Recent advances in the application of functional hydrogels in skin wound healing
Abstract
The effective treatment of skin wounds has long posed a significant challenge in the medical field, impacting patient comfort, quality of life, and the rate and outcome of wound healing. With continuous advancements in science and technology, novel materials have emerged, providing new possibilities for skin wound treatment. Among these, multifunctional hydrogels have shown considerable potential in promoting skin wound healing as a type of wound dressing material. This review systematically examines the progress in the application of multifunctional hydrogels in skin wound healing. Initially, the structure and composition of the skin are introduced. Subsequently, skin wounds are classified, and the wound-healing process is discussed in detail. Traditional and modern dressings are then categorized, with a particular emphasis on the characteristics and applications of hydrogel dressings. The various functions of hydrogels in skin wound healing, including antibacterial, antioxidant, hemostatic, adhesive, stimulus-responsive, and wound status monitoring, are reviewed. The paper concludes with a summary of the existing research gaps and provides insights into the future development directions of multifunctional hydrogels. This review aims to guide the preparation of hydrogel wound dressings and offer theoretical references for the exploration of next-generation functional hydrogels.
1 INTRODUCTION
The skin, the human body's largest organ, plays a crucial role in maintaining overall health and functionality.1 It serves as the body's first line of defense, protecting internal tissues from external threats, regulating temperature, preventing moisture loss, and perceiving external stimuli.2 However, the skin can suffer varying degrees of damage due to external trauma or internal factors. Such damage can manifest in cuts, abrasions, burns, and ulcers, posing risks of pain, discomfort, and even severe infection to patients.3, 4 In certain conditions, such as diabetes or prolonged pressure, the skin's healing capacity may be compromised, making wounds more prone to developing into chronic ulcers, and presenting more significant treatment challenges.5 Consequently, the effective treatment of skin wounds remains a significant challenge in the medical field. The development of safe and effective therapeutic approaches is essential not only to accelerate the wound healing process and alleviate patient pain and discomfort but also to reduce the risks of infection and other complications, thereby enhancing the quality of life of patients.
With the continuous advancement of science and technology, the emergence of novel materials has opened new avenues for wound treatment. In this context, multifunctional hydrogels have garnered significant attention in both research and clinical applications as highly promising novel wound dressing materials. The excellent biocompatibility, permeability, and diverse functionalities of these materials make them highly regarded options for wound management.6, 7 Hydrogel dressings form a soft, transparent gel film on the wound surface, effectively maintaining a moist environment that is conducive to tissue regeneration and wound healing. Compared to traditional dressing materials, hydrogel dressings offer superior permeability, facilitating the entry of cells and nutrients into the wound and thereby accelerating tissue repair processes.8 In addition to maintaining a moist environment, hydrogel dressings possess various additional functions. First, they exhibit excellent antibacterial properties, effectively inhibiting bacterial proliferation in wounds and reducing the risk of infection.9 Second, hydrogels have antioxidant properties, which help alleviate oxidative stress in tissues surrounding the wound and promote healing. Furthermore, hydrogels can quickly prevent bleeding, reducing blood loss from the wound. They also exhibit stimulus-responsive behavior, adjusting according to the wound status to provide more personalized treatment. Given their broad prospects in skin wound treatment, multifunctional hydrogel dressings stand out as an ideal wound treatment material. Their various outstanding characteristics offer patients faster and more effective healing, making them a highly valuable addition to wound care strategies.10, 11 However, despite the significant potential of hydrogel dressings in wound treatment, further clinical research and practical validation are necessary to better understand their effects on different types of wounds and to facilitate their widespread clinical application.
This review systematically examines the progress in the application multifunctional hydrogels in skin wound healing, aiming to provide the medical community with the latest research findings and clinical practice insights. Initially, the structure and composition of the skin are introduced. Next, different types of skin wounds, including acute and chronic wounds, are discussed, along with their characteristics and treatment strategies. Traditional and modern dressings are classified, with an emphasis on the characteristics and application advantages of hydrogel dressings. This review highlights the application of multifunctional hydrogels in wound healing, focusing on their antibacterial, antioxidant, hemostatic, adhesion-promoting, stimulus-responsive, and wound status monitoring properties. Finally, current research achievements are summarized, and future development directions for multifunctional hydrogels are outlined.
2 STRUCTURE AND FEATURES OF SKIN TISSUE
As the human body's largest organ, the skin plays a critical role in maintaining physiological equilibrium and shielding against external threats.12, 13 Its structure comprises the epidermis, dermis, and subcutaneous tissue. As the outermost layer, the epidermis includes the basal, granular, transparent, and cornified layers, which possess a self-renewal ability and prevent moisture loss. Beneath the epidermis, the dermis provides structural support to blood vessels, nerves, glands, and connective tissue, contributing to strength, elasticity, and various physiological functions, such as temperature regulation and sensory perception. Subcutaneous tissue, composed of fat and connective tissue, cushions, insulates, and stores energy while safeguarding internal organs and supporting skin integrity. The skin serves as a natural physical barrier against external hazards and can regulate temperature, transmit sensory information, and manage metabolism.14, 15
Despite its protective functions, the skin is susceptible to damage from trauma, surgery, and various physical and chemical agents.16 Factors such as ultraviolet radiation, chemicals, and environmental microorganisms pose potential threats to the skin. Mechanical injuries such as friction, abrasions, and cuts, commonly compromise skin integrity, increasing susceptibility to microbial infections, including bacteria, viruses, and fungi. Inadequate management of skin injuries can lead to systemic infections with potentially severe consequences.17, 18 Injuries often cause pain, swelling, and inflammatory responses, impacting an individual's quality of life. Therefore, timely and effective cleaning, treatment, and care are crucial for injured skin to minimize potential hazards and promote a favorable healing process.
3 THE HEALING PROCESS OF SKIN WOUNDS
The healing of acute wounds is a complex biological process that involves the coordinated efforts of keratinocytes, fibroblasts, and other cell types to facilitate migration and proliferation, triggering inflammation and granulation tissue synthesis, and ultimately achieving epidermal restoration.19 The specific healing process comprises four stages: hemostasis, inflammation, proliferation, and remodeling (as shown in Figure 1).20, 21 Hemostasis stage: Once a wound forms, platelets rapidly reach the injured site and adhere to endothelial cells, releasing clotting factors (platelet release factors and platelet aggregators) to promote platelet aggregation and the formation of thrombi and fibrin clots. This process stimulates vascular contraction to reduce blood flow and achieve rapid hemostasis, providing appropriate mechanical support to the injured area. Inflammation stage: The wound enters the inflammation stage, where inflammatory cells such as neutrophils and monocytes are recruited to the wound area to initiate an inflammatory response. This response aims to clear pathogens, cells, and other inflammatory mediators, stimulating tissue healing and guiding entry into the proliferation stage.22, 23 Proliferation stage: During the proliferation phase, neovascularization and cell aggregation occur at the wound site, promoting the regeneration of new tissue. Open wounds are gradually replaced by granulation tissue formed by the proliferation of epithelial cells, fibroblasts, and keratinocytes. Meanwhile, basal cells surrounding the wound tissue migrate to the wound surface to form new epithelial cells, ultimately completing re-epithelialization to form scar epithelium. Collagen is rapidly synthesized and deposited, and endothelial cells of blood vessel walls divide to form vascular buds, which then develop into blood vessels or capillaries. Remodeling stage: Finally, the healing process transitions to the remodeling stage, involving collagen rearrangement and scar formation to restore tissue structure and function.
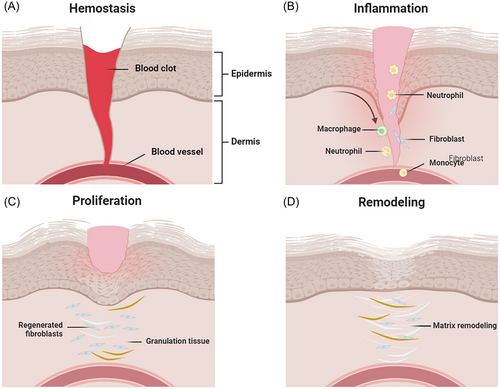
These stages are not strictly time-delineated and often exhibit overlapping characteristics. With continuous advancements in wound management techniques and scientific knowledge, significant improvements have been achieved in acute wound healing. A deeper understanding of the acute wound healing process will aid in developing more effective wound care plans, reducing complications and promoting healing. Integrating technology and scientific knowledge allows for better management of acute wounds, accelerating the healing process, reducing scar formation, and enhancing patients' quality of life.
Optimal skin wound healing requires the meticulous integration of complex biological and molecular events, including cell migration, proliferation, and extracellular matrix deposition and remodeling. Cellular responses to inflammatory mediators, growth factors, cytokines, and mechanical forces must be appropriate and precise. However, this orchestrated healing process is often compromised in chronic wounds, such as diabetic ulcers.24 Various pathogenic abnormalities, ranging from disease-specific intrinsic defects in the blood supply, angiogenesis, and matrix turnover to external factors such as infection and persistent tissue injury, contribute to healing failure.25 Chronic wounds may result from hyperglycemia, sustained inflammatory responses, or recurrent tissue damage. Uncontrolled inflammation can lead to extracellular polysaccharide matrix secretion, protecting bacteria from immune responses and antibiotics.26 Sustained hyperglycemia increases the levels of advanced glycation end products in the blood, inducing high levels of reactive oxygen species (ROS).27, 28 This disruption of the cellular redox balance disturbs metabolism and further damages vascular integrity and tissue regeneration. Chronic wounds often seriously impact patients' health, economy, and psychology, with annual U.S. expenditures exceeding one billion dollars.
Hence, adopting rational and appropriate wound care practices is paramount in managing acute or chronic wounds. A fundamental aspect of wound care is the selection and proper placement of suitable wound dressings, which play a crucial role in wound management by protecting wounds from potential external harm and facilitating cellular migration and proliferation within the wound.29
4 CATEGORIES OF SKIN WOUNDS
A skin wound refers to damage inflicted on body tissues by external forces, resulting in the discontinuity of skin tissue or mucosa. The formation of skin wounds typically involves tissue damage, vascular injury, and inflammation, thereby posing several potential hazards.30, 31 For instance, skin wounds are susceptible to pathogen invasion, leading to infectious diseases. Additionally, they may cause severe bleeding, which can be life-threatening, and induce the formation of scar tissue, affecting normal tissue structure and function and resulting in functional impairment or cosmetic issues. Therefore, timely and appropriate treatment of skin wounds is crucial to minimize potential hazards and promote effective wound healing. Systemic injuries are common and can be devastating, requiring lengthy recovery times.32, 33 Based on their clinical recovery time, skin wounds can be categorized as acute or chronic.
4.1 Characteristics and challenges of acute skin wounds
Acute skin wounds typically arise suddenly from events such as cuts, surgical procedures, and traffic accidents, often leading to significant skin trauma and bleeding. A rapid onset, predictable healing phases, short duration, and minimal complications characterize these wounds. However, several challenges persist in the healing process. Infection Risk: A potential for infection exists, particularly in wounds caused by lacerations, punctures, or burns. Immediate Care Needs: Timely and appropriate medical intervention is critical to prevent complications and promote healing. Pain Management: Effective pain management is essential for patient comfort and to facilitate healing. Scarring: Proper wound care is crucial for minimizing scarring, especially for deep or extensive wounds, which can be challenging. Acute skin wounds encompass various types, including incision wounds, partial-thickness injuries, and wounds involving significant tissue loss. While these different wound types may exhibit variations in the healing process, the fundamental phases of healing remain consistent.20 Understanding and effectively managing each stage of healing is paramount. Acute skin wounds generally heal within a specific timeframe and are influenced by factors such as the size and depth of injury to the epidermis and dermis layers and the patient's age and overall health, including conditions such as diabetes or venous insufficiency. Personalized wound management and comprehensive treatment approaches can optimize the healing of different types of wounds, minimize complications, and improve patients' recovery outcomes.34
4.2 Characteristics and challenges of chronic skin wounds
Chronic skin wounds deviate from the normal healing stages and remain open for extended periods, typically exceeding 4-6 weeks. These wounds commonly arise from underlying diseases, infections, immune dysfunction, or nutritional deficiencies, presenting significant challenges in management and treatment. The types of chronic skin wounds are described below. Pressure ulcers (bedsores), result from prolonged pressure on the skin and often occur in individuals with limited mobility, such as those who are bedridden or use wheelchairs. Diabetic ulcers are found on the feet of diabetic individuals due to poor blood circulation and nerve damage associated with the condition. Venous stasis ulcers arise from venous valve dysfunction in the legs, leading to increased pressure and fluid buildup, causing skin breakdown and ulceration. Arterial ulcers are caused by reduced blood flow due to arterial insufficiency and typically manifest as punched-out ulcers on the lower extremities. Infectious wounds are chronic infections that are prevalent in individuals with compromised immune systems and arise from cellulitis or infected surgical sites. Malignancy-related wounds arise from cancers invading the skin and underlying tissues; they are challenging to heal due to the aggressive nature of the malignancy.
The distinct features observed during the healing of these chronic skin wounds include several phases. Prolonged Inflammation: In contrast to acute wounds, chronic wounds often exhibit extended inflammation due to sustained pro-inflammatory cytokine activity, hindering progression towards the proliferative phase of healing. Impaired Angiogenesis: Chronic wounds frequently exhibit inadequate formation of blood vessels, resulting in reduced delivery of oxygen and nutrients to the wound bed, thereby impeding tissue regeneration. Excessive Protease Activity: Elevated levels of proteases, particularly matrix metalloproteinases, lead to the degradation of extracellular matrix components and growth factors, impeding healing progression. Biofilm Formation: Chronic wounds often harbor biofilms, resilient bacterial communities encased in a protective matrix, complicating infection control and diminishing treatment efficacy. Reduced Growth Factor Levels: Growth factors crucial for healing, such as platelet-derived growth factor and transforming growth factor-beta, are typically depleted in chronic wounds, thereby hindering tissue repair. Presence of Senescent Cells: Chronic wounds frequently contain senescent cells, which secrete inflammatory mediators and perpetuate a nonhealing state, contributing to delayed wound closure.35, 36
Managing chronic wounds poses significant clinical challenges due to their increasing incidence and associated morbidity. The etiology, treatment outcomes, and prognosis vary widely, complicating their definition and management. While some chronic wounds are termed complex wounds, even traumatic and acute wounds can be complex. However, chronic wounds typically refer to superficial, partial, or full-thickness skin injuries that heal through secondary intention. In the UK, the prevalence of chronic wounds is 1.47/1000.37 Some chronic wounds are complications of other diseases; for instance, diabetic foot ulcers are caused by diabetes. Individuals with diabetes are also predisposed to developing other chronic wounds, such as venous stasis ulcers.28, 38, 39 Despite undergoing a normal wound-healing process, chronic wounds are challenging to repair promptly and are highly susceptible to infection, which can lead to severe complications. In developed countries, an estimated 1-2% of individuals will experience chronic wounds during their lifetime, with approximately 6.5 million affected patients with chronic wounds in the U.S. alone.1, 40 In developing countries, the etiology of chronic wounds may differ significantly and is influenced by factors such as malnutrition, parasites, and chronic fungal infections.
However, impaired healing occurs across all chronic wounds, sometimes called “nonhealing” wounds by clinicians.35 Critical clinical issues in chronic wound treatment include impaired angiogenesis, prolonged inflammation, and persistent infection. New treatment modalities have been developed to address the complexities of chronic wounds, including bioactive wound dressings, skin equivalents for promoting epithelialization, matrix metalloproteinase inhibitors, and the topical application of growth factors. While attention to understanding and treating chronic wounds there has been renewed, significant gaps persist in comprehending their characteristics and mechanisms of impaired healing. Advances in basic research, improved wound dressings, and surgical techniques have led to progress in managing chronic wounds. However, challenges remain, necessitating further research to improve knowledge, develop effective treatments, reduce societal and economic burdens, and optimize health care management for chronic wounds, representing a potential silent epidemic.41
5 CLASSIFICATION OF SKIN WOUND DRESSINGS
According to records from ancient civilizations on clay tablets, the history of wound dressings can be traced back to approximately 2000 BC. Ancient civilizations used various materials to cover wounds to prevent infection and promote healing. For instance, ancient Egyptians utilized natural substances such as honey and animal fat to cover wounds, while ancient Greeks used beeswax and olive oil. Over time, people began using materials such as gauze and bandages to create different types of dressings.42 The first modern wound dressings emerged in the mid-1980s, featuring essential properties such as moisture retention and absorption of wound exudate (e.g., polyurethane foam, hydrocolloids, and iodine-containing gels).43 Currently, wound dressings have evolved into various types, including antimicrobial dressings, hydrogel dressings, and transparent dressings, to meet the therapeutic needs of different types of wounds. Modern multifunctional wound dressings protect wounds, promote healing and reduce the risk of infection.
5.1 Traditional skin wound dressings
Over the years, traditional wound dressings such as gauze, bandages, and cotton wool have been commonly used to maintain wound cleanliness and prevent pathogen infection because of their low cost, convenience of use, and ease of manufacturing.44 Gauze fibers are often employed to absorb exudate and fluids from open wounds but require frequent replacement to prevent the maceration of healthy tissues. Bandages, typically composed of natural cotton, cellulose fibers, or synthetic polyamide materials, are utilized to secure lighter dressings or as compression bandages. Due to wound exudation, traditional dressings necessitate frequent changes to prevent infection and are often prone to adhere to the wound site, resulting in secondary trauma upon removal.45 Furthermore, traditional wound dressings tend to be dry and cannot provide the moist environment necessary for accelerating wound healing processes. Modern dressings can replace traditional dressings because of their more advanced functionalities, better suitability for diverse wound requirements, and ability to promote wound healing.
5.2 Modern wound dressings
Modern wound dressings have undergone significant advancements, offering exceptional performance and versatility in clinical applications. They exhibit excellent biocompatibility, facilitating seamless integration with human tissues and minimizing allergic reactions and irritation. Typically composed of degradable materials, these dressings help obviate the need for secondary surgeries and alleviate patient discomfort. Moreover, they excel in moisture retention, creating an optimal healing environment that fosters cell regeneration and tissue repair. These attributes have propelled the widespread use of modern synthetic dressings in medical settings, enhancing the efficacy and comfort of wound care practices. Modern dressings are categorized into various forms, such as films, foam sheets, and hydrogels, which are distinguished by their manufacturing materials and specific therapeutic properties.
5.2.1 Hydrogel dressings for enhanced skin wound healing
Hydrogel, a highly hydrated three-dimensional network polymer resembling the extracellular matrix, exhibits excellent moisture absorption, maintaining a moist wound environment that promotes fibroblast proliferation and keratinocyte migration.46, 47 Its porous structure and breathability support oxygen transmission, aiding in tissue regeneration and wound healing, making it an optimal dressing choice.48 In 1962, Winter et al.49 reported that a moderately humid environment favored skin cell regeneration, forming the basis for the role of hydrogels not only in promoting wound healing but also in cooling wounds to alleviate patient pain. In 1989, Rosick et al. invented hydrogel dressings for wounds, initiating ongoing research efforts to enhance hydrogels by refining their pore size, biocompatibility, biodegradability, mechanical strength, and stability to meet diverse wound treatment needs.24, 50, 51 This continuous innovation has expanded the application of hydrogels in the medical field, establishing them as crucial tools for wound treatment.
Furthermore, hydrogels serve as platforms for controlled release systems, delivering drugs, cytokines, and stem cells to enhance wound healing outcomes.22, 52-54 They are classified into natural polymer hydrogels and synthetic polymer hydrogels based on their composition, as summarized in Table 1, along with their characteristics.
| Polymers | Physicochemical properties | Therapeutic features | Types of skin wounds | Refs. |
|---|---|---|---|---|
| HA | Noncrosslinked hybrid hydrogel | Antibacterial, free radical scavenging, and anti-inflammatory properties | Skin wounds of diabetes mice | [55] |
| Aldehyded HA | Crosslinked with thiol-ene click reaction, self-healing, and injectable | Free radical scavenging and angiogenesis | Acute wounds on rat skin | [56] |
| Methacrylated HA | UV photopolymerization, encapsulation of extracellular vesicles | Improving angiogenesis and nerve innervation | Pressure ulcers of diabetes mice | [57] |
| Thiolated HA | Michael addition reaction, electrostatic interactions, self-healing | Antibacterial, antioxidant, and anti-inflammatory properties | Skin wounds of diabetes mice | [58] |
| CS | Electrostatic interaction, Loaded with exosomes | Promotes fibroblast migration | Acute wounds on mouse skin | [59] |
| Methacrylated CS | UV photopolymerization, loaded with polydopamine | Anti-inflammatory and antibacterial properties | Acute wounds on mouse skin | [16] |
| Quaternized CS | Schiff base with aldehyde groups | Antibacterial properties, promotes collagen deposition | Acute skin wounds in rats; Acute wounds on mouse skin | [60, 61] |
| Thiolated CS | Loaded with Histatin1 | Accelerating migration, and angiogenesis | Acute skins wound in rats | [62] |
| Phenylboronic-modified CS | Dynamic phenylborate ester bond, dual pH- and glucose dual-responsive material | Loading insulin/L929 promotes angiogenesis and collagen deposition | Skin wound of diabetes rats | [63] |
| Phenolic acids modified CS | Schiff base/Michael addition | Antioxidant, and anti-inflammatory properties | Acute skin wounds in rats; burn wounds on mouse skin | [64, 65] |
| ODEX | Schiff base with amino groups | Anti- inflammatory propertiesion, promoting angiogenesis and the regeneration of collagen and epithelial tissue | Burn wounds on mouse skin | [65] |
| Catechol ODEX | Schiff base with amino groups, pH-responsive, and injectable | Antibacterial, antioxidant, and pro angiogenic properties | Skin wounds of diabetes rats | [66] |
| SA | Calcium ions, Loaded with AgNPs | Antibacterial, antioxidant, and proangiogenic properties | Acute skin wound in rats | [67] |
| Aldehyded SA | Schiff base with amino groups | Antibacterial, antioxidant, and hemostasis properties | Acute wound on mouse skin | [68] |
| SF | Hydrogen bonding crosslinking, injectable | Antibacterial and anti-inflammatory properties | Acute wound on mouse skin | [69] |
| Methacrylated SF | UV photopolymerization, Load with glycyrrhizic acid, Injectable | Anti-inflammatory properties and promotion of macrophage polarization (M2) | Skin wound of diabetes rats | [70] |
| GelMA | UV-cured handheld bioprinters, Load with VEGF | Promoting angiogenesis and epithelialization, reducing scarring | Skin wounds of pigs | [71] |
| Oxidized konjac glucomannan | Schiff base with amino groups, injectable | Clearing ROS, antibacterial and anti-inflammatory properties | Burn wounds on mouse skin | [72] |
| PVA | Dynamic bonds with borax, self-healing | Antibacterial, promote the migration of fibroblasts and angiogenesis | Skin wounds of diabetes rats | [73] |
| PELA | Temperature sensitive, load with MOF and curcumin | Antibacterial, anti-inflammatory, and immunomodulatory properties | Skin wounds of diabetes rats | [74] |
| PLGA-PEG-PLGA | Temperature sensitive, load with MXene nanosheets | Clearing ROS, antibacterial properties, and hemostasis | Skin wounds of diabetes mice | [75] |
| Polyacrylamide | Load with polypyrrole nanoparticles | Promoting cell adhesion, proliferation, elongation of electrically excitable cells, and regeneration of skin tissue | Acute skin wounds in rats | [76] |
| Poly(N-isopropyl acrylamide | Temperature-sensitive, load with AgNPs, adhesive | Antibacterial properties and promoting wound closure | Acute wounds on mouse skin | [77] |
- Abbreviations: L929 cells, mouse fibroblasts; HA, hyaluronic acid; SA, sodium alginate; ODEX, aldehyded dextran; SF, silk fibroin; CS, chitosan; GelMA, methacrylated gelatin; VEGF, vascular endothelial growth factor; ROS, reactive oxygen species; PVA, polyvinyl alcohol; PELA, poly (ethylene glycol)-poly(ε-caprolactone-co-lactide) hybrid; MOF, metal–organic framework; PLGA-PEG-PLGA, poly(lactic acid-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(lactic acid-co-glycolic acid).
Natural polymer hydrogel for skin wound healing
Natural polymer hydrogels are three-dimensional network structures formed from naturally derived polymer materials that possess excellent biocompatibility and can reduce host tissue immune and inflammatory responses during skin wound healing. Natural polymer hydrogels, including gelatin, alginate, chitosan,78 agarose, and hyaluronic acid,79 have been extensively studied and applied in skin wound healing. Additionally, these natural materials exhibit good degradability, allowing them to gradually degrade in the wound microenvironment, significantly minimizing the secondary damage caused by dressing changes to the wound. Moreover, natural polymers are rich in various active groups, such as amino, hydroxyl, and carboxyl groups, which can be chemically modified or functionalized to impart additional functionalities to natural polymers. For instance, altering the chemical structures of hydroxyl, carboxyl, or amino groups can adjust the swelling properties, drug delivery performance, and biological activity of hydrogels. By controlling the content and positioning of these active groups, the release rate and direction of the drugs loaded in the hydrogels can be regulated, achieving more precise drug delivery. Furthermore, these active groups increase the susceptibility of hydrogel to hydrolytic enzymes in the body, which ultimately degrade the hydrogels into harmless metabolic products, thereby reducing their negative environmental impact.
Despite the numerous advantages of natural polymer hydrogels for wound healing, they also exhibit several drawbacks. Their insufficient mechanical strength may prevent adequate support and protection, particularly for more extensive or more profound wounds. Excessive water absorption can increase the gel volume, dissolution, or rupture, affecting gel stability and durability. The degradation rate can be challenging to control due to the influence of wound environmental conditions, such as temperature, pH, and enzymes. Most natural polymer hydrogels lack antibacterial properties, posing a risk of wound infection. Researchers have employed various strategies to address these limitations, including crosslinking techniques, the incorporation of nanomaterials, chemical modifications or adjustments in component ratios, the introduction of antibacterial agents, and copolymerization with synthetic polymers. These approaches aim to enhance the performance of natural polymer hydrogels in wound healing applications, providing more reliable support and assurance for their clinical use.
Synthetic polymer hydrogel for skin wound healing
Synthetic polymers are high-molecular-weight materials synthesized through chemical methods, allowing their molecular structure and properties to be precisely controlled by adjusting the type of monomer and polymerization conditions. Common synthetic polymers include PVA, poly(ε-caprolactone), polyurethane, polyvinylpyrrolidone, polyethylene glycol, and polylactic acid. Due to their controllable physical and chemical properties, these polymers are widely used in the medical field, particularly as skin wound dressings. Compared to natural polymer hydrogel wound dressings, synthetic polymers can be molecularly designed to achieve specific mechanical strength, elasticity, water absorption, and degradation rates, which are crucial for meeting the treatment needs of different types of wounds. Moreover, the production process of synthetic polymers is more standardized, providing high purity and consistency, ensuring product stability and reproducibility, and reducing uncertainties in treatment. However, some synthetic polymers may still exhibit deficiencies in biocompatibility and biodegradability, potentially causing inflammatory responses or incomplete degradation. Bioactive molecules (such as peptides and adhesive proteins) or combinations with natural polymers, drugs, and antibacterial agents can be introduced to form multifunctional composite materials to enhance wound healing effectiveness. For instance, Irudayaraj et al.80 developed a hybrid hydrogel by combining PVA, gelatin, and borax loaded with exosome-coated oxygen nanobubbles. This hybrid hydrogel demonstrated potential in enhancing healing, promoting angiogenesis, facilitating exosome delivery, alleviating hypoxia, and reducing inflammation in a full-thickness wound model in male rats.
6 APPLICATION OF FUNCTIONAL HYDROGEL IN SKIN WOUND HEALING
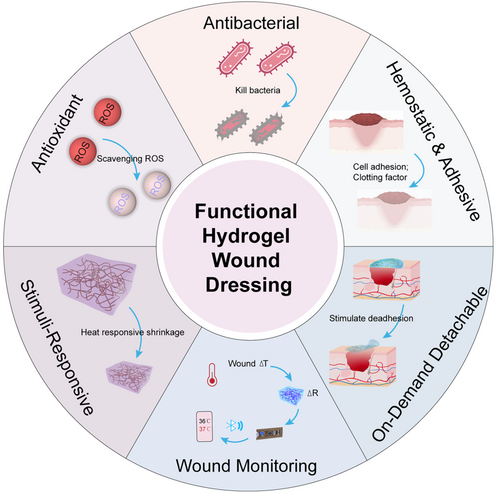
Advances in clinical wound healing requirements are intensifying demands on hydrogel materials employed as wound dressings. Initially serving the essential functions of physical coverage and isolation, hydrogel wound dressings are evolving toward a “functionalized” paradigm. This evolution aims to adapt to diverse wound conditions, swiftly enhancing healing outcomes. Diverse functional hydrogels with antibacterial, antioxidant, anti-inflammatory, hemostatic, and stimuli-responsive properties have emerged (Figure 2). These specialized hydrogels are tailored to address the intricacies of various wounds, providing enhanced therapeutic efficacy.32, 81, 82 Table 2 summarizes the preparation methods and functional characteristics of multifunctional hydrogel wound dressings.
| Composition | Crosslinking method | Functional characteristics | Main outcome | Refs. |
|---|---|---|---|---|
| PVA, borax, TA, guar gum | Borate ester bonds, hydrogen bonds | Self-healing; antioxidant and anti-inflammatory properties: clearing DPPH, upregulation of the M2 macrophage biomarker CD206; antimicrobial properties: E. coli, MREC, S. aureus, and MRSA; biocompatibility: L929 cells | Release the antibacterial and antioxidant TA, promote angiogenesis by inducing M2 macrophage polarization through the JAK/STAT pathway | [83] |
| GA-Ag NPs, carrageenan | Physical cross-linking (carrageenan heating-cooling) | Photothermal and antibacterial properties: E. coli, S. aureus; Biocompatibility: NIH-3T3 cells. | Kill bacteria at the wound site, clear inflammation, and promote wound healing | [84] |
| TA-strontium NPs, GelMA | UV photopolymerization; hydrogen bonds | Antioxidant: scavenging ABTS+; biocompatibility: HDFBs, HaCaTs, HUVECs | The release of Sr2+ and TA facilitates the recruitment and upregulat of the expression of extracellular matrix remodeling genes in macrophages | [85] |
| Caffeic acid grafted CS, Hydroxypropyl CS, ODEX, IGF1 | Dynamic Schiff base (amino and aldehyde groups) | pH-responsiveness; sprayable self-healing; self-adaptability; antibacterial properties: E. coli, S. aureus; antioxidant: scavenging DPPH | Upregulates the expression of angiogenic and anti-inflammatory cytokines, downregulates the pro-inflammatory cytokines TNF-α and IL-6, accelerates angiogenesis and skin tissue regeneration in wound healing | [86] |
| GA grafted polylysine, PEGDA, lipoic acid | Hot melt ring-opening polymerization | Instant wet adhesion (5 s); rapid hemostasis; antibacterial: E. coli, S. aureus; biocompatibility: L929 cells | Upregulates the expression of α-SMA and CD31 to promote angiogenesis | [87] |
| SFMA, glycyrrhizic acid, Zn2+ | UV photopolymerization; glycyrrhizic acid self-assembly | Injectability; immunoregulatory properties; biocompatibility: Raw264.7 and RS-1 cells | Stimulates the expression of angiogenic cytokines, promoting the polarization of macrophages toward the M2 phenotype | [88] |
| Methacrylated carrageenan, PNIPAm, PPy-PDA NPs, ZIF-8 | UV photopolymerization; electrostatic interaction | Dually thermoresponsive; photothermal–chemical and antibacterial properties: S. aureus and E. coli; photo-to-thermal converting ability; biocompatibility: L929 cells | Kills bacteria at the wound site, reduces the inflammatory response, promotes vascular regeneration, and enhances collagen deposition | [89] |
| Dopamine-grafted HA, Ti3C2@PDA NSs | H2O2/HbO2 oxidation coupling | Injectable; self-healing; antibacterial properties: S. aureus and E. coli; ROS scavenging capacity: H2O2 (Ti(SO4)2 solution), O2•– (NBT), and •OH (salicylic acid probe); RNS scavenging ability: scavenging DPPH and ABTS; hemostasis | Kills bacteria, clears ROS, alleviates hypoxia, promotes M2 macrophage polarization, and enhances angiogenesis | [90] |
| Methacrylate fish gelatin, fish gelatin, Protocatechuic aldehyde | UV photopolymerization; | Bioadhesive property; self-healing; antibacterial properties: S. aureus and E. coli; Antioxidant activity: scavenging O2•–, •OH, DPPH; immunomodulatory properties; biocompatibility: L929 cells | Clears bacteria at the wound site, enhances the transformation of M1 macrophages to M2 phenotype, and reduces inflammation. | [91] |
| Adipic dihydrazide modified gelatin, Oxidized Konjac Glucomannan, MXene@TiO2 | Schiff base reaction | Hemostasis; antioxidant: scavenging DPPH; Antibacterial activity: S. aureus and E. coli; biocompatibility: L929 cells | Kills bacteria at the wound site and eliminates excess reactive oxygen species, accelerating collagen deposition and angiogenesis | [92] |
| Dopamine-grafted collagen, aldehyded starch, Ca2+ | Schiff base reaction, hydrogen bonding, electrostatic interactions | Tissue adhesiveness (62 ± 4.8 KPa); high sealing performance (153.2 ± 35.1 mmHg); fast self-healing ability; quick hemostasis | Good biocompatibility promotes the regeneration of skin wound tissue | [93] |
| PNIPAM, HA, N,N’-Methylene bisacrylamide, glutaraldehyde | Free radical polymerization, nucleophilic addition reaction | Thermostimulated autoshrinkage and tissue adhesion; biocompatibility: L929 cells | Inhibit inflammatory response, promote angiogenesis and granulation tissue formation | [94] |
| Carborxymethy CS, Protocatechualdehyde, Fe3+ | Coordination bond, Schiff base bond | pH-sensitive; self-healing; pH monitoring capability; adhesive; antibacterial properties: S. aureus and E. coli; biocompatibility: L929 cells | Prevents bacterial infection of the wound, reduces wound inflammation, and accelerates wound healing | [95] |
| CS, HA | Electrostatic interaction | Antibacterial activity: S. aureus, MRSA, and E. coli; biocompatibility: L929 cells | Eliminates MRSA infection and promotes epidermal regeneration, collagen deposition, and angiogenesis | [55] |
| Thioctic acid, AgNPs | Ring-opening polymerization, coordination interactions | Self-healing; regenerative; hemostatic; antibacterial properties: MRSA, E. coli | Reduces ROS levels in the wound area to promote angiogenesis, reduce inflammation, and eliminate bacteria | [96] |
| Agarose, PVA, Ag, N-carboxyethyl CS | Hydrogen bonds, borate ester bonds, | Good transparency; on-demand dissolvable; self-healing; biocompatibility: L929 cells | Induces angiogenesis, enhances collagen deposition, controls wound infection, and thereby promotes wound healing | [97] |
- Abbreviations: GA-Ag NPs, gallic acid functional silver nanoparticles; HDFBs, human dermal fibroblasts; HaCaTs, human keratinocytes; HUVECs, human umbilical vein endothelial cells; IGF1, insulin growth factor-1; MREC, meropenem-resistant E. coli; NBT, nitroblue tetrazolium; PNIPAM, poly(N-isopropylacrylamide); PPy-PDA NPs, polypyrrole–polydopamine nanoparticles; Ti3C2@PDA NSs, polydopamine (PDA)-coated Ti3C2 MXene nanosheets; ZIF-8, Zn2+-derived zeolitic imidazolate framework.
6.1 Antibacterial hydrogel for skin wound dressings
Researchers are actively exploring advanced antibacterial materials in response to the increasing threat posed by drug-resistant pathogens. As porous 3D materials, antibacterial hydrogels have garnered considerable attention as viable alternatives for antibacterial applications.87, 98, 99 Depending on their matrix and antibacterial agent, antibacterial hydrogels are categorized into antibiotic-containing hydrogels, hydrogels incorporating inorganic nanoparticles, and hydrogels with inherent antibacterial properties.99 The advent of antibiotics marked a significant milestone in combating infectious diseases such as skin and soft tissue infections. Antibiotics exert their therapeutic effects through four primary mechanisms: inhibition of bacterial cell wall synthesis, disruption of critical metabolic pathways, interference with protein synthesis, and inhibition of nucleic acid synthesis.100 Despite the discovery of numerous antibiotics, less than 1% of antibiotics are currently utilized in clinical settings due to concerns regarding toxicity and limitations in host cell uptake.101, 102 To date, tetracycline,103-105 ciprofloxacin,106-110 gentamicin,111-113 and sulfamethoxazole114-116 have been widely used to prepare antibacterial hydrogel wound dressings. These antimicrobial agents are effectively incorporated into hydrogels through electrostatic interactions, hydrogen bonding, hydrophobic interactions, and van der Waals forces. These interactions critically influence the drug distribution, stability, release rate, and release mechanism within the hydrogel network. Understanding these interaction dynamics facilitates the optimization of hydrogels as drug carriers, enabling precise control over drug release kinetics and enhancing drug delivery efficacy. Furthermore, variations in hydrogel compositions and ratios significantly impact their physical and chemical properties, including mechanical strength, water absorption capacity, responsiveness to environmental stimuli (e.g., temperature and pH), and degradation rates. These properties, in turn, influence the release profiles of encapsulated drugs, thereby shaping the therapeutic efficacy of hydrogel-based wound dressings. For example, Ding et al.117 reported the fabrication of a dual-crosslinked interpenetrating network hydrogel based on poly(acrylic acid-co-hydroxyethyl methacrylate), synthesized via free radical polymerization of hydroxyethyl methacrylate and acrylic acid. This hydrogel was simultaneously loaded with the antibiotic gentamicin (Gen) as a dynamic physical crosslinker. The immobilization of Gen within the hydrogel matrix occurred through electrostatic interactions between the carboxyl groups of polyacrylic acid and the amino groups of gentamicin, thereby imparting pH-responsive characteristics to the drug release profile. This research documented sustained antibacterial efficacy against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) bacteria for a duration exceeding 28 days. Ji et al.118 reported a novel injectable and pH-responsive hydrogel wound dressing composed of oxidized sodium alginate and hyaluronic acid-ethylenediamine grafting. Tetracycline hydrochloride was incorporated into the hydrogel, attenuating the release rate of tetracycline and thereby exerting a substantial bacteriostatic effect that persisted throughout 120 h of antibacterial therapy. These antibiotics play a critical role in wound dressings, aiding in preventing and treating infections while promoting wound healing. However, the careful selection and use of antibiotics are essential to avoid the development of resistance and other potential adverse effects. Future research on antimicrobial hydrogel wound dressings should focus on identifying and evaluating novel antimicrobial agents, such as antimicrobial peptides (AMPs) and natural extracts.
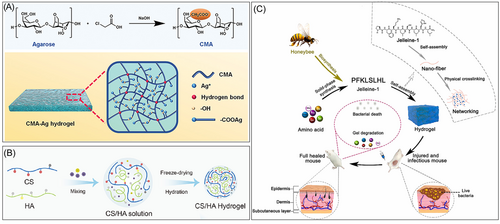
Due to their unique physicochemical properties—their ultrasmall size and high surface reactivity—nanomaterials have emerged as alternative tools to overcome antibiotic resistance. Common nanomaterials include metal-based nanoparticles (such as AgNPs119, 120 and copper nanoparticles121), metal oxide nanoparticles (composed of zinc oxide,122 cerium oxide,123 and yttrium oxide124), and other substances with antibacterial activity. They disrupt bacterial membranes, penetrate cells, and interact with cellular components such as DNA, ribosomes, and enzymes, disrupting standard cellular mechanisms.125 The bactericidal mechanisms of nanomaterials hinge on their core materials, size, and surface functionalization. AgNPs have been extensively applied in various wound dressings due to their broad-spectrum and efficient antibacterial activity. For instance, Mao et al.126 developed an agarose-based hydrogel dressing by complexing the hydroxyl groups of carboxymethyl agarose with Ag+ that exhibited significant antibacterial activity (Figure 3A). Wang et al.128 synthesized Gel-DA@AgNPs by reducing dopamine-modified gelatin, resulting in a hydrogel dressing with excellent hemostatic and antibacterial properties by combining it with agarose and borax. In addition to its inherent antibacterial properties, Ag can be combined or combined with various materials, such as nanoparticles. For example, Liu et al.129 prepared an antibacterial and hemostatic hydrogel by loading AuNPs into the cavities of halloysite nanotubes and mixing them with chitin. Li et al.130 prepared a chitosan-based hydrogel composite with Ag-Au NPs to treat bacteria-infected wounds. Despite the excellent antibacterial effects of these hydrogels, the physical and chemical instability of nanoparticles often limits their development. Surfactants such as polyvinylpyrrolidone and PVA can stabilize AgNPs but may also impact their size. Therefore, improving nanoparticle stability is crucial in designing antibacterial hydrogels based on AgNPs to control antibacterial activity while effectively minimizing toxicity.
Antimicrobial hydrogels with inherent activity generally refer to hydrogels containing natural antimicrobial components that exhibit fewer side effects than traditional antimicrobial hydrogels.131 CS, a natural cationic hydrophilic polymer, is characterized by its excellent biocompatibility, hemostatic properties, and antimicrobial activity.132-134 Its antimicrobial efficacy primarily stems from the amino and hydroxyl groups within its molecular structure that interact with anionic components on bacterial cell membranes, leading to membrane disruption and subsequent bacterial death. Additionally, chitosan can chelate metal ions within bacterial cells, interfering with metabolic processes. CS synergistically interacts with other natural polymers, such as HA, gelatin, and cellulose, commonly utilized in developing composite wound dressings and tissue engineering scaffolds. For example, Peng et al.135 developed a polysaccharide-based bioadhesive hydrogel using carboxymethyl chitosan and acrylic fiber, leveraging the inherent antimicrobial properties of CMSCs to achieve effective antimicrobial activity without the need for antibiotics. Chen et al.55 designed a non-cross-linked CS/HA hybrid antimicrobial hydrogel that exhibited excellent antibacterial effects against MRSA. In vivo, studies of the wounds of diabetic mice infected with MRSA indicated that CS/HA hydrogels significantly facilitated wound healing by eliminating MRSA infections and promoting epidermal regeneration, collagen deposition, and vascularization.
Furthermore, AMPs are abundant, short, cationic biomolecules produced by tissues and cells of various plant and invertebrate species.136 Over 880 different AMPs have been identified or predicted from nucleic acid sequences. AMPs are widely believed to bind to membranes, leading to bacterial destruction.137 AMPs exhibit broad-spectrum bactericidal activity against gram-positive bacteria, gram-negative bacteria, and fungi, making them a promising choice for developing antimicrobial composite materials.138, 139 For instance, Zhu et al.140 designed an antimicrobial hydrogel dressing composed of oxidized β-glucan and the AMPs CH3(CH2)6C(O)-GIKKIIKKI-NH2 (C8G2) through a Schiff base reaction. C8G2 is released in the mildly acidic environment of bacteria-infected wounds, inhibiting bacterial growth and reducing inflammation in vitro and in vivo, thereby accelerating the healing of MRSA-infected skin wounds. Wang et al.127 utilized the self-assembly of the natural AMPs Jelleine-1 nanofibers to develop an AMPs-based hydrogel that exhibited excellent antimicrobial activity and biodegradability. ε-Polylysine (ε-PL), a natural peptide formed by lysine residues linked via ε-amine and α-carboxyl groups, is widely utilized in the development of antimicrobial hydrogels due to its broad-spectrum antimicrobial activity, low toxicity, and excellent biocompatibility. The abundant functional groups on ε-PL facilitate chemical modification and customization for designing antimicrobial hydrogels with various functionalities, such as injectability, photocuring, and adhesion (as shown in Figure 3B). For instance, Qian et al.141 synthesized an injectable photocurable hydrogel by modifying ε-PL with methacrylic acid glycerol ester and γ-poly(glutamic acid), which possessed outstanding antimicrobial properties and biocompatibility.
6.2 Antioxidant hydrogel for skin wound dressings
ROS are byproducts of cellular oxidative metabolism produced by inflammatory cells such as neutrophils and other white blood cells,142, 143 playing a crucial role in wound healing. They are involved in regulating the inflammatory response, promoting the clearance of inflammatory cells, and resolving inflammation, thereby creating a favorable microenvironment for wound healing.142 However, excess ROS can lead to oxidative damage, apoptosis, and inflammation, ultimately hindering angiogenesis and inhibiting wound healing. In the case of a ROS imbalance, the addition of antioxidants can effectively promote enzyme repair and improve metabolism, ultimately restoring the average growth of cells.24 Common natural antioxidants include non-thiol compounds (plant polyphenols), thiol compounds, vitamins (ascorbic acid and vitamin A), and various enzymes (glutathione reductase and glutathione peroxidase) (as shown in Figure 4). These antioxidants exhibit excellent properties that help protect the body from ROS-mediated damage. Antioxidant hydrogels can eliminate excess ROS in wounds, reduce cellular oxidative stress, improve the pathological microenvironment of wounds, and achieve rapid wound healing. Plant polyphenols are a class of secondary metabolites with polyphenolic structures that are renowned for their potent antioxidant properties and are widely used in wound healing treatments. Common polyphenols include dopamine, tannic acid (TA), catechins, GA, and theaflavins.144 The phenolic hydroxyl groups in their structures have a strong ability to scavenge ROS and other free radicals, making them widely used in the preparation of antioxidant hydrogels.145
Typically, antioxidant properties are imparted to hydrogels through the direct addition of polyphenolic substances or by modifying polymers. For example, Gao et al.146 developed an antioxidant hydrogel wound dressing using TA, PVA, agarose, and hyperbranched polylysine. In studies of MRSA-infected wounds, this hydrogel exhibited excellent antioxidant and antibacterial capabilities, effectively killing bacteria in vivo, reducing inflammation, and promoting tissue regeneration. Additionally, Gou et al.147 developed an antioxidant hydrogel (OBGTP) loaded with tea polyphenols based on oxidized polysaccharides and adipic acid dihydrazide-modified gelatin. In a rat skin injury model, OBGTP exerted significant antioxidant effects, reducing the expression of inflammatory factors and promoting rapid wound healing. Additionally, phenolic groups (such as tyrosine, catechol, and hydroquinone) can undergo oxidative coupling under the influence of HRP/H2O2, hemin/H2O2, laccase, tyrosinase, sodium periodate, and sodium hydroxide to form injectable antioxidant hydrogels. The type and concentration of the oxidant, as well as the content of phenolic groups, affect the gelation rate, gelation time, and gel strength of phenolic-crosslinked hydrogels. For example, Tan et al.148 utilized HRP/H2O2 to catalyze the formation of antioxidant hydrogels (HA-DA@rhCol) from dopamine-modified hyaluronic acid (HA-DA) loaded with recombinant human type III collagen (rhCol). Their study indicated that a concentration of 1.5% HA-DA@rhCol resulted in a suitable gelation time (30 s) and injectability at 37°C. Furthermore, DPPH radical scavenging assays confirmed the in vitro antioxidant capacity of the HA-DA@rhCol hydrogel. Additionally, it effectively scavenged ROS, alleviated oxidative stress in chronic wound tissues, and promoted wound healing. Due to the mild reaction conditions, high specificity, and good biocompatibility of enzymatic crosslinking, it is particularly suitable for preparing hydrogel wound dressings. However, the activity of enzymes is susceptible to factors such as temperature and pH, necessitating specific storage and usage conditions.
6.3 Hemostatic and adhesive hydrogel for skin wound dressings
Wounds resulting from major trauma or surgery can lead to serious consequences such as hemorrhage, infection, shock, organ failure, and even death.2, 87 Therefore, achieving efficient and rapid hemostasis is crucial in wound treatment.149, 150 Hemostasis occurs in the early stages of wound repair, and hemostatic hydrogel dressings can facilitate rapid bleeding control, positively promoting wound healing. Current research indicates that hemostatic hydrogels physically seal wounds and enrich coagulation factors by absorbing wound exudates.151 Additionally, hemostatic and adhesive hydrogels can adhere seamlessly to the wound area for extended periods, reducing the risk of infection from the external environment. Numerous hemostatic dressings (such as HemCon, Surgicel®, and fibrin bandages) have been extensively developed for clinical treatment. However, weak adhesion to wet tissue surfaces often limits the hemostatic effectiveness of these materials.
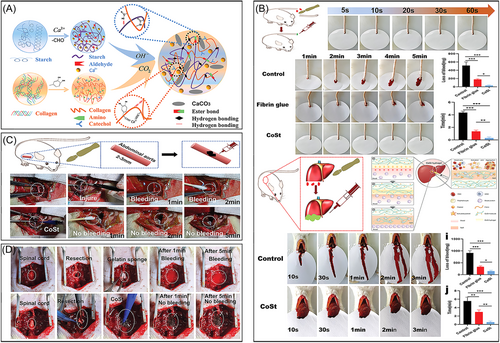
Inspired by the strong adhesion properties of marine mussels, researchers have developed a variety of phenolic-based adhesive hydrogel wound dressings. One of the most extensively studied adhesives from marine organisms is 3,4-dihydroxyphenylalanine, which is found in mussel foot proteins. DA can interact with various surfaces through physical (hydrogen bonding, π–π interactions, electrostatic interactions, and coordination) or chemical interactions (Michael addition and Schiff base reactions). Notably, during the free radical polymerization process, introducing high concentrations of DA may affect the polymerization of the hydrogel. Common strategies for preparing adhesive hydrogel wound dressings involve introducing functional groups or small molecules with adhesive properties to enable multiple interactions between the hydrogel and surrounding tissues. For instance, phenol hydroxyl and aldehyde groups can crosslink with thiol and amino groups on tissues via Michael addition and Schiff base reactions, resulting in stable tissue adhesion. Hemostatic hydrogels developed using a caffeic acid-modified gel (CaG), ODEX, and ZnO have shown excellent hemostatic performance in rat liver and heart bleeding models.152 Furthermore, Zn2+-catechol chelation endows the hydrogel with sustained anti-infection, anti-inflammatory, and antioxidant properties. Chen et al.153 developed a novel adhesive hydrogel using PVA, DA, and Cu2+, which possesses injectable and strong adhesive capabilities, serving as a physical barrier for hemostasis at wound sites. Similarly, Dai et al.93 reported a DA-based collagen–starch hydrogel (CoSt). In models of rat tail amputation, skin incisions, severe liver injury, abdominal aorta injury, and nerve transection injury, this hydrogel exhibited superior hemostatic efficiency compared to fibrin glue. The removal of adhesive hydrogels often causes discomfort, such as pulling and pain; damages newly regenerated skin tissue; and potentially leads to bleeding and infection (as shown in Figure 5). Moreover, the residual hydrogel may hinder wound repair. Emerging stimulus-responsive detachable adhesive hydrogels have garnered significant attention from researchers to mitigate these issues.
6.4 On-demand detachable hydrogel for skin wound dressings
Adhesive hydrogel wound dressings can function as moisturizers and hemostatic agents, stabilizing adhesion to irregular wounds and preventing interference from the external environment. The adhesion of nonbiodegradable adhesive hydrogel dressings is often irreversible, making their separation from the wound site during replacement difficult and potentially causing secondary injury or new infections.154 Even hydrogel dressings with relatively weak adhesion can cause varying degrees of damage to sensitive tissues, significantly limiting their application in treating skin wounds.155 Hydrogels with on-demand removal or dissolution capabilities can minimize adverse effects on wounds. Techniques such as pH, temperature, ion-induced phase separation, grafting of polymer chains with cleavable disulfide bonds, and terminal NHS esters can endow adhesive hydrogels with on-demand removal properties.
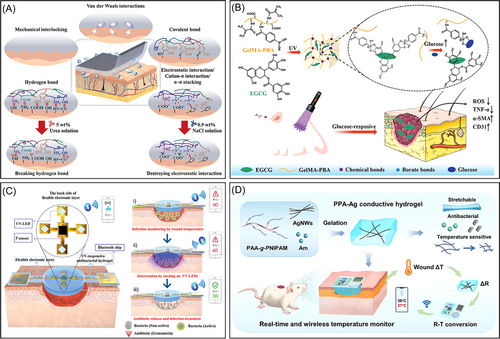
Additionally, the chemical composition of these hydrogels can be customized according to the type and condition of the wound, imparting unique physical and chemical characteristics to the on-demand adhesive hydrogels. For instance, Weng et al.156 developed an on-demand removable, adhesive, bioinspired hydrogel based on catechol chemistry that can be easily separated from porcine skin with the assistance of a 5 wt.% aqueous urea solution (as shown in Figure 6A). Zhou et al.160 created an on-demand, removable hydrogel using gelatin, TA quinone, and borax that effectively treated MRSA-infected wounds. The pH-sensitive borate ester (polyphenol-B) and Schiff base bonds crosslinked within the gel network enable on-demand removal and dissolution. However, the design and manufacturing of such on-demand removable or dissolvable hydrogel dressings can be complex and may result in low-toxicity byproducts, which could negatively impact tissues.
6.5 Stimulus-responsive hydrogel for skin wound dressings
Traditional hydrogels exhibit unique functionality and passively act at specific stages of wound healing. Modifying the chemical structure to impart various responsive characteristics (stimulus-responsiveness, injectability, self-healing, and conductivity) to hydrogels has emerged as a promising strategy for addressing antimicrobial, anti-inflammatory, and angiogenic needs throughout the wound healing process.161-164 Among these, stimulus-responsive hydrogels have garnered significant attention due to their abilities to respond to physical, chemical, or biochemical stimuli (such as temperature, pH, light, electric field, or magnetic field) by altering their physicochemical properties (e.g., solubility, shape, swelling behavior, network structure, and mechanical properties).17, 165, 166 Stimulus-responsive hydrogels can control the release of bioactive molecules (anti-inflammatory agents, epidermal growth factors, hemostatic agents, and antimicrobial substances) or adjust their physicochemical properties within the wound microenvironment, thereby accelerating the wound healing process. The pH of the skin plays a crucial role in maintaining normal function. The pH fluctuates during different stages of wound healing, gradually increasing from the surface to deeper layers. This variation can indicate the wound status, helping to predict the likelihood of healing or deterioration. Healthy skin maintains a slightly acidic pH (pH 4-6), chronic wounds exhibit a pH of around approximately 7.3–10, and acute wounds have a pH of approximately 7.4. As the wound heals, the pH returns to an acidic state.166, 167 Hydrogels with pH-responsive functionality can adapt to changes in the pH of the wound microenvironment by releasing loaded drugs or cytokines to promote wound healing. For instance, Schiff-base crosslinked hydrogels can dissociate under acidic conditions to release insulin, aiding in healing diabetic wounds.63, 86 Similarly, pH-responsive hydrogels that release TA achieve this property by deprotonating chitosan under alkaline conditions, thereby reducing its binding strength with TA.168 Tao et al.169 prepared a multifunctional hydrogel dressing loaded with zinc oxide nanoparticles through the use of phenylboronic acid-grafted CS, dibenzaldehyde-grafted polyethylene glycol, and TA. Due to the pH-responsive release of Zn2+ and TA, the hydrogel effectively killed MRSA, scavenged reactive oxygen and nitrogen species, mitigated oxidative stress, and induced the M2 polarization of macrophages.
Temperature-responsive hydrogels release drugs, antimicrobial agents, or cytokines through volume changes, resulting in short response times, simple operation, and adjustable response temperatures.89, 170-172 These properties have led to extensive research and application in wound healing and other fields. Temperature changes affect the interactions between hydrophobic and hydrophilic groups within the hydrogel, causing structural changes that result in volume expansion or contraction.173, 174 For instance, hydrogels with a lower critical solution temperature (LCST) contract when the temperature exceeds the LCST, aiding in the localized loading of substances and preventing accumulation in healthy tissues.171 Common thermosensitive polymers used in biomedicine include PNIPAM,175 poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide),176, 177 and poly(D, l-lactide)-poly(ethylene glycol)-poly(D, l-lactide).178, 179 For example, Haidari et al.180 developed a temperature-responsive hydrogel by copolymerizing NIPAM with AA and subsequently embedding AgNPs. Blacklow et al.77 created a temperature-responsive hydrogel dressing (AAD) using PNIPAM, alginate, and AgNPs. This AAD was attached to skin tissue via chitosan and carbodiimide-mediated reactions and contracted upon temperature triggering to facilitate active wound closure and AgNP release, thus accelerating wound healing. Similarly, Guo et al.181 designed a thermoresponsive hydrogel dressing based on quaternized chitosan, polydopamine-coated reduced graphene oxide (rGO-PDA), and PNIPAM. This hydrogel dressing can stably adhere to tissues and promote wound closure through contraction. However, PNIPAM hydrogels are typically nonbiodegradable and may cause localized inflammatory responses, impeding tissue repair. Therefore, many studies have attempted to use natural polymer crosslinkers (such as GelMA and SFMA) or incorporate natural polymers into the PNIPAM framework to enhance its biocompatibility.182
Diabetes has a high prevalence worldwide, with approximately 19%–34% of diabetic patients estimated to develop complications such as chronic wounds, posing a severe threat to their quality of life and safety.183-185 Due to the high glucose microenvironment in diabetic wound areas, which serves as a stimulus for controlled drug release, glucose-responsive hydrogel dressings have displayed unique advantages in treating chronic wounds. Currently, the glucose-responsive hydrogels developed include glucose oxidase (GOx) systems,186, 187 lectin systems,188, 189 and phenylboronic acid systems.190, 191 GOx is an endogenous redox enzyme that catalyzes the conversion of glucose into gluconic acid and H2O2, rapidly consuming glucose at the infected site while lowering the pH of the wound microenvironment.192, 193 However, GOx, a protein-like substance sensitive to the external environment, is prone to immune reactions in the human body. In the phenylboronic acid system, boronate ester bonds respond to high concentrations of glucose and H2O2, consistent with the microenvironment of diabetic wounds. Additionally, phenylboronic acid can bind to the diol structure in glucose to form boronic esters. In the high-glucose environment of diabetic wounds, glucose can competitively react with phenylboronic acid, leading to hydrogel dissociation or promoting drug release from the hydrogel. Currently, glucose-responsive hydrogel dressings based on the phenylboronic acid system offer advantages such as low toxicity, good stability, and no immune rejection reactions, and thus they are widely used for treating chronic diabetic wounds.194-196 For example, Zhou et al.157 developed a functional hydrogel utilizing dynamic boronate bonds that adaptively react with high glucose concentrations and hydrogen peroxide to alleviate oxidative stress and release deferoxamine in the early stages of wound healing. Sun et al.197 also designed a photocrosslinked GelMA hydrogel loaded with (-)-epigallocatechin-3-gallate (EGCG) through boronate bonds. This hydrogel releases more EGCG as glucose levels increase, thereby reducing inflammation, promoting angiogenesis, and facilitating tissue remodeling (as shown in Figure 6B). Additionally, phenylboronic acid esters exhibit responsiveness to both pH and ROS.198 Leveraging this characteristic, various responsive hydrogels have been developed to achieve more precise drug release within pathological microenvironments. For instance, Wang et al.199 constructed an injectable glycopeptide hydrogel with dual pH/ROS-responsiveness, enabling spatiotemporal drug release in response to these stimuli. Similarly, Yao et al.200 formulated a dual pH/ROS-responsive hydrogel loaded with platelet-rich plasma via hydrogen bonding and Schiff base formation. The Schiff base in the hydrogel dissociates in acidic and oxidative wound environments, releasing growth factors to effectively promote wound healing.
6.6 Hydrogel wound dressings for monitoring the skin wound status
Traditional wound dressings are physical barriers that maintain a moist environment. In contrast, hydrogels integrated with smart sensors enable real-time monitoring of wound conditions. These monitoring capabilities are crucial for the timely detection of infections, inflammation, and other complications, facilitating targeted wound management and treatment. The pH is a critical indicator of the wound status and is associated with various physiological processes, such as bacterial infection, angiogenesis, and collagen formation.201 Due to the release of fatty acids and amino acids by keratinocytes, the pH of healing wounds or normal skin is slightly acidic.202, 203 In acute skin wounds, neutrophils can lower the pH to prevent bacterial colonization. In chronic skin wounds, alkaline conditions may persist for months, increasing susceptibility to bacterial infections. Therefore, monitoring changes in the pH of skin wounds can be an effective method for assessing the wound status, providing alerts for the infection risk and assisting in wound management and treatment. Additionally, redox sensors can monitor oxidative stress levels at the wound site, providing critical information on the wound healing process. For example, Zhang et al.204 developed an amphiphilic ionic hydrogel wound dressing by incorporating GOx and HRP, enabling the simultaneous monitoring of pH and glucose levels in the treatment of diabetic wounds. Lu et al.205 crosslinked carboxymethyl cellulose with europium–ethylenediaminetetraacetic acid to prepare a functional hydrogel capable of monitoring the pH of diabetic wounds. The fluorescence intensity of this hydrogel has a linear relationship with pH, allowing real-time measurement of the wound pH.
Wound healing at joints, such as the fingers, elbows, and knees, is significantly affected by motion.206 Due to the frequent movement of joints, these wounds often exhibit slower recovery, posing more significant challenges for treatment. Traditional hydrogel dressings for joint wounds are prone to rupture and bacterial infection, and thus matching and monitoring irregular wounds is difficult. Hydrogels with self-healing and motion-sensing properties can autonomously repair damage and detect joint wound movement, effectively addressing these challenges.207, 208 For instance, Liu et al.209 developed a self-healing hydrogel dressing through dynamic borate ester bonds and supramolecular interactions that can effectively promote wound healing while simultaneously monitoring wound tearing at body joints in real time. Similarly, John et al.210 developed a bacteria-responsive hydrogel that reacts to bacterial enzyme-induced changes in dielectric properties, enabling the detection of the wound infection status.
Temperature is considered a crucial parameter for evaluating the wound healing status because it influences a range of chemical and enzymatic reactions and cytokine activities in the body.211 Monitoring the wound temperature can provide essential information related to healing, such as local blood flow, infection, and inflammation, thus providing a reference for developing personalized treatment plans.212 Previous studies have shown that local vasodilation, angiogenesis, and infiltration of inflammatory cells can increase the temperature of the wound area.213 Therefore, wound dressings with temperature-monitoring capabilities can help promptly detect the wound healing status, and intervention measures can be taken to prevent wound deterioration. For instance, Ma et al.158 designed an intelligent, flexible, electronic integrated wound dressing capable of real-time wound temperature monitoring, which is an early predictor of pathological infection (as shown in Figure 6C). In another study, Fan et al.159 developed a PNIPAM-based flexible wireless wound dressing with properties of conductivity, stretchability, antibacterial activity, and wireless temperature monitoring of the wound (as shown in Figure 6D). However, hydrogel wound dressings with monitoring capabilities also have certain limitations. For instance, their complex manufacturing process restricts their large-scale production and application. The sensitivity and stability of wound monitoring need to be improved in complex wound environments to ensure the accuracy of monitoring data. Additionally, the mechanical strength and biocompatibility of hydrogel materials require further enhancement to prevent damage or adverse reactions during use.
7 CONCLUSIONS AND PROSPECTS
The healing of skin wounds is a complex biological process that occurs in distinct stages. Traditional wound dressings, such as gauze and tape, function primarily as physical barriers and are limited in their ability to promote healing effectively. An ideal wound dressing should possess the following characteristics: maintain moisture in the wound area, allow for gas exchange, provide an effective protective barrier, prevent wound infection, and be both biocompatible and biodegradable.214 Multifunctional hydrogels, with their excellent biocompatibility, tunable biodegradability, and diverse functionalities—such as promoting cell proliferation, antibacterial activity, and inflammation regulation—have emerged as the most promising candidates for wound treatment. Therefore, modern medicine increasingly tends to use new types of dressings with better moisture control and wound protection functions, such as functional hydrogel dressings, to promote faster and more effective wound healing. These smart functional hydrogels wound dressings can actively promote the healing process of skin wounds through antibacterial, antioxidant, and anti-inflammatory properties. Additionally, by adjusting the chemical composition and preparation methods of the hydrogel materials, it is possible to design functional hydrogel wound dressings that meet the needs of different types of skin wounds.
This article reviews the advancements in multifunctional hydrogels for skin wound healing that offer a detailed introduction to the characteristics and treatment challenges of acute and chronic wounds. It categorizes traditional and modern dressings, emphasizing the characteristics and types of functional hydrogel dressings. Finally, it discusses the application of hydrogel wound dressings with various functional properties (antibacterial, antioxidant, adhesive, stimulus-responsive, and wound state monitoring) in treating skin wounds.
Despite significant progress, the application of hydrogel dressings still requires improvement due to the complex and dynamic nature of the microenvironment in skin wound healing. For instance, issues related to the biocompatibility and safety of hydrogel materials and nanomaterials must be addressed. Standard laboratory models often involve rats and mice, and further validation in larger animal models is needed. Additionally, the mechanisms through which multifunctional hydrogel wound dressings facilitate skin wound healing remain unclear.
- 1.
Explore the preparation processes of different hydrogels (such as nanocomposite hydrogels, bioengineered hydrogels, and 3D-printed hydrogels) to enhance material stability, drug-loading capacity, and controlled release properties, thereby meeting the treatment needs of various types of skin wounds;
- 2.
Investigate the use of artificial intelligence and machine learning to optimize hydrogel formulations and medical approaches for personalized wound care;
- 3.
Exploring the combined application of hydrogels with growth factors and gene therapy to enhance therapeutic effects and accelerate the wound-healing process;
- 4.
Conducting in-depth studies on the mechanisms of hydrogels in wound healing to further elucidate their potential applications in biology and biomedicine;
- 5.
In addition to addressing the treatment of skin wounds, emphasis should also be placed on developing functional hydrogel dressings for in vivo wound healing, such as wounds in the stomach, intestines, liver, and other organs.
AUTHOR CONTRIBUTIONS
Zhongwu Bei: Conceptualization (Equal), Funding acquisition (Equal), Investigation (Equal), Writing—original draft (Equal), Writing—review & editing (Lead); Jing Zheng: Formal analysis (Equal), Supervision (Equal), Writing—review & editing (Equal). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge financial support from the Postdoctoral Fellowship Program of CPSF under Grant Number GZC20241165. The authors thank www.biorender.com for providing scientific drawing materials.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




