Targeting the pathogenesis and boosting the therapeutic efficacy of Parkinson's disease by advanced nanoparticles
Abstract
With the aging of global population, the early diagnosis and treatment of neurodegenerative diseases such as Parkinson's disease (PD) have attracted considerable attention. Despite great advances achieved during the past decades, PD as the second largest neurodegenerative disease is still incurable. In the clinical practice, PD patients are mainly treated by drugs, and supplemented with deep brain stimulation or nerve nucleus destruction. The existing drugs can only relieve the symptoms of motor disorder, and cannot stop the progression of PD. Compared with small molecular drugs, nanoparticles exhibit multiple functions in the neuroprotection and neurorepair due to their tunable physical and chemical properties, easy modification and functionalization. Herein, we first briefly review the characteristics of nanoparticles crossing the blood–brain barrier, which is a primary challenge for the treatment of PD. Then, we summarize the pathologic mechanisms of PD and comprehensively discuss the novel PD therapy based on diverse nanoparticles, including alleviating oxidative stress, scavenging α-synuclein aggregates, chelating metal ions, delivering neurotrophic factors and genes, and transplanting stem cells. This review aims to highlight the great potential of advanced nanoparticles in the therapy of PD.
1 INTRODUCTION
Parkinson's disease (PD) is the second major neurodegenerative disease of the central nervous system (CNS), and mainly occurs in the middle-aged and elderly people. The currently available clinical drugs can only delay the progression of PD, but cannot reverse the course of the disease. The incidence, disability rate and mortality of PD is growing faster than other neurological diseases and the worldwide PD patients are expected to be about 10 million in 2030.1, 2 Although PD is considered as a motor disorder with symptoms of bradykinesia, myotonia, quiescent tremor and gait instability, individuals with PD usually experience the progression of nonmotor symptoms for several years before the beginning of motor symptoms,3 such as reduced sense of smell, sleep disturbances, fatigue, depression, anxiety, autonomic dysfunction, and numbness of limb pain.4 The PD patients will develop cumulative disability and loss of independence eventually.
PD is an incurable, progressive disease that often requires long-term drug treatment, and thus lead to drug resistance. Moreover, disease-related pathophysiology in the brain usually changes over time, which may also lead to the gradual decline in effectiveness of drugs.5 The existing treatment of PD can be briefly classified into pharmacological and nonpharmacological treatments. Although several drugs have been developed and used for treatment of PD, including carbidopa, levodopa, monoamine oxidation-B (MAO-B) inhibitors (e.g., rasagiline and selegiline), dopamine agonists (e.g., pramipexole and ropinirole), adenosine A2A receptor antagonists and amantadine,6 they can only relieve the PD symptoms. Nondrug interventions, such as deep brain stimulation and focused ultrasound, are commonly used to treat PD patients with motor disorders and resistant to drugs.7 Despite progress in PD therapy, it remains to be an incurable neurodegenerative disease.
Nanomaterials have the characteristics of small particle size, large specific surface area, high surface reactivity, multiple reactive centers, and good biocompatibility. They can be used as contrast agents for diagnosis of multiple diseases. As drug carriers, they can prolong the half-life of drugs and improve their utilization to achieve effective targeted delivery and reduce their side effects on normal tissues.8, 9 In addition, functionally engineered nanoparticles allow drugs crossing the blood–brain barrier (BBB) to enter into the brain effectively.10, 11 Over the past 30 years, several nanomaterials have been approved by Food and Drug Administration, including liposomes, polymers/micelles, iron oxides, mesoporous silica, and gold NPs.12 The flexibility in the design and modification of nanomaterials makes them very attractive in the treatment of various diseases in the complex biological microenvironment, such as cancer,13 cardiovascular diseases,14 and neurodegenerative diseases.11
In this review, we first briefly introduce the pathological mechanisms of PD and the BBB issue. Then, we focus on the recent advances in treatment of PD by targeting the pathological mechanisms with diverse nanoparticles, including mitigation of oxidative stress, removal of α-synuclein aggregates, metal ions chelation, stem cell-based cell replacement therapy, gene therapy, and neurotrophic factor (NTF) therapy (Figure 1). We also discuss the current challenges and the perspectives of advanced NPs and nanomedicines in the treatment of PD. We hope this review will provide guidance for the development of novel nanomedicines for the treatment of PD.
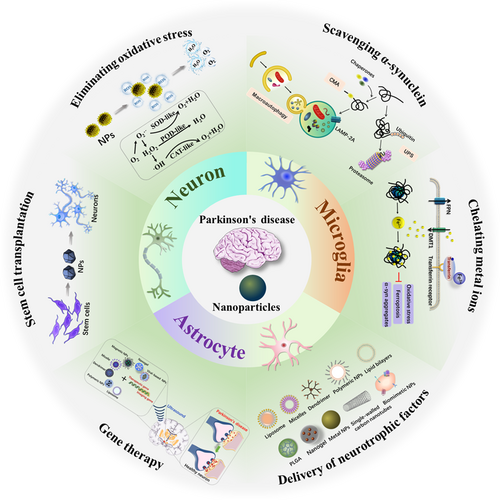
2 THE CELLS ASSOCIATED WITH THE PD
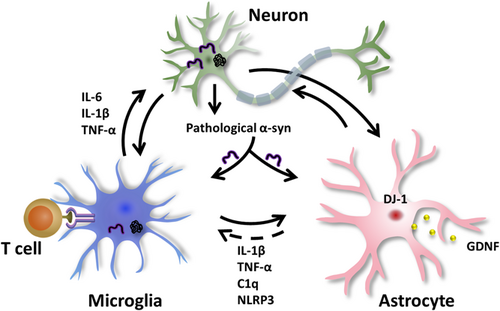
PD is typically characterized by the progressive loss of dopaminergic neurons in the substantia nigra and the appearance of Lewy bodies in the cytoplasm of neurons, which is a pathological hallmark and primarily composed of disordered α-synuclein aggregates.15 Moreover, it is widely believed that nonneuronal cells also make contributions to the progression of PD pathology.16 For example, astrocytes provide essential metabolic and functional support to neurons under normal circumstances. However, under certain conditions, the inflammatory microglia compromise their protective function, and mediate the transfer of astrocytes into the neurotoxic A1 phenotype in various neurological diseases.17 In this section, we briefly outline the interactions among neurons, microglia and astrocytes, and their contributions to PD pathology (Figure 2), which is useful for selecting appropriate targets during drug design and development.
2.1 Neuronal dysfunction in the PD
The characteristic features of PD include the loss of neurons in the substantia nigra and the presence of Lewy bodies in the neuronal cytoplasm. There are multiple molecular pathogenesis and pathways related to neuronal function involved in the pathogenesis of PD, such as misfolded α-synuclein aggregation, MAO-B catalyzes oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, dysfunction of the lysosomal and proteasome systems, abnormal metal ion homeostasis, neurotransmitter deficiencies, abnormal axonal transport and neuroinflammation.18 Besides, genetic factors are usually considered to be the etiology of familial PD, and there are around 5%–10% PD caused by genetic factors.3 For example, point mutation (A53T) at the SNCA is the most common familial inherited gene associated with PD.19 Meanwhile, the most interested genes of mutations are the SNCA, LRRK2 (which encodes leucine-rich repeat serine/threonine-protein kinase 2), PRKN (parkin RBR E3 ubiquitin protein ligase, PARK2), PINK1 (PTEN-induced putative kinase 1), GBA (glucosidase beta acid gene, which encodes glucocerebrosidase) and DJ-1genes,18 of which SNCA and LRRK2 are associated with dominant PD cases, while PRKN, PINK1, GBA, and DJ-1 are associated with recessive PD cases.20 In addition, environmental factors, including industrial or agricultural pollution (e.g., pesticides and herbicides), may also cause the PD.
As a key pathogenic protein, the intracellular aggregated α-synuclein has been found in all PD patients. In the pathogenic process, soluble α-synuclein monomers initially form oligomers, then gradually aggregate into small fibrils, and finally form large insoluble α-synuclein fibrils to produce pathological aggregates (Lewy bodies).3 In the healthy human brains, only about 4% of α-synuclein is phosphorylated at residues serine-129 (Ser-129). However, approximately 90% of α-synuclein phosphorylated at Ser-129 was detected in the Lewy bodies of PD patients.21 Extracellular α-synuclein oligomers are able to target cell and organelle membranes to disrupt their integrity because the N-terminal region of α-synuclein is able to strongly bind with lipid bilayers.22
It has been known that α-synuclein could not only enrich in the presynaptic terminal of neurons, but also strongly interact with plasma membranes and mitochondrial membrane. The accumulation of α-synuclein is also associated with endoplasmic reticulum stress and defects in organelles such as mitochondria and lysosomes.23 For example, González-Rodríguez et al.24 found that the dysfunction of mitochondrial complex I could initiate PD. In addition, the dysfunction of lysosome may also promote the aggregation of soluble α-synuclein oligomers, because the aggregated and misfolded α-synuclein cannot be timely and efficiently degraded through macroautophagy and chaperone-mediated autophagy (CMA). Reduced lysosomal glucocerebrosidase hydrolase (GCase) activity in the brains of PD patients caused by GBA mutations is also a common risk factor for PD.25, 26 Recent studies have shown that pathogenic α-synuclein species accumulated in neuronal lysosomes in the mouse brain, and the neurons released pathogenic α-synuclein via SNARE-dependent lysosomal exocytosis.27 The NOD-like receptor thermal protein domain-associated protein 3 (NLRP3) is a central driver for neurodegeneration, and its activation could trigger the neurodegeneration and cause the death of neurons.28
2.2 Microglia in the PD
As the primary innate immune cells in the brain, microglia act as a homeostatic sensor by monitoring the surrounding environment, clearing cell debris, and providing neurotrophy.29 In addition to brain immunity, microglia support brain development by pruning the synapses of developing neurons.30 Accumulating evidence indicates that activated microglia are the sources of multiple neurotoxic factors, including inflammatory factors and reactive oxygen species (ROS). They promote the progression of neuronal damage.31 Although low-concentrated ROS is the key signaling molecule under normal conditions, but neurons are particularly vulnerable to oxidative stress caused by excess ROS.32, 33 In addition, microglia cells can generate and release toxic oxygen and nitrogen species by modulating several enzyme systems, such as nicotinamide adenine dinucleotide phosphate oxidase, inducible nitric oxide synthase (iNOS), and myeloperoxidase. Furthermore, the damaged microglia exacerbate lipopolysaccharide-induced proinflammatory responses, NLRP3 activation, and astrocyte proliferation, thus increasing degeneration of dopaminergic neurons and motor dysfunction.34 Microglia enhance the phagocytosis of α-synuclein aggregates through the interaction between their toll-like receptor 2 (TLR2) and α-synuclein.35 As a return, they can be activated by α-synuclein through either the TLR4 or TLR2 pathway.36 Many studies have reported that microglial activation and neuroinflammation may be the key regulators for the loss of dopaminergic neurons in PD patients.37
Similar to a double-edged sword, the microglia can also alleviate the symptoms of PD by releasing some anti-inflammatory factors38 and phagocytosing α-synuclein aggregates.39 For example, microglia can form functional networks, reduce the load of a-synuclein aggregates by donating healthy mitochondria to each other, relieve the inflammatory response and the cytotoxicity of α-synuclein aggregates, and eventually degrade α-synuclein aggregates in a collaborative way.40 In addition, microglia may also play a crucial role in PD immunotherapy by targeting α-synuclein.3 For example, the activated microglia strongly express the major histocompatibility complex (MHC), which is involved in processing and presenting autoantigens to T cells.41 In a word, microglia play an important role in the progression of PD and can be an important target.
2.3 Astrocytes in the PD
Astrocytes are the most abundant subtypes of glial cells in the brain and are critical for maintaining the normal neuronal function of a healthy brain. As a component of BBB, astrocytes release various NTFs (e.g., glial-derived neurotrophic factor [GDNF]), which are particularly crucial for developing and survival of dopaminergic neurons. Astrocytes can also affect neurons by controlling the concentration of extracellular potassium ions (K+) and intracellular calcium ions (Ca2+) to prevent their overexcitation. In gray matter, astrocytes control the number of synapses, regulate the blood flow to ensure adequate energy expenditure, and provide lactic acid to neurons for energy metabolism.42
However, increasing evidence suggests that the damage of astrocytes is related to the pathology of PD. For example, reactive astrocytes may contribute to PD pathology through their roles in glutamate-mediated excitatory toxicity, K+ buffering, and Ca2+ homeostasis.43 The trauma and infection of the CNS may activate astrocytes and exacerbate PD pathogenesis.44 There exist two states of reactive astrocytes, which are defined as “A1” and “A2.” They are similar to the “M1” and “M2” phenotype of macrophages.45 The activated microglia can secrete tumor necrosis factor alpha (TNF-α), interleukin-1 alpha (IL-1α), and complement component 1q to regulate the transformation of astrocytes into the toxic A1-reactive astrocytes,46, 47 as shown in Figure 2. Meanwhile, A1 astrocytes can produce cytokines such as TNF-α, IL-1β, IL-6, and interferon-γ, thus losing the promotive ability for neuronal survival, growth, synaptogenesis, and phagocytosis, and lead to neuronal and oligodendrocyte death.48, 49 In contrast, A2 astrocytes play a protective role by secreting NTFs that promote survival and growth, synaptic repair and promote angiogenesis by regulating angiogenesis-related vascular endothelial growth factor (VEGF) and transforming growth factor β (TGFb).50
In addition, genetic mutations of DJ-1, α-synuclein, calcium-independent phospholipase A2, ATP13A2 (also known as PARK9), PINK1, and Parkin are involved in the damage of astrocyte functions, including inflammatory response, glutamate transport, and secretion of NTFs.51 For example, the defects or mutations of DJ-1 in astrocytes impair their neuroprotective effects, including anti-inflammatory function52 and mitochondrial quality control.53 The deficiency of the ATP13A2 gene, which encodes transmembrane lysosome type P5 ATPase, exacerbates astrocyte-mediated neuroinflammation through activation of NLRP3 inflammasomes.54 PINK1 and Parkin encode mitochondrial targeted protein kinase and E3 ubiquitin ligase respectively, and are involved in mitochondrial quality control pathways for elimination of damaged mitochondria.
Previous studies have demonstrated that mitochondrial dysfunction is the main cause of astrocytes transforming from the resting state to the neurotoxic A1 state.55 Transfer of mitochondria between dopaminergic neurons and astrocytes is a critical way to remove the damaged mitochondria without activating neuroinflammation because the damaged mitochondria in neurons can be released and transferred to astrocytes for processing and recycling.56, 57 The astrocytes can provide neurons with functional mitochondria, enhance neuronal adenosine-triphosphate (ATP) replenishment and modulate neuronal antioxidant defenses.58 In addition, glutathione (GSH) is one of the main defenses against ROS, depletion of GSH in astrocytes can selectively inhibit mitochondrial complex I, thereby affect mitochondrial function by triggering ROS-induced cascade reaction, leading to the damage of dopaminergic neurons.59 It should be noted that reprogramming astrocytes into functional dopaminergic neurons by transcription factors and microRNAs (miRNAs) has recently attracted extensive attention.60 However, the long-term viability and functional integrity of neurons derived from them, and the effect of depleting astrocytes on neuronal functions need to be further explored.
3 DESIGN OF NANOPARTICLES FOR CROSSING THE BBB
PD patients are mainly treated by chemical drugs in clinical practice. However, an obstacle for chemotherapy of PD is the BBB, which is mainly composed of microvascular endothelial cells, astrocytes, and pericytes.61 In addition, other components such as smooth muscle, basement membrane, and microglia also maintain an intact BBB to ensure its protective effect on brain tissue. The BBB prevents almost all large molecules and 98% of small molecules to enter into the brain.62 It is a highly selective semipermeable boundary of endothelial cells, allows the selective and active transport of some small molecules and various nutrients, ions, and macromolecules, such as glucose and amino acids that are essential for maintaining neural functions.63
Many studies have focused on how to overcome the BBB issue and efficiently deliver drugs into the brain. There are several approaches for crossing the BBB, specifically, different types of endocytic transport systems are expressed on the brain capillary endothelial cells (Figure 3), such as cell-mediated transport (CMT), receptor-mediated transport (RMT) and adsorption-mediated transport (AMT).11 CMT mainly recognizes and transports many indispensable nutrients and ions (such as glucose, amino acids, nucleosides, vitamins, choline, electrolytes, etc.).64 RMT, which is also known as clathrin-dependent endocytic transport, involves many specific receptors, such as the low-density lipoprotein (LDL) receptor, the transferrin receptor, diphtheria toxin receptor, and insulin receptor.62 They are mainly responsible for the recognition and transport of endogenous macromolecules. AMT mainly transports positively charged macromolecules. For example, the cationic penetrating proteins, which are a kind of amphiphilic cationic polypeptide, can bind to anionic sites on the surface of endothelial cell membranes through the electrostatic interactions to penetrate the BBB. Fan et al.65 prepared a human H-ferritin nanocarrier for targeting transferrin receptor 1 (TfR1), which is highly expressed on brain endothelial cells and malignant brain tumor cells to successfully mediate nanocarrier crossing the BBB.65 Similarly, dos Santos Rodrigues et al.66 designed transferrin-modified multifunctional liposomes to cross the BBB by receptor-mediated transcytosis. The angiopep-2 peptide, which is highly expressed in the brain capillary endothelial cells and glioma cells for targeting LDL, is also a promising candidate to assist nanodrugs crossing the BBB.67, 68 Glucose-coated nanocarriers, which can bind to glucose transporter-1 expressed on the brain capillary endothelial cells, were also used to cross the BBB.69, 70 In addition, a few type of circulating cells and their derivatives, such as macrophages,71 red blood cells, neutrophils, and exosomes,72 can carry drugs and across the BBB.
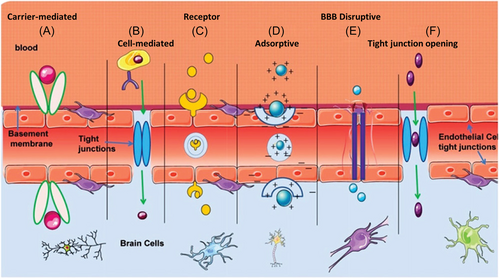
Over the past few decades, several noninvasive therapeutic delivery strategies have been developed to cross the BBB, such as intranasal delivery, which allows the drug to be transported along the olfactory and trigeminal axons via transneural pathways.73 In addition, focused ultrasound, which is a promising technology for drug delivery, can open the BBB transiently, locally, and reversibly. To date, focused ultrasound has been used to assist in the delivery of various compounds into the brain, such as nanoparticles,74 antibodies (e.g., trastuzumab75), chemotherapy drugs (e.g., temozolomide76), nucleotide small interfering RNA (siRNAs),77 neurotrophins,78 viral and nonviral vectors.79 Recent works have demonstrated that focused ultrasound can be used to efficiently deliver nanoprobes or nanomedicine into the brain for imaging of immunosuppressive M2− tumor-associated macrophages in orthotopic glioma,80 significantly reducing chemo-/radio-resistance of glioblastoma,81 and improving the radiation therapy82 and immunotherapy of orthotopic glioblastoma.83 Moreover, the focused ultrasound has been used for the treatment of brain tumors in clinical practice with encouraging results.75 Both the fundamental research and clinical practice show that focused ultrasound has great potential in the delivery of small molecular drugs and nanoagents for imaging and therapy of brain diseases.
In addition, with the emergence of nanotechnology, various nanoparticles have been considered as promising drug carriers or as drugs themselves due to their unique properties, such as small size, high drug loading efficiency, good stability, easy modification, biodegradability, and high biocompatibility. Nanoscale materials have been extensively explored for drug delivery, such as metal nanoparticles, polymeric nanoparticles, lipid nanoparticles, exosomes, and so on.
4 TREATMENT OF PD BY ADVANCED NANOPARTICLES
Although the pathogenesis of PD is still unclear, it is generally believed that oxidative stress, mitochondrial damage, neuroinflammation, α-synuclein toxicity, and iron overload are the main causes of PD.84 Herein, we present the application of advanced nanoparticles in the treatment of PD by targeting these causes.
4.1 Alleviation of oxidative stress
Substantial evidence supports that oxidative stress is a prominent feature in the pathogenesis of PD. Reduced activity of antioxidative enzymes and nonenzymatic antioxidants in the brain of PD patients could be responsible for the disease progression. For example, it has been demonstrated that the activity of glutathione peroxidase (GSH-Px), which can reduce the oxidized glutathione (GSSG) into GSH in the brain of PD patients, was significantly reduced to result in the accumulation of hydroxyl radical (•OH).85 Abraham et al.86 demonstrated that the activities of superoxide dismutase (SOD), catalase (CAT), GSH-Px, and glucose-6-phosphate dehydrogenase were significantly decreased in the PD patients.86 Dopaminergic neurons are particularly vulnerable to oxidative stress due to the production of numerous oxidants, including H2O2, •OH, superoxide radical (•O2−), and DA semiquinone radicals, during enzymatic and nonenzymatic reactions when dopamine is released from synaptic vesicles into synaptic cleavages or cytosol.87 Oxidation can cause damages to lipids, proteins, DNA, and RNA in the brains of PD patients88 and the accumulation of byproducts from lipid peroxidation has been found in the serum and cerebrospinal fluid of PD patients.89
In recent years, nanoparticles with ROS scavenging capability and enzyme-like activity have been developed for PD treatment (Table 1). Carbon, metal, and metal oxide nanoparticles are often used to protect neurons by scavenging ROS. For example, polyhydroxylated fullerene derivative (C60(OH)24) can protect neurons from 1-methyl-4-phenylpyridine (MPP+) induced mitochondrial dysfunction and oxidative stress by effectively eliminating superoxide, hydroxyl, and lipid radicals.90 Ren et al.91 demonstrated that graphene oxide quantum dots exhibited neuroprotective effects by effectively scavenging MPP+-induced ROS, mitochondrial damage, cell toxicity, and apoptosis. Lei et al.92 used ultra-thin graphite-carbon nitride (g-C3N4) nanosheets to enhance the peroxidase activity of hemin and restore dopamine-dependent behavior of PD nematode model by reducing ROS-mediated neurotoxicity.
| Nanoparticles | Applications | Mechanism | References |
|---|---|---|---|
| C60(OH)24 | Scavenge radicals. | Act as a phase 2 enzyme inducer to promote the expression of Nrf2 and the activity of γ-glutamyl cysteine ligase, increase the level of glutathione. | [90] |
| Graphene oxide quantum dots | Decrease ROS, α-synuclein, mitochondrial damage, and apoptosis. | CAT-like activity and regulation of metabolism. | [91] |
| Ultrathin graphitic carbon nitride nanosheet (g-C3N4) | Scavenge ROS and inhibit ROS-induced apoptosis. | POD-like activity. | [92] |
| Prussian blue nanozyme (PBzyme) | Inhibit pyroptosis and scavenge ROS. | Reduce the activation of NLRP3 inflammasomes and caspase-1, downregulate GSDMD cleavage. | [93] |
| CuxO nanoparticles | Scavenge ROS. | Multiple enzyme-mimicking activities, such as CAT, GPx, and SOD. | [94] |
| PtCu nanoalloys | Scavenge ROS and inhibit α-synuclein spread. | POD-like, CAT-like, and SOD-like activities. | [95] |
| Fe3O4 nanoparticles | Reduce oxidative stress and apoptosis. | CAT-like activity. | [96] |
| CeNP-PEG | Scavenge ROS and promote microglia to polarize from M1 into the M2 phenotype. | Downregulate the activation of NF-κB and nuclear translocation of P65 protein. | [97] |
| Yb3+, Er3+ Co-doped CeO2–x upconversion nanoparticles | Scavenge ROS and increase antioxidant capacity. | CAT-like and GPx-like catalytic activity. | [98] |
| Chiral Se@CeO2 superparticles | Inhibit oxidative stress and reduce the amount of α-synuclein. | GPx-like, CAT-like, SOD-like, and POD-like activity. | [99] |
| Mn3O4 nanoflowers | Prevent ROS-mediated neurological disorders. | SOD-like, CAT-like, and GPx-like activity. | [100] |
| Lactoferrin (Lf)-modified Au-Bi2Se3 nanodot | Scavenge ROS and protect mitochondria. | SOD-like, CAT-like, POD-like, and GPx-like activity. | [101] |
| Polydopamine and selenocystine nanocomposite | Scavenge ROS to relieve oxidative stress. | CAT-like, SOD-like, and GPx-like activity. | [102] |
| Albiflorin-glycyrrhizic acid-nanogel | Relieve oxidative stress and provide anti-inflammation property. | Regulate the mRNA level of oxidative stress and the signaling molecules in the PI3K–AKT signaling pathway. | [103] |
| RVG29 conjugated 4,4′-dimethoxychalcone (RVG-nDMC) | Reverse oxidative stress and inflammation. | Inhibit TH ubiquitination, regulate glucose metabolism, and decrease mRNA levels of proinflammatory cytokines. | [104] |
| Engineered exosomes contain polymeri-zation of l-arginine derivatives (MSCEXO/PMA) | Reduce intracellular ROS level and regulate the microglia to M2-phenotype. | Gradually consume ROS during chemotaxis, reduce ROS levels, produce beneficial NO. | [105] |
- Abbreviations: CAT, catalase; GPx, glutathione peroxidase; mRNA, messenger RNA; NF-κB, nuclear factor-κB; NO, nitric oxide; Nrf2, nuclear factor erythropoie-like 2; PD, Parkinson's disease; PI3K–AKT, phosphoinositide 3-kinase–protein kinase B; POD, peroxidase; ROS, reactive oxygen species; RVG, rabies virus glycoprotein; SOD, superoxide dismutase.
Some inorganic metallic nanoparticles also exhibit excellent antioxidative capability, such as monometallic (e.g., gold nanorods and quercetin encapsulated by mesoporous silica, AuNRs@QCT),106 and polymetallic nanoparticles (e.g., PtCu nanoalloys).95 Ma et al.93 showed that Prussian blue nanoenzyme, as a pyroptosis inhibitor, can alleviate PD by removing ROS to reduce the activation of microglial NLRP3 inflammasomes and caspase-1 (Figure 4A). In addition, metal oxide nanoparticles, including iron oxide,96 cerium oxide,97 Yb3+ and Er3+ codoped cerium oxide,98 and manganese oxide107 are also commonly used for neuroprotection due to their excellent enzyme-like activity. Singh et al.100 reported a Mn3O4 nanozyme with SOD-, CAT- and GPx-like activities for protecting SH-SY5Y cells from MPP+-induced cytotoxicity. Hao et al.94 prepared uniform CuxO nanoclusters with ROS scavenging capability to rescue the memory loss of PD mice.
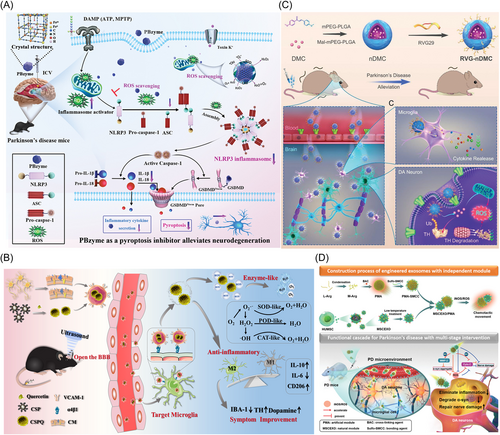
Metal chalcogenides NPs also show excellent capability of scavenging ROS. Lactoferrin-modified Au–Bi2Se3 nanodots can also improve the memory and activity of PD mice by their multienzyme-like properties, such as SOD, CAT, GPx, and POD, which can maintain mitochondrial function and control ROS levels in cells.101 Quercetin-modified ultra-small Cu2−xSe nanoparticles (CSPQ nanoparticles), which showed better capability of scavenging free radicals than nanoparticles and quercetin alone, also can alleviate oxidative stress through their multiple enzyme activities. Cell membrane-modified CSPQ nanoparticles can target microglia by the interactions between α4β1–VCAM-1 to polarize microglia into M2 phenotype, and significantly improve the movement disorder of PD mice (Figure 4B).38
Other nanoparticles have been also developed to modulate ROS level for PD therapy. For example, natural compounds curcumin-loaded polysorbate 80-modified cerasome nanoparticles,108 curcumin analog-based nanoparticle,109 polydopamine and selenocystine nanocomposite,102 glycyrrhizic acid-zinc alginate nanogel encapsulated albiflorin.103 Zhang et al.104 developed the BBB-penetrating peptide (rabies virus glycoprotein 29 [RVG29]) conjugating with natural autophagy inducer 4,4′-dimethoxychalcone to form a novel delivery system (RVG-nDMC), which can alleviate oxidative stress in dopaminergic neurons and reverse tyrosine hydroxylase ubiquitination (Figure 4C).104 Amphiphilic nanoparticles modified natural exosomes, which were derived from human umbilical-cord mesenchymal stem cells (MSCs), also showed excellent capability of capturing chemical attractants (ROS and iNOS) through l-arginine derivatives on nanoparticles (Figure 4D).105
4.2 Elimination of α-synuclein aggregates
The hypothesis of prion-like α-synuclein suggests that once α-synuclein aggregates were formed in neurons, they would be released into the extracellular space and taken up by neighboring neurons, resulting in the accumulation of α-synuclein in new host neurons.110, 111 As an inherent and soluble protein, α-synuclein consists of 140 amino acid residues, mainly locates in the presynaptic terminal of neurons and participates in the regulation of synaptic activity by regulating the release of synaptic vesicles.112 In the healthy physiological state, α-synuclein mainly exists in the form of monomer. Nevertheless, under the pathological conditions, mismodification of α-synuclein (such as nitrification, oxidation, and phosphorylation) leads to degeneration and formation of insoluble oligomers or fibrous structures, even aggregates.113 α-Synuclein oligomers can bond to lipids and aggravate oxidative stress, increase the permeability of mitochondria, lysosomes and vesicle membranes, and interfere with ion homeostasis and protein clearance pathways.114-116 Moreover, about 90% of pSer129-α-synuclein was detected in the brain of PD patients. It is the main component of Lewy bodies (the key pathological feature of PD).117
CMA, ubiquitin-proteasome system (UPS) and autophagy-lysosomal pathway (ALP) are the key pathways of repairing or eliminating abnormal proteins.118 When α-synuclein is subjected to mutations, posttranslational modifications, or external stimuli (oxidative stress, ultraviolet radiation, toxic compounds, etc.), it usually shows conformational changes. The altered α-synuclein is first recognized and repaired by the molecular chaperones, and the unrepairable α-synuclein is degraded by the UPS system or by the molecular chaperone autophagy. The ubiquitin-proteasome degradation of proteins mainly involves the following steps: (i) ubiquitin activator E1 activates ubiquitin molecules in an ATP-dependent manner; (ii) ubiquitin activator E1 transmits the activated ubiquitin molecule to ubiquitin-conjugating enzyme E2; (iii) ubiquitin ligating enzyme E3 binds the E2 bound ubiquitin to the targeted protein to form a specific ubiquitin protein; (iv) The 26S proteasome specifically recognizes the ubiquitin-tagged substrate protein and promotes its degradation.119 In addition, restoration of 20S proteasome activity in the UPS also promotes the clearance of pathological α-synuclein, thereby protecting neurons.120
As the accumulation of unrepaired and undegraded α-synuclein, the proteins become toxic and they are ultimately degraded by ALP (i.e., macroautophagy).113 In a word, soluble proteins are cleared through UPS and CMA pathways (Figure 5A), while these oligomers and protein aggregates, which cannot pass through the narrow pores of the proteasome, are degraded by the autophagy pathway.121
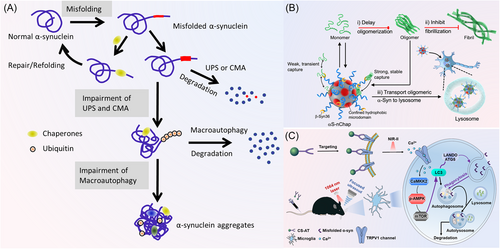
The activation of above clearance pathways has significant effects on the treatment of PD. Promoting chaperonin-mediated autophagy can reduce the level of abnormal high-molecular-weight and phosphorylated α-synuclein species, then alleviate α-synuclein-induced neurodegeneration.122 During CMA, cellular soluble proteins are recognized by the chaperone heat shock proteins and delivered to the lysosomes for degradation through the lysosomal-associated membrane protein 2A and the lysosomal HSPA8.123 The in vitro studies demonstrated that increasing the activity of heat shock proteins, such as Hsc70,124 Hsp70,125 Hsp90,126, 127 and Hsp104,128 can prevent the formation of α-synuclein fibrils, disaggregate fibrils, and even ameliorate α-synuclein induced synaptic deficits.129 Some small heat shock proteins, such as αB-crystalline and Hsp27, can prevent α-synuclein aggregation by their transient interaction.130 Wu et al.131 customized a nanochaperone (αS-nChap) to specifically recognize α-synuclein and prevent its oligomerization by dynamically capturing and stabilizing α-synuclein. Meanwhile, oligomeric α-synuclein can be captured to prevent fibrosis, and transported to the lysosomal degradation system (Figure 5B).
Many studies have demonstrated that some nanoparticles can regulate the autophagy (macroautophagy) of neurons, microglia, and astrocytes to promote the degradation of α-synuclein aggregates and the treatment of PD (Table 2). For example, Liu et al.109 developed a curcumin analog-based nanoscavenger (nano-CA), which can stimulate the major autophagy regulator transcription factor EB (TFEB) to facilitate the clearance of α-synuclein monomers, oligomers, and aggregates in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse.109 How to controllably enhance the cellular autophagy is of great importance for treatment of PD. In a recent work, Cu2−xSe-anti-TRPV1 nanoparticles were used as a photothermal switcher to controllably activate autophagy of microglia through the second near-infrared-II laser irradiation. The enhancement of autophagy level by Cu2−xSe-anti-TRPV1 nanoparticles was regulated by ATG5 and Ca2+/CaMKK2/AMPK/mTOR signaling pathways. The enhanced autophagy promoted the phagocytosis and degradation of α-synuclein, and ultimately improved the therapeutic effect of PD (Figure 5C).39
| Nanoparticles | Applications | Mechanism | References |
|---|---|---|---|
| Nanochaperone (αS-nChap). | Regulate the assemble of α-synuclein. | Capture and stabilize monomeric α-synuclein, capture oligomeric α-synuclein to prevent fibrillization and degrade α-synuclein oligomers by lysosome. | [131] |
| α-Synuclein fibril-specific nanobody (PFFNB2). | Decrease α-synuclein related pathogenesis. | Specifically bind to α-synuclein PFFs and dissociate α-synuclein fibrils. | [132] |
| Graphene quantum dots. | Prevent neuron-to-neuron transmission of α-synuclein pathology, reduce Lewy body and Lewy neurite formation. | Inhibit the fibrillization of α-synuclein, directly interact with mature fibrils and trigger its depolymerization. | [133] |
| Curcumin analog-based nanoscavenger. | Eliminate α-synuclein. | Stimulate the nuclear translocation of TFEB and thus trigger both autophagy and calcium-dependent exosome secretion. | [109] |
| Quercetin-modified Cu2−xSe nanoparticles. | Promote the degradation of α-synuclein aggregates in neurons. | Modulating SQSTM1/p62-dependent selective autophagy by activating Nrf2. | [134] |
| Cu2−xSe-anti-TRPV1 nanoparticles. | Promote the degradation of α-synuclein aggregates in microglia. | Enhance autophagy regulated by ATG5 and Ca2+/CaMKK2/AMPK/mTOR. | [39] |
| RuIII complex trans-[ImH] [RuCl4(Me2SO)(Im)] (NAMI-A). | Inhibit the aggregation of α-synuclein and the affinity between α-synuclein and membrane. | Disassemble preformed fibrils or binds to residues at N-, C-termini, and NACore regions involved in protein aggregation and membrane binding. | [135] |
| Polydopamine-assembled curcumin nanoparticles. | Reduce oxidative stress and decrease α-synuclein accumulation. | Scavenge ROS, prevent α-synuclein fibrillation and cause fibrils to disaggregate. | [136] |
- Abbreviations: Nrf2, nuclear factor erythropoie-like 2; PD, Parkinson's disease; PFFs, preformed fibrils; ROS, reactive oxygen species; TFEB, transcription factor EB.
In addition to the above pathways, several other approaches have been shown to clear α-synuclein. Recently, Butler et al.132 designed a nanoantibody PFFNB2, which can specifically recognize α-synuclein PFF rather than α-synuclein monomer, to significantly dissociate α-synuclein fibrils. In addition, treatment with PFFNB2 inhibited PFF-induced phosphorylation of α-synuclein serine 129 (pS129) in the primary mouse cortical neurons. Kim et al.133 demonstrated that graphene quantum dots can cross the BBB and protect neurons against dopamine loss induced by α-synuclein preformed fibrils, Lewy body/Lewy neurite pathology and behavioral impairment.
4.3 Chelation of metal ions
Brain imaging and pathological studies have shown that iron accumulation was specifically observed in the substantia nigra of PD patients.137 The abnormal accumulation of iron in PD is likely due to the imbalance of iron homeostasis caused by changes in iron regulatory proteins, such as divalent metal transporter 1 (DMT1), ferroportin (FPN), and transferrin.138 For example, increased DMT1 levels may lead to the increase of iron input to cells, and FPN is reduced in MPTP and 6-hydroxydopamine (6-OHDA) induced PD models.139
Excess iron can induce oxidative stress through the formation of •OH, leading to the peroxidation of DNA, proteins, and lipids. This is closely associated with α-synuclein accumulation, mitochondrial dysfunction, neuroinflammation, and disruption of the ubiquitin-protease system.140 The abnormal iron accumulation-induced cell death is most likely due to the imbalance of iron homeostasis caused by the dysfunction of iron regulatory proteins.141 This iron overload-dependent cell death is usually known as ferroptosis. Iron chelation therapy of early PD patients has been clinically tested to evaluate the pathophysiological roles of iron in the PD occurence.142 Another study showed that decrease of reactive iron by transgene expression of ferritin or oral administration of the metal chelator chloroquine protected mice against the neurotoxicity of MPTP.143 Hence, chelating iron ions, or inhibiting ferroptosis by iron chelation has shown the potential in treatment of PD. For example, therapeutic iron-chelating nanoparticles were constructed by using a zwitterionic poly(2-methacryloyloxyethyl phosphorylcholine) and HIV-1 transactivating transcriptor, the experimental results showed that these nanoparticles can reverse the pathological and behavioral defects of PD mice.144 You et al.145 reported a type of novel polymeric nanoparticles containing deferoxamine modified by brain-targeting peptide RVG29. As shown in Figure 6A, these nanoparticles effectively reduced dopaminergic neuronal damage and reversed neurobehavioral deficits by significantly reducing iron levels and oxidative stress in the substantia nigra and striatum of PD mice (Figure 6B–G).145
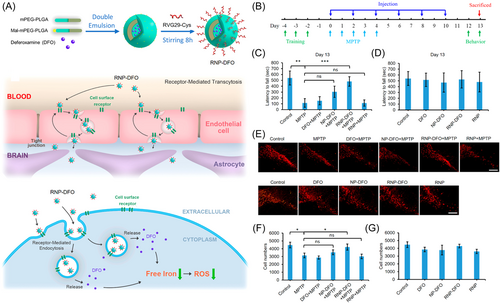
In addition to iron, the imbalance of zinc, manganese, and copper ions are also associated with PD. As the second most abundant transition metal in the brain, zinc ions (Zn2+) can directly inhibit the activity of tyrosine hydroxylase and antagonize the binding of Fe2+ ions, thus negatively regulate dopamine synthesis.146 Chelation of zinc ions can alleviate PD-associated lysosomal alterations and dopaminergic neurodegeneration by inhibiting zinc-mediated cytotoxicity.147 The disorder of manganese metabolism is also associated with PD, because manganese can affect neurotransmitter synthesis, and release, particularly in the basal ganglia.148, 149 For example, manganese may compete with Ca2+ influx, increase neurotransmitter release and the rhythmic firing activity in dopaminergic neurons, which may cause their dysfunction.150 Xin et al.151 demonstrated that Slc39a14 (manganese transporter) knockout mice showed a high accumulation of manganese in the brain and motor neurobehavioral dysfunction, which can be alleviated by Na₂CaEDTA (a manganese chelator).
4.4 Delivery of NTFs
NTFs, including GDNF, brain-derived neurotrophin (BDNF), cerebral dopamine neurotrophic factor, and mesencephalic astrocyte-derived neurotrophic factor, are protein molecules that support neuronal development, maturation, and survival.152, 153 NTFs regulate the functional activity of neurons through axonal regeneration, synthesis and release of neurotransmitters, and expression of their transporters.154 NTFs-based therapies hold great promise for treating neurodegenerative diseases such as Alzheimer's disease, PD, amyotrophic lateral sclerosis, and Huntington's disease. However, most NTFs face the problems of short half-life, poor pharmacokinetics, and low efficiency of crossing the BBB in vivo.155 Systemic administration of NTFs usually results in severe side effects because of their difficulties in crossing the BBB, and free NTFs are readily removed from circulation by extracellular peptidases, tissue binding, receptor-mediated clearance, and thus rapid degradation by glomerular filtration.156 The clinical treatment with NTFs is therefore high cost and high risk, because NTFs are injected directly into the brain of PD patients.157 Given the outstanding performance of NTFs in the treatment of PD, the development of effective strategies for delivery of NTFs is crucial for their clinical application.
A promising approach is the use of nanocarriers, which can not only stabilize NTFs, prolong their blood circulation time and half-life in the brain but also control the release of NTFs. Functionalization of NTFs with targeting ligands can improve their capability of crossing the BBB and their efficiency in the brain.158 The following factors should be considered for efficient delivery of NTFs into brain by nanoparticles, such as the stability of NTFs in body fluids, the capability of NTFs crossing the BBB and diffusing in the brain, and their bioavailability for targeted cells.155 In the past few decades, many studies on the delivery of NTFs by nanosystems have shown promising results. For example, Xu et al.159 used a nanocapsule to deliver nerve growth factor (NGF) into the CNS of healthy mice and nonhuman primates to promote NGF-mediated nerve regeneration, tissue remodeling, and functional recovery. Wu et al.160 found that GDNF liposomes can be effectively delivered to the brain for repairing dopaminergic neurons under the assist of small molecular agonist SC79, which can cross the BBB. In another translational study, a single administration of microencapsulated NTF was used to sustainably control the release of GDNF in the brain. This method protected GDNF from degradation, and promoted the survival of nigral dopaminergic neurons and recovery of motor in Parkinsonian monkeys.161 In addition to delivering exogenous NTFs, the use of nanotechnology to increase the endogenous NTFs has also shown great potential.162 In conclusion, NTFs delivered by nanotechnology is a promising approach for the treatment of PD.
4.5 Gene therapy
As mentioned previously, gene mutation is one of the pathogeneses of PD. Therefore, introducing therapeutic genes (DNA/RNA), silencing, or correcting the defective genes can be used to treat PD. PD-related genes mainly include SNCA, LRRK2, PINK1, Parkin, and DJ-1. Among of them, SNCA is the first gene encoding α-synuclein, which is the major component of Lewy bodies associated with hereditary PD.19 LRRK2 mutation is the most commonly inherited gene in PD, accounting for 3%–41% of familial PD cases.163 PINK1 and Parkin genes are involved in mitochondrial maintenance, and associated with mitochondrial dysfunction and oxidative stress. DJ-1 mutations cause 1%–2% autosomal recessive Parkinsonism, and oxidative stress damage. The strategy of specific knockdown of target genes by siRNA can be used to treat neurodegenerative diseases such as PD. Meanwhile, nanoparticle-based gene delivery has emerged as a potential treatment for PD (Figure 7A).164 This method may avoid harmful side effects, such as immunogenicity and carcinogenicity.165 For example, the siRNA (SNCA) delivered by polyethyleneimine nanoparticles can effectively reduce α-synuclein expression in PD models.166 In addition, Liu et al.167 showed the excellent performance of exosome-curcumin-phenylboric acid-poly (2-(dimethyl amino) acrylate) nanoparticles modified with RVG29 in the targeted delivery of SNCA siRNA for clearance of α-synuclein aggregates in the PD neurons.
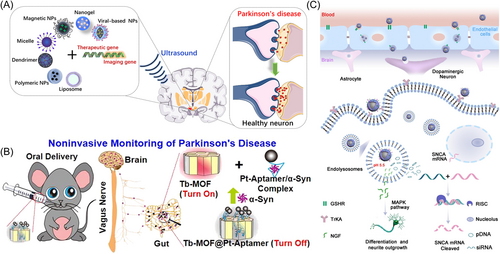
Unlike siRNAs, miRNAs are a class of short noncoding RNAs that regulate gene expression by binding with cellular transcripts, leading to translational repression or degradation of targeted messenger RNAs.168 Given the important role of miR-124 in neurogenesis, synaptic morphology, and neurotransmission, as well as regulation of mitochondrial dysfunction, oxidative damage, and neuroinflammation in PD, plasma miR-124 could be a potential biomarker of PD.169 miR-124 can promote neuroprotection, neurogenesis, and functional motor recovery in preclinical models of PD.170 For example, miR-124 was delivered by polymeric nanoparticles to improve motor symptoms in 6-OHDA mice.171 Huang et al.172 prepared RVG29-conjugated poly(lactic acid-co-glycolic acid) (PLGA) NPs to deliver miR-124 to the brain, and the nanoparticles alleviated PD symptoms by relieving neuroinflammation.
NPs could be also used to deliver DNA fragments for monitoring and therapy of PD (Table 3). For example, Miao et al.179 developed Tb-based luminescent metal-organic framework containing Pt-aptamer for noninvasive monitoring of α-synuclein (Figure 7B). In addition, a novel brain-targeted zinc oxide quantum dot gene delivery system (ZnO@polymer-NpG) was developed to deliver plasmid DNA (pDNA) and NGF into the brain. The released pDNA can silence α-synuclein by suppressing the expression of SNCA (Figure 7C), and protect neurons.175 Similarly, Gao et al.176 fabricated polydopamine nanoparticles and coated with magnesium oxide and anti-SNCA plasmids. The nanoparticles exhibited brain targeting capability and antioxidative activity, and showed good neuroprotective effect. In their another study, chitosan-pegylated polylactic acid nanoparticles coated with NGF, acteoside, and pDNA significantly reversed the loss of dopaminergic neurons in the substantia nigra and striatum of PD mice.180 They also prepared actively targeted gold nanoparticles carrying pDNA, which can inhibit the expression of α-synuclein.177, 178
| Nanoparticles | Applications | Type of gene | References |
|---|---|---|---|
| RVG peptide–modified exosome curcumin/phenylborate-poly (2-(dimethylaminoethyl acrylate) nanoparticles/small interfering RNA targeting SNCA (REXO-C/ANP/S). | Clear the α-synuclein aggregates and inhibit immune activation. | Small interfering RNA targeting SNCA. | [167] |
| Thioketal-linked polypeptide copolymer/poly(l-lysine)-TK-poly(l-leucine) and poly(γ-glutamic acid) loaded siRNA (LSLGMA). | Regulate the phenotype of microglia to M2 and anti-inflammatory. | siRNA for downregulating the expression of NF-κB. | [173] |
| Amphiphilic polymer poly (propylene sulfide)-polyethylene glycol loaded hydrophobic curcumin and superparamagnetic iron oxide nanoparticles/MSC-derived exosome (PR-EXO/PP@Cur). | Clear α-synuclein aggregates, alleviate neuroinflammation and restore neuronal function. | Endogenous miR-133b which can promote the growth of nerve axons and restore neuronal function. | [174] |
| Glutathione-modified ZnO quantum dots composites (ZnO@Polymer-NpG). | Suppress SNCA expression, induce neurite outgrowth and differentiation. | pDNA for arising siRNA to silence cellular SNCA gene. | [175] |
| Polymeric nanoparticles formed by poly(lactic acid-co-glycolic acid) and perfluoro-1,5-crown ether, coated with protamine sulfate. | Promote neurogenesis and brain repair. | MicroRNA-124 for promoting the neuronal commitment and maturation of neural stem/progenitors cells, induce migration of neurons. | [171] |
| Poly(lactic-co-glycolic acid) nanoparticles. | Inhibit neuroinflammation and enhance neuroprotection. | MicroRNA-124 for targeting MEKK and NF-κB pathways. | [172] |
| MgO-based polydopamine nanoparticles modified with polyethylene glycol, lactoferrin, and puerarin (MgOp@PPLP). | Suppress the expression of α-synuclein and reduce the level of oxidative stress and inflammation. | Anti-SNCA plasmid. | [176] |
| Gold nanoparticle composites. | Decrease α-synuclein aggregates. | Plasmid DNA to inhibit α-synuclein expression. | [177, 178] |
- Abbreviations: MSC, mesenchymal stem cell; NF-κB, nuclear factor-κB; PD, Parkinson's disease; siRNA, small-interfering RNA.
Another well-studied gene therapy is based on the NTF gene (e.g., BDNF or GDNF gene). For example, brain-penetrating pegylated NPs loaded with GDNF gene were demonstrated to restore dopamine levels and dopaminergic neuronal density in the striatum of 6-OHDA-induced PD rats.181 In addition, a novel microvesicle (MB)–liposome complex was used for delivery of GDNF/BDNF gene to promote normal dopamine secretion and recovery of motor behavior in PD models.182 All these examples demonstrate that nanotechnology is an attractive approach for delivery of genes to treat PD.
4.6 Stem cell transplantation
Due to the progressive loss of dopaminergic neurons in the substantia nigra striatum in PD and the fact that stem cells may differentiate into specific neuronal groups (such as DA neurons), cell replacement therapy has become a viable option for the treatment of PD.183 In the 1980s, the approach of transplant fetal midbrain tissue with dopaminergic neurons, which can restore physiologically synaptic release of dopamine in the denervated striatum and reverse motor deficits, aroused great interest in the clinical community.184
To date, different types of stem cells, mainly including neural stem cells (NSCs), embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and MSCs, have been used to improve the symptoms of various neurodegenerative diseases.185 NSCs are stem cells derived from embryonic brain tissue or from patient cells with the self-renew capability. They can differentiate into neurons, astrocytes, and oligodendrocytes.185, 186 ESCs are stem cells with high differentiation and strong self-renew capability of differentiating into any type of cells in principle, including dopaminergic neurons.187 Similar to ESCs, human iPSCs can differentiate into dopaminergic neurons. However, iPSCs cannot spontaneously form dopaminergic neurons after transplantation, which means that they must be differentiated into sufficient progenitor cells before transplantation.188 MSCs are a class of adult stem cells with multidirectional differentiation potential, and exist in the interstitium of various tissues and organs. Stem cells used for nerve repair and regeneration can differentiate not only into neurons,189 but also microglia190 and astrocytes.191 However, cell replacement therapy is often affected by low graft survival, moderately limited cell integration, and delayed therapeutic effect, which limits its further application.192
The rich properties of nanoparticles, including promoting the secretion of various bioactive factors for anti-inflammation, antiapoptosis, and immunoregulation, regulating cell metabolism, protecting dopaminergic neurons, and reversing motor dysfunction, make them very attractive in stem cell regulation. On the one hand, the use of nanoparticles to carefully modulate the microenvironment and regulate stem cell growth is valuable for the treatment of neurodegenerative diseases. On the other hand, based on the unique properties of nanoparticles, the transplanted stem cells could be accurately tracked by in vivo imaging after the stem cells are labeled by nanoparticles, and the therapeutic effects of stem cell transplantation could be evaluated.185 For example, He et al.193 reported that bioactive black phosphorus nanosheets can effectively improve the antioxidative capacity and the survival rate of different types of stem cells by upregulating the nuclear factor erythropoie-like 2-dependent antioxidative pathway, including human placental chorionic MSCs, iPSCs, and NPCs. Shi et al.194 demonstrated that chiral nanoparticles with high G-factor values significantly upregulated Map2, Yap1, and Taz genes, resulting in mechanical force, cytoskeletal protein action, and accelerated differentiation of mouse NSCs into neurons. Huang et al.195 synthesized a PBAE-PLGA-Ag2S-RA-siSOX9 (PPAR-siSOX9) nanoformula with high gene/drug deliverability to promote the neuronal differentiation of NSCs. It has been demonstrated that hydroxyapatite nanorods promoted neural differentiation of NSCs into GABAergic neurons through Ca2+/c-Jun/TLX3 signaling, which was further confirmed by the in vivo study.196 Many innovative studies on the functional nanoparticles for tracking stem cells in PD have also been reported. For example, gold NPs were used to monitor the migration and homing patterns of exosomes derived from bone marrow MSCs (MSC-exo) in various brain pathologies after intranasal administration.197 However, the safety and controllability of using nanoparticles and stem cells to treat neurodegenerative diseases need to be studied more extensively.
5 CONCLUSIONS AND PERSPECTIVES
In summary, great advances in the neuropathology and the progress for treatment of PD has been achieved in the past decades. We summarized the related neurodegenerative mechanisms of PD and its novel treatment approaches based on nanoparticles. Encouraging results suggest that rational design of nanoparticles with tunable and unique properties can enhance the therapeutic efficacy. The nanoparticles can not only provide large surface area for loading more drugs and altering the drug pharmacokinetics, functionalizing with targeting ligands (e.g., antibodies, targeting peptides) to improve drug performance, but also exhibit the intrinsic oxidation–reduction properties, which can be used to regulate the cellular signaling pathways, functions and the surrounding microenvironment. Moreover, their optical, thermal and magnetic properties can be used for diagnosis by molecular imaging.
Despite great advances achieved, there is still no nanoparticle, drug or method that can completely cure PD so far. The difficulties in the treatment of PD could be due to the following reasons. (1) The exact cause of PD is still unknown because of its multifactorial pathophysiology feature. There are many mechanisms are unclear, such as the origin of PD, the role of α-synuclein in the progression of PD, and the role of intestinal flora in PD.198 (2) The complicated pathological regions of the brain are involved, including the substantia nigra, striatum, globus pallidus, caudate nucleus, and cerebral cortex. There is a lack of drugs or methods specifically target these relevant brain regions in the PD therapy. For the clinical applications of nano-agents, they face several critical issues, such as mass production with high quality control, bioeffects and biosafety, capabilities of crossing the BBB and targeting at damaged or degenerative neurons, and so on.
In view of the above difficulties, the following issues should be considered during development of nanoagents for PD diagnosis and therapy. (1) PD is a chronic disease, and its diagnosis mainly depends on clinical symptoms. However, the brain neurons have been severely damaged before the appearance of symptoms. It is of great significance to achieve early screening and diagnosis of PD by developing advanced and specific molecular imaging technology. In this context, multimodal nanoprobes with complementary functions would shed the light on this aspect and make great contributions. (2) Due to the complex relationship between various pathogenesis of PD, it would be very important to develop advanced nanoparticles which can simultaneously target the causes of different pathogenesis. Deep understanding of PD pathogenesis and its causes is helpful to design and screen such versatile nanoparticles. Moreover, since PD is directly related to the degeneration, damage and loss of neurons, it would be great if the fabricated NPs can efficiently cross the BBB and target these neurons. (3) In the CNS, both astrocytes and microglia can strongly interact with neurons. They not only play important roles in maintaining neuronal healthy, but also in the occurrence and progression of PD. Therefore, in addition to treating the damaged and degenerated neurons, targeting them will be another option for synergistic treatment of PD. (4) Since the pathogenesis of PD is related to autoimmune diseases and immune disorders, the immunotherapy of PD is a new direction, which will bring new opportunity for nanoparticles and new hope for the treatment of PD. (5) In addition to the efficacy of nanoparticles, the most important issue is their biosafety. As mentioned previously, the biodistribution, pharmacokinetics, metabolism, clinical toxicity, and immunogenicity of nanoparticles should be extensively studied in different animal PD models before clinical test. Although there is a long way for the final clinical application of nanoagents in PD therapy, we believe that advanced multifunctional nanomaterials would bring bright future for this incurable disease, and for the treatment of other diseases.
AUTHOR CONTRIBUTIONS
Hanghang Liu: Conceptualization; data curation; methodology; investigation; formal analysis; resources; project administration; visualization; writing—original draft. Menglong Hua: Conceptualization; data curation; methodology; investigation; formal analysis; resources; project administration; visualization; writing—original draft. Qing Zheng: Conceptualization; data curation; methodology; investigation; formal analysis; resources; project administration; visualization; writing—original draft. Yifan Gao: Conceptualization; data curation; methodology; investigation; formal analysis; resources; project administration; visualization; writing—original draft. Zhen Li: Conceptualization; funding acquisition; resources; supervision; writing—review and editing. All authors have read and approved this manuscript.
ACKNOWLEDGMENTS
Zhen Li acknowledges the support from the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (22KJA310004), the National Natural Science Foundation of China (81971671), and the National Key Research and Development Program of China (2022YFA1104300). The authors also are grateful for support from the Jiangsu Provincial Key Laboratory of Radiation Medicine and Protection, the Priority Academic Development Program of Jiangsu Higher Education Institutions (PAPD).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this review because no new data were created or analyzed in this manuscript.




