Targeting staphylococcal bone infections
A novel quinolone antibiotic was developed by Shiladitya Sengupta and colleagues using molecular-docking simulation, and one lead compound, VCD-077, was selected based on this strategy.1 Antimicrobial resistance is increasing, driven by widespread antibiotic overuse in medical and agricultural settings.2 Paradoxically, despite the urgent need to develop a new generation of antibiotics, their marketability is suppressed, partly because the new antibacterial drug may quickly develop new resistance and the development of new antibiotics is sluggish.3, 4 The ideal next-generation antibiotic should demonstrate excellent activity against drug-resistant bacteria, good biosafety, flexible drug delivery, and the ability to retard the development of resistance. In this regard, recently researchers have focused on developing novel antibiotics by improving drug performance, availability, targeted enrichment, and reducing systemic toxicity.
Reporting in Nature Biomedical Engineering, Shiladitya Sengupta and colleagues now show that VCD-077 screened by molecular-docking simulations exhibits excellent activity against drug-resistant bacteria and its biofilms, reduces the development of bacterial resistance and is compatible with bone cement for local delivery and treatment of staphylococcal bone infections in rats (Figure 1).1
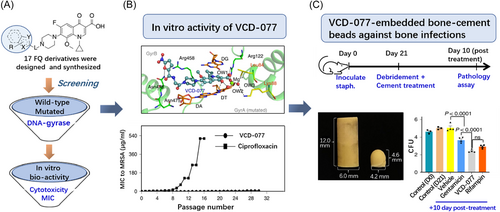
Bone infections are rising rapidly due to accidental fractures and the global aging population.5 Sengupta and co-authors designed a series of quinolone antibiotics using molecular-docking simulation. They selected VCD-077 from 17 candidate antibiotics, which can effectively against clinical drug-resistant staphylococcal and retard the development of resistance. The minimal inhibitory concentration (MIC) of ciprofloxacin, Staphylococcus aureus sensitive antibiotics, increased stepwise to 64.0 μg/ml by the 20th passage to S. aureus. While S. aureus were still sensitive to VCD-077 even at the 28th passage. Specifically, VCD-077 is potent against S. aureus and Staphylococcus epidermidis biofilms because it is a weak acid with a single pKa of 5.8, that is, it still maintains antibacterial activity at pH 5.5–7.4. In contrast, gentamicin lost part of its activity at acid pH 5.5. Therefore, VCD-077 has a unique advantage in the prevailing weakly acidic infected bone environment. Notably, VCD-077 is physicochemically compatible with polymethylmethacrylate bone cement, exhibits desired pharmacokinetics without loss of mechanical properties of bone cement. VCD-077-impregnated bone cement showed more powerful anti-infective activity compared with either FDA-approved rifampin or gentamicin-impregnated bone cement. Overall, the authors show that VCD-077-impregnated bone cement could be used as rational next-generation drugs or coatings to treat bone infections.
The high efficacy of Sengupta and co-authors' VCD-077-impregnated bone cement suggests that flexible local administration can be achieved at ideal safe concentration, and is effective against resistant strains, as well as delaying the development of new resistance. However, before VCD-077-impregnated bone cement can be considered for the treatment of clinical bone infection, three main challenges were compelled to be discussed. First, the authors used rodents in this experiment, which may be differences between the efficacy of antibiotics-impregnated bone cement in rodents and human bones. Studies using larger animal infection models (such as pigs and monkeys) would employ to corroborate the impressive antimicrobial activity in rat models. Second, significant biosafety assessment under good laboratory practice guidelines should be performed. And third, new antibiotic development should focus on specific screening molecules and combinations with emerging nanoparticle-based delivery systems that can target both resistant and persistent bacterial populations.6 Developing novel antibacterial agent delivery systems to improve the bioavailability of antibiotics is a promising strategy for increasing the lifespan of newly developed antibiotics. Integration of such novel antibiotics and combinations into nanoparticle-based delivery systems or coating implants might transform the landscape of infectious diseases.
AUTHOR CONTRIBUTIONS
Drafting the article: Zhang Xiangchun. Revising the manuscript: Xulin Hu and Hongping Chen. All authors have read and approved the final manuscript
ACKNOWLEDGMENTS
This research was supported by the National Key Research and Development Program of China (2021YFD1601102), Central Public-interest Scientific Institution Basal Research Fund (No. Y2022QC24), and Innovative Program of Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2021-TRI, CAAS-ZDRW202011).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




