing mice as well as human programmed cell death protein-1 (PD-1) transgenic mice. Xing et al.52 reported that the early phase 1 study demonstrated that 99mTc-labeled anti-PD-L1 sdAb (99mTc-NM-01) single-photon emission computed tomography (SPECT)/CT imaging is a feasible and safe diagnostic procedure, delivering an acceptable radiation dose and presenting favorable biodistribution and image characteristics correlating with PD-L1 immunohistochemistry results in 16 patients with non-small cell lung cancer (NSCLC). Nanobodies-based probes have become powerful and ideal tools that are well suited for noninvasive nuclear imaging, in vivo optical imaging, or as intracellular tracers for basic research to detect cancer-specific biomarkers, providing great convenience in the clinical detection and prognostic assessment of cancers. However, there are several barriers that limit the clinical translation of nanobodies. For noninvasive nuclear imaging, the high kidney uptake of nanobodies poses a risk of nephrotoxicity and hampers imaging of lesions close to the kidney and bladder. Size increase via conjugation with fusion to PEG or albumin may be a solution strategy to overcome this limitation. Another prerequisite for the clinical adaptation of nanobodies is humanization. Despite the high homology to the human (V) region, camel-derived nanobodies may have significant associated immune responses.53 The nanobodies employed for the diagnosis of some cancer biomarkers are listed in Table 1.
| Name | Target | Species | Diseases | Application fileds | Conjugation | LOD | Range | Time | References |
|---|---|---|---|---|---|---|---|---|---|
| NbCEA5/aCEA-nb-800 | CEA | dromedary | Colorectal cancer (CRC)/pancreatic cancer | Fluorescence | IRDye800CW | 15 min–3 h | [54] | ||
| Nb2D5-HAP | Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM-5) | Alpaca | CRC | Chemiluminescent enzyme immunoassay (CLEIA) | Human placental alkaline phosphatase (HAP) | 0.85 ng/mL | 0.31–640 ng/mL | [55] | |
| [99mTc]Tc-NB4 | Epithelial cell adhesion molecule (EpCAM) | Human colorectal adenocarcinoma/human acute promyelocytic leukemia | SPECT/CT | 99mTc | 12 h | [56] | |||
| [68Ga]Ga-NOTA-3CMP75 | Membrane type 1-matrix metalloproteinase (MT1-MMP) | Llama | Triple-negative breast cancer (TNBC) | PET/CT | Gallium-68 (68Ga) | 45 min | [57] | ||
| 89Zr-anti-CLDN18.2 VHH-ABD | Isoform 2 of claudin 18 (CLDN18.2) | Gastric cancer | PET/CT | 89Zr | 4 h | [58] | |||
| Tb-NB1-H | Epidermal growth factor receptor (EGFR)/EGFRvIII | Breast cancer/glioblastoma | Fluorescence | Quantum dot (QD) | 80 ± 20 pM (16 ± 4 ng/mL) | [59] | |||
| GPN2-Nluc | Glypican-3 (GPC3) | Alpaca | Hepatocellular carcinoma (HCC) | Bioluminescence enzyme immunoassay (BLEIA) | Nanoluciferase | 1.5 ng/mL | [60] | ||
| [68Ga]Ga-NOTA-ABDG2 | GPC3 | Alpaca | HCC | PET | 68Ga | T1/2 = 1.1 h | [61] | ||
| 68Ga-NOTA-Nb109 | PD-L1 | Lung cancer/human glioma cancer/human colorectal cancer/human lungcancer/melanoma/colon cancer | PET | 68Ga | 1 h | [44, 62-65] | |||
| Nb-C4bpα-AP | AFP | HCC | Ratiometric fluorescence enzyme immunoassay (RFEIA) | Alkaline phosphatase (AP) | 0.013 ng/mL | [66] | |||
| A1-SBP | AFP | Alpaca | HCC | Nb-SBP-mediated fluoroimmunoassay (NS-LFIA) | Streptavidin-binding peptide (SBP) | 0.237 ng/mL | 0.49–125 ng/mL | [67] | |
| Nb-A1 and phage-A2 | AFP | HCC | P-ELISA | Phage | 0.237 ng/mL | [68] | |||
| Nb2 and Nb40 | PSA | Alpaca | Prostate cancer | Immunosensor | AuNPs | 0.08 ng/mL | 0.1–100 ng/mL | [69] | |
| Nb3@nPCN-224 | Human Epididymis Protein 4 (HE4) | Ovarian cancer | Photoelectrochemical (PEC) immunosensor | Porphyrin-based metal-organic framework (MOF) nanosphere (nPCN-224) | 0.560 pg/mL | 1.00 pg/mL –10.0 ng/mL | [70] | ||
| Nb151HA | Serum ferritin (SF) | Hepatoma | ELISA | HA-tag | 1.01 ng/mL | 9.0–1100 ng/mL | [71] | ||
| A1 and E8 | Cadherin 17 (CDH17) | Alpacas | Gastric cancer | Fluorescence | IR800 dye | 3–24 h | [72] | ||
| JK36AF680 | Cluster of differentiation 38 (CD38) | Llama | Multiple myeloma (MM) | Fluorescence | Alexa Fluor 680 | 6 h | [73] | ||
| VHH1E12 | Platelet-derived growth factor receptor beta (PDGFRB) | Llama | CRC | Fluorescence | IRDye-800CW | 6 min | [74] | ||
| 99mTc-CD3813 | CD38 | MM | SPECT/CT | 99mTc | 1 h | [75] | |||
| 64Cu-radiolabeled CD4-Nb1/CD4-Nb1-Cy5.5 | Human CD4 | Alpaca | CD4 T-cell leukemia HPB-ALL cells | PET/magnetic resonance imaging (MRI) | 64Cu/Cy5.5 | 10–20 min | [76] | ||
| 68Ga-NODAGA-SNA006 | CD8 | Lung cancer | PET/CT | 68Ga | 15 min | [77] | |||
| Nb15 | Signal regulatory protein alpha (SIRPα) | Alpaca and dromedaries | Glioblastoma (GBM) | SPECT/CT | 99mTc | 1 h | [78] | ||
| [68Ga]Ga-NOTA-MMBC3 | B cell maturation antigen (BCMA) | Alpaca | MM | PET/CT | 68Ga | [79] | |||
| RAD201(99mTc-NM-02) | HER2 | Breast cancer | SPECT/CT | 99mTc | 2 h | [80, 81] | |||
| [68Ga]Ga-NOTA-mal-hPD-L1 Nb | Human PD-L1 | Melanoma | PET | 68Ga | 3 h | [82] | |||
| 3132 | LAG-3 | Llama | Mouse colorectal cancer/melanoma/mouse lung cancer | SPECT/CT | 99mTc | 1 h | [83] | ||
| [111In]In-MSAP.2Rs15d | HER2 | Dromedary | Breast cancer | SPECT/CT and fluorescence | 111In | Stable for 24 h | [84, 85] | ||
| NB7, NB8, B13, NB37 | Prostate-specific membrane antigen (PSMA) | Camel | Prostate cancer (pCa) | Fluorescence | AlexaFluor680 | 3 h | [86] |
3.1.2 Nanobody as a reagent in infectious diseases diagnosis
Infectious diseases are a class of diseases caused by various pathogens that can be naturally spread between humans and animals. There is an urgent and realistic need to promote rapid, reliable, and convenient diagnostic techniques for the detection and identification of pathogens.87 Early and accurate diagnosis of infectious diseases could facilitate the control of disease worsening and the spread of pathogens. Currently, nanobodies have been extensively applied in detecting a variety of viruses, including human immunodeficiency virus-1 (HIV-1) and respiratory syncytial virus (RSV).88, 89 The spread of SARS-CoV-2 in recent years has led to the devastating global coronavirus disease 2019 (COVID-19) pandemic. Maeda et al.90 presented a panel of nanobodies that can quantify the spike proteins of five SARS-CoV-2 variants of concern (VOCs) including Omicron via ELISA, lateral flow, kinetic, flow cytometric, microscopy, and western blot analysis assays and the structural analyses showed that the P86 clone targets epitopes that are conserved and contacts the N-terminal domain (NTD), which are two hidden crevasses in the SARS-CoV-2 spike (S) proteins that are rarely accessed by conventional antibodies. The results demonstrated the application of nanobodies in a variety of immunoassays for surveilling viruses in the environment and monitoring infected individuals. However, it is important to note that nanobodies recognize mostly conformational epitopes, their application as probes in western blot may have low success rate.91 Gransagne et al.92 set up a sandwich ELISA assay using two nanobodies (VHH NTD E4-3 and VHH G9-1), allowing to detect SARS-CoV-2 nucleocapsid (N) protein as little as 4 ng/mL in solution. The ELISA assay could also detect the nucleoprotein (N) in human nasal swabs and SARS-CoV-2 variants (B.1.1.7/alpha, B.1-351/beta, and P1/gamma). Girt et al.93 identified the optimal combination (C5-Fc-SS-biotin as the capture agent and F2-Fc-horseradish peroxidase (HRP) as the probe agent) for the detection of SARS-CoV-2 in an ELISA, giving the LOD of 514 pg/mL for purified spike protein, 33 pg/mL for purified RBD, 16 tissue culture infectious dose 50 (TCID50)/mL for pseudovirus and 69–16 ffu/mL for heat-Empigen inactivated SARS-CoV-2, respectively. Nanobody pairs provide a simple and accurate ELISA for monitoring and quantifying heterologous SARS-CoV-2 proteins during their production in the biotechnology industry, demonstrating the promise of nanobodies in diagnostics. Further benefits like sensitivity may be gained by higher-order multimers capture/detection agents. Moreover, SARS-CoV-2 antigen detection plays a vital role in pandemic containment by mass tests of infected individuals.
Bacterial pathogens need to be identified quickly and efficiently, as bacterial infections may cause significant morbidity and mortality.94 Nanobodies represent an alternative strategy for diagnostic applications in bacterial infections, opening up access to targeted antigens previously unavailable to conventional antibodies during bacterial infections.94 Staphylococcal enterotoxins (SEs) secreted by Staphylococcus aureus are one of the significant causes of food poisoning, which is a severe threat to human health.95 Staphylococcal protein A (SpA) on the surface of cell walls of nearly all S. aureus or secreted into the culture supernatant exhibits a solid nonspecific interaction with the Fc-terminus of conventional antibodies, resulting in false positive during immunoassays and limiting its practical application of the method. Nanobodies without the Fc region can be used to replace the conventional antibody, to circumvent the SpA interference, and solve the bottleneck problem.95 Ji et al.95 developed a sandwich ELISA to detect Staphylococcal enterotoxin B (SEB) premised on nanobody, displaying an extensive quantitative range from 1 to 512 ng/mL with a LOD of 0.3 ng/mL. Hu et al.96 developed an Nb147-based sandwich ELISA for the surveillance of S. aureus contamination, possessing a LOD of 1.4 × 105 colony-forming units (CFU)/mL in milk or 10 CFU/mL after an 8 h enrichment step. Sun et al.97 developed a sandwich chemiluminescent immunoassay (CLIA) for the detection of SEB using a nanobody-alkaline phosphatase (Nb-ALP) fusion protein, which reduced analysis time and enhanced the sensitivity while avoiding the chemically coupled probes. The working range of the sandwich CLIA based on fusion protein, Nb37-ALP, was 3.12–50.0 ng/mL with SC50 = 8.59 ng/mL and the LOD was 1.44 ng/mL, indicating that Nb37-ALP is a precious tool for rapid and sensitive application in immunoassays. The nanobody-based assays described above fill the lack of immunoassays for intact S. aureus.
Nanobodies were shown to be capable of deeply penetrating the conserved epitopes of antigens, cryptic in intact parasites, which are inaccessible to classical antibodies.98 Human toxocariasis (HT) is a cosmopolitan zoonotic disease caused by the roundworm Toxocara canis (T. canis), a roundworm that colonizes the intestinal lumen of dogs. Morales-Yánez et al.99 designed an electrochemical magnetosensor to quantify T. canis excretory/secretory antigens (TES), aiming to identify active cases of HT. The assay has a LOD of 10 pg/mL in serum, a sensitivity that any other TES antigen detection test has never achieved. Ding et al.100 developed a lateral-flow immunoassay based on gold nanoparticles for the detection of Schistosoma japonicum soluble antigen (SEA) based on a nanobody (VHH-52). The LOD value for SEA detection was 3.4 ng/mL, laying the foundation for the application of nanobody in colloidal gold immuno-chromatographic techniques. The sensitivity and simplicity of its application make this method suitable for large-scale screening and could be conducted to meet the possible needs for surveillance in endemic countries. The nanobodies employed for the diagnosis of some infectious diseases are listed in Table 2.
| Name | Target | Species | Pathogens | Application fileds | Conjugation | LOD | Range | Time | References |
|---|---|---|---|---|---|---|---|---|---|
| Phage-Nb-SH | Vibrio parahaemolyticus (V. parahaemolyticus) | Bactricamel | V. parahaemolyticus | Colorimetric immunosensor | AuNPs | ~103 cfu/mL | Within 100 min | [101] | |
| 89Zr-VHH21 | Ly6C/G | Alpaca | Influenza A virus | PET | 89Zr | 24 h | [102] | ||
| HRP- hcAb A7.2 | Spike protein | SARS-CoV-2 | ELISA | HRP | [103] | ||||
| R1a-B6 | Hemagglutinin (HA) | Alpaca | Group 1 influenza A viruses | ELISA | 1.2 μg/mL | [104] | |||
| M13OTA and M13AFP | OTA/AFP | Dynamic light scattering (DLS) immunosensor | Multi-branched gold nanoflower (AuNF)/gold-coated magnetic nanoparticles (MNP@AuNP) | 1.37 and 57 pg/mL | [105] | ||||
| P543-Fc | Spike protein | Alpacas | SARS-CoV-2 | Low than 20 ng/mL | [90] | ||||
| VHH72 | Spike protein | Llama | SARS-CoV-2 | Organic electrochemical transistor (OECT)-based sensor | 1.2 × 10−21 M | [106, 107] | |||
| VHH-72-13C | Viral particles | Llama | SARS-CoV-2 | Electrochemical sensor | 1.2 × 104 viral RNA copies/mL | In 10 min | [108] | ||
| Nb | Spike protein | Camel | SARS-CoV-2 | Photoelectrochemical (PEC) immunosensor | 5 fg/mL | 0.015–15,000 pg/mL | [39, 109] | ||
| HRP-conjugated BiNb-3C5 | E2 glycoprotein | Chikungunya virus (CHIKV) | ELISA | HRP | 5–1000 ng/mL | [110] | |||
| sGP7, sGP49, RBD8, and RBD10 | Ebola virus secreted glycoprotein (sGP)/SARS-CoV-2 spike protein RBD | Ebolavirus/SARS-CoV-2 | Rapid electronic detection (Nano2RED) | Streptavidin-coated gold nanoparticles (AuNPs) | 1.3 pM for sGP and 1.3 pM for RBD | Within 5–20 min | [111, 112] | ||
| ZE | Nucleoprotein (NP) | Llama | Ebolavirus | Colorimetric and fluorescent visualization | Ascorbate peroxidase (APEX2) and mNeonGreen | 2–5 pfu/0.001 ng | [113] | ||
| SpyCatcher002-mClover3-HisTag/E12 | C-reactive protein (CRP) | Llama | Inflammation | Biosensor | Spy-Tag | 0.21 ng/mL | [114] | ||
| Nb13×2:eGFP and Nb1:AP | P9-1 | Llama | Mal de Río Cuarto virus (MRCV) | Sandwich ELISA | AP and eGFP | 0.236 ng/mL | [115] | ||
| ROTADIAL/monovalent SbP-labeled 2KD1 Nb | Viral structural protein 6 (VP6) | Llama | Rotavirus group A (RVA) | ELISA | Soybean peroxidase (SbP) | 1.4 × 103 AU-1 viral particles/100 μL | Less than 2 h | [116] | |
| Bt-C2–B6 | SARS-CoV-2 nucleocapsid protein | Llama | SARS-CoV-2 | MagPlex fluid array assays | Biotin | 50 pg/mL of recombinant nucleocapsid and of killed virus down to 1.28 × 103 pfu/mL | [117] | ||
| Bt-H6 sdAb | Lassa NP | Llama | Lassa virus | MagPlex-based homogeneous sandwich immunoassay | Biot | 39 ng/mL | [118] | ||
| BtNb32/HANbD6(32/D6) | Nonstructural protein 1 (NS1) | Llama | Zika virus | ELISA | Biotin | 0.80 ng/mL | [119] | ||
| NbPCV11 and NbRBC48 | Porcine circovirus type 2 (PCV2) | PCV2 | Hemagglutination assay | 104.09 TCID50/mL | Within 30 min | [120] | |||
| Fenobodies (NDV-fenobody-4) and RANbodies (NDV-RANbody-49) | Newcastle disease virus (NDV) | Bactrian camel | NDV | Sandwich ELISA | 22 of HA titers and 10 ng of purified NDV particles | [121] | |||
| QD-sdAbE2-1 and AF488sdAbE2-2 | E2 protein | Camelus bactrianus | Classical swine fever virus (CSFV) | Direct immunofluorescence assay (DIFA) | QD/AF488 | [122] | |||
| PPV-VP2-Nb56-HRP/PPV-VP2-Nb12-EGFP | Viral particles 2 (VP2) | Bactrian camel | Porcine parvovirus (PPV) | Sandwich ELISA | HRP and EGFP | 100 TCID50/100 μL | [123] | ||
| TxB (L-45 + S-E3) and GDH (4-L + 4-S) | Glutamate dehydrogenase (GDH) and toxin B (TcdB) | C. difficile infection (CDI) | NanoBiT (NanoLuc Binary Technology) split-luciferase assay | 44 fM | 32 min | [124] | |||
| Nb-01 | Five Salmonella serovars, including Salmonella Enteritidis (S. Enteritidis), Salmonella Typhimurium (S. Typhimurium), Salmonella London (S. London), Salmonella Paratyphi B (S. Paratyphi B) and Salmonella Hadar (S. Hadar) | Bactrian camel | Salmonella | Streptavidin-bridged sandwich ELISA (SAB-ELISA) | Biotin | 6.31 × 103 CFU/mL, 9.15 × 103 CFU/mL, 4.23 × 103 CFU/mL, 7.31 × 103 CFU/mL and 7.25 × 103 CFU/mL towards S. Typhimurium, S. Enteritidis, S. London, S. Paratyphi B and S. Hadar, respectively | Within 180 min | [125, 126] | |
| S. Enteritidis SE-Nb9 and HRP-Nb1 | S. Enteritidis | Bactrian camel | S. Enteritidis | Sandwich ELISA | HRP | 5 × 104 CFU/mL | [127] | ||
| Microcystis aeruginosa | Microcystis aeruginosa (M. aeruginosa) | Llamas | M. aeruginosa | Immunofluorescence/cell FLISA/thermal lens spectrometry (TLS) | GFP | 1.2 cells/mL | [128] | ||
| Biotinylated-Nb147 | Inactivated S. aureus | Alpaca | S. aureus | Sandwich ELISA | Biotin | 1.4 × 105 CFU/mL | [96] | ||
| CL9P4 | Vibrio cholerae O1 | Camel | Vibrio cholerae | Sandwich ELISA | 104 CFU/mL | [129] | |||
| B7/B1 | SEB | Bactrian camel | S. aureus | ELISA | 0.3 ng/mL | 1–512 ng/mL | [95] | ||
| Nb37-ALP | SEB | S. aureus | CLIA | ALP | 1.44 ng/mL | [97] | |||
| 2vb10-6×His | Brucella lumazine synthase (BLS)-B subunit of Shiga toxin type 2 (Stx2B) fusion protein | Llama | Stx-producing Escherichia coli (STEC) | ELISA | 4-8 ng/mL | [130, 131] | |||
| Nb 2TCE49 and Nb 1TCE39-ALP | TES antigens | Alpaca | T. canis | Sandwich immunosensor | ALP | 1 pg/mL | [132, 133] | ||
| Biotinylated nanobodies 2TCE49 and monovalent nanobodies 1TCE39 | TES antigens | T. canis | ELISA | HRP | 0.163 ng/mL | [133] | |||
| Biotinylated nanobodies 2TCE49 and monovalent nanobodies 1TCE39 | TES antigens | T. canis | Electrochemical assay | Biotin | 0.01 ng/mlL | [134] | |||
| Biotinylated-bivalent Nb2TCE49 and Nb 1TCE39-HRP | TES antigens | T. canis | Electrochemical magnetosensor | Biotin/HRP | 10 pg/mL | [99] |
3.1.3 Nanobody as a reagent in other diseases diagnosis
According to the report of the World Health Organization (WHO), the prevalence of cardiovascular disease is incessantly progressing and has doubled in the past two decades. Matrix metalloproteinase-2 (MMP-2) is an endopeptidase that plays acritical pathogenic role in cardiovascular diseases and cancers due to its role in atherogenesis, plaque instability, platelet activation, cancer growth, cancer growth metastasis, and some other pathologic conditions.135 Marturano et al.135 developed a nanobody (VHH-136), chemically conjugated to a fluorescent probe, which allowed to detect of human MMP-2 on cells in suspension using flow cytometry and on intact or permeabilized cells using immune-cytochemistry, showing great interest for basic and clinical research for the noninvasive visualization and quantification of MMP-2 in vivo.
The aggregation and proliferation of α-Synuclein (αSyn) are hallmarks of several neurodegenerative diseases, including Parkinson's disease (PD), Lewy Body dementia, and multiple system atrophy.136 Gerdes et al.136 designed a new Fluorescent Reporter (FluoReSyn) for reporting the presence or absence of human αSyn (hαSyn) by fusing the nanobody NbSyn87 to a fluorescent protein (Alexa647). The results demonstrated that the biosensor, FluoReSyn, is a valuable instrument with the capability of reporting small amounts of hαSyn in cell lines, primary neurons, and human cerebrospinal fluid samples, indicating the great potential of FluoReSyn for clinical research and diagnostics. Sterile alpha (SAM) and Toll/interleukin-1 receptor (TIR) motif containing 1 (SARM1) is a signaling enzyme possessing multicatalytic functions, regulating axonal degeneration (AxD), an early event in neurodegenerative diseases, through its nicotinamide adenine dinucleotide (NAD) metabolic activity.137 The TIR within SARM1 is the catalytic NADase domain, the Armadillo repeat motif (ARM) domain serves an autoinhibitory function, and the enzymatic activity is activated in vitro by nicotinamide mononucleotides (NMN). To address the problem that the structure of the NMN-activated form of SARM1 has not been fully determined, Hou et al.137 generated a unique nanobody, Nb-C6, that binds specifically to the NMN-activated SARM1 and stabilizes the ARM domain, imaging, and quantifying the activated SARM1, facilitating its activation and allowing the structural determination of NMN-activated full-length SARM1by cryo-EM. The nanobody, Nb-C6, not only was a valuable tool to visualize and localize active SARM1 and to activate SARM1 in cells but also provided an important understanding of the structural changes during the activation process to shed light on the activation mechanism of SARM1.
Amyloid beta oligomers (Aβo) are a viable biomarker for human Alzheimer's disease (AD). Habiba et al.138 used nanobody targeting Aβ1-40 (PrioAD12) and nanobody targeting Aβ1-42 (PrioAD13) oligomer antibodies to detect the level of Aβo in the retina, blood, and brain of 3-month-old APP/PS1 mice with immunohistochemistry, evidently offering a real possibility of establishing a screening platform in retinal imaging of Aβo and a reference diagnostic platform in blood assay for Aβo.139
3.2 Nanobody as a reagent in small-molecule detection
Nanobodies for analytical applications are particularly attractive as they can withstand harsh measuring conditions, such as high temperatures or the presence of organic solvents that would denature conventional antibodies, and are commonly used for the extraction of food contaminants.9 On the other hand, structural data reveal that the small-molecule-binding pocket in conventional antibodies is usually located at the VH and VL interface.140 Therefore, single-domain antibodies may have some limitations in analyzing low molecular weight analytes due to the lack of a VL domain.141 The current study showed the nanobodies have diversity in the morphology in recognizing haptens, such as the extended CDR3 (and CDR2/3) forms sufficient binding pockets for low molecular weight hapten ligands,142 the hapten is sandwiched between two VHH domains and their CDR3 loops in a 2:1 stoichiometry,143 or hapten inserted itself under and through the CDR1 loop.144, 145 More efforts are needed to investigate the structure of nanobodies and the structural data and recognition mechanisms of small molecules.
3.2.1 Nanobody as a reagent in toxin detection
Mycotoxins are secondary metabolites produced by fungal species that colonize crops, causing severe health hazards and considerable economic losses to humans and animals, as well as to industry and agriculture. Common mycotoxins with concern include aflatoxins (AFs), ochratoxins (OTs), zearalenone (ZEN), fumonisins (FBs), and other trichothecenes due to their frequent occurrence and high toxicity.146 Sensitive and effective determination technologies for mycotoxins surveillance are essential steps to ensure food safety and public health. Zhao et al.147 established a rapid magnetic beads-based directed competitive ELISA (MB-dcELISA) utilizing Nb28 and its mimotope ME17 for the detection of aflatoxin B1 (AFB1), with 50% inhibitory concentration and the detection limit of the MB-dcELISA were 0.75 and 0.13 ng/mL, respectively, and a linear range of 0.24–2.21 ng/mL. The MB-dcELISA combination of nanobody and mimotope was not only rapid and straightforward but also performed better in the validation study, providing a new strategy for the detection of AFB1 and various toxic small molecular weight compounds. Su et al.148 constructed two OTA-specific fluonanobodies (FluoNbs), one of which is a nanobody fused at the carboxyl-terminal of superfolder green fluorescence protein (SGFP), SGFP-Nb, displaying better fluorescence performance, selected as the tracer for OTA, to develop a FluoNb-based nanosensor (FN-Nanosens) via the fluorescence resonance energy transfer. The FN- Nanosens showed a LOD of 5 pg/mL and a linear detection range of 5–5000 pg/mL, with highly selective for OTA and good accuracy and repeatability in recovery experiments, showing that FN-Nanosens is an ideal immunosensing tool with ultrahigh sensitivity, providing a new homogeneous detection mode for the analysis of ultralow levels of small-molecule contaminants.
Microcystin-LR (MC-LR) is a kind of highly toxic biohazard produced by several bloom-forming cyanobacteria that seriously pollute the ecological environment and agro-products. Xu et al.149 designed a newly recombinant genetically engineered antibody (AVHH-MVHH) based on the previously obtained anti-MC-LR single-chain antibody and nanobody to establish an indirect competitive ELISA (IC-ELISA), with the LOD of 0.0075 μg/L, which is far below the maximum residue limit standard of 1.0 μg/L for MC-LR in drinking water proposed by WHO, showing good accuracy, repeatability, stability, applicability, and ultrasensitive binding activity. The nanobodies employed for the detection of some small molecules are listed in Table 3.
| Name | Target | Species | Small molecule | Application fileds | Conjugation | LOD | Range | Time | References |
|---|---|---|---|---|---|---|---|---|---|
| G8 | AFB1 | Mycotoxins | Immunosensor | Succinylated HRP (sHRP) | 20.0 fg/mL | 50.0 fg/mL–20.0 ng/mL | [150] | ||
| Nb28-Nluc | OTA | Mycotoxins | BLEIA | Nanoluciferase | 3.7 ng/mL | Within 3 h | [151] | ||
| Nb2-12 | 1-(4-Carboxybutyl)-1′-methyl-[4,4′-bipyridine]-1,1′-diium (PH-Q)-concanavalin A (ConA) | Alpaca | Paraquat | Time-resolved fluorescence immunochromatographic assay (TRFICA) | Time-resolved fluorescent europium (III) [FM] | 0.0090 ng/mL | 0.0201–0.165 ng/mL | [152] | |
| Nb-CC4-ALP | 1-Naphthol (1-NAP) | Bactrian camel | Carbaryl | Ratiometric fluoroimmunoassay (RFIA) | ALP | 0.01 ng/mL | 0.05–66.35 ng/mL | [153, 154] | |
| Nb-B15 and Nb-C21 | Hemiustilaginoidin F-H1-bovine serum albumin (BSA) | Alpaca | Ustilaginoidins | ic-ELISA | IC50 values were 11.86 and 11.22 μg/mL | 3.41–19.98 and 1.17–32.13 μg/mL | [155] | ||
| Anti-SEB sdAb ACVE and A3H2/anti-ricin sdAb D12f and F6H2Y | SEB/ricin | Vertical flow assay (VFA) | AuNPs | 0.12 μg/mL for SEB/0.11 μg/mL for ricin | [156] | ||||
| P2 | Cry2A toxin | Bacillus thuringiensis | ELISA | QD-Bead | 0.41 ng/mL | [157] | |||
| Nb28 | OTA | Mycotoxins | Bioluminescence immunosensor | 0.01 ng/mL | 0.04–2.23 ng/mL | [158] | |||
| Nb28 | AFB1 | Small molecular contaminants (SMCs) | Noncompetitive magnetic-chemiluminescent enzyme-linked immunoassay (Nc-MCLEIA) | 0.006 ng/mL | [159] | ||||
| Nb-jd8 | Fenitrothion (FN) | Alpaca | Organophosphorus pesticides (OPs) | ELISA | AP | 0.14 ng/mL | [160, 161] | ||
| Nb3-biotin | 3-PBA | Pyrethroids | ELISA | Biotin | 0.02 ng/mL | [162] | |||
| VHH T3-15 | Tetrabromobisphenol A (TBBPA) | Alpaca | Brominated flame retardant (BFR) | ELISA | Biotin | 0.75 ng/mL | Less than 30 min | [163, 164] | |
| C1 | Cyantraniliprole and chlorantraniliprole | Bactrian camel | Anthranilic diamide insecticides | ELISA | HA tag | 0.1 ng/mL | 0.2–5.7 ng/mL | [165] | |
| “Quenchbody (Q-body)” | Methotrexate (MTX) | Llama | Chemotherapeutic agent | Fluorescent biosensor “Quenchbody (Q-body)” | TAMRA | 0.56 nM | [144, 166] |
3.2.2 Nanobody as a reagent in pesticide residues detection
Pesticides can keep crop health and increase crop yields, but they pose a potential hazard to human health and the environment. Thus, research and sensitive analytical methods have been established to control these chemicals in both raw materials and processed foods. Phenoxybenzoic acid (3-PBA) is a common urinary metabolite of numerous pyrethroids pesticides, a class of broad-spectrum synthetic insecticides as an alternative to organophosphate insecticides.167 It is widely employed as a biomarker for human or environmental pyrethroids exposure. El-Moghazy et al.167 developed a novel sensitive electrochemical competitive immunosensor for the rapid detection of 3-PBA by coupling between the nanofiber and nanobody technologies in a range of 0.8–1000 pg/mL, with a detection limit of 0.64 pg/mL, showing excellent properties and stability over time. The novel nanosensor exhibited stunning analytical performance of easy use, low cost, reusability, and brief analysis, which has the potential to be an alternative to conventional methods for point-of-care detection.
4 CONCLUSION AND OUTLOOK
Due to their superior molecular properties, nanobodies are being developed for broad applications spanning biological research, in vivo or in vitro diagnosis, and target therapies as highly versatile molecules. The number of articles on novel or ingenious nanobody formats and new applications is currently exploding. In particular, the publication output on anti-SARS-CoV-2 nanobodies has entered a phase of exponential growth since the outbreak of COVID-19. Retrieval of nanobodies from the immune libraries remains a dominant method due to the high enrichment of nanobodies in vivo. However, the cost, feeding grounds, and antigenic properties limit its application. Nonimmune libraries have thus been developed to address concerns of the immune libraries. The naïve and semisynthetic/synthetic libraries are promising alternatives to rapidly selecting diverse and highly functional binders. Using transgenic mice to generate more target nanobodies is likewise attracting the interest of scientists.168 Numerous selection techniques independent of the approaches to constructing libraries have been well documented. Among them, phage display technology remains the classical method for the facile and rapid screening and panning of specific nanobodies. P. pastoris is currently the most promising host for large-scale fermentation to produce nanobodies. Nanobody discoveries are ideally designed with the ultimate application of the nanobody in mind, and most display or expression platforms can be applied to any library type depending on the envision or imagination of users. The characteristics of antibodies are a crucial point in the development of inexpensive and sensitive diagnostic test kits. Nanobodies could become resistant to extreme temperatures and chemical denaturation, and their small size allows them to detect antigens at a greater functional density, increasing the sensitivity of diagnostic tests. Nanobodies could also be assembled in more complex constructs composed of multiple nanobodies and enzymes, drugs, or other reporter molecules to satisfy different demands. The unique advantages of nanobodies will undoubtedly make them play an even more critical role in various fields to tackle new applications.
AUTHOR CONTRIBUTIONS
Qianling Su: Conceptualization (equal); data curation (equal); investigation (equal); resources (equal); software (equal); validation (equal); visualization (equal); writing—original draft (equal); writing—review & editing (equal). Wei Shi: Data curation (equal); formal analysis (equal); resources (equal); supervision (equal); validation (equal); visualization (equal); writing—original draft (equal); writing—review & editing (equal). Xianing Huang: Data curation (equal); investigation (equal); resources (equal); visualization (equal); writing—original draft (equal); writing—review & editing (equal). Shihua Yin: Conceptualization (equal); data curation (equal); supervision (equal); writing—original draft (equal); writing—review & editing (equal). Xiaomei Yang: Conceptualization (equal); funding acquisition (equal); methodology (equal); project administration (equal); supervision (equal); writing—original draft (equal); writing—review & editing (equal). Xiaoling Lu: Conceptualization (equal); funding acquisition (equal); project administration (equal); supervision (equal); writing—original draft (equal); writing—review & editing (equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was financially supported by the Project of National Key Research and Development Plan “National Key R&D Program of China” Grant/Award Number: 2019YFE0117300; the International (Regional) Cooperation and Exchange Program of National Natural Science Foundation of China, Grant/Award Number: 82220108003; National Natural Science Foundation of China, Grant/Award Number: 82260614; Guangxi Science and Technology Base and Talents Project, Grant/Award Number: GuiKe-AD20238062 and GuiKe-AA20325001.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




