Oral hard tissue defect models for evaluating the regenerative efficacy of implant materials
Abstract
Oral hard tissue defects are common concomitant symptoms of oral diseases, which have poor prognosis and often exert detrimental effects on the physical and mental health of patients. Implant materials can accelerate the regeneration of oral hard tissue defects (such as periodontal defects, alveolar bone defects, maxilla bone defects, mandible bone defects, alveolar ridge expansion, and site preservation), but their regenerative efficacy and biocompatibility need to be preclinically validated in vivo with animal-based oral hard tissue defect models. The choice of oral hard tissue defect model depends on the regenerative effect and intended application of the tested implant material. At the same time, factors that need to be considered include techniques for constructing the particular defect model, the scaffold/graft material used, the availability of animal model evaluation techniques and instrumentation, as well as costs and time constraints. In this article, we summarize the common oral hard tissue defect models in various animal species (such as periodontal model, jaw defect model, and implantation defect model) that can be used to evaluate the regenerative efficacy and biocompatibility of implant materials.
1 INTRODUCTION
Oral and maxillofacial hard tissues include teeth and jaws. Inflammation, trauma, tumors, and other pathological factors often lead to oral and maxillofacial hard tissue defects,1 which not only affect the patient's masticatory and speech functions but also detrimentally impact their social interactions and mental health.2 At the same time, studies have shown that the pathophysiological healing process of oral and maxillofacial hard tissue injuries includes two consecutive interrelated stages: early inflammatory response and subsequent osteogenic repair.3 The repair of oral and maxillofacial hard tissue defects is challenging, and there are various problems associated with current therapeutic modalities commonly used in clinical practice, resulting in suboptimal regeneration, long healing time, and poor quality of newly formed bone. Oral and maxillofacial hard tissue defects are often afflicted by inflammation and infection, which is not conducive to the regeneration and repair of bone tissue.
At present, it is still challenging to repair larger than critical-sized hard tissue defects clinically. The gold standard that is used as a point of reference and comparison is autologous transplantation due to histocompatibility and nonimmunogenicity, which is more conducive to osseointegration.4 Additionally, allografts and demineralized bone matrices have also been used to treat hard tissue defects, but there are risks of immunological rejection and pathological transmission.5 At present, the commonly used methods for repairing hard tissue defects include guided bone regeneration (GBR),6 bone grafting,7 and the controlled delivery of growth factors.8 These methods, used alone or in combination, have greatly improved the repair of hard tissue defects, but they also have some inherent deficiencies such as poor predictability and limited indications.
Bone tissue consists of cortical bone and cancellous bone with a marrow compartment. In addition to mineral storage and calcium homeostasis,9 bone tissue also has important hematopoietic and immune functions.10 Bone marrow contains osteoblasts, osteocytes, osteoclasts, bone marrow mesenchymal stem cells (BMSCs), adipocytes, and vascular endothelial cells, as well as other diverse cell lineages, all of these play key roles in the maintenance of stem cells11 and the generation of the various hematopoietic lineages.12 Although bone tissue has the excellent regenerative capacity, there is still a limit. Current treatment modalities often rely on scaffolding/grafting materials to facilitate the repair of the defect area.13 At the same time, peptide-based nanomaterials also have much potential in the repair of defect areas due to their good biocompatibility, versatility, and biological activity.14 Recently, polyetheretherketone (PEEK) has attracted extensive attention in repairing large bone defects due to its excellent biocompatibility, as well as good thermal and chemical stability, together with excellent mechanical properties similar to natural bone.15 Newly developed hydrogels are also considered as among the best candidates for bone tissue engineering owing to their unique 3D network structure with high water content and functional properties.16
The use of implant materials to promote the repair of hard tissue defects has many advantages, such as the newly formed bone tissue being able to fully integrate with the existing skeletal system, as well as facilitating the repair of large hard tissue defects.17 Implant materials are usually fabricated by techniques such as electrospinning,18 and may be combined with transplanted cells (such as osteoblasts or stem cells)19 and various bioactive components (e.g., bone morphogenetic proteins [BMPs])20 to further stimulate hard tissue formation at the implant site. At the same time, the regenerative effects of the implanted materials can also be evaluated within the oral hard tissue defect model under healthy or diseased states.
In this review, we critically examined the various live animal models of oral hard tissue defects that can be used to evaluate the regenerative effects of implanted materials. Specifically, we discussed the commonly used periodontal models, jaw defect models, and implantation defect models, as well as the various animal species utilized in these models. In simple terms, periodontal models include periodontal defect model and periodontitis model, which are defects caused by trauma or inflammation around teeth. Jaw-based models including alveolar, maxilla, and mandible bone defect refer to bone defects in the maxilla or mandible. Implantation defect models including alveolar ridge expansion model and site preservation, respectively refer to bone defects at the alveolar ridge and at the tooth extraction site with alveolar bone preserved.
2 PERIODONTAL MODELS (INCLUDING PERIODONTAL DEFECT MODEL AND PERIODONTITIS MODEL)
2.1 Nonhuman primate model
Nonhuman primates are very similar to humans in terms of tooth anatomy and periodontal tissue anatomy. Experimentally generated defects in these animals are the first choice for evaluating the efficacy and safety of periodontal regenerative therapies. Defects that do not have the capacity for spontaneous regeneration and which are identical on contralateral sides can readily be created. Nonhuman primate models are recommended for clinical translation experiments involving invasive procedures and testing of potentially harmful new devices and drugs.21 In a study by Emerton et al.,22 periodontal defects were made by using wire ligation in 12 baboons (Papio hamadryas). The teeth on both sides of the mandibular of the oral cavity were randomly treated with β-tricalcium phosphate (β-TCP) or β-TCP containing different concentrations of recombinant human growth and differentiation factor-5 (rhGDF-5). The result showed that the incorporation of rhGDF-5 onto β-TCP particles can effectively promote periodontal tissue regeneration.22 Other studies reported that loading of recombinant human transforming growth factor-β3 in Matrigel,23 or the binary application of recombinant human osteogenic protein-1 (hOP-1) and transforming growth factor-β3 (hTGF-β3) in Matrigel-matrix can significantly enhance periodontal tissue regeneration in a nonhuman primate (Papio ursinus) model.24 The study of Hammarstrom et al.25 investigated the use of propylene glycol alginate (PGA), hydroxyethyl cellulose, and dextran as carriers for enamel matrix preparations. Only the combination of PGA with amelogenin fraction could result in significant regeneration of periodontal tissues in monkeys.25 The oral structures of nonhuman primates share various similarities to that of humans and are afflicted with similar oral microbial pathogens, plaque, calculus, and periodontal disease. Cynomolgus monkeys were infected with the human pathogen Porphyromonas gingivalis, and plaque-like bone loss was observed after 5 months.26 Although periodontitis in nonhuman primates is similar to the human disease, the high costs and specialized housing conditions of these animals limit their widespread application in basic and translational periodontal disease research.27
2.2 Porcine model
A study by Chen et al.28 on a porcine model showed that aligned PLGA/Gelatin electrospun sheet (APES) has a directional fiber orientation that guides cell proliferation, while native dental pulp extracellular matrix (DPEM) retains the inherent fiber structure and ECM proteins. It was demonstrated that both APES/treated dentin matrix (TDM) and DPEM/TDM can promote odontoblast differentiation of dental stem cells in vitro. Sandwich composites (APES/TDM/DPEM) seeded with stem cells were shown to produce tooth root-like tissues at 12 weeks after transplantation into porcine jaws.28 The oral and maxillofacial structures of minipigs closely resembles that of humans. Minipigs at 6 months of age usually develop gingivitis with inflammatory cell infiltration in the gingival tissue, which progressed to severe periodontitis by 16 months of age with similar histopathology as the human tissue. Periodontitis in minipigs has been associated with infection with Porphyromonas gingivalis, Streptococcus mutans, and Semiactinomycetes. Minipigs are suitable for periodontal and oral examinations, but they are expensive and difficult to maintain, and few periodontal studies use miniature pigs as models.27
2.3 Canine (Dog) model
The study of Matsuura et al. depicts a single-walled intraosseous defect that was surgically created on the mesial aspect of the mandibular canine (Figure 1D).32 In the study of Ivanovic et al.,35 a three-walled intraosseous defect was created on the second and fourth premolars of a dog model. After a 3-month healing period, acute-type defects were filled with the deproteinized bovine bone mineral (DBBM) with or without a collagen carrier matrix, or Bio-Oss® particles as controls. After 12 weeks postsurgery, the results showed that DBBM particles induced periodontal regeneration.35 The study of Tovar et al.36 created standardized class III bifurcation defects in the maxillary second and third premolars of dogs, with analyses being carried out at 6, 12, and 24 weeks. The experimental defects (n = 5) were covered with collagen membrane (CM) (positive control), scCO(2)-treated CM (experimental), or no membrane (negative control). The results showed that the scCO(2)-treated CMs exhibited similar performance in guided tissue regeneration (GTR) to untreated samples in class III bifurcation defects, whereas all defects implanted with the membrane managed to achieve regeneration of native periodontal tissue.36 In another study,37 24 class II furcation defects dissected from four male beagle dogs were arbitrarily assigned to three treatment groups: lithium-calcium-silicate (Li2Ca4Si4O13, LCS), β-TCP, and blank control. Each group has eight samples. The in vivo study demonstrated that in a beagle dog periodontal defect model, LCS significantly promoted the regeneration of periodontal defects compared with β-TCP. Hence, LCS is a promising bioactive material for periodontal regeneration, thus providing a promising therapeutic strategy for the treatment of periodontitis-related diseases.37 In the study of Luo et al.,38 36 inflammatory Class II furcation defects were surgically created in the bilateral mandibles of dogs. Then ESEHT (Extraction Socket-Derived Early Healing Tissue) from 2-week healed extraction sockets and suitable alveolar bone from the interdental septa or surrounding alveolar walls were explanted from the Beagle dog (proper alveolar bone [PAB]). The results showed that ESEHT and PAB displayed similar effects in increasing the percentage of regenerated cementum and regenerated bone, which was significantly higher than that of the blank control group.38 Anzai et al.39 created single-walled periodontal defects in the mesial portions of mandibular first molars of beagle dogs, following by treatment with β-TCP alone or Fibroblast Growth Factor-2 (FGF-2) plus β-TCP. The results demonstrated the effectiveness of the simultaneous use of FGF-2 and β-TCP as osteoconductive materials in periodontal regeneration, following severe damage from progressive periodontitis.39 Ogawa et al.40 implanted Nano-β-TCP scaffolds, FGF-2-loaded nano-β-TCP scaffolds, and noncoated collagen scaffolds in a dog model of single-wall subosseous defect. Histological observations were performed 10 days and 4 weeks after surgery, with histological specimens showing an approximately fivefold improvement in periodontal tissue repair after implantation of FGF-2-loaded nano-β-TCP scaffolds, as compared with collagen scaffolds.40 Yamamoto et al.41 created intraosseous defects in dogs, which were treated with bovine-derived xenograft (BDX) plus enamel matrix derivative (EMD), BDX alone, or neither (control group). These findings suggest that the use of BDX with EMD is effective in enhancing the formation of new bone and cementum and that this combination is effective in treating intraosseous defects.41 Yang et al.42 prepared single-walled periodontal intraosseous defects to assess the periodontal regenerative potential of treated dentin matrix particles/dental follicle cells (TDMP/DFCSs) in beagle dogs. The outcome demonstrated the potential of TDMP/DFCSs in periodontal regeneration.42 Nagai et al.43 generated class II bifurcations in the mandibular premolars of eight adult male beagle dogs. Periodontal regenerative treatment was then administered using collagen sponge graft material with or without topical platelet-derived factor releasate (PR). The results showed that the collagen sponge graft material with PR promoted new attachment for periodontal tissue regeneration therapy.43 Acemannan is a polysaccharide obtained from the Aloe vera plant, which can stimulate both soft and hard tissue healing. In an in vivo study,44 an acemannan sponge was used to treat in canine premolar class II bifurcation defects. The acemannan sponge significantly accelerated the regeneration of periodontal tissue in class II bifurcation defects.44 Nunez et al.45 created three-walled intraosseous periodontal defects, 3 mm wide and 4 mm deep, in mandibular canine second and fourth premolars. Enhanced periodontal regeneration was observed upon implanting collagen sponges embedded with 750,000 cementum-derived cells.45 Kwon et al.46 surgically created bilateral 4 × 5 mm (w × d), single-walled, critical-sized, intraosseous periodontal defects on the mandibular second and fourth premolars of beagle dogs. The defect sites were then implanted with rhGDF-5/β-TCP or rhPDGF constructs. The rhGDF-5/β-TCP showed great potential in supporting and accelerating periodontal wound healing/regeneration.46 As shown in Figure 1F, Kawamoto et al. show digital photographs and computed tomography (CT) images of a class II bifurcation defect in a dog.34 Dogs provide a suitable live animal model for studying periodontal defects and periodontitis. In dogs, subgingival plaques primarily affect anaerobic gram-negative cocci, similar to human subgingival plaques. However, the relatively expensive nature of the canine model, coupled with demanding animal care requirements such as companionship, exercise, space, and grooming, would limit the application of dogs in periodontal research.
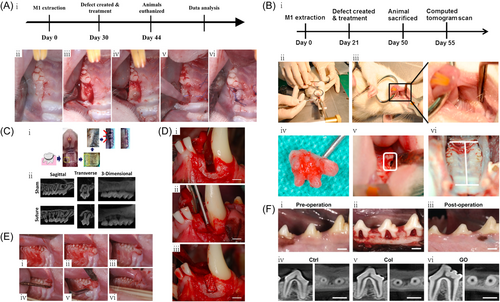
2.4 Rodent—rat
As shown in Figure 1A, Chi-yu Lin et al. depict the experimental protocol and generation of bone defects in a periodontal rat model.29 In the study of Spolidorio et al.,47 a periodontitis model was created with rat mandibular first molars. After removal of the ligature, rats treated with normal rabbit serum exhibited decreased myeloperoxidase activity, decreased alveolar bone loss, and increased number of blood vessels. Thrombocytopenia resulted in delayed alveolar bone regeneration, reduced blood vessel numbers, and a modest decrease in myeloperoxidase activity. The findings showed that thrombocytopenia leads to delayed periodontal healing in the rat experimental periodontitis model.47 To overcome the limited bioactivity and regenerative potential of currently available membranes, the study of Nasajpour et al.48 developed the poly(caprolactone) (PCL) composite membrane containing zinc oxide (ZnO) nanoparticles. Using a rat periodontal defect model to evaluate the membrane function, the results confirmed that the engineered membrane possessed both osteoconductive and antibacterial properties, thus demonstrating its great potential in periodontal tissue engineering.48 He et al.49 reported that periodontal tissue regeneration can be enhanced by regulating macrophage (M phi) and cell recruitment in high-stiffness hydrogels implanted in critical-size periodontal defects of male Sprague–Dawley (SD) rats.49 Cai et al.33 seeded mesenchymal stem cells onto polylactic-co-glycolic acid/polycaprolactone electrospinning scaffolds, which were precultured under various in vitro conditions, followed by implantation of cell-scaffold constructs into rat periodontal defects, with bare scaffolds serving as controls. The results showed that chondrogenic differentiation of MSCs before implantation can be an effective strategy for enhancing periodontal regeneration (Figure 1E).33 Oortgiesen et al.50 created periodontal defects in 48 rats, which were treated according to one of the following strategies: Bio-Gide(®), Bio-Gide(®)-ALP, Bio-Gide(®)-ALP/Bio-Oss(®), Bio-Gide(®)/Bio-Oss(®)-ALP, Bio-Gide(®)-ALP/Bio-Oss(®)-ALP, or empty. The results show that immobilization of ALP on GTR/GBR materials (Bio-Gide(®) and Bio-Oss(®)) can improve the performance of these materials in GTR/GBR procedures.50 The study of Pin-Hsien Wu et al. depicts the periodontal rat animal model and the creation of bone defects (Figure 1B).30 Fujishiro et al.51 induced experimental periodontitis in rats by lifting full-thickness gingival flaps and ligating silk threads around the mandibular first molar. At 14 days after induction of experimental periodontitis, the bifurcation area was treated with Emdogain gel or PGA. The results demonstrated that wound-healing macrophages may express BMPs after Emdogain gel application, which play an important role in the regeneration of periodontal tissue at bifurcations.51 Takeuchi et al.52 extracted the maxillary first molars of 45 Wistar rats. After healing, bilateral periodontal defects were surgically created within the mesial second molars, followed by treatment of defects with RADA16 or Matrigel. The results suggest that the application of self-assembling peptide (SAP) hydrogel promoted the healing of surgical periodontal defects by enhancing cell recruitment and possibly angiogenesis.52 Cai et al.53 implanted stromal cell derived factor (SDF)-1α-loaded gelatin sponges into rat periodontal defects for 1 and 6 weeks, with blank materials and blank defects as controls. The SDF-1α construct significantly improved the regeneration of periodontal defects.53 Yoshida et al.54 created periodontal defects within the mesial maxillary first molars of 40 Wistar rats, which were then filled or unfilled with 1.5% SPG-178. Every 2 days, the animals received injections of parathyroid hormone (PTH) (1–34) or saline. The results demonstrated that intermittent systemic PTH and locally delivered neutral SAP hydrogels promoted periodontal healing.54 A study by Babo et al.55 tested an aggregate partitioning system consisting of (A) a platelet lysate (PL)-based construct, and (B) calcium phosphate cement (CPC) composites incorporated with PL-loaded hyaluronic acid microspheres that were aggregated to promote alveolar bone regeneration. This two-layer system was evaluated in a rat three-walled periodontal defect model. The results demonstrated the positive therapeutic effects of immobilizing PL-based structures on the root surface in an aggregated separation procedure, which facilitated the regeneration of functional periodontal tissues.55 Matsugami et al.56 cultured rat periodontal ligament-derived cells (PDLC) on various SAP hydrogels in vitro, and evaluated the vitality/proliferation of the cultured cells. In vivo, normalized periodontal defects were formed in the mesial of Wistar rats maxillary first molars and were treated with various SAP hydrogels. The results showed that topical application of functionally designed SAP hydrogels, especially PRG (integrin binding sequence), promoted periodontal healing.56 Cui et al.57 reported that MXene (Ti3C2Tx) exerted a strong therapeutic effect on osteogenic differentiation and periodontal defect repair in an experimental rat model of periodontal fenestration defect. Hoz et al.58 created fenestrated defects buccal to the root of the mandibular first molar using an extraoral approach on 18 male Wistar rats that were divided into three groups. These consisted of two controls (untreated defects or those treated with gelatin matrix scaffolds [GMS] only) and the experimental group that was treated with 5 μg/dose of GMS-embedded Cementum protein 1-derived peptide (CEMP1-p1). The results showed that the synthetic peptide CEMP1-p1 promoted periodontal regeneration in the rat fenestration model. In the study of Bizenjima et al.,59 50 Wistar rats were assigned to streptozotocin-induced diabetic or nondiabetic groups. Periodontal defects were created surgically in the maxillary first molars. Defects were then treated with hydroxypropylcellulose (HPC) or FGF-2 and HPC. FGF-2 treatment of surgical periodontal defects in the diabetic rats exerted beneficial effects on new bone formation.59 Bone defect surgery was then performed mesially on the maxillary first molar root. After root planing and ethylenediaminetetraacetic acid conditioning, EMD was applied to the root on one side of the maxilla. It was observed that periodontal healing was impaired in streptozotocin-induced diabetic rats. During the short-term healing process in this pathological model, EMD exerted no beneficial effects on new bone and cementum formation and did not ameliorate the adverse effects in the systemically compromised animals.60 In another study on a diabetic rat model, Corrêa et al.61 created bilateral fenestrated defects on the buccal side of mandibular first molars at 7 days after diabetes mellitus (DM) induction. After surgery, each animal defect was arbitrarily assigned to two subgroups: untreated (control) and EMD implantation. DM may exert significant adverse effects on the newly formed bone mineral density (BD). EMD may provide greater defect filling (DF) under diabetic or normal conditions; however, it may not significantly increase neo cementum formation (NCF) in DM animals.61 The periodontal anatomy of the molar area of rats shares some similarities with humans, and rats are often used as experimental models of periodontitis because they are much cheaper and easier to care for. However, rats cannot recapitulate all aspects of the pathophysiology of periodontitis in humans.
2.5 Rodent—mouse
As shown in Figure 1C, Xu Qin et al. depict the ligation-induced mouse model of periodontitis.31 Qian et al.62 fabricated a novel silver-modified/collagen-coated electrospun poly(lactic-co-glycolic acid/polycaprolactone) (PLGA/PCL) scaffold (PP-pDA-Ag-COL). In a murine model of periodontitis, the PP-pDA-Ag-COL scaffold improved alveolar bone regeneration and was effective in treating periodontitis. This demonstrated that the PP-pDA-Ag-COL scaffold exhibited enhanced pro-osteogenic and antibacterial properties, and had much potential for treating alveolar/craniofacial bone regeneration.62 In the study of Ma et al.,63 the small molecule galanin (GAL) was utilized as a novel scaffold coating agent for promoting the healing and regeneration of craniofacial tissues. Using immunohistochemistry, GAL and GAL receptors were detected in the vicinity of healthy periodontal tissue and blood vessels, whereas the onset of periodontal disease resulted in the downregulation of GAL. Thus, GAL coating promoted periodontal regeneration and repaired periodontal defects generated in periodontitis model mice.63 The low cost of mice and the availability of multiple transgenic strains allow for in-depth mechanistic studies. Nevertheless, their small size and thus the amount of harvested tissues required for analysis requires a large number of animals (Tables 1 and 2).
| Research model classification | Species | Advantages | Disadvantages |
|---|---|---|---|
| Animal models for preclinical evaluation | Primate | Human-like oral structure, oral microbial pathogens, plaque, calculus, and periodontal disease. | Expensive animal costs and special housing conditions. |
| Porcine | Oral and maxillofacial structures resemble that of humans in terms of anatomy, physiology, and disease development, and the histopathology is identical as that of human tissues. | Expensive and has feeding issues. | |
| Canine (Dog) | Develop natural or empirical periodontitis comparable to humans. | Animals are expensive and have complex maintenance requirements. | |
| Basic research animal models | Rodent—rat | The periodontal anatomy of the molar region has some similarities to that of humans and can serve as an experimental model of periodontitis that is less expensive and easier to handle. | Cannot recapitulate all aspects of human periodontitis progression. |
| Rodent—mouse | The low cost of mice and the availability of multiple transgenic strains allow for in-depth mechanistic studies. | Small size and not easy to operate. |
| Research model classification | Species | Defects-types of defects and how these were made | Scaffold/graft materials used | References |
|---|---|---|---|---|
| Animal models for preclinical evaluation | Primate | Allow plaque to accumulate around the wire ligature and form chronic disease. 2 months later, the ligature was removed to form a bilateral periodontal defect. | β-Tricalcium phosphate granules (β-TCP), 0.5, 1.0, or 2.0 mg recombinant human growth and differentiation factor-5 (rhGDF-5)/g β-TCP. | [22] |
| Class II furcation defects were made bilaterally in the molars of baboons. | Recombinant human transforming growth factor-β3 in Matrigel(®). | [23] | ||
| Defects developed at the bilateral furcation of the mandibular molars in baboons. | Single applications of 25 μg of recombinant human osteogenic protein-1 (hOP-1) and 75 μg of transforming growth factor-β3 (hTGF-β3) in Matrigel matrix were compared with a 20:1 binary application of 25 μg hOP-1 and 1.25 μg hTGF-β(3). | [24] | ||
| The oral, mucoperiosteal flaps were lifted from the canines on each side of the maxilla to the first molar. The buccal alveolar bone plate, exposed periodontal ligament, and bone cement were removed. | Various porcine enamel matrix preparations with or without carriers. | [25] | ||
| Porcine | Miniature porcine mandibular defect. | Well-aligned PLGA/Gelatin electrospun sheets (APES) were fabricated, and combined with treated dentin matrix (TDM) and native dental pulp extracellular matrix (DPEM) to form APES/TDM and DPEM/TDM. | [28] | |
| Canine (Dog) | A three-walled intraosseous defect was created on the second and fourth premolars of a dog model. | The deproteinized bovine bone mineral (DBBM) with or without a collagen carrier matrix, or Bio-Oss® particles as controls. All defects were covered with Bio-Gide® membranes. | [35] | |
| Standardized Class III gingival defects were created on the upper second and third premolar teeth of dogs. | Experimental defects (n = 5) were covered with collagen membranes (positive control), scCO(2)-treated collagen membranes (experimental), or no membranes (negative control). | [36] | ||
| Class II furcation defects were prepared from four male beagle dogs. | Lithium calcium silicate (Li2Ca4Si4O13, LCS) bioceramics. | [37] | ||
| Surgical creation of 36 inflammatory grade II furcation defects in the bilateral mandibles of dogs. | Extraction Socket-Derived Early Healing Tissue (ESEHT) was harvested from extraction sites and appropriate alveolar bone (PAB) was harvested from the interdental septa or around the fossa wall of canine bilateral mandibles. | [38] | ||
| Establishment of a single-walled periodontal defect in the mesial portion of the mandibular first molar on both sides. | β-TCP alone or FGF-2 plus β-TCP. | [39] | ||
| Canine model of single-walled bone defect. | The β-TCP was crushed into nano-sized particles (84 nm), which were then dispersed. Nanoscale β-TCP scaffolds were prepared by coating the surface of collagen scaffolds with nanoscale β-TCP dispersions. | [40] | ||
| Creation of intraosseous defects in dogs. | Enamel matrix derivative (EMD) and bovine-derived xenograft (BDX) combination. | [41] | ||
| Single-walled periodontal intraosseous defects in beagle dogs. | Human dental follicular cell sheets (hDFCSs) and human-treated dentin matrix particles (hTDMP) were prepared. | [42] | ||
| Class II furcations were established in the mandibular premolars of eight adult male Beagles. | Platelet-derived factor releaser (PR), PR is freshly prepared from blood drawn from dogs before treatment. | [43] | ||
| Canine premolar class II bifurcation defects. | Polysaccharide acemannan was obtained from the Aloe vera plant. | [44] | ||
| Basic research animal models | Rodent—rat | Rat periodontal defect model. | A polycaprolactone (PCL) composite membrane was fabricated by electrospinning of PCL and ZnO particles. | [48] |
| Experimental periodontal defects in Fischer rats. | Mesenchymal stem cells were seeded onto polylactic-co-glycolic acid/polycaprolactone electrospinning scaffolds, followed by preculturing under multiple in vitro conditions. | [33] | ||
| Experimental periodontitis in rats were made by lifting full-thickness gingival flaps and tying silk threads around the mandibular first molar. | Emdogain gel or propylene glycol alginate. | [51] | ||
| Rat periodontal defect. | Stromal cell-derived factor 1 (SDF-1) was loaded onto gelatin sponge. | [53] | ||
| Periodontal defects within the mesial maxillary first molars of 40 Wistar rats. | Defects were filled or unfilled with 1.5% neutral SAP nanofiber hydrogel (SPG-178). Animals received injections of PTH(1-34) or saline every 2 days. | [54] | ||
| A standardized periodontal defect was formed in the mesial of Wistar rats maxillary first molars. | Rat periodontal ligament-derived cells (PDLC) cultured on various self-assembling peptide (SAP) hydrogels. | [56] | ||
| An experimental rat model of periodontal fenestration defects. | Two-dimensional nanomaterial MXene (Ti3C2Tx). | [57] | ||
| A fenestration defect was created using an extraoral approach on the buccal side to the root of the mandibular first molar. | GMS-embedded Cementum protein 1-derived peptide (CEMP1-p1). | [58] | ||
| Periodontal defect caused by maxillary first molar surgery. | Hydroxypropylcellulose (HPC) or fibroblast growth factor (FGF)−2 and HPC. | [59] | ||
| Supra/subosseous combined periodontal defect in diabetic rats, surgically created bone defect on the mesial root of the first maxillary molar. | EMD. | [60] | ||
| Seven days after diabetes mellitus (DM) induction, a bilateral fenestration defect developed on the buccal side of the first mandibular molar. | EMD. | [61] | ||
| Alveolar bone defects created in male Sprague–Dawley rats. | 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside (THSG). | [29] | ||
| Rodent—mouse | Murine periodontitis model. | A novel scaffold (PP-pDA-Ag-COL) was generated by electrospinning a basic PLGA/PCL matrix followed by in situ reduction, polydopamine coating, and collagen I impregnation with silver nanoparticles (AgNPs). Three intermediate materials were involved in the fabrication of the scaffolds, namely, PLGA/PCL (PP), PLGA/PCL-polydopamine (PP-pDA), and PLGA/PCL-polydopamine-Ag (PP-pDA-Ag) were used as control bracket. | [62] |
- Abbreviations: PLGA, poly(lactic-co-glycolic acid); PTH, parathyroid hormone.
3 JAW DEFECT MODELS (INCLUDE ALVEOLAR, MAXILLA, AND MANDIBLE BONE DEFECTS)
3.1 Primate model
In a study by Boyne et al.,64 mandibles were removed from eight primates to simulate hemimandibular resection defects after traumatic bone loss or tumor surgery. Recombinant human (rh) BMP-2 in a collagen carrier was then placed into the hemimandibular resection defects and then fixed in place utilizing an orthopedic titanium mesh. In another cohort of nonhuman primates, the recovery area was functionally stimulated 5 months after recombinant human bone morphogenetic protein-2 (rhBMP-2) implantation by placing an intraoral titanium implant. To assess the function of rhBMP-2 in the elderly, a separate group of macaque monkeys over 20 years of age underwent the same procedure of mandibular resection, followed by an evaluation of the role of the tapered implants by chewing 5 months after rhBMP-2 implantation. The histomorphometric analyses in all these studies suggest that the use of rhBMP-2 for bone repair without the use of bone graft material provides a new approach for bone remodeling in clinical facial bone defects.64
3.2 Porcine model
Konopnicki et al.65 produced 3D-printed scaffolds with β-TCP and polycaprolactone (PCL). Six 2 × 2 cm defects were made in mandible (N = 2 minipigs). Altogether, six constructs were implanted into the defects and six untreated defects were used as controls. 3D-printed β-TCP and PCL scaffolds that were seeded with pBMSC and implanted into the mandibular defects of pigs showed excellent bone penetration depth.65 A critical size defect was created in the mandible of minipigs and implanted with bone porcine blocks (BPB) and BPB/bone marrow stromal stem cells (BMSSCs). The results showed that using BPB as a scaffold to induce bone regeneration was enhanced by the seeding of BDPSCs in tissue engineering constructs.66 Jensen et al.67 prepared three standardized bone defects in the two mandibular angles of 12 adult minipigs. Defects were grafted with autologous, inorganic bovine bone or synthetic β-TCP. The result demonstrated that defects of the three treatment groups eventually regenerated with newly developed bone and bone marrow. The graft material exhibited thorough osseointegration.67 Stevanovic et al.68 evaluated the effects of hydroxyapatite ceramics and poly(lactide-co-glycolide) and novel polyethyleneimine composite scaffolds in vivo for bone rehabilitation of porcine mandibular defects, as compared with traditional allograft bone (BioOss). The scaffolds were prepared in three steps using the polymer foam template method. Three-month-old pigs were utilized, and the defect was created in the canine, premolar, and molar regions of mandibles. The results demonstrated that the hydroxyapatite ceramic/polyethyleneimine composite scaffold displayed better regenerative efficacy in the treatment of porcine mandibular defects compared with conventional allografts in terms of bone density and histological characteristics of bone tissue.68
3.3 Canine model
Rothamel et al.69 evaluated the biodegradation profile of two distinctive native CMs in the dog model. Histological examination was performed 4, 8, 12, and 24 weeks after implantation in the anterolateral maxilla. The results showed that in vivo, both membranes were absorbed without inflammation within 8 weeks (Bio-Gide) or 12 weeks (Remotis), and induced early vascularization. Both examined membranes could support underlying bone formation.69 Lanao et al.70 developed composites of CPC and poly(lactic-co-glycolic) acid (PLGA) with different microsphere morphologies (hollow vs. dense). Besides blank CPC-PLGA composites, the loading of microspheres with platelet-derived growth factor (PDGF) and insulin-like growth factor (IGF) was also investigated. A total of four different CPC composites were implanted into single-wall mandibular defects in Beagle dogs to evaluate their efficacy in promoting alveolar bone regeneration. The results show that the CPC-dPLGA composite exhibited excellent bone formation and it is a biologically advantageous material for use as a standard material.70 Matsui et al.71 implanted octa-calcium phosphate collagen (OCP/Col) composites or collagen discs into canine alveolar cleft models (n = 6). Macroscopically, the OCP/Col-treated alveolar bone was significantly enlarged, and the radiopacity of the OCP/Col-implanted area was comparable to the original alveolar bone. Upon histological analysis and microcomputed tomography study, it was observed that the OCP/Col-treated area was largely filled with newly formed bone. OCP/Col-derived bone was shown to be composed of outer cortical and inner cancellous structures with dense trabeculae, similar to the original bone structure.71
3.4 Rabbit model
In the rabbit model (Figure 2B), Kun Liu et al. depict the implantation of scaffold in the mandibular alveolar bone defect.73 The study by Xu et al.78 developed a gelatin/β-TCP composite nanofiber membrane composed of gelatin and β-TCP. The healing of mandibular defects in rabbits implanted with the biomimetic gelatin/β-TCP composite nanofiber membrane was assessed. The results revealed that the composite membrane significantly improved bone formation compared with a pure gelatin membrane (Figure 2G).78 A study by Nannmark et al.79 created bilateral bone defects in the maxilla of eight rabbits which were filled with 60% collagenated porcine bone (CPB)/40% collagen gel or 80% CPB/20% collagen gel. The results demonstrated that different CPB/collagen ratios did not affect the bone tissue response to CPB. Both materials displayed osteoconductive properties and began to be resorbed at 8 weeks postsurgery.79 In another study by Nannmark et al.80 bilateral bone defects of 5 × 8 × 3 mm were created in the maxilla of 14 rabbits, which were then filled with prehydrated and collagenized porcine bone (PCPB) + collagen gel (test) or with PCPB alone (control). Animals were killed at 2, 4, and 8 weeks after surgery for histological and morphometric evaluation. The results showed that the PCPB has osteoconductive properties and is resorbed over time.80 To evaluate the stimulatory effects of the material matrix on bone tissue regeneration in situ within a rabbit mandibular alveolar bone defect model, Shao et al.81 fabricated a mechanically strong Mg-substituted wollastonite (CSi-Mg10) which was implanted into bone defects and compared with implants using typical calcium phosphate and calcium silicate porous bioceramics. The in vivo results showed that the CSi-Mg10 scaffold had significantly higher osteogenic capacity after 16 weeks postimplantation. These results suggest that the customized CSi-Mg10 3D robocast scaffold can be used to repair alveolar bone defects.81 Hassan et al.82 generated critical-sized alveolar bone defects in the maxilla of 24 New Zealand rabbits divided into three groups, one of which was treated with a 5 μg/kg rhBMP-2 nanoparticle (NP) preparation. It was found that preparing NPs from a mixture of PLGA and PCL in a 4:2 (w/w) ratio yielded the best-sustained release pattern. Therefore, this formulation was selected for scanning electron microscope imaging and in vivo evaluation. The results showed that the bulk of defects processed with rhBMP-2 in NPs within 6 weeks have well-developed bone.82 Li et al.83 created mandibular defects in 48 New Zealand rabbits. The mandibular defects were implanted with antigen-extracted xenogeneic cancellous bone (AXCB), AXCB/rhBMP-2 and autologous bone, or left blank. It was concluded that AXCB/rhBMP-2 showed good osteogenic effects in bone defect repair. AXCB is a good carrier of rhBMP-2 that promotes bone formation.83 Kamal et al.84 developed unilateral alveolar cleft defects in 16 New Zealand rabbits. The alveolar cleft defects were filled with β-TCP or β-TCP/composite xenodentin. Compared with β-TCP alone, β-TCP/composite xenodentin exhibited enhanced bone regeneration in the reconstruction of alveolar cleft defects in rabbits.84 Moroi et al.85 made 3 × 5 mm mandible bone defects on both sides of adult male white rabbits and covered with uHA/poly-L-lactic acid (PLLA) mesh or titanium mesh on the right side, but with no mesh on the left side. The results showed that the bone area ratio of the titanium group and uHA/PLLA group was significantly greater than that of the control group at 2 and 4 weeks after the operation. However, there was no significant difference in BMP-2 levels between the uHA/PLLA group and the titanium group.85 Zhu et al.86 implanted nano-hydroxyapatite/collagen (nHAC)/concentrated growth factor (CGF) and nHAC into the rabbit mandible. It was observed that the nHAC/CGF material could induce new bone formation.86 Sawada et al.87 applied gelatin hydrogels with or without BMP-2 solution to experimental alveolar fissures prepared from rabbit maxilla. As a control, some alveolar fissures were left untreated. Significant bone regeneration was observed in alveolar fissures treated with BMP-2-incorporated gelatin hydrogel compared with other groups.87 In a study by Yin et al.,88 an α-tricalcium phosphate (doped and undoped 6% strontium; α-TCP-Sr6, α-TCP) bioceramic scaffold was developed for the repair of alveolar clefts of rabbits. The results showed that both α-phase TCP scaffolds exhibited significant new bone ingrowths compared with the β-phase scaffolds for inducing the osteogenic capacity of rabbit alveolar clefts.88 As shown in Figure 2C, Qin et al. depict the autologous tooth transplantation preparation, surgery, and micro-CT observation after implantation.74 Soares et al.89 created critical-sized segmental defects bilaterally in the jaws of 18 rabbits. The defects were filled with DBBM-C containing 10% (w/w) collagen, lyophilized bovine medullary bone (LBMB), or left untreated. Both graft materials yielded improved bone regeneration and more complex architecture after healing, as compared with the untreated defect. In the LBMB samples, there was a clear integration between the host bone and the implanted graft.89 Zhang et al. depict the representative 3D m-CT images of a rabbit mandibular defect and quantitative analysis of bone mineral density (BMD) and bone volume/total volume ratio (BV/TV ratio) at 4 and 12 weeks after implantation (Figure 2F).77 Turri et al.90 created a 5 × 5 mm defect in the edentulous space between the maxillary incisors and molars of 18 rabbits. CaS and DBBM particles were then placed in the defect. Compared with the DBBM group, the CaS group showed enhanced bone regeneration during all three healing phases.90 Lundgren et al.91 prepared defects in the edentulous areas on the maxilla of 22 rabbits. The rabbits were treated with Gore-Tex(®) reinforcement (GTAM) (ePTFE) barrier that was placed to cover the defects, fully enclosed with perforated titanium foil and uncovered control defects, respectively. The results showed that the amount of regenerated bone tissue was approximately the same as in the control group under the collapsed GTAM barrier. The highest degree of regeneration was obtained in the defects below the titanium foil.91 Guo et al.92 evaluated the performance of composite nano-hydroxyapatite/polyamide (n-HA/PA) biomaterials under different osteogenic conditions in vivo. Scaffolds were implanted into critical-sized bone defects in the mandibular angle and body of rabbits. The results demonstrated that the n-HA/PA biomaterial possessed mechanical strength advantages over n-HA and exhibited significant bone regeneration in bone marrow-poor sites when coimplanted with BMSCs.92 The alleviation of irreversible bone resorption accompanied by atrophy and the reconstruction of soft tissues surrounding the missing tooth remains a formidable challenge for implant therapy. A study by Li et al.93 developed biphasic materials consisting of electrospun membranes of fish collagen/poly(lactide-glycolide) and nano-hydroxyapatite/poly(lactide-glycolide) scaffolds to facilitate oral rigidity and soft-tissue regeneration. At the same time, injectable autologous platelet-rich fibrin (i-PRF) was prepared and used in combination with the scaffold. The combination of i-PRF and biphasic material was implanted into the palatal defect of rabbits and was found to be more conducive to the simultaneous healing of oral soft and hard tissues.93
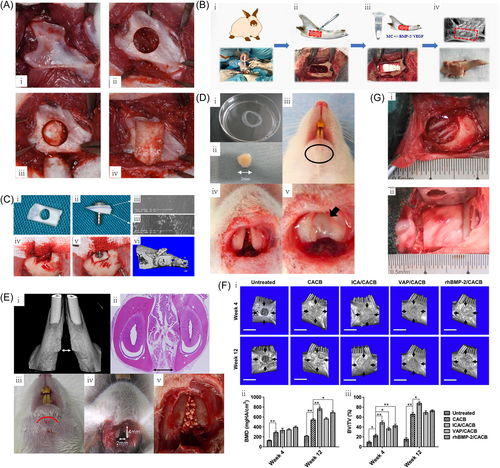
3.5 Rat
Furuhata et al. depict a surgical plan for creating a circular bone defect in the mandibular angle (Figure 2A).72 In the study of Boda et al.,94 electrospun nanofibrous films of PLGA-collagen-gelatin (2:1:1 weight ratio) were mineralized in modified simulated body fluid (mSBF) and cryogenically cut into 20 µm fragments. Implantation of mineralized nanofiber fragments (2 mg) with and without E7-domain-conjugated BMP-2 (E7-BMP-2) peptide into the rat maxilla following the production of a critical defect with the dimension of 2 mm × 2 mm (diameter × depth) in the alveolar socket. The results indicated that the combination of mineralized nanofiber fragments and E7-BMP-2 peptide could be an effective treatment option for alveolar bone defects.94 Zellin et al.95 created standardized, critical-sized mandibular transosseous defects bilaterally in rats, which were randomly covered with different types of membranes. After 6 weeks of healing, some membranes (such as Gore-Tex Augmentation Material, Millipore™, and Resolut “long term”) yielded good improvements in bone healing.95 Nascimento et al.96 prepared a mandibular defect model in the distal roots of mandibular first molars in Wistar rats and the results showed that the used MTA was able to induce bone regeneration and its effects can be further optimized when combined with calcium hydroxide purum.96 The study of Subramaniam et al.97 developed a collagenase (HAP/CS/HA-Col) scaffold loaded with hydroxyapatite, calcium sulfate hemihydrate, and collagenase loaded with hyaluronic acid as a bone substitute for alveolar bone regeneration. Bone formation in vivo was evaluated in rats with alveolar bone defects and enhanced bone regeneration with the HAP/CS/HA-Col composite was verified by micro-CT images and histological staining.97 Han et al.98 established a rat mandibular defect model to study the barrier/osteoinductive dual effects of hydroxyapatite (HAp) nanowire-modified polylactic acid (PLA) films. There was more newly formed bones in the HAp/PLA group that were of better quality.98 Li et al.99 prepared mangiferin-loaded poly(D,l-lactide-co-glycolide) (PLGA) scaffolds by freeze-drying technology utilizing ice particles as pore-forming materials. The scaffolds were implanted into alveolar bone defects in diabetic rats and hematoxylin and eosin staining was utilized to assess bone repair. The implanted scaffolds increased histological scores for bone regeneration and improved the healing of alveolar defects in diabetic rats.99 Figure 2D depicts the implantation technology of osteogenic matrix cell sheets being utilized in the mandibular symphysis.75 Zhao et al.100 created a 5 mm diameter full-thickness mandibular bone defects in rats and then utilized a tissue-engineered bone construct of β-TCP and genetically modified bone marrow stromal cells (BMSCs) overexpressing bone morphogenetic protein-2 (BMP-2). The results showed that BMP-2 gene therapy together with β-TCP scaffolds can be used to promote mandibular repair and bone regeneration.100 In the study of Wang et al.,101 SD rats were utililized in an alveolar bone injury model to evaluate the effects of double-tube biphasic bioceramics/PEEK in bone repair. The dual-tube biphasic bioceramic/PEEK composite material was found to enhance the repair of alveolar bone defects, enhance bone mineral density, and accelerate alveolar bone repair.101 In a study by Oliveira et al.,102 90 rats were allocated into the application of laser irradiation: laser group and control group. The result demonstrated that low-level laser therapy (LLLT) was found to improve the osteoconductive potential of deproteinized bovine bone (DBB) and hydroxyapatite/β-tricalcium phosphate (HA/β-TCP) grafts, as well as enhanced bone formation in ungrafted areas.102 Nguyen et al.103 surgically produced critical-sized alveolar defects in 60 SD rats. Four scaffold treatment arms were applied and new bone formation was assessed by radiomorphology and histomorphology at 4, 8, and 12 weeks postsurgery. The results showed that absorbable collagen sponges and hydroxyapatite-tricalcium phosphate scaffolds induced more bone formation than untreated controls. rhBMP-2 added a small but significant histomorphological osteogenic advantage to hydroxyapatite-tricalcium phosphate scaffolds.103 Yagyuu et al. depict the rat mandibular joint and implant technique (Figure 2E)76 (Tables 3 and 4).
| Research model classification | Species | Advantages | Disadvantages |
|---|---|---|---|
| Animal models for preclinical evaluation | Primate | The bone healing mechanism is similar to that of humans, and the jaw defect models are easy to prepare and operate. | The cost of the experiment is too high, and the number of samples is difficult to guarantee. Moreover, the self-recovery capacity of large animals used for preclinical evaluation is weak, which is not conducive to the short-term observation of bone regeneration, and this can easily cause the experimental period to be too long. |
| Porcine | |||
| Canine (Dog) | |||
| Basic research animal models | Rabbit | These are is cheap and widely used, has strong anti-infection capacity and good ability to tolerate surgery, as well as easy to manage during the peri-operative period. | The constructed bone defect repair model can only be used for preliminary research on the molecular mechanisms of bone defect repair and for short-term evaluation and biocompatibility evaluation of bone repair materials. The human bone reconstruction process cannot be fully simulated. Small animals used for basic research are too small and inconvenient to handle. |
| Rat |
| Research model classification | Species | Defects-types of defects and how these were made | Scaffold/graft materials used | References |
|---|---|---|---|---|
| Animal models for preclinical evaluation | Primate | Mandibles were removed from eight primates to simulate hemimandibular resection defects. | Recombinant human (rh) BMP-2 in a collagen carrier and fixed with an orthopedic titanium mesh. | [64] |
| Porcine | Six 2 × 2 cm defects were produced in mandible (N = 2 minipigs). | 3D-printed frameworks were generated with β-tricalcium phosphate (β-TCP) and polycaprolactone (PCL). | [65] | |
| A critical size defect was created in each mandible of minipigs. | Porcine bone blocks (BPB) and BMSSC seeded on BPB to form BPB/BMSSC. | [66] | ||
| Three standardized bone defects in the two mandibular angles of 12 adult minipigs. | Autologous, inorganic bovine bone (ABB) or synthetic β-tricalcium phosphate (β-TCP). | [67] | ||
| Canine (Dog) | Canine alveolar cleft model. | Octacalcium phosphate collagen (OCP/Col) composite. | [71] | |
| Basic research animal models | Rabbit | Bilateral bone defects were created in the maxilla of eight rabbits. | 60% of bone substitute of collagenized porcine bone (CPB)/40% collagen gel or 80% CPB/20% collagen gel. | [79] |
| Bilateral bony defects were created in the rabbit maxilla, 5 × 8 × 3 mm. | Prehydrated and collagenized porcine bone (PCPB) with or without collagen gel. | [80] | ||
| Rabbit mandibular alveolar bone defect model. | Wollastonite (CSi-Mg10). | [81] | ||
| Alveolar bone defect of critical size in rabbit maxilla. | NPs from a mixture of PLGA and PCL in a 4:2 ratio (w/w) with sustained release of BMP-2. | [82] | ||
| Rabbit mandibular defect. | Antigen-extracted xenogeneic cancellous bone (AXCB) incorporated with or without rhBMP-2. | [83] | ||
| After incision along the mandible of rabbits, 3 × 5 mm bone defect were made on both sides. | uHA/PLLA mesh and titanium mesh. | [85] | ||
| Rabbit mandibular defect. | Nano-hydroxyapatite/collagen (nHAC) combined with concentrated growth factor (CGF). | [86] | ||
| A 5 × 5 mm defect was created in the edentulous space between the maxillary incisors and molars of rabbits. | Calcium sulfate (CaS) and deproteinized bovine bone mineral (DBBM) granules. | [90] | ||
| Defects were formed in the edentulous areas on the maxilla in 22 rabbits. | Gore-Tex(R) reinforced material (GTAM) (ePTFE) barrier, fully enclosed or perforated titanium foil. | [91] | ||
| Rodent—rat | Critical-sized 2 mm × 2 mm (diameter × depth) alveolar socket defects were created in rat maxilla. | Electrospun nanofibrous films of PLGA-collagen-gelatin (2:1:1 by weight ratio) were mineralized in modified simulated body fluid (mSBF) and cryogenically cut into 20 µm fragments. Then different amounts of heptaglutamic acid E7 domain-conjugated BMP-2 peptides can be loaded. | [94] | |
| Bilateral fabrication of normalized, transosseous, critical-sized mandibular defects in adult rats. | Different types of biodegradable films. | [95] | ||
| Rat mandibular defect. | Mineral trioxide aggregate (MTA) and calcium hydroxide P.A. | [96] | ||
| Rat alveolar bone defect. | Hydroxyapatite, calcium sulfate hemihydrate, and collagenase loaded with hyaluronic acid (HAP/CS/HA-Col). | [97] |
- Abbreviations: BMP, bone morphogenetic protein; BMSSC, bone marrow stromal stem cells; NP, nanoparticle; PLLA, poly-L-lactic acid; rhBMP-2, recombinant human bone morphogenetic protein-2.
4 IMPLANTATION DEFECT MODELS (INCLUDE ALVEOLAR RIDGE EXPANSION AND SITE PRESERVATION)
4.1 Nonhuman primate model
Suzuki et al.104 created bilateral bone defects in the edentulous mandibular alveolar ridge of nonhuman primates, which were grafted with two different types of bone graft materials. Each bone defect was closed and sutured by a skin-matrix barrier membrane-covered flap in front of the mucoperiosteal tissue. At 4 years posttreatment, alveolar bone at all sites was clinically normal, and mature cortical and cancellous bone formation was observed histologically in both bone graft materials.104 Boller et al.105 created defects (15 mm × 8 mm × 5 mm) on both sides of the mandibles of nine baboons. A poly(ester urethane) (PEUR)/ceramic CR scaffold with different concentrations of rhBMP-2 were implanted at the defect sites. Incremental new bone formation within the mandible was inspected in a dose–response manner. Ridge widths remained the same in all groups without additional protective films.105
4.2 Porcine model
Gomez et al.106 created two normalized intrabony defects (15 mm × 8 mm × 8 mm) on each side of the mandible of 18 Göttingen minipigs. Groups were nested within subjects and randomly distributed among the four groups with or without graft and membrane. From a temporal perspective, three-dimensional assessment showed progressive bone ingrowth, the existence of granular bone grafts, bridging of the defect wall, and preservation of mandibular structure over time. Volume analysis illustrated no substantial differences between all groups at 4 weeks after surgery (p > 0.127). The proportion of new bone formation was higher in the control and cerabone® bone graft/porcine pericardium-based membrane (CJ) groups than in the bovine bone graft/bilayer CM (BOB) and Bio-Oss® bone graft/porcine pericardium-based membrane (BOJ) groups at 8 and 12 weeks postoperatively (p < 0.039). Compared with the bovine bone graft, the natural bovine bone graft group demonstrated greater graft resorption potential over time, with significant differences observed between 4 and 8 weeks postoperation (p < 0.003).106
4.3 Canine (Dog) model
Figure 3A illustrates how a model of the defective mandible was constructed, followed by subsequent implantation of the fabricated scaffolds into the previously formed defects, which were allowed to heal for 3 months.107 In the study of Birang et al.,111 four cylindrical cavities (6 × 6 mm) were prepared in bilateral mandibles of 3 dogs. These defects were randomly assigned to four different treatment groups-filled with EMD/bone ceramic (BC) and covered with or without nonabsorbable membrane, covered with membrane only, and control (untreated), then left to heal for 2, 4, or 6 weeks. The results showed that EMD/BC may improve bone formation in bone defects more than the membrane overlay alone, and there was no significant additive effect on total bone formation with membrane use.111 Sanz-Martin et al.112 created cheekbone defects in the mandibles of beagle dogs. Augmentation procedures were then performed after 3 months using a bone replacement graft (BRG), a resorbable CM (MBG), or a combination of the two procedures (CBG). BRG and CBG were equally effective and superior to MBG in increasing horizontal tissue contours.112 Liu et al.113 extracted the mandibular third premolars to second molars bilaterally from six Beagle dogs. After 8 weeks of healing, six standardized box-shaped defects were created on the buccal mandible and were randomly implanted with porcine hydroxyapatite (PHA), fluorinated porcine hydroxyapatite (FPHA) or left blank, followed by covering with CM. The results showed that FPHA achieved better alveolar ridge width maintenance and bone regeneration results as a bone substitute in lateral ridge augmentation compared with PHA.113 The ability of mineralized collagen/Mg-Ca alloy combined scaffolds in enhancing osteogenesis was evaluated in a canine alveolar preservation model. Cone beam CT, X-ray microscopy, and biomechanical analyses showed that the combined mineralized collagen/Mg-Ca alloy scaffold was more effective than the mineralized collagen alone in reducing alveolar ridge resorption and preserving the alveolar site. The composite scaffold can promote bone regeneration.114 In a study by Du et al.,115 nano-hydroxyapatite/coralline (nHA/coral) blocks and VEGF/nHA/coral blocks were implanted arbitrarily in a split-mouth design in canine mandibular box defects. This study illustrated that nHA/coral blocks are the best scaffold for transplantation of mandibular defect blocks and that additional VEGF coating by physisorption can promote angiogenesis during the initial stages of bone healing, suggesting that prevascularized nHA/coral blocks have the capacity to serve as bioactive materials for bone regeneration in large-area alveolar bone defects.115 Han et al.116 developed an injectable bone cement nHAC/CSH consisting of nano-hydroxyapatite/collagen (nHAC) and calcium sulfate hemihydrate (CSH). An alveolar bone defect was created around an immediate implant in a dog model. Each defect was then randomly assigned to three groups. The ITB group (dBMPC+nHAC/CSH) showed significantly higher bone-implant contact and bone mineral density than the injectable bone cement nHAC/CSH or control group within the peri-implant defect area at 3 months postimplantation.116 Fukuba et al.117 created a critical-sized saddle defect (8 mm long × 4 mm deep) in a bilateral procedure 2 mm from the canine mesial side, at 12 weeks after extraction of the maxillary incisors in six dogs. Then acid gelatin/β-TCP sponge (IEP 5.0) soaked with rhFGF-2 was applied to the defects in the acid group, and the IEP 9.0 was applied to the basic group. The new bone area was measured by micro-CT analysis, the recent bone height and the total tissue height calculated by tissue section in the basic group were much lower than that in the acidic group. Controlled release of rhFGF-2 induced significantly more alveolar bone formation compared with the short-term application of rhFGF-2.117 Ku et al. illustrates the drilling of an 8.0 mm hole in dog mandible (Figure 3B).108 Hoshi et al.118 created a bilateral 10 × 5 mm (W × D) saddle-shaped defect at 3 mm mesial to the maxillary canine at 12 weeks after tooth extraction. The defects were filled with gelatin/β-TCP sponge incorporating rhFGF-2 at experimental sites, while saline-infiltrated gelatin/β-TCP sponge was implanted at control sites. The results showed that the combined use of rhFGF-2 and gelatin/β-TCP sponge promoted cristae enlargement in canine saddle-shaped bone defects.118 Von Arx et al.119 evaluated different grafting procedures to enhance the lateral, extension (8 × 10 × 14 mm) and chronic bone defects of the mandibular alveolar ridge. Experimental sites received TCP pellets or demineralized freeze-dried bone allograft (DFDBA) pellets. The study supported the use of autografts with barrier membranes to augment lateral ridges that extend alveolar bone defects.119 A total of 11 male Beagle dogs were used in the investigations. In the study by Hsu et al.,120 experimental ridge defects were produced to form atrophic ridges. Subsequently, vertical bone augmentation (VBA) was attempted by GBR using titanium mesh and allograft. In the hemi-mandibles, rhBMP-2/absorbable collagen sponge was thoroughly mixed with the allograft before surgery. The results illustrated that the presence of rhBMP-2 in the composite grafts can increase the vertical gain compared with non-rhBMP-2 sites.120 Chao et al.121 surgically prepared 50 saddle-shaped alveolar defects (10 mm × 10 mm × 4 mm) on the edentulous alveolar ridge of 10 Beagle dogs. After implant placement, five different materials were implanted in the vertically exposed implant fixtures defect. The results showed that the structure of HAp/TCP/Col + 0.2 mg/mL rhBMP-2 without a barrier membrane can be a promising tool for peri-implant eminence.121 The study of Wikesjo et al.122 generated bilateral, critical-sized, peri-implant defects (5 mm) in 12 Hound Labrador mongrel dogs. Animals received implants coated with different concentrations of rhBMP-2 or an uncoated control. The results showed that rhBMP-2 coating onto the surface of titanium porous oxide implants generated clinically significant local bone formation, including vertical enhancement of the alveolar ridge and osseointegration.122 Shafieian et al.123 drilled four cylindrical defects in the mandibles of five mongrel dogs randomized to the four groups. In vivo, the platelet rich plasma (PRP)-enriched human adipose-derived mesenchymal stem cells seeded on HA/TCP particles induced significant bone formation in the bone defects (p < 0.05).123 Fahmy et al.124 prepared resorbable silica-calcium-phosphate composite bioactive ceramic particles (SCPC50) that were coated with rhBMP-2 and then implanted into the canine mandibular saddle-shaped defects (12 × 7 mm). The results demonstrated that coating with SCPC50-rhBMP-2 further accelerated bone regeneration and markedly improved vertical bone height. Hence, SCPC50-rhBMP-2 can be used as a substitute for autologous bone transplantation.124 Cehreli et al.125 extracted bilateral mandibular second premolars in dogs. The poly(l-lactide)-hydroxyapatite (PLLA-HA) composite was placed at the left surgical site and the right extraction site was utilized as a control. The experimental PLLA-HA composite can be safely used as a small defect filler for applications such as the repair of alveolar defects, ridge augmentation, and sinus lift procedures.125 Lee et al.126 extracted mandibular premolars from mongrel dogs and then surgically created three lateral ridge defects on each side of the dental arch. After 4 weeks postsurgery, guided cristae augmentation was performed on each defect with the six treatment regimens. The P+D (L- with DBBM PRF) group showed newly formed bone under histological and micro-CT analysis. Compared with nonabsorbable and absorbable barrier films, l-PRF was more effective in bone regeneration.126 Stavropoulos et al.127 created three saddle-shaped bone defects on both sides of the mandibular edentulous area. Then defects are filled with canine demineralized freeze-dried bone (DFDB) and covered with PGA: TMC membranes of four different porosities, CM, and DFDB alone (control). The results showed that PGA: TMC membranes protected DFDB-filled defects and yielded a greater amount of bone regeneration than the other two groups.127 Machtei et al.128 demonstrated the bone-enhancing effects of BCP/BCS grafts in split extraction socket defects.128 In the study of Shafieian et al.,129 cylindrical penetrating defects were drilled in the mandibular plates of five mongrel dogs and were filled randomly with four different materials. The results showed that PRP-assisted hADSCs generated bone tissue regeneration in canine alveolar bone defects.129 Kim et al.130 extracted the mandibular premolars unilaterally in six dogs and induced three cristae defects. Each defect site was randomly assigned to deproteinized porous bovine bone mineral (BM) alone, a combination of BM and bioabsorbable porcine-derived bilayer CM, or neither membrane nor bone graft as a control group. Gross evaluation of 3D CT-reconstructed images of the canine mandible at 16 weeks after implantation showed the greatest bone gain in group B with excellent buccal alveolar ridge thickness. The results showed that BM led to more successful bone regeneration during GBR, in particular, CM was used in combination as a barrier to promote the regeneration of canine alveolar ridge defects.130 In a study by Jang and Choi,131 eight alveolar extraction sockets from four dogs were arbitrarily assigned into two groups to ascertain a split-mouth architecture with the following treatment: control group, sockets filled with commercially purchased synthetic HA; Test group, sockets filled with grainy pounds. The results showed that the natural ceramic powder derived from GBP is suitable for canine alveolar bone reconstruction.131 Inomata et al.132 extracted all premolars in beagle dog mandibles bilaterally. After a 12-week healing period, two bony defects (5 mm long; 5 mm wide; 7 mm deep) were created on the mandibles, and the buccal bone plate was excised. The bone defects were then randomly assigned to four groups. It was found that the application of β-TCP to alveolar bone defects and covering of open wounds with free buccal mucosa or collagen sponge may contribute to ridge augmentation.132 Shi et al.133 created four enlarged mandibular extraction sockets in mongrel dogs, with two on each side, being made in each animal. It was observed that implantation of SGCS/PRP in fresh extraction sockets promoted bone formation in this canine model. The addition of PRP to SGCS promoted bone regeneration during the early stages of healing.133 Thoma et al.134 treated surgically created spinal defects with four treatment modalities. The results showed that the combination of rhBMP-2 and bulk allograft yielded the largest crest width of the four treatment regimens used in this canine crest augmentation model.134 Lindhe et al.135 raised the full-thickness flap in five Beagle dogs, which included two premolars in the maxilla and two premolars in the mandible and removed the distal root. Allografts implanted in porcine collagen (BPCAP; -TCP core coated with nanocrystalline biomimetic hydroxyapatite) were used to fill the fresh extraction sockets at the premolar site. It has been documented that biphasic allografts did not undergo significant resorption during tissue remodeling, but facilitated substantial bone formation within the postextraction site.135 Min et al.136 extracted the mandibular right premolar four (PM4) from eight beagle dogs and implanted anti-BMP-2 mAb + bovine inorganic bone mineral with collagen (ABBM-C) and native porcine bilayer CM. ABBM-C and CM were functionalized with anti-BMP-2 mAb (test group) or an isotype-matched control mAb (control group). The results showed that the functionalization of the ABBM-C-framework and CM apparently led to new bone formation within the healing alveolar bone.136 Tien et al.137 extracted the distal roots of three mandibular premolars from six beagle dogs, and the entire buccal and lingual bone walls were surgically removed. Grafted DPBM with or without coverage by a CM or no grafts were then applied to the defect. The results demonstrated that grafting with a CM preserves the alveolar ridge size in two-walled extraction sockets with substantial bone regeneration.137 Chao et al.138 surgically prepared 24 saddle-shaped alveolar defects (10 mm mesial-distal and 4 mm apical-coronal) in the alveolar ridges of four male Beagle dogs treated with four different materials. The results showed that the BMP-2 peptides can accelerate bone regeneration around implants.138 As shown in Figure 3D, Xu et al. depict the surgery procedure by which a bone defect was created after mandibular premolar extraction and subsequent installation of the implant into the distal region of the bone defect.110

4.4 Rabbit model
As shown in Figure 3C, Zhang et al. illustrate the process of predictable alveolar bone augmentation and synchronized dental implantation by the PLGA/HA/β-TCP composite scaffold.109 Li et al.139 selected 24 New Zealand rabbits aged 8–12 weeks that were randomly assigned to four groups: the three experimental groups received PPP, CGF, and PRF gel implantation after bilateral mandibular anterior tooth extraction, while for the control group, no material was implanted. In summary, the application of PPP, CGF, and PRF in the tooth extraction socket effectively promoted bone regeneration. In the long-term, CGF exhibited more potent osteoinductive and tissue regeneration capacities compared with the control139 (Tables 5 and 6).
| Research model classification | Species | Advantages | Disadvantages |
|---|---|---|---|
| Animal models for preclinical evaluation | Primate | These have oral-maxillofacial anatomy and bone healing mechanisms similar to humans and is easy to operate. | Expensive and require a larger breeding space. There is also a risk of zoonotic disease transmission. Poor self-healing capacity, easily infected, and are not suitable for short-term observation of bone regeneration effect. |
| Porcine | |||
| Canine (Dog) |
| Research model classification | Species | Defects-types of defects and how these were made | Scaffold/graft materials used | References |
|---|---|---|---|---|
| Animal models for preclinical evaluation | Primate | Bilateral bone defects in the edentulous mandibular alveolar ridge of nonhuman primates. | One side is grafted with bioactive glass (xenograft) and the opposite side is grafted with human demineralized bone matrix (xenograft). Each bone defect was closed and sutured with a skin-matrix barrier membrane-covered flap anterior to the mucoperiosteal tissue. | [104] |
| Defects (15 mm × 8 mm × 5 mm) were created on both sides of the mandibles of nine baboons. | A poly(ester polyurethane) (PEUR)/ceramic CR scaffold with different concentrations of rhBMP-2 were implanted at the defect sites. | [105] | ||
| Porcine | Two normalized intrabony defects (15 mm × 8 mm × 8 mm) were produced on each side of the mandibles of 18 Göttingen minipigs. | Groups were nested within subjects and randomly assigned to the following groups: (i) Negative control (No graft and membrane), (ii) Bovine bone graft/Bilayer collagen membrane (BOB) (iii) Bio-Oss® Bone graft/Porcine pericardium (BOJ) and (iv) Cerabone® Bone graft/Porcine pericardium (CJ). | [106] | |
| Canine (Dog) | Four cylindrical cavities (6 × 6 mm) were prepared on both sides of the mandibles of 3 dogs. | Combination of enamel matrix derivatives (EMD) and bone ceramics (BC). | [111] | |
| Cheekbone defects in the mandibles of beagle dogs. | Bone replacement graft (BRG), resorbable collagen membrane (MBG), or a combination of both procedures (CBG). | [112] | ||
| Mandibular third premolars to second molars were extracted bilaterally from six male Beagle dogs. After 8 weeks of healing, 6 standardized box-shaped defects were created on the buccal mandible. | Combination of porcine hydroxyapatite (PHA) and fluorinated porcine hydroxyapatite (FPHA) with collagen membrane (CM). | [113] | ||
| Canine alveolar preservation model. | Mineralized collagen/Mg-Ca alloy combination scaffold. | [114] | ||
| Canine mandibular critical sized defect model. | Nano-hydroxyapatite/coralline (nHA/coral) blocks and VEGF/nHA/coral blocks. | [115] | ||
| Alveolar bone defect around immediate implant in dogs. | Injectable bone cement nHAC/CSH composed of nano-hydroxyapatite/collagen (nHAC) and calcium sulfate hemihydrate (CSH). | [116] | ||
| 12 weeks after extraction of the maxillary incisors in 6 dogs, critical-sized saddle defects (8 mm long × 4 mm deep) were created with a bilateral procedure, 2 mm from the canine mesial side. | 0.3% rhFGF-2 soaked acid gelatin/β-TCP sponge (IEP 5.0) and rhFGF-2 soaked alkaline gelatin/β-TCP sponge (IEP 9.0). | [117] | ||
| Beagle dogs were used to produce experimental ridge defects to form atrophic ridges. | Recombinant human bone morphogenetic protein (rhBMP)−2/absorbable collagen sponge. | [120] | ||
| Fifty saddle-shaped alveolar defects (10 mm mesial and distal and 4 mm coronal) on the edentulous alveolar ridge of 10 Beagle dogs. | 0.2 mg/ml rhBMP-2 with hydroxyapatite (HAp)/β-tricalcium phosphate (TCP)/collagen (Col) composites. | [121] | ||
| Saddle defect in dog mandibles (12 × 7 mm). | Porous silica-calcium-phosphate composite (SCPC50) loaded and unloaded with rhBMP-2. | [124] | ||
| Bilateral mandibular second premolars were extracted from dogs to prepare mandibular defects. | Poly(l-lactide)-hydroxyapatite (PLLA-HA) composite. | [125] | ||
| Three saddle-shaped bone defects on both sides of the mandibular edentulous area of five dogs. | Canine demineralized freeze-dried bone (DFDB) and covered with PGA: TMC membranes of four different porosities, collagen membrane and DFDB alone (control). | [127] | ||
| Unilateral extraction of mandibular premolars resulted in 3 ridge defects in 6 dogs. | Deproteinized porous bovine bone mineral (BM) alone (group A), a combination of BM and a bioabsorbable porcine-derived bilayer collagen membrane (CM) (group B), or neither membrane nor bone graft, as a control group (group C). | [130] | ||
| Alveolar bone defects were formed in 8 alveolar bone extraction sockets in 4 dogs. | Bioceramic natural hydroxyapatite (HA) bone substitute developed from goose coracoid bone pellets (GBPs). | [131] | ||
| In beagle dogs, all premolars in the bilateral mandibles were extracted. After a 12-week healing period, two bony defects (5 mm long; 5 mm wide; 7 mm deep) were created on the mandible, and the buccal bone plate was excised. | β-tricalcium phosphate (β-TCP) covered with free buccal mucosa or collagen sponge. | [132] | ||
| Four enlarged mandibular extraction sockets were created in each dog on each side. | The combination of SGCS and PRP. | [133] | ||
| Surgically-created spinal defects in dogs. | The combination of rhBMP-2 and bulk allograft. | [134] | ||
| Full-thickness flaps of five beagle dogs were lifted, including two maxillary and two mandibular premolars, and the distal roots were removed. | Allograft implanted with porcine collagen (BPCAP; -TCP core coated with nanocrystalline biomimetic hydroxyapatite) for filling fresh extraction sockets in premolar sites. | [135] | ||
| Extraction of mandibular right premolar four (PM4) in 8 beagle dogs. | Implanted with anti-BMP-2 mAb + inorganic bovine bone mineral with collagen (ABBM-C) and native porcine bilayer collagen membrane (CM). | [136] | ||
| The distal roots of three mandibular premolars were extracted from 6 beagle dogs, and the entire buccal lingual bone wall was surgically excised. | Three treatment regimens were assigned to the following groups: No graft (None), Grafted DPBM (BG), and Grafted DPBM covered with collagen membrane (BG+M). | [137] | ||
| Twenty-four saddle-shaped alveolar defects (10 mm mesio-distal and 4 mm apical-coronal) were present in the alveolar ridges of four male Beagle dogs. | Hydroxyapatite (HAp)/β-tricalcium phosphate (TCP)/collagen (Col) composite with different concentrations of bone morphogenetic protein (BMP)-2 derived-peptide (BMP-2 peptide) were implanted. | [138] |
- Abbreviation: PRP, platelet rich plasma.
5 ADVANTAGES AND DISADVANTAGES OF BASIC RESEARCH AND PRECLINICAL EVALUATION ANIMAL MODELS
Basic research animal models are usually small in size, which minimizes requirements of space, facilities, and major housing requirements associated with larger preclinical evaluation animal models. This allows the inclusion of a large number of animals, thereby facilitating the collection of sufficient data for proper analysis and extrapolation on a more economical basis. Basic research animal models are easier to handle than preclinical evaluation animal models. For example, preparation and recovery from surgery often takes less time. Basic research animal models can tolerate more complex surgical procedures than preclinical evaluation animal models, with fewer complications. Moreover, basic research animal models are more resistant to disease and infection, reducing the risk of animal attrition during experiments, and (in contrast to primates) pose lesser risks of zoonotic transmission. They also tend to have a more uniform genetic background, with much lesser variation between individual animals in terms of biological responses, which means fewer experimental replicates needed to obtain statistically valid data. Nevertheless, validation in preclinical evaluation animal models are still required before proceeding to human clinical studies. Finally, it must be noted that the utilization of nonhuman primates encounters much greater ethical challenges than basic research animal models.140
6 ADVANTAGES AND DISADVANTAGES OF THE APPLICATION OF ORAL HARD TISSUE DEFECT MODELS
Oral hard tissue defect models include periodontal models, jaw defect models, and implantation defect models. The periodontal models have the advantages of easy determination of the defect location and less intraoperative blood loss, but these also have certain disadvantages such as difficult operation and long preparation time, usually taking 7 days, 14 days, or longer to prepare. Compared with periodontal models, the preparation time of the jaw defect models and implantation defect models are shorter, and the preparation can be completed on the same day of the experiment, but the defect is often not easy to locate because the defect is deep and covers the muscle, with limited field of view during preparation. Both models bleed more and are more technically challenging. These three defect models all correspond to the oral and maxillofacial defects that may occur clinically and are common defect models of the oral and maxillofacial region.
7 CONCLUSIONS
Oral hard tissue defect models include periodontal models, jaw defect models, and implantation defect models. Each of these defect models have utilized a variety of different animal species to evaluate the regenerative effects of implant materials, including small animal models for basic research and large animals for preclinical studies. Sometimes, it is better to start with small animals first, and then conduct large animal experiments, which is more conducive to time and cost savings, as well as being more ethically acceptable. The animal oral hard tissue defect models summarized in this review have certain reference significance for evaluating the regenerative efficacy of clinical oral hard tissue defect models. The intended clinical application of implant materials can be used as a selection criteria for particular defect models. And implanted materials provide a promising therapeutic strategy for the regeneration of clinical oral hard tissue defects. In summary, there is a diverse variety of different oral hard tissue defect models that can be used to effectively evaluate the regenerative effects of implanted materials, which have much value for both preclinical research and clinical applications.
AUTHOR CONTRIBUTIONS
Xiaowen Sun and Xuehui Zhang designed the review. Xiaowen Sun wrote the paper. Boon Chin Heng and Xuehui Zhang revised the paper. All authors provided final approval of the paper. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (2021YFB3800800), the National Natural Science Foundation of China (82022016, 52273258, 51973004), and the Beijing Municipal Natural Science Foundation 7222226.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




