Biomedical applications and prospects of temperature-orchestrated photothermal therapy
Abstract
Photothermal therapy (PTT) has been regarded as a promising strategy considering its advantages of high inherent specificity and a lower invasive burden. Since the photothermal killing of cells/bacteria showed different patterns of death depending on the varying temperature in PTT, the temperature change of PTT is vital to cell/tissue response in scientific research and clinical application. On one hand, mild PTT has received substantial attention in the treatment of cancer and soft/hard tissue repair. On the other hand, the high temperature induced by PTT is capable of antibacterial capacity, which is better than conventional antibiotic therapy with drug resistance. Herein, we summarize the recent developments in the application of temperature-dependent photothermal biomaterials, mainly covering the temperature ranges of 40–42°C, 43–50°C, and over 50°C. We highlight the biological mechanism of PTT and the latest progress in the treatment of different diseases. Finally, we conclude by discussing the challenges and perspectives of biomaterials in addressing temperature-orchestrated PTT. Given a deep understanding of the interaction between temperature and biology, rationally designed biomaterials with sophisticated photothermal responsiveness will benefit the outcomes of personalized PTT toward various diseases.
1 INTRODUCTION
Temperature, as an important sign, affects a series of biochemical reactions and kinetic effects from cells and tissues to organs and systems.1, 2 The normal temperature of the human body is about 37°C, which is necessary to ensure normal metabolism and life activities. Fever often indicates the presence of certain diseases, including infectious diseases (bacteria, viruses, parasites) and noninfectious diseases, among which high and prolonged fever may cause tissue damage and necrosis. Every coin has two sides. It was found that elevated temperature could be used to treat diseases.3 In the 19th century, researchers observed that the hyperthermia induced by injecting cancer patients with live bacteria caused partial regression of tumors.4 In 1927, an Austrian doctor Jauregg treated syphilis infection of the central nervous system in some people by inoculating them with the blood of malaria patients to induce a high fever.5, 6 According to the treating area, hyperthermia is mainly divided into systemic hyperthermia and local hyperthermia. Systemic hyperthermia heats a patient's entire body around 42°C and is rarely used in clinical now, because of its various complications and large injury to patients.7 In recent years, with further research on hyperthermia, many methods have been invented to make the heating site and temperature controllable,8, 9 including focused ultrasound (FUS),10-12 radiofrequency ablation (RFA),13-15 microwave ablation (MVA),16, 17 hyperthermic perfusion,18, 19 and so on. Hyperthermic perfusion today is generally combined with chemotherapy to treat specified sites of tumors, and its indications are relatively limited. In hyperthermic intraperitoneal chemotherapy, the perfusion of heated solution with chemotherapy drugs in it enables to eliminate minimal residual of cancer after surgically removed or the metastatic tumors unable to eradicate through the operation.20, 21 Owing to the properties of focusability and penetrability, FUS uses ultrasonic waves as its energy source and focuses the low-energy ultrasonic wave in vitro on the lesions in vivo. The instant temperature over 65°C at the focal point achieves the purpose of killing tumor tissue.10-12 RFA generates heat through the ion oscillation and friction in tumor tissue by using alternating current, and its focal temperature can reach 90–100°C. The extraordinarily high temperature generated by FUS and RFA increases their insecurity while using them. The feature of MVA is similar to RFA, but the difference is the source to generate heat. MVA can create larger ablation zones than RFA and has been reported in the treatment of some early-stage superficial cancer, such as tongue cancer.13, 22 These methods can cause irreversible necrosis of tumor cells, thus lessening or eliminating tumors. However, the means of traditional localized hyperthermia mostly heat tumors by applicators or antennas, which can send out microwaves or radio waves. For deep tumors, applicators, and antennas need to be implanted into the tumor tissue.23 These operations tend to cause damage to tumor-adjacent tissue, inducing side effects to downgrade the life quality of patients.
Photothermal therapy (PTT) is a new kind of hyperthermia method for various diseases. It transports different kinds of photothermal agents (PTAs) in the region of disease, converting near-infrared (NIR) light energy into regional hyperthermia. PTT is efficient to treat disease and minimizes thermal damage to surrounding normal tissue. Due to its superior temperature controllability, PTT is safer to use than traditional hyperthermia methods mentioned above.24 There have been a large number of preclinical studies and preliminary clinical trials on PTT in the treatment of tumors, proving its effectiveness and clinical translation potential. For example, gold nanoshell-based PTT has gained positive outcomes in clinical trials of prostate tumor treatment.25 Compared with traditional tumor therapeutic methods, the advantages of PTT are as followings: (a) Noninvasiveness. The operation of PTT is relatively simple. Using injectable nanomaterials as carriers,26 the distribution of PTAs in targeted tissue can be completed. Then, the initiation of the therapeutic process can be controlled by NIR light, preventing damage to healthy tissue and reducing the pain of patients. This characteristic enables PTT as a potential way to cure the tumor located in the critical area, which is difficult to completely remove by surgical operation.27, 28 (b) Synergy. Combining PTT with chemotherapy, photodynamic therapy (PDT), immunotherapy, and other treatments can increase efficacy and reduce side effects.29 By tailored designing of multifunctional biomaterials, it can also achieve the therapeutic effect from tissue repair to the tumor via orchestrating temperature. Therefore, traditional hyperthermia treatments are appropriate for superficial and early-stage lesions, for they can wholly ablate the target tissue. But for deep-seated tumors, traditional ways are difficult to completely remove lesions without damaging normal tissue. Furthermore, distant metastatic tumors cannot be handled only by local hyperthermia, some other therapies are necessary to use synergically. Apart from the application of the antitumor treatment, PTT has also been studied in wound healing, anti-infection, and other diseases.30
The ideal PTA should possess the features of high photothermal conversion efficiency (PCE) and tumor-targeting performance.31, 32 Nanoparticles show good performance in both aspects making it possible to carry out systematic PTT by intravenous injection.33-35 As shown in Figure 1, the classification of PTAs can be broadly divided into inorganic PTAs and organic PTAs. Among them, inorganic PTAs mainly include noble metal nanoparticles (gold nanorods,36-38 gold nanocrystals,39 gold nanostars,40 gold nanoshells41, 42), carbon nanomaterials (graphene,43 carbon nanotubes44, 45), and other inorganic nanoparticles (black phosphorous,46, 47 MoS248-50 and silica,51 and so on). Organic materials include organic dyes (indocyanine green [ICG],52 Prussian blue53) and polymer nanoparticles (such as polypyrrole,54, 55 semiconducting polymer nanoparticles56). In practical experiments, different kinds of PTAs are often assembled into complex structures to enhance the photothermal effect or achieve other different purposes. Another element of PTT is NIR light, in which NIR-I (750–1000 nm) and NIR-II (1000–1700 nm) are usually applied in PTT.57, 58 A semiconducting copolymer nanoparticles with dual-peak absorbing capacity in both NIR-I and NIR-II termed poly(diketopyrrolopyrrole-alt-cyclopentadithiophene)-ran-(diketopyrrolopyrrole-alt-thiadiazoloquinoxaline) were reported, demonstrating the therapeutic effect of NIR-II in the treatment of deep tumors.59 At the same time, biomaterials with the property of NIR luminescence can be doped in PTAs for NIR fluorescence (NIRF) imaging-guided PTT, realizing a precise treatment.60-62
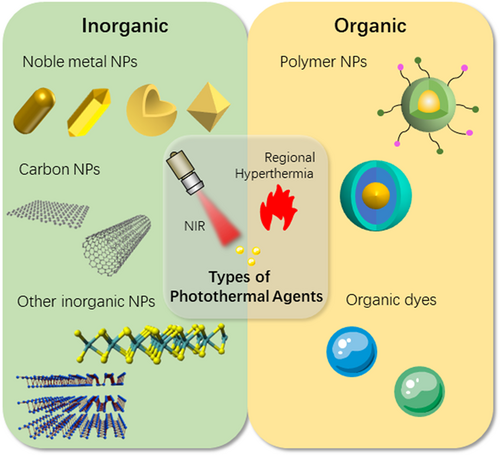
The effects of hyperthermia on cells and tissues are primarily controlled by the range of temperature change and the time of temperature rise. At the cellular level, when the temperature reaches around 41°C, a series of rapid changes in gene expression patterns are initiated, such as the production of heat shock proteins (HSP), which can alleviate the effects of heat damage and develop heat tolerance.63 When exposed to a temperature above 42°C, apoptosis and necrosis of cells begin, and tissue damage occurs. Irreversible tissue damage may be caused by 15–60 min of exposure to 42–45°C, or about 5 min of exposure to temperatures above 50°C.64, 65 As the temperature increment goes up, rapid protein denaturation at temperatures above 60°C leads to almost instantaneous coagulative necrosis and irreversible tissue damage.66 Tumor cells are less tolerant to heat than normal tissue, which is one of the bases of hyperthermia for tumor therapy.67 At the tissue level, the formation of local hyperthermia increases the permeability of tumor blood vessels and cell membranes. In addition, for bacteria, localized hyperthermia (above 50°C) can lead to lethal damage through bacterial protein denaturation and irreversible bacterial destruction and ultimately kill the bacteria.68, 69 As illustrated in Figure 2, PTT can be used for the treatment of tumors, tissue defects, and anti-infection, depending on the effects at varying temperatures.
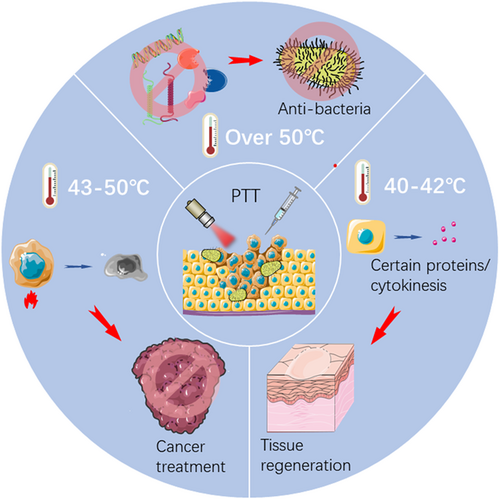
This review focuses on the various therapeutic effects of temperature-dependent PTT and its role in the treatment of different diseases in recent years. The main content contains tissue regeneration at 40–42°C, cancer treatment at 43–50°C, and antibacterial treatment over 50°C. The rational design of biomaterials for accurate PTT and its biological mechanism in the treatment of different diseases are analyzed. The perspectives and challenges faced by PTT in clinical translation and possible solutions are discussed. The limitations and development directions of PTT are elaborated, and some advanced strategies based on PTT are put forward.
2 BIOMEDICAL APPLICATIONS OF TEMPERATURE-DEPENDENT PTT
According to the effects of different temperatures on cells and tissues mentioned above, we summarize the biomedical applications of PTT in different temperature ranges. The following three sessions of commonly used temperature ranges for disease treatment can be introduced.
2.1 The temperature of 40–42°C for tissue regeneration
The temperature between 40°C and 42°C does not cause any damage at the cellular level. Instead, these mild heating treatments have beneficial health effects, such as increasing blood flow and the rate of ion diffusion across cell membranes. It is universally acknowledged that some tissues (like tendons) are more likely to stretch when exposed to moderate heat, thus helping to relax muscles, relieve pain, and improve local and systemic circulation.70, 71 That may be the reason why people are likely to relax by bathing in a hot spring. At the same time, this temperature level causes normal tissue cells to synthesize certain proteins or release cytokines, such as HSP47,72 HSP70, HSP90,73-75 BMP2, BMP7, and so on. BMPs are considered a crucial pathway in osteoblast differentiation.76-78 By adding some specific ions (like CO32−), the expression of MMP2 and MMP979 can also be increased, which can degrade the extracellular matrix and plays an important role in keratinocyte migration, granulation tissue remodeling, and angiogenesis.
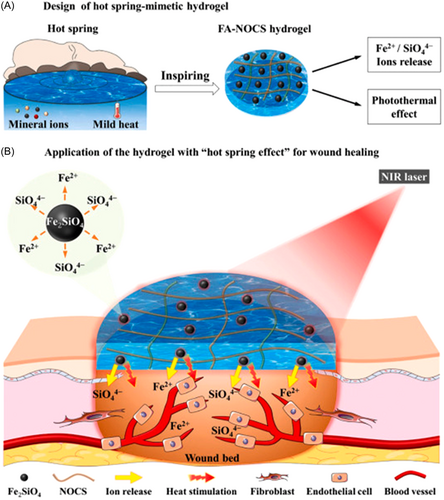
In chronic skin wound healing, heat treatment at 40–41°C induced tube-formation of endothelial cells and promoted vascularization, which was proved in hindlimb ischemia mouse models.80, 81 Based on these principles, a new type of hydrogel with a “hot spring effect” has been reported (Figure 3). Fayalite (FA) was used as PTA and incorporated into N, O-carboxymethyl (NOCS) chitosan to obtain bioactive composite FA-NOCS hydrogels. Fayalite ceramic powders not only have the function of photothermal conversion but also can release Fe2+/SiO44+ ions. In the full-thickness excisional wound model of diabetic mice, the staining intensity of CD31 in the FA-NOCS with irradiation group was higher than that of the blank group and other control groups, indicating that the formation of new blood vessels in the wound area was significantly enhanced. Meanwhile, as a new-type wound dressing, hydrogels possess the distinguished properties of biocompatibility and inflammatory exudates absorption ability, thus promoting the effect of wound healing.83 Further research on the mechanism showed that Fe2+/SiO44+ bioactive ions mainly promoted the expression of VEGF, HIF-1α, basic fibroblast growth factor, and basic fibroblast growth factor receptor genes, while thermal stimulation promoted the expression of VEGF, eNOS, and HSP90 genes.82 Other metallics with both photothermal and angiogenic effects can also be applied to skin wound healing, such as Cu, Au, Ag, and so on.84-86 Taking advantage of copper for tissue repair, Xiao et al. further studied the influence of ultrasmall CuS@BSA nanoparticles for the differentiation of mesenchymal stem cells (MSCs). The result in vivo demonstrated that both CuS@BSA NPs and NIR-induced mild heating (42°C) promoted wound healing through the differentiation of MSCs, and the combination of the two factors was more effective than alone.87
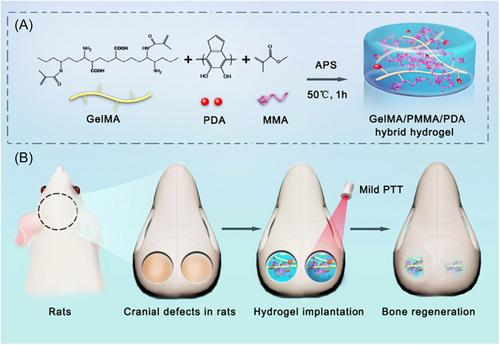
Similarly, PTT can be used in bone defect regeneration and repair. The self-healing ability of bone tissue is limited when the bone defect exceeds a critical value or the defect is caused by a tumor, infection, or osteoporosis.88-91 Experiments have proved that a mild PTT hydrogel platform with the temperature raised to 40–42°C can greatly promote cell proliferation in rat models of skull defects, and significantly increase the healing area of large bone defects after a few weeks (Figure 4).92 The reason for mild PTT for enhanced bone repair may relate to the increased expression levels of BMP2 and HSP47.93 Gold nanoparticles (GNPs) can promote the osteogenic differentiation of MSCs by activating the intracellular p38 mitogen-activated protein kinase (MAPK) pathway.94-96 In addition to converting light energy into heat, some PTAs themselves can also promote bone tissue repair. For example, black phosphorus (BP) is a nice type of nanomaterial with good infrared response used as PTA,88 especially the ultrasmall BP quantum dots (BPQDs).97 Phosphorus is a fundamental element constituting about 1% of total human body weight, and primarily exists in bone tissue.98 Because of the ability of BP degrades naturally into nontoxic PO43− in a physiological environment, it would serve as a biodegradable PTA in promoting the osteogenesis of bone tissue.88, 99, 100 In a practical case, researchers incorporated BPs into poly(lactic-co-glycolic acid) (PLGA), forming a biodegradable photothermal osteogenesis material termed BPs@PLGA membranes. After periodical PTT at 40.5 ± 0.5°C, the expression of osteogenesis-related proteins, such as type I collagen, osteocalcin, osteopontin (OPN), and other proteins like HSP47 and HSP70 were promoted in vitro. And a significant difference in bone regeneration was observed after 5 and 10 weeks of PTT in vivo.101 Besides, gene engineering techniques have been used to produce a cell line with high expression of BMP2 termed C3H-BMP-2high, which was stored in a NIR reactive scaffold containing GNPs. Therefore, NIR can be used as a gene switch and implement the precise spatiotemporal control of BMP2.102
The experiments above demonstrate the ability of PTT to promote tissue repair. However, the use of PTT for simple wound healing or tissue regeneration is limited, because tissue defects are often caused by tumors or accompanied by bacterial infection. As a consequence, researchers have come up with the idea of designing bifunctional biomaterials for both tumor treatment and tissue repair. For example, a nanohydroxyapatite/graphene oxide/chitosan (nHA/GO/CS) scaffold was designed to treat osteosarcoma. Tumor tissue was eliminated first at around 48°C. After that, a mild temperature (42 ± 0.5°C) was employed to promote the osteogenesis of human bone marrow mesenchymal stem cells and soft tissue regeneration.103 This change in temperature provides a new thought for the design of a PTT-based multifunctional scaffold. Except for bone tumors with bone tissue engineering, melanoma with skin tissue engineering, breast cancer with adipose tissue engineering, and osteochondroma with cartilage tissue engineering are possible directions of PTT based on different temperatures in the future. We have summarized the related cases of PTT application for tissue regeneration in Table 1.
| Temperature | Applications | Therapeutic type | Photothermal agents | Laser irradiation | Performance in vitro | Performance in vivo | References |
|---|---|---|---|---|---|---|---|
| 40°C | Chronic wound healing | PTT | Fayalite | 0.36 W/cm2 808 nm 15 min/day | Stimulate viability of endothelia cells and fibroblasts | Enhance chronic wound healing by stimulating new blood vessel formation | [82] |
| 42°C | Skin regeneration | PTT | CuS | 0.8 W/cm2 980 nm 5 min |
Induce MSCs differentiating to fibroblasts | Stimulate extensive collagen deposition and improve the regeneration of the skin | [87] |
| 41.0 ± 1.0°C | Bone regeneration | PTT | BPs | 0.5 W/cm2 808 nm 450 s |
Upregulate the expressions of cellular HSPs | The bone regeneration was facilitated after 10 weeks | [101] |
| 48°C/42 ± 0.5°C | Osteosarcoma treatment and tissue regeneration | PTT | GO | 0.6 W/cm2 808 nm 5 min/ 1.5 W/cm2 808 nm 10 min |
Apoptosis and necrosis of human osteosarcoma cells was significantly promoted/The HSP47, HSP70 levels and RUNX2, OCN, OPN protein levels of hBMSC was promoted | The tumor decreased significantly in volume/the new bone formation was promoted | [103] |
- Abbreviations: BP, black phosphorus; GO, graphene oxide; HSP, heat shock proteins; MSC, mesenchymal stem cell; PTT, photothermal therapy.
2.2 The temperature of 43–50°C for tumor therapy
The temperature between 43°C and 50°C is usually used for tumor therapy because it can promote the permeability of blood vessels and cell membranes in lower and cause rapid irreversible damage of cells in higher temperatures of this range. PTT at 43–48°C is also known as mild temperature for tumor therapy. At the cell level, it can induce cell death through either extrinsic or intrinsic mitochondrial pathways of apoptosis, but it is sublethal and reversible.104 The mild high temperature can increase the permeability of vascular, thus the delivery and accumulation of drugs improve in tumor sites.105 However, mild-temperature PTT also activates the cell protective pathways of cancer cells, such as the secretion of HSP and autophagy activation, which protect cancer cells from heat damage and severely impair the therapeutic effect of PTT.106, 107 So, mild PTT is often combined with other therapies for synergistic effects instead of being used alone.108 In the previous study, PTT-chemotherapy,109-111 PTT-PDT,112, 113 PTT-immunotherapy,114 PTT-radiotherapy (RT)115, 116 and PTT-gene therapy (GT)65, 117-119 were widely studied. At the same time, through further exploration of the molecular mechanism of tumor cells, other advanced strategies have been developed to improve the anticancer efficiency of PTT at mild temperatures, such as HSP inhibitors,120, 121 autophagy regulation agents,122 and targeting enhanced strategy.123-125
The main mechanism of using PTT alone in the treatment of tumors involves inducing tumor cell apoptosis under mild high temperatures. In this case, multiple irradiations are often required and the treatment time is correspondingly increased. For example, the titanium oxide nanofibers under irradiation raised tumor temperature to about 45°C in vivo. After the continuous irradiation for 5 min a day, the survival time of the mice was significantly prolonged, although the tumors were not completely eliminated.126 To solve the problem that mild temperature cannot induce complete apoptosis of tumor cells, the concept of organelle-targeted hypothermia therapy was proposed. Considering that the nucleus is the control center of cell growth and development and the genetic material and proteins in the nucleus are sensitive to heat, seeking PTAs targeted to the nucleus is expected to induce tumor cell death at mild temperatures.127, 128 Nanoscale coordination polymers (NCPs) are considered a promising nanocarrier platform for tumor imaging and therapy. Jiang et al. reported Hf-heptamethine indocyanine dye (HI-4COOH)-based NCPs as intrinsic nucleus-targeting PTA for mild temperature PTT. This method resulted in remarkable antitumor efficacy without recurrence in vivo.125 In addition to nuclear targeting, membrane targeting,129 endoplasmic reticulum targeting,130 mitochondrial targeting,123, 131 cytoskeletal targeting,132 multiorganelle targeting,124 and tumor angiogenesis endothelial cells (αVβ3 integrin) targeting133 are also hot research topics of mild-temperature PTT and prevention of tumor recurrence and metastasis so far.
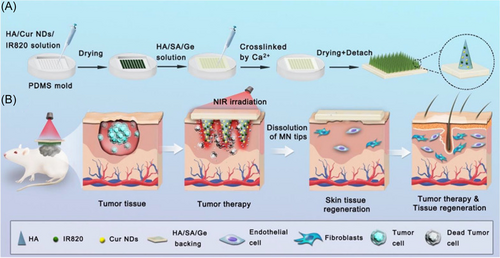
The combination therapy is widely used in mild-temperature PTT to reduce the number of treatments and completely eliminate tumor tissue. The combined therapy includes PTT-co-chemotherapy, PTT-co-PDT, PTT-co-immunotherapy, and so on. Chemotherapy is one of the most common methods for tumor treatment, and its effectiveness has long been proven by a large number of clinical practices. However, its limitations lie in serious side effects and the occurrence of drug resistance.134, 135 To solve these problems, localized mild-temperature PTT could be used to enhance the permeability of cell membranes and blood vessels. The increment of drugs entering tumor cells improves the effectiveness of chemotherapy, resulting in a reduction in the dose and frequency of chemotherapy.136 At the same time, PTT can achieve accurate temporal and spatial control of chemotherapy drugs by adjusting the position, time, and power density of NIR irradiation, while the use of chemotherapeutic agents can compensate for the insufficient thermal damage. Layer double hydroxide (LDH) is a two-dimensional (2D) nanomaterial with a strong drug loading capacity,137 and it has excellent PCE and biological imaging capability.138, 139 In the report of Liu et al., 5-fluorouracil (5-FU) and albumin-bound paclitaxel (nAb-PTX), were loaded into a copper-doped LDH for the treatment of breast cancer. The dose of chemotherapeutic drugs used in this study was 8–50 times less than that normally used. With the PTT temperature at ~44°C, the biomaterial showed a good tumor inhibition effect.140 In the treatment of some superficial tumors, such as melanoma, a microneedle-based photothermal/chemo cotherapy platform was proved to be an efficient way to eliminate the tumor and treat wound defect simultaneously (Figure 5).141, 142 Another application of PTT-chemotherapy cotherapy is the elimination of multidrug resistance (MDR) of tumors.143-145 P-glycoprotein (P-gp) is overexpressed on MDR tumor cells, which boosts the transport of chemotherapy drugs and reduces their accumulation in cells.146 The study revealed that mild heat inhibited the expression of P-gp and enhanced the retention of anticancer drugs in cells.147 In addition, PTT can be used to induce the controlled release of a temperature-sensitive drug delivery system to further improve the drug effect and enhance the curative effect.148-150
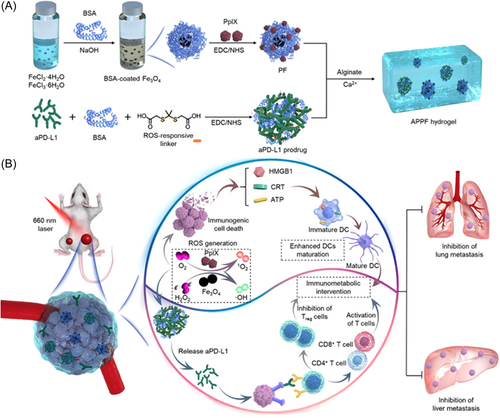
Immunotherapy shifts the emphasis of cancer treatment from killing tumor cells to taking advantage of patients' own immune system, by inducing the body to develop adaptive immunity and eliminating tumor cells spontaneously.151 Unfortunately, due to the immune escape mechanism of tumor cells, most patients have primary resistance and acquired resistance to immunotherapy, resulting in poor treatment effects and tumor recurrence.152, 153 In combined therapy, photothermal-induced immunogenic cell death (ICD) releases damaged-associated molecular patterns (DAMPs), consequently increasing the immunogenicity of the tumor microenvironment.11, 154 Immune checkpoint blockade treatment is one of the most commonly used immunotherapies, and most of the inhibitors are designed to aim at programmed death receptor-1 (PD-1) and its ligand (PD-L1).155, 156 Yu et al. combined BMS-202 (an inhibitor of PD-1/PD-L1) and IR-780 to treat metastatic pancreatic cancer. Ding et al. apply aPD-L1 prodrug and Fe3O4 nanoparticles to breast cancer. Li et al.157 assembled thymopentin (TP5), an immunomodulator that can alter the function of mature T cells, and ICG to treat pancreatic tumors. Except for primary tumor, this strategy alleviates distant tumors and prevent metastasis simultaneously (Figure 6).158 The inhibited expressions of hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF) reduced the possibility of tumor metastasis.159 Photothermal synergistic immunotherapy can also be used to develop cancer vaccines and induce long-lasting T-cell responses for the treatment of various types of tumors.160-163 In the experiment of Chen et al., they developed a vaccine-like drug by combining ICG, PLGA as the capsule, and imiquimod (R837) as an immunoregulator to activate an immune response. It is worth mentioning that the immune-memory effect can protect mice from cancer relapse after the elimination of primary tumors for 40 days.164 Moreover, PTT can combine with anti-inflammation therapy to alleviate the inflammation microenvironment of the tumor and reverse the immunosuppression for enhancement antitumor therapy. Zhang et al. utilized a kind of organic small chromophore (TTM) as PTA and nonsteroidal anti-inflammatory drugs (NSAIDs) named Aspisol (AL) as an anti-inflammation agent. The outcome demonstrated significant inhibition of distant tumors by combination therapy.165
Aside from the schemes mentioned above, some other new strategies gradually shine in mild-temperature PTT with further research on the molecular mechanism of tumors. Cancer cells show rapid nutrition consumption to maintain rapid proliferation. Cancer starvation therapy is to “starve” cancer cells by blocking glucose transport by using glucose oxidase or interfering with the process of glycolysis.166-168 The inhibition of Glut1 can reduce ATP produced by metabolism and thereby intervene in the synthesis of a series of products including HSP and overcome the thermal tolerance of tumor cells and promote PTT.169 Another new strategy is autophagy inhibition. Autophagy is a dynamic cellular pathway that can degrade and recover misfolded proteins or damaged organelles to maintain a stable intracellular state, while disruption of autophagy balance can lead to cell imbalance.170 The heat produced by PTT leads to damage to intracellular components, and the autophagy process of tumor cells is activated to protect themselves. The development of autophagy inhibitory materials such as chloroquine-loaded PDA was also proved to be an effective method to improve the efficacy of PTT.106 In short, mild-temperature PTT is a promising and effective therapeutic method. PTT works synergistically with other treatment methods to enhance curative effects remarkably and reduce toxic side effects without damaging healthy tissues. At the same time, the bioimaging capabilities of PTAs can show the condition of their aggregation and distribution, improving the accuracy of treatment.
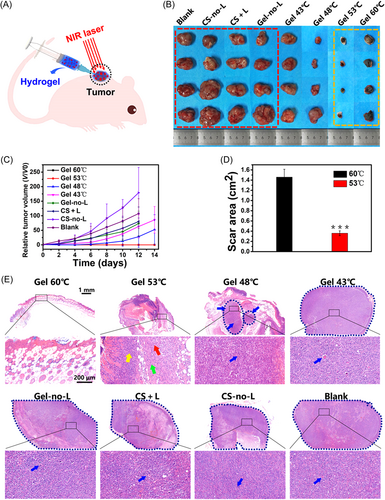
The temperature around 50°C is the traditional PTT temperature. Studies have reported that when the temperature rises above 48°C, the irreversible denaturation of the protein occurs within 4–6 min.66 Compared with mild-temperature PTT, the time and frequency of traditional PTT are greatly shortened. But the consequence is that the healthy tissue around the tumor takes a great risk of burns. To determine the ablation temperature of the tumor, a study compared the effects of PTT without other treatments combined at different temperatures, as shown in Figure 7. Under the same conditions, the photothermal temperature below 48°C showed tumor recurrence and regrowth after 7 days. In the groups of 53°C and 60°C, it was observed that the two groups did not have tumor recurrence after treatment, but skin burns occurred in both groups. Unfortunately, the area of skin damage caused by treatment at 60°C was four times larger than that at 53°C.61 It proved that the appropriate PTT temperature may be around 50°C. Recently, a novel biomedical material named protein-based photothermal bioplaster (PPTB) is designed to treat malignant skin tumors. By the complexation of adhesive proteins and gold nanorods, PPTB possesses both excellent adhesion performance and PCE. In the research of Zheng et al., the tumor-bearing mice were exposed to an 808 nm laser (0.6 W/cm2) for 15 min, with the temperature, rise above 50°C, once a day for 3 days. Consequently, the tumor growth was significantly suppressed and no recurrence was observed during the observation for 2 weeks. It is worth mentioning that PPTB is found to accelerate the healing and regeneration of skin defects.171 Moreover, many kinds of nanoparticles with stable photothermal performance are designed for PTT, such as MoS2/LaF3,172 Ag@Ag2O,173 and so on. Furthermore, given the importance of imaging technology in cancer treatment, the imaging-guided therapy method has attracted extensive attention.174 Liu et al.175 doped Fe(III) in polyaminopyrrole to serve as both magnetic resonance imaging contrast agent and PTA.
There have also been several cases of combination therapy at this temperature, showing greater efficiency than implementing PTT alone. For example, Huang et al. reported that 91.7% of tumor cells were killed in synergistic chemo-photothermal therapy with Bi as PTA and DOX as a chemotherapy drug. While in mono-chemotherapy and mono-photothermal therapy, the rates of killed tumor cells were only 73.9% and 81.6%, respectively. In vivo antitumor experiments, the body weight of mice was not affected by various treatments for 18 days compared with the control group, which further verified the low side effects of this treatment.176 Because the tumor involves soft/hard tissue damage, a multifunctional treatment platform to meet the needs of tumor treatment and tissue repair is an urgent need.177, 178 With the continuous research on PTT, the newly recent research showed the development of multifunctional treatment systems. For example, CuS nanoparticles were loaded in a three-dimensional (3D) network of oxidized dextran (ODex), linked by the amino groups of polyethylene glycol (PEG), forming an injectable and self-healing hydrogel. With the irradiation of 808 nm NIR light, the local temperature of the tumor increased to 50°C, and the tumor in the abdomen of the mice disappeared completely. In addition, this biological material can promote the proliferation and migration of human umbilical vein endothelial cells (HUVECs) and human dermal fibroblasts (HDFs). Meanwhile, hydrogels maintain a sustained release of Cu2+ and create a moist microenvironment, helping skin wound healing.179 To compensate for the lack of penetration depth of NIR-I, PTAs that absorb NIR-II have been extensively studied.63, 180, 181 Xu et al. designed NIR-II-activated hollow nanocarbons termed HPP. The surface of hollow carbon nanospheres was modified by PEG-graft-polyethylenimine (PEG-g-PEI) polymer to increase the biocompatibility of the material. With the irradiation of NIR at 1064 nm, the material exhibited good photothermal performance, rapidly raised the temperature of the local area of the tumor to 50°C within 7 min, and significantly inhibited the growth of the tumor.182 For the convenience of comparison, we have summarized some specific cases of PTT application for tumor therapy in Table 2.
| Temperature | Applications | Therapeutic type | Photothermal agents | Laser irradiation | Performance in vitro | Performance in vivo | References |
|---|---|---|---|---|---|---|---|
| 44°C | Breast cancer treatment | PTT-chemotherapy | Cu-doped layer double hydroxide | 0.75 W/cm2 808 nm 3 min |
4T1 cells viability was reduced | The tumor growth was dramatically suppressed with much lower dose of drugs | [140] |
| 43°C | MDR lung cancer treatment | PTT-chemotherapy | DAP-F | 0.8 W/cm2 808 nm 10 min |
Mild hyperthermia can inhibit cisplatin resistance in A549DDP lung cancer cells | Tumor volumes were almost completely reduced and exhibited extensive nuclear condensation and fragmentation | [145] |
| 45°C | Cancer treatment | PTT-RT | Ti2C3@Au | 0.75 W/cm2 1064 nm 10 min |
A salient DNA damage of 4T1 cells was achieved when treated with Ti3C2@Au nanocomposites under X-ray irradiation | Effectively improved the tumor hypoxia microenvironment and enhanced RT | [183] |
| 44.2°C | Cancer treatment | PTT-PDT | BiAgOS semiconductor | 1.43 W/cm2 808 nm 10 min |
The production of ROS was enhanced and induced the apoptosis of HeLa cells and U14 cells | Inhibited tumor growth effectively and displayed obvious apoptosis and necrosis of tumor cells | [184] |
| 44°C | Pancreatic cancer treatment | PTT-immunotherapy | IR-780 | 0.5 W/cm2 808 nm 10 min |
The cellular uptake of nanoparticles increased and showed significant anti-proliferation effect in Pan 02 cells | Promoted dendritic cells maturation in spleen and increased the recruitment of endogenous immune cells, therefore, suppressed primary and metastasis tumor | [164] |
| 42-45°C | Cancer treatment | PTT-GT | CNTs | 1 W/cm2 808 nm 5 min |
Enhanced levels of HeLa cell apoptosis and downregulated survivin expression | Showed significant suppression of tumor growth | [65] |
| 50°C | Cancer treatment | PTT | Hollow carbon nanospheres | 0.6 W/cm2 1064 nm 7 min |
Almost all the 4T1 cells were eradicated | The tumor size was significantly inhibited | [182] |
- Abbreviations: PTT, photothermal therapy; ROS, reactive oxygen species; RT, radiotherapy.
2.3 Over 53°C for antibacterial treatment
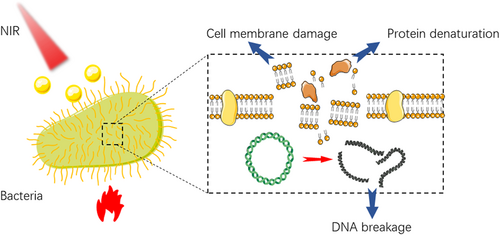
When the temperature exceeds 53°C, it is easy to cause large-area necrosis of normal tissues rapidly. While as an antibacterial method, PTT does not cause bacterial mutation and has a wide antibacterial spectrum, hence this high temperature is suitable for antibacterial treatment.185 In the treatment of bacterial infections, the discovery, and development of antibiotics have greatly improved this situation. Due to the strong mutation ability of the bacteria and the abuse of antibiotics, the emergence of drug-resistant bacteria (such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium (VREF) has become a headache problem nowadays.186, 187 Another dilemma is that bacteria attach and secrete extracellular polymeric substances on the interface, enabling the formation of a 3D biofilm.188, 189 Similar to the effect of high temperature on cells, PTT kills bacteria through protein denaturation, cell membrane damage, DNA breakage, and so on (Figure 8). The difference is that bacteria generally have a better ability of thermotolerance than cancer cells. When PTT is used to inactivate bacteria, the temperature needs to rise above 55°C to produce a sufficient killing effect on bacteria.190, 191 Hydrogel is one of the most common platforms for antibacterial therapy of PTT, on account of its remarkable biocompatibility.192, 193 For example, Qi et al. constructed an injectable self-healing cationic guar gum (CG) hydrogel platform loading with PDA@Ag nanoparticles as PTA. The CG/PDA@Ag (CPA2) hydrogel was raised to 56.2°C after 808 nm NIR irradiation (1.0 W/cm2) for 3 min and the wound site displayed almost no bacteria on the agar plate. What's more, after killing bacteria and accelerating the repair of wounds, the CPA2 can be wholly absorbed, avoiding additional removal.194 In the field of stomatology, some researchers also tried to use PTT in treating infected tooth root canals to improve the prognosis.195
There are also many other bioactive materials being developed. A biomimetic material RBC@Fe3O4 was created by wrapping Fe3O4 nanoparticles in the red blood cell (RBC) membrane, in which the RBC membrane can absorb bacterial toxins. RBC@Fe3O4 nanoparticles exhibited higher antibacterial performance than single Fe3O4 in vitro. The treatment temperature of the experimental group was increased to 59.2°C after the irradiation and the appearance of normal tissues was observed 14 days later, while other groups still exhibited serious skin damage.196 In another study, ICG was loaded in zeolitic imidazolate frameworks-8 (ZIF-8), which shows superior stability in a physiological environment. In the MRSA-induced murine subcutaneous abscess mice model, PTT for about 30 min in the experimental group killed more than 90% of MRSA, demonstrating the prominent activity of ZIF-8-ICG in vivo to eliminate multidrug-resistant bacterial infections.197
Similarly to tumor treatment, various synergic therapies are being used in antibacterial treatment. PDT is a kind of treatment method which uses a specific wavelength laser to irradiate photosensitizers (PSs), so that light and oxygen molecules combine to produce reactive oxygen species (ROS), therefore, causing damage to bacteria. Triggered by light, PDT has almost the same advantages as PTT, like light-sensitive, noninvasive and fewer side effects.198 Since the generation of ROS requires oxygen, PTT can promote blood flow to bring more oxygen in the period of combination therapy, and enhance the benefit of PDT.199 In the experiment of Shi et al., a self-assembled nano-system of ICG and polycationic brushes (sPDMA@ICG NPs) was developed to PTT-PDT for treating periodontitis. The high temperature of 51.2°C in vitro proved to kill bacteria effectively and prevent the resorption of alveolar bone.200
When chemotherapy was combined with PTT, the previously discovered inefficient antibiotics can be reused. PTT was combined with traditional antibiotics to act on drug-resistant bacteria, hoping to find a way to reuse and prolong the working life of the old antibiotics.201, 202 In the study of Tan et al., red phosphorus (RP) is used as a PTA in combination with a variety of traditional antibiotics including tetracycline, roxithromycin, penicillin, and four kinds of aminoglycosides. The anti-MRSA effect was observed even under 50°C. This is related to the PTT inhibiting the activity of the modifying enzyme.203 The VREF can also be killed when the antibiotics were combined with PTT.204 Besides, some metallic particles from PTAs can also exert antibacterial effects while possessing photothermal properties, such as Fe2+,205 copper atoms,10 silver nanostructures,206, 207 and so on. Moreover, we have summarized some specific cases of PTT application for antibacterial therapy in Table 3.
| Temperature | Applications | Therapeutic type | Photothermal agents | Laser irradiation | Performance in vitro | Performance in vivo | References |
|---|---|---|---|---|---|---|---|
| 59.2°C | Antibacterial treatment | PTT | Fe3O4 | 2.5 W/cm2 808 nm 5 min |
Viable colonies were little on LB agar plates | The photothermal sterilization was obvious and regenerated normal tissue was observed | [196] |
| 56.2°C | Antibacterial treatment | PTT | CG/PDA@Ag | 1.0 W/cm2 808 nm 3 min |
The bacterial cell membrane showed severe damage, and the bacteria was distorted or even ruptured | The wound treated with PTT possessed the smallest open wound after 12 days, new epidermal and dermal tissues can be observed | [194] |
| 51.2°C | Periodontitis treatment | PTT-PDT | sPDMA@ICG NPs | 2.0 W/cm2 808 nm 10 min |
The destruction effect and penetration ability through the bacterial membrane are stronger | The progress of periodontitis was almost prevented completely, the epithelial cells arranged normally and no obvious inflammatory changes | [200] |
| 45°C | Antibacterial treatment | PTT-antibiotics | RP | 1.0 W/cm2 808 nm 30 min |
With the addition of PTT, the gentamycin resulted in an approximate 53-fold decrease in the MRSA burden compared with the antibiotic alone | The combined treatment decreased the MRSA on the wounds for about 10 times, compared with the mono-antibiotic group | [203] |
- Abbreviations: CG,cationic guar gum; ICG, indocyanine green; MRSA, methicillin-resistant Staphylococcus aureus; PDA, polydopamine nanoparticles; PDT, photodynamic therapy; PTT, photothermal therapy; ROS, reactive oxygen species; RP, red phosphorus; RT, radiotherapy.
In short, PTT is mainly used in temperature ranges including 40–42°C, 43–50°C, and over 50°C, manifesting strong therapeutic effects and potential for clinical translation for tumor treatment, tissue repair, and antibacterial therapy.
3 PROSPECTIVE AND DISCUSSION
Researchers have already conducted a large number of experiments and achieved certain results in the field of PTT. However, there are still some problems and challenges that need to face. Meanwhile, some new directions can be explored for further development. Although various PTAs have been developed for PTT, PTAs still have different shortcomings and problems which hinder clinical translation. An ideal PTA should have characteristics including good biocompatibility, high PCE, modifiability, and long half-life. Organic PTAs have the inherent advantages of good biocompatibility, excellent biodegradability, and high modifiability. While their shortcomings are low PCE and easy to decompose.52, 208 Compared with organic PTAs, inorganic nanoparticles have high PCE and strong stability, but they are not biodegradable.209-211 Despite many experiments taking cytotoxicity assay for related materials on cells or in vivo, these short-term experiments cannot determine its potential long-term toxicity to the organism. In recent years, a kind of supramolecular bioorganic PTAs have come into researchers' view, and derived the concept of “supramolecular photothermal effects.” The PTAs and other functional structures can self-assemble into an integrated treatment platform through noncovalent intermolecular interactions, thus enhancing the stability of organic PTAs and their PCE.212-214 For example, biliverdin (BV), as an intermediate product of biological metabolism, possess the feature of favorable biocompatibility. After being assembled with metal-binding short peptides, the BV nanosystem acquired further performance of higher PCE and broader NIR absorption.215 Moreover, peptides can control the pathway of energy conversion and disassembly of nano drugs, by altering the coding sequence of amino acids.216, 217 The supramolecular strategy has overcome the shortage of PTAs to a certain extent. But it is still a newly emerging strategy, and needs further research to be applied to clinical practice.
Another important parameter for PTT, tissue penetration is a vital issue for disease treatment. Due to the lack of deep-tissue penetration performances of NIR-I, more biomaterials that respond to NIR-II need to be developed. Under the coverage of 5 mm chicken breast muscles, NIR irradiation at 1064 nm can still increase the temperature of the deep region significantly compared with irradiation at 808 nm.218 Therefore, more NIR-II-responsive PTAs should be constructed, and other strategies to increase the depth of PTT need to be actively put forward.
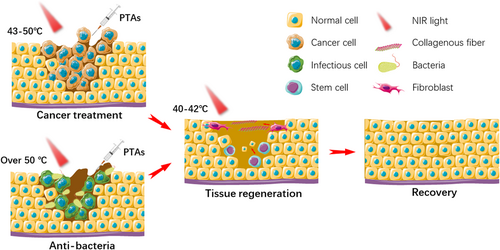
With the development of multidisciplinary cooperation, multifunctional biological materials were designed and prepared to achieve multiple goals. The multifunctional biological materials capable of PTT were applied including bone tumor treatment and bone tissue engineering,219, 220 breast cancer treatment and adipose tissue engineering,221, 222 skin tumor treatment and skin tissue engineering,223 antibacterial treatment and tissue engineering,224 and so on. As shown in Figure 9, tumor tissue or bacteria could be killed and eliminated at treatment temperature and then tissue repair can be promoted at the mild temperature of 40–42°C. However, cancer treatment and tissue repair are treated on the same platform showing the problem of uncontrollability for cancer treatment and tissue repair process, for the reason that we have not studied the physiological mechanism of PTT completely.225 Therefore, it is also necessary to develop some strategies that can inhibit tumors and promote tissue repair at the same time in PTT.
In terms of clinical applications, there are already some clinical trials underway. ICG is the only PTA approved by Food and Drug Administration (FDA).226 In 2011, a pilot clinical trial tried to treat late-stage metastatic breast cancer patients using 0.25% ICG as PTA and glycated chitosan (GC) as immunoadjuvant for laser immunotherapy. In eight patients available for evaluation, the clinical benefit response rate was 75% after the noninvasive irradiation of 805 nm laser. Laser irradiation, ICG, and GC injection were performed every 4 weeks. The power density of irradiation was 1 W/cm2, and each targeted tumor was irradiated for 10 min. After the treatment, there were no significant adverse events except for limited local thermal injury.227 ICG was also used in treating melanoma and was proven to be safe and effective (NCT00453050).228 In recent years, gold nanoshells are also being used as PTA in preclinical studies to treat prostate cancer. Patients were infused with gold-silica nanoshells and given laser irradiation the next day. After the irradiation of 810 ± 10 nm NIR, with the power density of 4.5–6.5 W/cm2 for 3 min. 87.5% (14/16) of lesions were negative for tumors in the ablation zones and the patients did not have severe complications or harmful changes in the genitourinary system.25 Another phase II clinical trial reported by American Urological Association indicated that 72% of biopsies were negative for prostate cancer, drawing the conclusion that nanoparticle-directed FLA is a promising and feasible ablative remedy (NCT02680535).229 Besides, devices for monitoring the extent of thermal coagulation during PTT like a clinical transrectal diffuse optical tomography system are being developed and perfected for accurate PTT.230 In the application of antibacterial, Yue et al. are starting a phase I clinical research, using gold-silver-cuprous oxide (Au-Ag-Cu2O) composite nanogel combined with PTT to treat severe drug-resistant microbial keratitis. This trial is still in the stage of recruiting volunteers (NCT05268718), but we are looking forward to the outcome. Thus, PTT is gradually adapting from laboratory to clinical translation for tumor therapy and has achieved promising results. By innovating next-generation technologies, such as manufacturing safe and efficient PTAs, improving laser equipment, and developing accurate monitoring systems, PTT will eventually be translated into clinical application successfully and expand the scope of application by multiple strategies. The clinical applications are summarized in Table 4.
| Clinical application | PTAs | Therapeutic effect | References | NCT |
|---|---|---|---|---|
| Metastatic breast cancer treatment | ICG | The clinical benefit response rate was 75% | [227] | - |
| Melanoma treatment | ICG | The probability of 12-month overall survival was 70% | [228] | NCT00453050 |
| Prostate cancer treatment | gold-silica nanoshells (AuroShells) | 87.5% (14/16) of lesions were negative for tumor in the ablation zones | [25] | - |
| Prostate cancer treatment | gold-silica nanoshells (AuroShells) | At 3 months, 35 of 45 (77.8%) lesions met the criteria for success focal ablation | [229] | NCT02680535 |
| Microbial keratitis | Au-Ag-Cu2O composite nanogel | - | - | NCT05268718 |
- Abbreviations: ICG, indocyanine green; PTA, photothermal agent.
4 CONCLUSION
In this review, we summarized recent research on the therapeutic effects of temperature-orchestrated PTT (including tissue regeneration, antitumor therapy, and antibacterial treatment). Mild PTT is a promising and thriving treatment because of the low risk of tissue burns. Meanwhile, it often needs to be used in combination with other therapies to synergistically enhance therapeutic effects and reduce side effects. It is worth mentioning that the construction of sophisticated biomaterials can perform orchestrated functions of antitumor/antibacterial treatment and tissue repair, simultaneously. Although some limitations still exist for PTT in clinical translation applications, like the shortcomings of existing PTAs and the imperfection of therapeutic and regeneration platforms, these problems are being gradually resolved with the progress of research. Overall, we are confident that precise temperature control will broaden the applications of PTT in disease treatment after the challenges are well resolved under the continued efforts of clinicians and researchers. Along with the constant development and innovation of technology to design the next generation of biomaterials, PTT-related biomedical applications from the laboratory to the clinic will be realized in the near future.
AUTHOR CONTRIBUTIONS
Nuo Xu: Formal analysis (equal); writing – original draft (equal); writing – review and editing (equal). Xu Zhang: Formal analysis (equal); validation (equal); writing – review and editing (equal). Tingting Qi: Formal analysis (equal); funding acquisition (equal); writing – review and editing (equal). Yongzhi Wu: Formal analysis (equal); writing – review and editing (equal). Xi Xie: Formal analysis (equal); investigation (equal); validation (equal). Fangman Chen: Formal analysis (equal); writing – review and editing (equal). Dan Shao: Conceptualization (equal); funding acquisition (equal); supervision (equal); writing – review and editing (equal). Jinfeng Liao: Conceptualization (equal); funding acquisition (equal); project administration (equal); supervision (equal); writing – review and editing (equal). All authors have read and approved this manuscript.
ACKNOWLEDGMENT
This work was financially supported by the National Natural Science Foundation (32171354, 31972925, and 82072049), the Fundamental Research Funds for Central Universities, Sichuan Science and Technology Program (2021YJ0123), Sichuan Cadre Health Research Project (2021-805), Excellent Youth Fund of Sichuan Cancer Hospital (YB2021028).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Animal experiments are not invloved in this article.
Open Research
DATA AVAILABILITY STATEMENT
No new data were created or analyzed in this review. Data sharing is not applicable to this article.




