Nanobiotechnology-mediated radioimmunotherapy treatment for triple-negative breast cancer
Abstract
Breast cancer has become the most common and greatest threat to women's health in the world. As the most dangerous subtype of breast cancer, triple-negative breast cancer (TNBC) can lead develop drug resistance, resulting in poor therapeutic effects. The synergistic effect of radiotherapy and immunotherapy can not only improve the therapeutic effect of in situ TNBC but also induce the strong abscopal effect of radiotherapy to remove the tumor cells from the metastatic tumor and prevent tumor recurrence. However, many preclinical studies have pointed out that the application of radioimmunotherapy still has such problems as large toxic and side effects of drugs and asynchronous administration scheme. With the increasing popularity of nanobiotechnology in tumor therapy, there are more and more research results related to nanoparticles, such as inorganic nanoparticles, organic nanoparticles, and nanoparticle–hydrogel complexes in radioimmunotherapy. In this review, we briefly summarize the related clinical treatment methods of TNBC and review the related nanobiotechnology used in the preclinical studies of TNBC radioimmunotherapy. It is mainly characterized by the controlled release of drugs locally, enhanced radiosensitization, and avoidance of systemic toxicity. However, further studies are needed to improve the convenience and effectiveness, so as to explore new options for the clinical treatment of TNBC.
1 INTRODUCTION
Breast cancer has become the most common and greatest health threat to women worldwide. It is mainly divided into three categories: luminol, human epidermal growth factor receptor-2 (Her-2) overexpression, and triple-negative breast cancer (TNBC).1 According to the National Cancer Institute, breast cancer accounts for 15% of all new cancer cases and usually affects women aged 65–74.2 As the most dangerous subtype of breast cancer, TNBC accounts for about 10%–20% of the diagnosed breast cancer. Its lack of simultaneous expression of estrogen (ER), progesterone (PR), and Her-2 receptors leads to its lack of specific therapeutic drug targets.3 Due to the highly malignant nature of TNBC, it can lead to drug resistance, tumor recurrence and metastasis, thus reducing the 5-year survival rate and quality of life of patients with poor prognosis.4-6 At present, due to the shortcomings of the low efficiency of antitumor drugs and large damage to normal tissues, the antitumor effect of the clinically adopted treatment scheme is not ideal.7 Clinically, surgery is still the main intervention form of comprehensive treatment for breast cancer.8, 9 However, the high local recurrence rate of breast cancer is still a clinically fatal problem. In addition to these traditional therapeutic strategies, many studies continue to propose new clinical treatments for TNBC.8, 10
Related studies have shown that radiotherapy combined with immunotherapy can improve innate and adaptive immunity to eliminate tumors in situ and at metastatic sites, induce the body to establish long-term immune memory effect, and prevent tumor recurrence, thus becoming a new strategy to improve the survival rate of patients with TNBC.11-15 In Phase 2 clinical study, local tumors were better controlled with TNBC radiotherapy combined with immunotherapy, but the clinical incidence of systemic tumor rejection was extremely low.16-19 However, the potential toxicity between radioimmunotherapy remains a major challenge in stimulating optimal synergy.20-22 Moreover, since the tumor microenvironment is often in a state of hypoxia, it will increase the radiation resistance of tumor cells and affect the effect of radiotherapy.23, 24
In recent years, its ability to accurately regulate antitumor immunity has attracted increasing attention due to the exploration of nanobiotechnology in tumor immunotherapy.25 The development of nanobiotechnology provides effective solutions for the delivery and precise regulation of tumor immunotherapy, especially in drug delivery, greatly reducing systemic toxicity and the incidence of adverse immune events.26, 27 As a delivery carrier, nanoparticles (NPs) have a series of advantages over immunotherapeutic agents in the delivery process, including prolonging cycle time, targeted delivery, promotion of uptake and presentation, controlled release, and multiple drug delivery methods.28-31 NPs are used as carriers for drug delivery and controlled release so that the maximum concentration of immunotherapeutic drugs can be gathered at the tumor location in TNBC radioimmunotherapy.25, 32, 33 The NPs with special radiosensitization effect not only can enhance the radiotherapy effect but also combine with the immunotherapy of TNBC to induce the antitumor immune response of the body, which can eliminate the residual or metastatic tumor cells and prevent tumor recurrence.34-39
This paper reviews the relevant nanobiotechnologies used in preclinical studies of TNBC radioimmunotherapy, to provide a better clinical treatment method for patients with TNBC. In this paper, the existing clinical treatment options for patients with TNBC are summarized firstly, the superior development prospect of radioimmunotherapy in TNBC treatment is highlighted, and the specific mechanism, as well as the advantages and disadvantages in the current application, are analyzed. Then, we focus on the description of nanobiotechnology-mediated radioimmunotherapy, which is mainly applied to the local controlled release of immunotherapeutic drugs in tumors, enhancement of radiosensitization, and avoidance of systemic toxic reactions. However, further studies are needed to improve the convenience and effectiveness, so as to explore new options for the clinical treatment of TNBC.
2 CLINICAL TREATMENTS FOR TNBC
Compared with other types of breast cancer, TNBC has limited therapeutic regimens and is prone to relapse and metastasis with a poor prognosis.9 Since the expressions of ER, PR, and HER2 are negative, it is generally considered that TNBC has no intracellular estrogen signal transduction, and is insensitive to endocrine therapy.40, 41 Clinical treatment methods for breast cancer mainly include: surgical resection, chemotherapy, radiotherapy, immunotherapy, and other target treatments (Table 1).
| Therapy methods | Scheme selection | Side effect | Applicable conditions |
|---|---|---|---|
| Surgery | Standard treatments | Incomplete surgical resection and wound infection | Early and middle period |
| Chemotherapy | Standard treatments | Drug toxicity and drug resistance | Surgical adjuvant therapy and neoadjuvant therapy |
| Radiotherapy | Adjuvant treatments | Radiation injury and second tumor | Surgical adjuvant therapy and neoadjuvant therapy, palliative care |
| Immunotherapy | Novel clinical treatments (PD-1/PD-L1 and CDLA-4) | irAEs and drug resistance | Chemotherapy drug resistance and metastatic stage |
| Other treatments | Novel clinical treatments (PARPi and ADCs) | Unsure therapeutic index and safety profile | Chemotherapy drug resistance and metastatic stage |
- Abbreviations: ADC, antibody–drug conjugates; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; irAEs, immune-related adverse events; PARP, poly(ADP-ribose) polymerase inhibitor; PD-1, programmed death 1; PD-L1, programmed death-ligand 1; TNBC, triple-negative breast cancer.
2.1 Surgery
The surgery includes breast-conserving surgery, mastectomy, and peripheral lymph node dissection.42 Due to the increasingly younger age of patients with TNBC tumors, breast-conserving surgery and modified radical surgery are currently the most commonly used clinically.1 Breast-conserving surgery maximizes breast preservation while eliminating lesions with less surgical trauma than modified radical mastectomy. However, no matter which surgical approach is adopted, there is the problem of incomplete tumor cell resection, which leads to tumor recurrence. To completely remove the residual cancer cells and reduce the postoperative recurrence rate, it is generally recommended that the patients undergo preoperative neoadjuvant therapy or postoperative adjuvant therapy.41
2.2 Chemotherapy
Chemotherapy generally works by affecting the life cycle of cells, which can inhibit the abnormal proliferation of tumor cells and kill tumor cells. As a neoadjuvant therapy before surgery, chemotherapy can reduce the tumor size and improve the surgical resection effect. As an adjuvant therapy after surgery, chemotherapy can eliminate residual tumor cells and prevent a recurrence.41 In recent years, the research focus has been on the selection of chemotherapeutic drugs, such as anthracyclines, paclitaxel, ixabepilone, and platinum drugs.9 In some clinical studies, bevacizumab has been combined with chemotherapy drugs to treat TNBC disease, but the survival time of patients has not been significantly prolonged.8 On the other hand, paclitaxel, as a first-line chemotherapy drug for TNBC, has no targeted distribution effect after systemic administration, resulting in a low concentration of the drug in the tumor tissue and easy to cause adverse reactions.40 However, TNBC is prone to develop drug resistance to a variety of chemotherapeutic drugs, and after drug resistance, treatment options are small, resulting in a poor clinical prognosis. The residual metastatic lesions will eventually lead to tumor recurrence.43 In addition, any cytotoxic drug may cause serious adverse reactions, including bone marrow suppression, gastrointestinal reactions, or involving vital organs.8
2.3 Radiotherapy
Radiotherapy is recommended as adjuvant therapy for patients with TNBC after operation to improve the prognosis and life quality.44 Radiotherapy is divided into external ionizing radiotherapy and internal radioisotope radiotherapy. External radiation ionizing radiotherapy is widely used in clinics, while internal radioisotope radiotherapy has received few clinical studies.37 Radiation can not only lead to the death of tumor cells but also change the tumor environment into an “inflammatory” environment so that the tumor microenvironment is conducive to the recruitment of effector T cells.45 Radiation therapy after TNBC surgery helps to reduce the risk of tumor recurrence in breast tissues and their adjacent lymph nodes and reduces mortality.46-48
However, radiotherapy has a limited role in the clinical treatment of TNBC.49 First, high-dose radiation is usually required to effectively remove cancer cells, which inevitably damages the surrounding normal tissues.37 The common and painful side effects of radiation therapy are dermatitis, lymphedema, leukopenia, and even secondary tumor initiation.38, 50 These reactions were graded sequentially according to severity, ranging from dry or erythematous and dry desquamation to more severe wet desquamation and ulceration.51 Second, due to the rapid progress of tumor cells and the distortion of blood vessels in the tumor, the tumor microenvironment is in a state of hypoxia for a long time, and this hypoxia phenomenon of solid tumors will lead to the tolerance of the tumor to radiotherapy.49
2.4 Immunotherapy
To escape from various immune monitoring and clearance in vivo, tumor cells usually produce a variety of molecular and cellular mechanisms.52, 53 Tumor immunotherapy regimens include immune checkpoint blockers (ICBs), therapeutic antibodies, cancer vaccines, cell therapy, and small molecule inhibitors. Immunotherapy does not directly target and kill tumor cells, but uses immune-related small molecular drugs or immune cells to enhance the recognition and removal ability of the autoimmune system, and antagonize the immune inhibition-related molecular proteins on the surface of tumor cells to achieve the purpose of removing tumor cells.54 TNBC has a lower gene mutation load and weaker immune function compared with other breast cancer cell subtypes, thus many patients with TNBC are resistant to immunotherapy. In addition, the tumor microenvironment of TNBC is also characterized by immunosuppression, evasion of immune detection and drug resistance.52, 55 The fastest-developing clinical research focus in tumor immunotherapy is the inhibitor of programmed cell death protein 1 and its ligand (PD-1/PD-L1). According to surveys, PD-L1 was highly expressed in 51% of patients with TNBC, PD-1 was highly expressed in 70% of patients, and PD-L1 and PD-1 were highly expressed in 45% of patients.56-58 PD-L1 was found to be highly expressed in TNBC tumor tissues and to inhibit the activation of tumor-infiltrating CD8+ T cells.59 PD-L1 inhibitors can specifically bind to PD-L1 on tumor cells to inhibit its expression so that T cells with inhibited functions can recover their recognition function on tumor cells. Clinically, PD-L1 inhibitor combined with albumin-paclitaxel can be used as the first-line drug to treat unresectable, locally advanced or metastatic TNBC.12, 60, 61
However, systemic administration is easily accompanied by severe side effects, leading to the occurrence of immune-related adverse events (irAEs).62, 63 irAEs affect almost all organs or tissues and the exact mechanism of irAEs is unknown. Skin toxicity occurs in one-third of patients, but most of them are mild, with more maculopapules and pruritus.64 Rare irAEs, including neurotoxicity and cardiovascular toxicity, highlight the potentially life-threatening nature of irAEs.17 Second, there are many complex interactions between molecular and physical mechanisms in the tumor microenvironment, and immunotherapy drugs have limited responses and are prone to drug resistance. What is more, the delivery characteristics of immune drugs themselves are usually poor, which limits the penetration of systemic administration.13, 65-67
2.5 Other treatments
At present, there are also clinical studies on new targeted drugs against TNBC, including poly (ADP-ribose) polymerase (PARP) inhibitors, antibody–drug conjugates (ADC), which are revolutionizing the therapeutic landscape and providing new opportunities for early-stage patients with TNBC.68 PARPs are a family of related enzymes that contribute to the recognition of DNA single-strand breaks and facilitate DNA repair to maintain genomic stability.69 There is evidence to support the possibility of synergy between PARP inhibitors and DNA damage chemotherapy. However, the toxicity of this combination will limit clinical administration.70 ADCs are a new treatment modality with increasing applications in advanced-stage breast cancer.71 ADC consists of recombinant monoclonal antibodies covalently bound to a cytotoxic agent via a synthetic linker. Preclinical evidence also suggests that ADCs alter inflammatory TME effects by inducing immunogenic cell death (ICD), favor the infiltration of cytotoxic T cells and antigen-presenting cells, and exhibit synergistic antitumor activity when coadministered with ICIs.71, 72 PARPi and ADCs hold great promise for the treatment of patients with TNBC. However, more research is still needed to improve the therapeutic index and safety profile of anticancer agents.
3 RADIOIMMUNOTHERAPY FOR TNBC
Radioimmunotherapy is mainly used for the treatment of tumors with distant metastasis and large systemic toxicity, which seriously affects the survival rate.73 The heterogeneity of TNBC disease greatly affects individual treatment choices, so there is an urgent need to develop new treatment options.74 A large number of preclinical and clinical studies have shown that the combination of immunotherapy and radiotherapy may be a promising strategy for synergistic efficacy enhancement.18 Chang et al.75 significantly enhanced the ICB effect and improved immune regulation by implementing the triple therapy of radiotherapy, in situ tumor vaccination, and phosphoinositide 3-kinase δ (PI3Kδ) inhibition immune tolerance on the 4T1 mice model. We propose the following scheme that postoperative local radiotherapy combined with immunotherapy could be performed for TNBC that met the indications of early and middle stages of surgical resection, and local radiotherapy combined with immunotherapy is more appropriate for TNBC patients with distant metastasis.76
3.1 Mechanism of radioimmunotherapy
The tumor antigens caused by radiation can potentially provide tumor antigen stimulation and induce an antitumor-specific immune response.77, 78 However, the limited ability to activate immune response prevents them from causing a systemic immune response.79 An interesting phenomenon has been found in the process of local tumor radiotherapy: abscopal effect (Figure 1).80-82 Radiotherapy can induce different forms of tumor cell death, thereby releasing dangerous signals such as proinflammatory cytokines, chemokines, and tumor antigens, and producing a large number of new tumor antigens that are presented to T-lymphocytes.83 Immunotherapy drugs or immune cells can strongly stimulate the immune response system and synergistically stimulate the abscess formation effect, thereby realizing local destruction of tumor cells and targeted elimination of distant metastasis.84, 85 For example, PD-1/PD-L1 can enable T cells with inhibitory function to recover their recognition function for tumor cells so that they can exert effects on antitumor through the autoimmune system and the immune memory cells produced can also prevent the recurrence of the tumor. In view of the clinical success of ICBs in early and metastatic breast cancer, the combination of radiotherapy and immunotherapy to enhance local and systemic immune response has aroused great interest.86
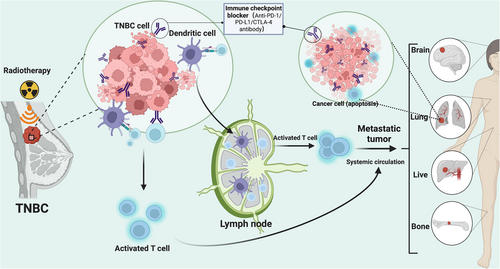
3.2 Application of radioimmunotherapy in TNBC
The combination of local external radiotherapy and immunotherapy can improve the function of dendritic cells, enhance the activation of T cells, and increase the sensitivity of tumor cells to radiotherapy by increasing the release of tumor antigens and cross-presentation of antigen-presenting cells.87-89 Oba's team differentiated induced pluripotent stem cells (iPSCs) into iPSC-derived DCs (iPSC-DC) in mice and tested the antitumor effects of intratumoral injection of iPSC-DCs and radiotherapy in a homologous in situ mice tumor model resistant to treatment with PD-L1. The results showed that iPSC-DCs combined with radiotherapy promoted the initiation of CD8+ T cells, synergistically delayed the growth of treated tumors and distant nonirradiated tumors, caused tumors with poor immunogenicity to respond to PD-L1 therapy, and produced tumor-specific immune memory.90 In Phase 2 clinical trial evaluating the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic TNBC, the HO's team set the treatment at a radiotherapy dose of 3 Gy administered in five daily doses. Pembrolizumab was intravenously administered at a dose of 200 mg for 3 days during the first radiotherapy segment and every 3 weeks 3 days until disease progression. The primary study endpoint was the elimination of nonradiotherapy site tumors at Week 13. The results showed that of the 17 enrolled female breast cancer patients, nine achieved the study endpoint, three achieved partial response, and one was in a stable condition. The results of this clinical study undoubtedly provide new and powerful clinical evidence for TNBC immunotherapy combined with radiotherapy.13
Radioisotope radiotherapy means that the radioisotope is placed into or near the tumor in vivo and the radiation energy of it is mainly derived from radioisotope molecules, such as 131I,111In.91 However, radioisotopes are not targeted so that the systemic administration of radioisotopes may have toxic effects on normal tissues and organs of the body. The development of novel radioimmunotherapy conjugates is relevant to the era of personalized medicine, especially in the context of multimodal treatment strategies for poorly prognostic cancers that are resistant to conventional therapy.92, 93 Kelly et al.94 developed a stable radiolabeling method for the production of 111In-DOTA-hTAB004 and 225Ac-DOTA-hTAB004 with high radiolabeling efficiency, purity, and stability, and in vivo treatment results showed tumor regression and significantly increased survival in treated mice compared to control group.
3.3 Side effects of radioimmunotherapy
Radiotherapy combined with immunotherapy has considerable potential for clinical application, but its limitations and side effects cannot be ignored.73 Since immunotherapy alters the balance between immunity and immune tolerance, the increased likelihood of tumor recognition is accompanied by a higher immune response rate in normal tissues. In a Phase 2 trial of radioimmunotherapy for metastatic TNBC,95 28 patients were enrolled and treated. Clinical benefit rate was seen in six patients (21.4%), and the median overall survival increased by more than 2-fold in patients with clinical benefit. In terms of safety,treatment-related adverse events (AEs) of any cause were reported in 22 patients (78.6%). The most common AEs were any-grade constitutional (35.7%), hypothyroidism (21.4%), diarrhea (14.3%), nausea (10.7%), dyspnea on exertion (10.7%), alkaline phosphatase elevation (10.7%), and hyperthyroidism (10.7%). Grade 3 or higher treatment-related AEs were reported in 10 patients (35.7%). Immune-related AEs at any grade occurred in 13 patients (46.4%). The most common immune-related AEs were hypothyroidism (21.4%) and hyperthyroidism (10.7%). Grade 3 or higher immune-related AEs were reported in two patients (7.1%), including one pneumonitis (3.6%), and one transaminitis (3.6%). Two patients (7.1%) discontinued treatment due to Grade 3 pneumonitis and Grade 2 transaminitis. What is more, some nontumor-specific antigens may activate self-reactive T cells and will likely attack and damage normal tissues. To solve these problems, locally controllable target therapy is necessary for the local release and control of drug or radioisotope molecules.96
In recent years, the exploration of nanobiotechnology in tumor immunotherapy has attracted increasing attention, especially since it can greatly reduce the systemic toxicity and the incidence of irAEs. As a delivery carrier, NPs have lots of advantages over immunotherapy drugs, including prolonging cycle time, targeted delivery, promotion of uptake and presentation, controlled release, and multidrug delivery platform.28 Controlled release can prolong the action time of the drug without degradation, and it is more beneficial to promote its absorption and utilization.33 In addition, the nanomaterials with the ability of radiosensitization can enhance the radiotherapy effect. The NPs can combine with immunotherapy while performing radiotherapy, thereby inducing the body to produce an antitumor immune response to eliminate residual or metastatic TNBC tumor cells and generate immune memory to prevent tumor recurrence.34
4 NANOBIOTECHNOLOGY FOR TNBC RADIOIMMUNOTHERAPY TREATMENT
At present, the research and application of nanobiotechnology in the field of health care have developed rapidly, especially the application of NPs in tumor radioimmunotherapy.25 The development of nanobiotechnology provides effective solutions for the delivery and precise regulation of tumor immunotherapy, especially in greatly reducing systemic toxicity and the incidence of irAEs (Table 2).26, 27
| Cell line | Radioimmunotherapy | Types of nanoparticles | Effect | Ref. |
|---|---|---|---|---|
| MDA-MB-231 cells | 15 Gy dose at 160 kV using an x-ray source and abscopal effect | Gold nanoparticles (AuNPs) | Effect of AuNPs enhanced radiotherapy on survival and AuNP-driven events in TNBC that may be relevant in developing multimodal radiotherapies that may include radiosensitizers as well as immunotherapy. | [39] |
| GL261 tumor | 2 Gy x-ray dose and α-PD-1 | CpG@ Au NPs | When combined with an anti-programmed death 1 antibody, irradiated CpG@Au led to consistent abscopal responses that efficiently suppressed distant tumors in a bilateral GL261 tumor-bearing model. | [97] |
| 4T1 cells | Radiotherapy (3 × 8 Gy) and anti-PD-1 and anti-CTLA-4 antibodies | MION | Single-fraction HT added to radiotherapy + immunotherapy improves local tumor control via enhanced cell kill/cytoreduction and recruitment of CD3+ T-lymphocytes, with a modest potential to reduce lung metastases. | [98] |
| MDA-MB-231 cells | 5 Gy radiation and NK cells | Selenium-containing nanoparticles | The enhanced antitumor effect of PSeR/DOX NPs/Radiotherapy in vivo was the coaction of radiotherapy, radiation-facilitative chemotherapy, and seleninic acid-mediated immunotherapy | [99] |
| 4T1 cells | X-ray (4 Gy) and abscopal effect | Hybrid nanoplatform (MGTe) | MGTe was able to enhance the radiotherapy and further improve the antitumor immunological effects, indicating the satisfactory synergistic effect of MGTe between radiotherapy and immunotherapy for tumor suppression. | [100] |
| 4T1 cells | X-ray radiation and anti-CTLA-4 | Liposomes | This work promotes tumor oxygenation via sequential delivering catalase and exogenous H2O2 into tumors using well-established liposomal carriers, showing great potential for clinical translation in radioimmunotherapy of cancer. | [101] |
| MDA-MB-231 cells | 8 Gy x-ray dose and abscopal effect | Liposomes (M/LM-liposome) | Radiotherapy induces potent ICD in tumors pretreated with M/LM-liposome, which completely ablates the local tumors and meanwhile induces a systematic antitumor immune response, which can result in potent abscopal effect for complete elimination of metastasis and prolonged survival time of mice. | [102] |
| 4T1 cells | 131I and anti-CTLA-4 | 131I-Cat-containing alginate hydrogels | The 131I-Cat/CpG-containing hydrogel showed marked antitumor efficacy and the ability to eliminate distant metastatic tumors, eliciting long-term immune memory against tumor recurrence when combined with the systemic administration of anti-CTLA-4. | [103] |
- Abbreviations: Au NP, gold nanoparticles; Cat, catalase; CpG, cytosine-phosphate-guanine; CpG@Au NP, CpG-decorated Au nanoparticles; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; DOX, doxorubicin; ICD, immunogenic cell death; IT, immunotherapy; MGTe, glutathione decorated Te nanoparticles with tumor cell membranes and bacterial outer membranes; MION, magnetic iron oxide nanoparticle; M/LM-liposome, mannose and levamisole hydrochloride; NK, natural killer; NPs, nanoparticles; PD1, programmed death 1; PSeR, seleninic acid polymer; Rad, radiotherapy; TNBC, triple-negative breast cancer.
4.1 Inorganic NPs used for TNBC radioimmunotherapy
4.1.1 Properties and function of inorganic NPs
The main composition of inorganic NPs is inorganic substances, which can be divided into metal NPs, inorganic oxide NPs, inorganic semiconductor NPs, and so on. Metal NPs, mesoporous silicon dioxide, and carbon NPs applied in biomedical research are all inorganic NPs.104 Inorganic NPs have good application prospects in targeted drug administration and controlled release of drugs when used as drug delivery carriers.105, 106 Based on multifunctional metal NPs, researchers are committed to developing NPs with high-Z metal elements as radiosensitizers to improve the efficacy of radiotherapy by applying more energy to tumor location.107 Most of the metal NPs with radiation sensitization are high atomic number elements, such as gold, platinum, barium, and bismuth.108 Different metal NPs utilize different molecular mechanisms, such as the generation of intracellular reactive oxygen species (ROS), which can increase oxidative stress and specific apoptotic tumor cell death.25 Metal NPs mediated radiotherapy is highly dependent on O2 and ROS at the radiotherapy site to kill tumor cells. However, the microenvironment of TNBC tumors is in a state of hypoxia, which seriously affects the antitumor effects of the NPs combined with radiotherapy.
4.1.2 Mechanism of inorganic NPs applied for TNBC radioimmunotherapy
Metal NPs are used in a variety of cancer treatment studies and may offer numerous opportunities for therapeutic and diagnostic analysis due to their magnetic, optical, thermal, and electrical properties.104 They are introduced into tumor tissues and irradiated with high-energy rays due to its high mass energy absorption coefficient for x-rays. After absorbing the rays, the NPs will produce various effects, such as the photoelectric effect and Compton effect. Then, it will release various particles, such as photoelectrons, Compton electrons, and Auger electrons, which can react with organic molecules or water in tumors to generate a large number of free radicals and improve the radiation treatment effect.109, 110 On the one hand, electrons can be taken out from cell target molecule DNA for oxidation to prevent the electrons from being absorbed again, which can lead to potential chemically lethal damage to tumor cells. On the other hand, it can also inhibit sulfhydryl compounds such as glutathione (GSH) in cells, which can increase cell sensitivity to radiation and improve the radiotherapy effect. In this way, the toxic and side effects on adjacent normal tissues can be significantly reduced while increasing tumor radiation damage. Some metal NPs containing high atomic number metal elements can not only promote cell damage effect through photoelectric interactions but also load a plurality of drugs to selectively kill tumor cells, which can improve the radiation sensitivity of cells. At the same time, it can achieve the synergistic treatment of other treatment options and radiotherapy to overcome the defects of a single radiotherapy.111
4.1.3 Application of inorganic NPs in TNBC radioimmunotherapy
The original intention of tumor radiotherapy is to kill as many tumor cells as possible while minimizing damage to normal tissues around the tumor. However, its damage to normal tissues cannot be ignored due to the nonspecificity of radiation, which limits the application of radiotherapy in clinical treatment due to side effects. As a new type of research hotspot NP, gold NPs (Au NPs) have the characteristics of high concentration, small size, good dispersibility, and excellent colloidal stability. What is more, it has ideal biocompatibility and low toxicity in vivo and has been applied to clinical trials of many tumors. Au NPs combined with radiotherapy can increase the mortality of tumor cells.39 Combined with the characteristics of targeted drugs, a large number of experiments have confirmed that the radiosensitization effect of Au NPs is far superior to other radiosensitizers.35 Radiosensitive Au NPs were used as nanocarriers for cytosine-phosphate-guanine oligo-deoxynucleotides (CpG ODNs) in the study of Cao et al.97 CpG-decorated Au NPs (CpG@Au NPs) were injected into the primary tumor as planned, and α-PD-1 was administered by intraperitoneal injection followed by 2 Gy radiotherapy 24 h after injection, while that distal tumor did not undergo related treatment. The results showed that CpG@Au NPs could efficiently reconstruct M2-type tumor-associated macrophages into M1 phenotype, increase the immunosuppressive microenvironment, and improve the efficacy of radiotherapy in vivo. The CpG@Au NP-mediated immune regulation by macrophages activates innate immunity, which could coordinate adaptive immunity under x-ray irradiation, and had an excellent antitumor effect on both primary and distant tumors when combined with α-PD-1 (Figure 2).
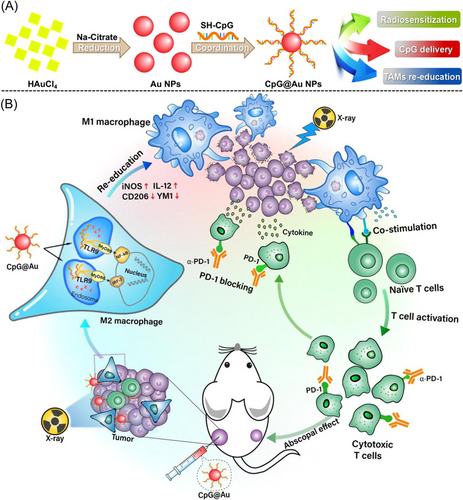
The radiosensitization of Au NPs strongly depends on tumor targeting. Kefayat et al.112 modified the common targeting drugs to ovine serum albumin Au NPs (BSA-Au NPs) and determined the optimal targeting modification drug according to the tumor targeting and biological distribution. The study found that glutamine and folic acid-modified BSA-Au NPs had significant tumor targeting and radiosensitization in 4T1 breast tumor-bearing mice. All the NPs showed biocompatibility, which could significantly improve tumor targeting and therapeutic effect. In addition to binding to tumor-targeting drugs, the Au NPs can be used in combination with other sensitizers to enhance the efficacy of radiotherapy. Hybrid anisotropic nanostructures composed of Au and TiO2, also known as dumbbell-shaped Au-TiO2 NPs, were developed by Cheng's team. The NPs showed a synergistic therapeutic effect in the radiotherapy of the TNBC tumor model because of the high atomic number of metal, dielectric oxide had strong asymmetric electrical coupling at the interface, and a large amount of ROS could be generated to induce cancer cell apoptosis finally so that tumor growth was significantly inhibited.113 Other metal ions have also been investigated for related applications, such as copper ions and zinc particles. Wang et al.114 constructed a mixed-valence (Cu+/Cu2+) Cu-based nanoscale coordination polymers. The polymer simultaneously and independently induced Cu+-triggered hydroxyl radical production and Cu2+ triggered GSH elimination, which could synergistically produce a potent ICD induction effect with radiation therapy. The polymer can be used as a radiosensitizer to enhance the effects of radioimmunotherapy at the same time and has important significance for radioimmunotherapy of TNBC primary tumors and metastatic tumors.
4.2 Organic NPs used for TNBC radioimmunotherapy
4.2.1 Properties and functions of organic NPs
Organic NPs are based on lipids, proteins, polysaccharides, and organic high-molecular polymers. The application of organic nanomaterials in biomedicine has also been developed rapidly. In particular, the research on lipid NPs is increasing and it has been widely used in recent decades as a carrier of drugs.105 Liposome is usually spherical, with a size of 25–1000 nm, and can efficiently load hydrophilic or hydrophobic drugs, which can protect the drugs from external environment destruction. At the same time, the characteristics of easy modification and functionalization enabled it to identify specific targets, further applying liposomes to targeted drug delivery systems. Liposome has a cell-like structure, thus it is mainly swallowed by the reticuloendothelial system, thus activating the autoimmune function of the body.115 Liposomes are widely used as the carrier of anticancer drugs, especially based on the following principles: targeting of antitumor drugs, selectivity of drugs released, and retention of drugs in target locations.116 Liposomes can delay the release of drugs in the body and improve the efficacy without increasing toxicity.117 Liposome is often used for tumor chemotherapy, such as doxorubicin liposome and cisplatin liposome. What is more, liposome also has cell affinity and tissue compatibility. Liposomes have no damage to or inhibition of normal cells and tissues and can be adsorbed on the periphery of target cells for a long time so that drugs can enter the target cells, or fuse in cells and be digested and released by lysosomes.115 A variety of lipid-based NP delivery systems have been developed, including liposomes, nanomicelles, nanoemulsions, solid lipid NPs, lipid drug conjugates NPs, and so on.118
4.2.2 Mechanism of organic NPs used for TNBC radioimmunotherapy
Lipid-based NP delivery platforms can overcome the barriers of tissue and target tumors while prolonging therapeutic plasma drug concentrations and improving the bioavailability of multiple antitumor drugs.118 Liposome preparations have been applied in the clinical treatment of tumors, especially in chemotherapy with the approval of the Food Drug Administration. Liposomes are usually used as drug carriers to protect drugs from degradation and target tumor sites. The release of tumor-associated antigens (TAAs) and its cross-presentation in tumor-infiltrating dendritic cells directly affect the activation of cytotoxic T cells in radioimmunotherapy.119 However, the production and release of TAAs after local radiotherapy in tumors are limited, and TAAs partially internalized by dendritic cells are degraded in lysosomes. The liposome-coated radiosensitizer not only enhances the radiotherapy reaction of tumor cells but also generates and releases enough TAAs to induce the activation of immune cells. The abscopal effect by synergistic effect with immunotherapy can improve the efficacy of radiotherapy and reduce the damage to human health tissues.120 The liposome can also be used as a carrier of radioisotopes to target and transport them to tumor positions. In addition, liposomes are also a good choice for carrying immunotherapy agents.36 For example, PD-1, PD-L1, and CpG can be used in tumor immunotherapy, and the synergistic effect with liposomes can significantly improve the therapeutic effect.
4.2.3 Application of organic NPs in TNBC radioimmunotherapy
Song's team innovatively proposed a strategy to alleviate tumor hypoxia by delivering exogenous H2O2 into the tumor and then catalase (Cat)-induced decomposition of H2O2. The essence of this strategy is to utilize the drug delivery function of liposomes and inject an additional dose of H2O2@liposome 4 h after the intravenous preinjection of Cat@liposome (Figure 3). The continuously released H2O2 can be decomposed by Cat@liposome, so as to have a lasting effect on enhancing the oxygenation in the tumor environment. Radiation of 8 Gy was administered after 24 h of injection, and combined radiotherapy with anti-CTLA-4 immunotherapy. In marked contrast, for mice treated with Liposome@Cat plus Liposome@H2O2, to promote their tumor oxygenation, obviously enhanced efficacy was achieved in the radioimmunotherapy with x-ray plus anti-CTLA-4, compared to that achieved by conventional radioimmunotherapy.101
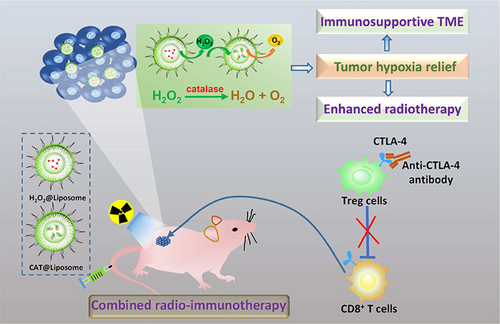
In addition, relevant research has targeted the design of a liposome-based drug delivery system, which can provide an effective immune induction system for immunotherapy.121, 122 Intravenous formulations of R848 (toll-like receptor 7 and 8 agonists) were developed by Zhang et al.123 using thermosensitive liposomes as a delivery vehicle. Topical administration of R848 demonstrated superior efficacy when coadministered with αPD-1. Eight of 11 breast cancer-bearing mice achieved complete exon deletion line tumor regression within 100 days and improved survival at the treatment. Munakata et al.124 used immunotherapeutic liposome to form spherical nucleic acid in a TNBC-targeted immunotherapy regimen, which contained immunostimulating oligonucleotide as an adjuvant and encapsulated lysate from a TNBC cell line as an antigen. It showed that it significantly increased the number of cytotoxic CD8+ T cells and decreased the number of myeloid suppressor cells in the TME during the treatment of the TNBC mice model.
4.3 NP hydrogel applied for TNBC radioimmunotherapy
4.3.1 Properties and functions of NP hydrogel
Nanobiotechnology offers a number of advantages for drug delivery, including high loading yields, combination therapy, controlled release, extended cycle, and targeted delivery.125, 126 NP-hydrogel drug delivery systems can improve the therapeutic index of drugs by changing the pharmacokinetics and biological distribution characteristics of drugs, so it is more conducive to the precise regulation of drugs in tumor sites and the spatial distribution of drug molecules.127 With the development of nanobiotechnology, therapeutic NP have been increasingly combined with other biological materials, especially the loading of NPs into hydrogels, which has attracted extensive attention.128, 129 Hydrogel is a polymeric material with excellent biocompatibility, safety, low cost, and low immunogenicity. It can be synthesized from a variety of natural materials, such as hyaluronic acid, fibrin, chitosan, alginate (ALG), and gelatin. Hydrogel has a multilayer, thin-walled, and porous structure, which can carry a variety of drugs or NPs to high local aggregation in tumor position and realize the controlled release in time and space.66
The main mechanisms of controlled release of the hydrogel can be divided into three categories: hydrogel swelling, passive diffusion out of hydrogel along the concentration gradient, and hydrogel degradation. Due to the biodegradability of hydrogel, NPs and drugs are typically released by passive diffusion and hydrogel degradation.130-132 With the appropriate composition, the hydrogel not only maintains the structural integrity and functionality of the contained NPs but also provides additional engineering flexibility to improve overall therapeutic efficacy.133 The NPs can be mixed with a liquid hydrogel solution and then injected into the tumor position.134 Finally, it is embedded into a controlled release platform of a hydrogel drug delivery system under the changeable conditions of temperature, pH value, ion exchange, and so on.135, 136 Injectable hydrogel has more advantages in continuous drug administration and drug action time extension. It can target local drug administration to achieve the therapeutic effect at a lower drug concentration, which is used for reducing the toxicity and side effects of patients, and improving the comfort, convenience, and compliance of patients.137, 138
4.3.2 Mechanism of NP hydrogel applied for TNBC radioimmunotherapy
Radiotherapy can induce different forms of tumor cell death, which can release dangerous signals such as proinflammatory cytokines, chemokines, and tumor antigens. Those signals can trigger local and systemic immune responses by producing a large number of new tumor antigens that are presented to T lymphocytes.83 NP hydrogel can target immunotherapy drugs to tumor areas and achieve controlled release of drugs in time or space by exogenous or endogenous stimulation.62, 103 At the same time, the hydrogel designed with special materials can also act as a radiosensitizer to increase the sensitivity of tumor cells to radiation. In addition, when radioisotope therapy is combined with immunotherapy, the radioisotope can produce significant toxic effects on normal tissues in vivo. Hydrogel is a local drug delivery platform for a combination of radiotherapy and immunotherapy, which greatly reduces side effects. The injectable hydrogel was used as the drug delivery platform to directly implant isotope molecules into the tumor, which can avoid damage to normal tissues and organs. The hydrogel can also promote tumor immunity by capturing and transferring tumor antigens released during radiotherapy to antigen-presenting cells.73, 80
4.3.3 Application of NP hydrogel in TNBC radioimmunotherapy
Chen et al.139 found that the properties, degradation rate, and drug release kinetics of hydrogel can be adjusted by simply changing the gel mixing ratio. The hydrogel matrix prepared according to the specific ratio not only avoided the initial burst release but also achieved the sustained release of specific drugs for up to 80 days in vitro. In the research of Kim's team, to overcome the backflow difficulty of injectable hydrogel during injection and the immediate uncontrollable diffusion of toxic therapeutic drugs, a temperature-sensitive hydrogel was developed as an injectable carrier for the delivery of therapeutic agent microspheres. The gel exhibited two states: aqueous fluid at low temperature and polymeric gel at body temperature (37°C). The solution gelatinized almost instantaneously at body temperature to prevent the microspheres from flowing back and being released immediately during the injection.140
Sodium ALG is a water-soluble natural polysaccharide material with biocompatibility, which is crosslinked with multivalent cations to form a hydrogel. Chao et al.141 prepared 131I-Cat/CpG/ALG injectable liquid-phase hydrogel using sodium ALG, which included the radioisotope 131I, CAT, CpG oligonucleotide immunoadjuvant, and ALG. After the liquid hydrogel was implanted into the tumor site by using the syringe, the liquid gel quickly became a solid hydrogel platform to control the release of the loaded molecules in the presence of endogenous Ca2+. 131I radioisotope would radiate energy at the tumor site to cause DNA damage of tumor cells, while CAT was able to decompose hydrogen peroxide into oxygen. Thus, it was able to increase the hypoxia environment in the tumor microenvironment for a long time, and enhance the effect of radiotherapy to promote the death of tumor cells. The CpG oligonucleotides would activate toll-like receptor 9 expressed in congenital immune cells, so as to produce a strong proinflammatory immune response and increase the activities of cytotoxic CD8+ T cells and mononuclear macrophages in tumors and circulation, which enhanced the abscopal effect of radiotherapy and remove metastatic tumor cells.98 For mice treated with either 131I-Cat/ALG-based radioisotope radiotherapy or surgery, rapid tumor progressions were observed after they were rechallenged with a secondary tumor 40 days later. In marked contrast, there was no visible secondary tumor growth in mice after their initial tumor was eliminated by 131I-Cat/CpG/ALG treatment. The result showed that 131I-Cat/CpG/ALG therapy triggered a systemic antitumor immune response that was not only suitable for the clearance of solid tumors but also suitable for the clearance of metastatic tumors and the prevention of tumor recurrence (Figure 4).49, 103
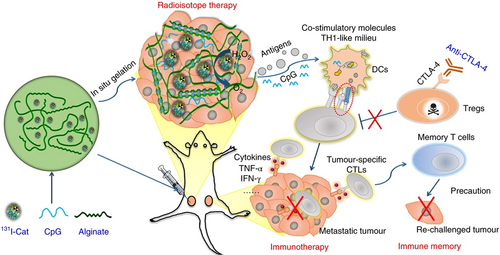
5 CONCLUSIONS AND PERSPECTIVES
Breast cancer has become the most common and greatest threat to women's health in the world. As the most dangerous subtype of breast cancer, TNBC is easy to lead to the generation of drug resistance and tumor recurrence and metastasis because of the lack of ER, PR, and Her-2 receptors at the same time. Clinically, surgery combined with chemoradiotherapy is still the main intervention form for the comprehensive treatment of TNBC. However, the antitumor effect is not ideal due to the shortcomings of low efficiency and large toxicity of drugs. Related studies have shown that the combination of radiotherapy and immunotherapy to improve innate and adaptive immunity can be used as a new strategy to improve the survival rate of patients with TNBC. In addition to treating local tumors and metastases, it can also induce the body to establish long-term immune memory cells and prevent tumor recurrence.142 However, the potential toxicity of radiation and asynchronous dosing regimens between radiotherapy and immunotherapy remains a major challenge to find the best synergy between the two treatments. In recent years, due to the exploration of nanobiotechnology in tumor immunotherapy, its ability to accurately regulate antitumor immunity has attracted increasing attention. Since NPs can actively or passively target tumor tissues, the use of NPs as a delivery system for drugs has always been a hot topic. The combination of NPs and radioimmunotherapy overcomes the clinical obstacles, which limit tumor treatments. Nanobiotechnology can combine immunotherapy with radiotherapy to improve the therapeutic effect of tumors, reduce the related toxic and side effects, and improve the survival rate and quality of life of patients. The scheme has a prominent antitumor treatment effect on TNBC tumor animal models in preclinical studies and provides a new idea for the clinical treatment of TNBC patients.
However, new side effects that may occur after combined administration should be considered, and the safety of nanobiotechnology should be emphasized while considering the effectiveness of the regimen.88 For example, implantation of hydrogel inevitably leads to slight chronic inflammation and foreign body reaction around the hydrogel, which is also the typical manifestation of many NPs.135 At present, there are a variety of NPs used for tumor treatment and the research on its action mechanism is not deep enough. It plays a different role in tumor radioimmunotherapy due to the difference in their synthesis, morphological characteristics, action mechanism, degradation, and absorption. In addition, the precise dose, treatment sequence, and administration route should be considered when designing the platform loaded with NPs.143 However, there are no relevant results from clinical trials and no standard treatment scheme for nanobiotechnology combined with radioimmunotherapy, which greatly hinders the application of NPs. However, the research on the application of nanobiotechnology used in tumor treatments has attracted much attention from the academic community and it has made rapid progress. In the future, more research efforts will be invested in this area to find the most appropriate scheme for NPs cooperating with tumor treatment. What is more, combined with Big Date Ere, perhaps we can customize the personalized drug delivery system based on the genomic data of different patients and taking into account factors such as patients' immune function, radiation sensitivity, and adaptability to dose, so as to bring better treatment effects for patients suffering from TNBC in the future.144
AUTHOR CONTRIBUTIONS
Mei Zhu: Conceptualization (equal); methodology (equal); writing – original draft (equal). Xi Yang: Conceptualization (equal); methodology (equal); writing – original draft (supporting). Jia You: Investigation (supporting); resources (supporting). Lingnan Zheng: Investigation (supporting); resources (supporting). Cheng Yi: Conceptualization (supporting); project administration (supporting); supervision (supporting). Ying Huang: Conceptualization (supporting); project administration (equal); supervision (supporting). All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the Sichuan Provincial Nature Science Foundation (No. 2022NSFSC1379) and Sichuan Science and Technology Program (No. 2022YFSY0054). We also acknowledge the contribution of BioRender with which part of our figures was created: https://biorender.com.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




