Advances in intravital imaging of liver immunity using optical microscopy and labeling methods
Abstract
The use of optical microscopy and labeling methods in intravital imaging allows for direct tracking of cell behavior and dynamic changes at the molecular level in the physiological or pathological microenvironment of living animals, revealing the spatiotemporal information of individual cells in the immune response. The liver is an immunological organ that contains unique innate and adaptive immune cells, including Kupffer cells (KCs) and different types of T cells, and is involved in coordinating multiple immune responses in the body. Using intravital imaging to visualize the movement behaviors and functions of immune cells during the reaction processes of the liver under physiological and pathological conditions has shed new light on the understanding of liver immunity, which is of great significance for the diagnosis and treatment of liver diseases. This review introduces various window models and labeling methods for the liver in intravital optical imaging and describes how it provides movement behavior and functional information about different types of immune cells, such as KCs and T cells, in the liver. Additionally, we highlight recent advances in intravital optical imaging of liver diseases, such as nonalcoholic fatty liver disease, infections, and tumors. This review aims to be a useful resource for comprehending the developments and achievements in intravital imaging of the liver and uncovering spatiotemporal information of immune response in a living microenvironment.
1 INTRODUCTION
As the largest solid organ in the body, the liver not only has important physiological functions, participating in physiological processes such as protein synthesis, metabolism and toxin removal, but is also considered to have unique immunological functions (Figure 1a) [1]. The hepatic lobule is the basic structural and functional unit of the liver and features a longitudinal vessel in the center, the central vein (CV). The portal tract within the hepatic lobules is generally considered to comprise the interlobular vein, interlobular bile duct, and interlobular artery. The portal tract is connected to the CV through a network of capillaries called hepatic sinusoids (Figure 1b). Different from the traditional understanding of the hexagonal structure of hepatic lobules and the triplex structure of the portal tract, the latest research of our group showed that the hepatic lobules of mouse liver are roughly oval in shape [2], and that the portal area features a sophisticated quadruplex vessel structure of lymphatic vessels intertwined with interlobular veins [3]. The unique dual blood supply system of the liver continuously exposes it to circulating blood. In addition to screening and eliminating a variety of exogenous antigens flowing through the blood [4], the liver has to be immunotolerant to a large number of antigens from the gastrointestinal tract [5]. Therefore, it is very important to maintain immune surveillance and autostabilization of the liver under normal physiological conditions. The various types of innate immune cells in the liver, which include the largest tissue-resident macrophages—the Kupffer cells (KCs)—as well as Dendritic cells (DCs), Natural Killer T (NKT) cells and granulocytes, directly detect and capture circulating pathogens, participate in immune modulation, mediate the elimination of pathogens and the recruitment of leukocytes, and present antigens to lymphocytes in the liver (Figure 1c) [6-8].
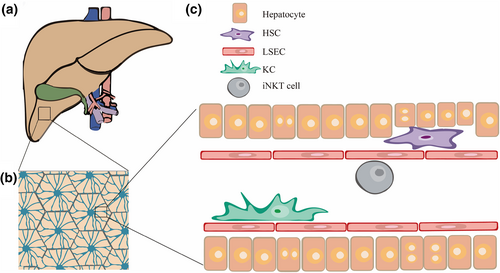
Schematic diagram of the composition of the liver vasculature. (a) Schematic diagram of the liver: yellow, liver; green, gallbladder; blue, hepatic vein; and red, hepatic artery. (b) Schematic diagram of the hexagonal hepatic lobule: blue, liver sinusoids. (c) Immune cells in the liver sinusoids.
In the last two decades, high-resolution multiphoton excitation microscopy and laser confocal microscopy have become indispensable tools in biomedical research. In 2002, Science published three articles introducing the application of optical microscopic imaging technology in the dynamic intravital imaging of immune organs [9-11], declaring the arrival of the era of intravital immune optical imaging. Intravital optical imaging of immune processes provides researchers the ability to visualize and capture dynamic biological information with single-cell resolution in multiple organ systems in vivo, such as the movement and migration of immune cells and cell contact between cells in the tissue microenvironment [12]. Compared with traditional biological methods, intravital optical imaging can be used to study complex biological processes involving multiple cells and multiple molecules in physiological and pathological environments. Such studies require visual investigative methods that are capable of simultaneously labeling and imaging multiple immune cells and molecules in the same environment. Additionally, compared with positron emission tomography, magnetic resonance imaging, and computed tomography, optical imaging technologies with high spatial and temporal resolution and multicolor imaging capabilities, such as confocal microscopy, multiphoton microscopy, photoacoustic imaging systems, and light field microscopy [13], have great application prospects in high-precision tracing of cell movement behavior and monitoring of molecular functions in vivo. Despite performing important physiological functions, some cells, such as neutrophils, tissue-resident immune cells, and hematopoietic stem cells, are not suitable for ex vivo study due to their short lifespan, difficulty with expansion, and sensitivity to microenvironmental factors [14]. As the only method currently available to directly and immediately attain spatiotemporal information from the intact mammalian immune system at a cellular/subcellular level [14], intravital optical imaging has yielded remarkable advances in our understanding of somatic cell biology.
Intravital visualization of the immune response under both physiological or pathological conditions of the liver can be used to monitor the contact between different immune cells and the spatiotemporal dynamics information of their movement behavior. Therefore, we anticipate that intravital imaging will profoundly enrich the understanding of regional immune characteristics of the liver by supporting deeper analysis of liver immune function.
In this paper, we review the advances in intravital optical imaging of liver immunity. After a brief introduction of window models and labeling methods for this imaging, we focus on the spatiotemporal dynamics of liver immune cells obtained by intravital optical imaging in recent years, as well as the progress of immunological research in liver disease models. We believe that this paper will provide readers with a better understanding of (1) various methods used for intravital imaging and (2) how these methods will support new discoveries in liver immunology.
2 WINDOW MODELS AND LABELING METHODS IN INTRAVITAL OPTICAL IMAGING OF THE LIVER
Confocal microscopy and multiphoton microscopy are the most widely used intravital optical imaging methods. In contrast to wide-field microscopy, confocal scanning microscopy performs optical section imaging of specimens by adding a pinhole aperture on the optical path to prevent the nonfocal plane beam from being collected, thereby producing high-resolution fluorescence images [15]. On this basis, spinning disk confocal microscopy uses an array arrangement of multiple pinholes to simultaneously generate hundreds of focal plane beams for parallel detection, significantly improving the scanning speed [16]. Regarding the imaging of deep tissues (hundreds of microns in depth), multiphoton excitation microscopy is used more frequently because it offers the advantages of reduced photobleaching, increased penetration depth, more robust and stable optical alignment, and increased sensitivity to weak fluorophores [17]. With its high levels of contrast and spatial resolution, photoacoustic imaging has also been widely used in biomedical research in recent years, achieving submicron resolution at an imaging depth of ~1 mm due to the ultrasonic imaging principle [18].
2.1 Window model for intravital optical imaging of the liver
The anatomical position of the liver makes it susceptible to the influence of factors such as heartbeat and respiratory jitter during intravital imaging. Furthermore, the imaging depth of high-spatiotemporal-resolution optical imaging technology is limited, so early intravital imaging of the liver is usually performed to expose the liver lobe directly; however, this method can only carry out imaging observations for a few hours. In recent years, a number of research groups have developed liver window models, including the purse window model, frame window model, and drawer window model, to meet their urgent needs for long-term dynamic observation of liver immunity and high-resolution intravital imaging of the liver lasting several days to more than a month.
2.1.1 Intravital optical imaging with direct exposure of the liver
The simplest and easiest way to image the liver in vivo is to directly image the liver lobe through a simple abdominal incision that is exposed and externalized. This imaging method causes great damage to the body and is only suitable for imaging within a short period of time (e.g., within 6 h) [19-21]. As early as 2004, Geissmann et al. studied the crawling behavior of NKT cells (labeled by green fluorescent protein [GFP]) in mouse liver with confocal imaging using direct exposure of the liver. Their study found that NKT cells stopped crawling when activated by an antigen, revealing a new type of intravascular immune surveillance behavior [22].
2.1.2 Long-term optical imaging based on the abdominal window
The implantable imaging window allows for extended imaging time and proper tissue function while maintaining the quality and depth of imaging. The pioneering work performed by Jacco van Rheenen's team at the Royal Netherlands Academy of Arts and Sciences in 2012 established an abdominal window imaging model using the “pouch suture” method combined with a titanium ring, which can be used to observe mouse liver over a long period (5–6 weeks) with less damage [23]. However, the problem of pulsing due to lung breathing and heart beating still exists during liver imaging. This has led to the development of many improved imaging window models. For example, Jacquemin et al. replaced the titanium alloy rings with organosilicon materials to improve the softness of materials and avoid inflammation caused by wear at contact sites [24]. The plastic 3D-printed abdominal imaging window developed by Kuss et al. is lightweight, quickly manufactured, and can be mounted directly on the microscope carrier scaffold without fixing the window step [25], which improves the elimination of cell motion artifacts caused by dither during confocal imaging. However, these methods all require the use of tissue glue to fix the liver to the window model, which limits the imaging area of the liver; additionally, its limited adhesive strength may lead to detachment of the liver from the window in long-term experiments.
2.1.3 Dual-mode fluorescence/photoacoustic imaging based on a drawer-type abdominal window
A drawer-type abdominal window with an acrylic/resin coverslip model (DAWarc) was developed by our group. This model not only solves the liver focal plane dither caused by breathing and heart beating through the drawer structure designed to physically fix the hepatic lobes, but also balances the penetration efficiency of light and sound signals with the acrylic/resin cover, making it compatible with long-term live fluorescence and photoacoustic imaging [26]. Using the DAWarc model, long-term intravital fluorescence/photoacoustic imaging was performed for several days to reveal the spatial location and movement behavior of invariant NKT (iNKT) cells, as well as the distribution and morphological information of KCs in the microenvironment of liver metastases [26]. Without the need to fix liver tissue with tissue glue, this model facilitates intravital imaging of the liver, which is conducive for long-term dynamic monitoring of changes in immune cells and liver blood vessels during disease progression and the relationship between the two.
2.2 In vivo optical labeling of liver immune cells
Using different transgenic mouse strains, dyes, or antibodies [20], simultaneous labeling and multicolor imaging to observe different types of immune cell populations in vivo are conducive to the dynamic study of multicellular interactions and the regulation of cell functions in physiological and pathological microenvironments.
2.2.1 Transgenic mouse models of liver immune cells labeled with fluorescent proteins
Fluorescent reporter mice are constructed by tapping reporter genes into marker genes, achieving the purpose of marking different types of cells in vivo. For example, Clec4f-Cre-tdtomato mice were constructed by expressing nuclear localization signal-tagged tomato fluorescent protein under the promoter of Clec4f, a KC-specific gene [27]. By inserting enhanced GFP (EGFP) cDNA into the CXCR6 coding region, CXCR-GFP mice with GFP-labeled iNKT cells in the mouse liver were constructed [22]. By expressing Cre recombinase under the guidance of the Lecithin retinol acyltransferase (Lrat) promoter, Lrat-Cre1Rshw/Mmjax mice containing retinol lipid droplet-labeled hepatic stellate cells (HSCs) were also constructed [28]. The development of different types of transgenic fluorescence reporter mice serves as a potent tool for visualizing cell behavior in vivo.
2.2.2 Models of liver immune cells labeled with fluorescent dye-conjugated antibodies
Mouse liver immune cells can be specifically labeled by injecting fluorescent dye-conjugated antibodies into mouse tail veins. The following lists a few of the many examples of this model type: liver macrophages labeled by fluorescent dye-conjugated F4/80 antibodies; different types of T cells labeled by CD4 or CD8 antibodies; liver sinusoidal endothelial cells (LSECs) labeled by CD31 antibodies; and NK cells labeled by NK1.1 antibodies. Fluorescent dye-conjugated antibodies are the most widely used for in vivo specific labeling, but they are expensive and may hinder some cell functions. In addition to antibodies, cells can also be specifically labeled by nanoparticles coated with fluorescent dyes. The pomegranate-type fluorescent nanoparticles (size, ~300 nm) that were developed by Deng et al. can be used to label KCs through nonspecific uptake [29] (Table 1).
| Cell types | Transgenic reporter mouse | Antibody labeling methodsa |
|---|---|---|
| Kupffer cell | Clec4f-Cre-tdtomato mice [27] | Dye-conjugated anti-F4/80 or anti-Clec4f antibody |
| Invariant natural killer T cell | CXCR6-GFP mice [22] | Not reported |
| Neutrophil | Lys M-EGFP mice [30]b | Dye-conjugated anti-Ly6G antibody |
| Liver sinusoidal endothelial cell | Tie2 mice [31]c | Dye-conjugated anti-CD31 antibody |
| HSCs | Lrat-Cre1Rshw/Mmjax mice [28] | Not reported but has autofluorescence |
| CD8+ T cell | CD8a-Cre mice [32] | Dye-conjugated anti-CD8 antibody |
| CD4+ T cell | ThPOK-CreERT2.hCD2 mice [33] | Dye-conjugated anti-CD4 antibody |
- a For labeling cells in vivo using dye-conjugated antibodies, a total of 1–2 g antibodies were injected intravenously into mice approximately 10 min before imaging.
- b Constructed by knockin of the enhanced GFP (EGFP) gene into the mouse lysozyme M (lys) site and combined with the targeting vector.
- c The Tie2-Cre (Tek-Cre) transgene mouse has the endothelial-specific receptor tyrosine kinase (Tek or Tie2) promoter directing expression of Cre recombinase.
3 INTRAVITAL OPTICAL IMAGING OF LIVER IMMUNE CELLS
3.1 Intravital optical imaging reveals movement behavior and dynamic functional information of liver immune cells
3.1.1 Liver resident macrophages—KCs
The liver contains the largest population of tissue-resident macrophages, the KCs, which develop from the yolk sac and account for approximately 20% of nonparenchymal liver cells [34]. Located in the hepatic sinuses, KCs have the amazing ability to quickly clear pathogens from the blood flowing through these sinuses, thereby performing effective immune surveillance. Because of their special morphology, strong phagocytosis, and easy labeling, an abundance of intravital imaging studies have been conducted on KCs. Zeng et al. labeled KCs with intravenous injection of Alexa Fluor 750-conjugated anti-mouse F4/80 antibody and visualized their ability to capture GFP-labeled bacteria in hepatic sinuses using spinning disk confocal microscopy. This study reported that 60%–80% of intravenous Staphylococcus aureus bacteria were captured and isolated from liver sinusoids by KCs within 5 min [35] and that this bacterial capture relied on complement receptor of the immunoglobulin superfamily (CRIg). CRIg was found to bind directly to lipteichoic acid, a cell wall component of Gram-positive bacteria, indicating that it is a functional pattern recognition receptor that can directly recognize bacterial components. This relatively independent process mediated by CRIg enables KCs to capture and prevent systemic bacterial transmission with high efficiency. Additionally, Sun et al. revealed the mechanism by which liver KCs actively filter the transmission of fungal pathogens in blood using confocal microscopy to image the dynamic interaction between KCs and fungi in the liver [36]. The imaging results visually showed that GFP-labeled yeast cells flowed in through the blood of infected mice stagnated during capture by KCs labeled with phycoerythrin (PE)-conjugated anti-F4/80 antibody (Figure 2a), suggesting that the clearance process of the fungus was related to the active filtration by KCs. In another study, the clearance rate of Cryptococcus neoformans was significantly reduced in KC-depleted mice compared with that in normal mice, further demonstrating the filtration function of KCs on fungi (Figure 2b) and suggesting that enhancing KC phagocytosis could improve the clinical status of patients with invasive fungal infection [36].
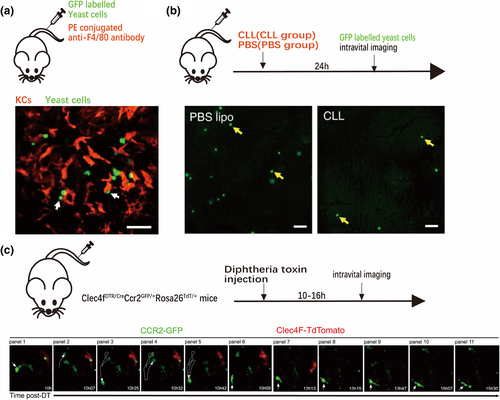
Imaging results of KCs phagocytosing fungi and morphological changes in monocytes after consumption of KCs. (a) Capture of green fluorescent protein (GFP)-labeled yeast cells (green) flowing through the blood by KCs labeled with PE-conjugated anti-F4/80 antibody (red) [36]. (b) Significant reduction in fungal clearance (fixation phenomenon) after KC removal with disodium chlorophosphate liposomes (CLL group) [36]. (c) Time-series imaging performed by intravital two-photon imaging of the liver showing transformation of monocytes into the characteristic volume and shape of KCs [38]. (a and b) of this figure are reproduced with permission from reference [36], and (c) is reproduced with permission from reference [38].
In addition to working alone, KCs can also act in concert with LSECs to effectively remove particles from circulation. When applied systematically, 30%–99% of nanoparticles are captured in the liver, mainly by KCs and LSECs [37]. By adjusting the size of the nanoparticles, the path of uptake can also be adjusted. In terms of nonspecific uptake, single nanoparticles with a diameter of >100 nm or polymerized nanoparticles with a diameter of <100 nm, interact more strongly with KCs, while other smaller nanoparticles have a higher proportion of uptake by LSECs [37]. Knowing the nonspecific uptake ability of KCs, our group developed a self-assembled “off/on” nanopomegranate particle (size ~300 nm) that was efficiently taken up by KCs (>98%) in the liver following intravenous injection. By applying this nanoparticle, fluorescence/photoacoustic dual-mode imaging was executed to reveal the strategic arrangement and function of KCs in living liver [29].
In addition to KCs, there are monocyte-derived macrophages in the liver. When KCs are depleted by diseases or artificial means, monocytes will quickly infiltrate the liver and transform into KCs in volume and shape (Figure 2c). Such behaviors enable monocytes to do the following: interact with other cells in the liver sinusoids by extending pseudopodia; promote changes in factors that drive the differentiation and development of KCs in the microenvironment; and eventually become macrophages imprinted with the KC identity, including the expression of transcription factors ID3 and LXR-α, and cell surface markers F4/80 and Clec4f [38, 39].
3.1.2 iNKT cells
Accounting for approximately 30% of intrahepatic lymphocytes, iNKT cells can reside in tissues for a long time [40]. Intravital imaging of CXCR6-GFP mice has revealed that iNKT cells in the liver crawl along the hepatic sinuses to perform intravascular patrol work (Figure 3a) and quickly stop moving in response to homologous antigens [22]. Under treatment with iNKT cell agonists or inflammatory cytokines, 70%–90% of iNKT cells were observed to become stationary for a few minutes [22], suggesting that the rapid arrest behavior of iNKT cells is a feature of activation. Thus, by monitoring the spatiotemporal characteristics of iNKT cells, researchers can better understand their activation in vivo. Wang et al. observed the dynamic relationship between iNKT cells (CXCR6-GFP cells) and KCs (labeled by Alexa Fluor 647-conjugated anti-F4/80 antibody) through real-time confocal imaging of the liver in nonalcoholic fatty liver disease (NAFLD) model mice established by feeding with a methionine- and choline-deficient (MCD) diet [41]. The imaging results showed that iNKTs were recruited to the liver and slightly aggregated on the first day of MCD feeding, while KCs began to gather together slightly on the second day (Figure 3b). Upon removal of KCs with liposome disodium chlorophosphite, iNKT cells did not accumulate until the fifth day of feeding. Multiphoton microscopy was then used to image the colocalization contact sites among iNKT cells, KCs and Nile red dye-labeled free lipids in the livers of NAFLD mice. This confirmed that rather than KCs, which are mainly involved in scavenging and phagocytosis, the main cells that phagocytose free lipids released by necrotic hepatocytes are activated iNKT cells [41], providing new insights into the pathogenesis of NAFLD and inspiring new therapeutic targets.
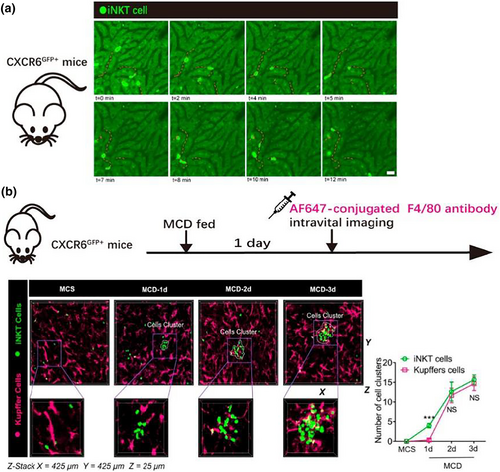
Imaging results of iNKT cell patrol behavior and aggregation of iNKT cells and KCs in the NAFLD model. (a) Confocal microscopic images of a live liver of a CXCR6-GFP mouse in which iNKT cells (bright green) moved along the liver sinusoid at an average rate of 16 μm/min [22]. (b) Left: hepatic intravital microscopy of CXCR6-GFP mice fed a normal or MCD diet; Right: quantification of the number of iNKT cell clusters and KC clusters in untreated or MCD-fed CXCR6-GFP mice [41]. (a) of this figure is reproduced with permission from reference [22], and (b) is reproduced with permission from reference [41].
Additionally, using CXCR6-GFP mice, Liew et al. performed intravital imaging through spinning disk confocal microscopy, showing three stages of iNKT cells during aseptic liver injury repair [42]: (1) repulsion, during which iNKT cells did not enter the site of injury and even performed a 180° U-turn when patrolling near the site; (2) retention, which occurred at approximately 8 h after injury, when self-antigens and arrest cytokines such as interleukin-12 (IL-12) and IL-18 remained in production by iNKT cells at the boundary of the injury site; and (3) infiltration, which occurred at the injury site 48–72 h after injury. By delving into the behavior of iNKT cells that differ from that of neutrophils, macrophages and platelets recruited at the initial stages of injury, this research discovered that self-antigen-driven iNKT cells act as detectors and coordinators of the transformation from inflammation to tissue recovery, playing an indispensable role in the liver injury repair process [42]. While damage to the liver causes changes in iNKT movement behavior, damage to distant organs can also affect the dynamics of iNKT behavior [43]. Wong et al. used CXCR6-GFP mice for intravital imaging through spinning disk confocal microscopy and found that iNKT crawling behavior in the liver of mice with transient cerebral artery embolization was initially no different from that of normal mice; however, with cerebral ischemia reperfusion, the number of crawling cells was significantly reduced. This movement behavior pattern was not observed during cytokine or antigenic stimulation, or in posterior limb ischemia‒reperfusion model mice, suggesting that this behavior is mediated by neurotransmitters [43] and providing new insights into the cross-talk between the central nervous system and the immune system.
3.1.3 Effector T cells in the liver
The liver has been regarded as an organ conducive to the induction of strong immune tolerance [44], which is also considered to be the main reason for the persistence of chronic liver disease [45]. Despite this, the liver can also establish a rapid and potent T-cell response in acute inflammatory infections [46].
T-cell activation in acute infections is characterized by complex mechanisms. Under activation by different antigen-presenting cells, CD8+ T cells may exhibit different behaviors. Benechet et al. showed that when hepatitis B virus (HBV) antigen was expressed by hepatocytes (hepatocyte antigen-presenting group), antigen-specific CD8+ T cells formed loose but persistent intravascular clusters around the portal vein bundle, with a significantly increased proportion of motile cells. These cells differed from effector T cells in the transcription level and epigenetics, secreting little or no interferon (IFN)-γ and showing no cytotoxicity. Instead, they expressed programmed cell death protein 1. However, when the antigen was presented by KCs (KC antigen-presentation group), CD8+ T cells fully differentiated into effector T cells with the ability to secrete IFN-γ and cytolytic-like granzyme, forming dense extravascular clusters with most cells at rest and scattered among the hepatic lobules. Long-term continuous imaging monitoring showed that the T-cell clusters in the KC antigen-presenting group began to depolymerize, with migration of the cells from the liver on the 5th to 7th day of antigen presentation, while the cell clusters in the hepatocyte antigen-presenting group remained, suggesting persistence of the antigen-presenting effect in the hepatocyte antigen-presenting group. This study revealed differences in the roles of different antigen-presenting mechanisms in the initiation of CD8+ T cells in the liver [47].
As the main effector of tumor elimination, cytotoxic T cells (CTLs) recognize the major histocompatibility complex polypeptide antigen complex on the surface of tumor cells via the T-cell receptor (TCR). After making stable contact, the CTLs secrete IFN-γ and perforin to kill the tumor cells [48]. Our research group established a method that quantitatively evaluates the ability of CTLs to kill tumor cells in vivo and was used to prove that the ability of CTLs to kill tumor cells is limited in the immunotolerant environment of the liver [49].
To establish a tumor visualization model with multicolor labeling in vivo, Liu et al. screened a melanoma cell line that stably expressed the genetically encoded caspase-3 molecular probe (C3) and used it to generate liver metastasis model mice. Next, the tumor-bearing mice received in vitro-activated tdTomato fluorescent protein-labeled ovalbumin T-cell receptor I CTLs by adoptive transfer. The process of CTL-specific recognition and contact with tumor cells in the liver, as well as the temporal and spatial dynamics of caspase-3 activation, were dynamically monitored by intravital microscopic molecular imaging (Figure 4a). The results showed that the cumulative contact time required for CTLs to induce apoptosis of tumor cells was 98.9 ± 6.3 min. Most (82.6%) apoptotic tumor cells had contact with multiple CTLs, and at most six CTLs made successive contact with a single tumor cell (Figure 4b) causing the apoptosis of tumor cells. Therefore, the killing of tumor cells in living liver tissue, which leads to tumor cell apoptosis, is the result of long-term cumulative contact among multiple CTLs acting synergistically [49]. Quantitative evaluation and comparison of the tumor cell killing capacity of CTLs revealed that their ability to kill tumor cells in the liver was significantly limited compared with that in the spleen and was related to the high expression of the immunosuppressive IL-10 in liver tissue. Thus, intravital immuno-optical molecular imaging can be used to accurately evaluate the effectiveness of tumor immunotherapy in vivo and help understand the effect of organ immune status on cellular immunotherapy.
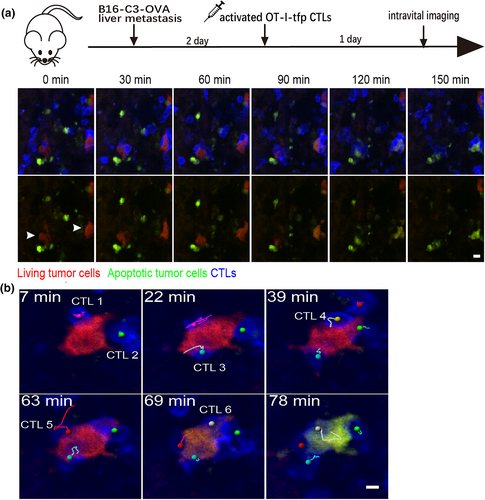
Intravital imaging of CTLs in contact with tumor cells and tumor cell death due to multicellular contact [49]. (a) Intravital imaging showing the loss of fluorescence resonance energy transfer (FRET) signaling in tumor cells with prolonged contact with CTLs. Scale bar: 10 μm. (b) Intravital imaging of six CTLs (visualized as magenta, green, cyan, yellow, red, and gray track markers) coming into contact with a tumor cell and inducing apoptosis. Scale bar: 5 μm. The images in this figure are reproduced with permission from reference [49].
3.1.4 Neutrophils in the liver
Infiltration of neutrophils into the liver is one of the early responses to tissue injury or systemic inflammation [50]. Damaged cells in the liver release endogenous damage-related molecular proteins, such as formyl peptide, to activate innate immunity [51]. Formyl peptide receptor (FPR) signaling is important for guiding neutrophils to migrate to the site of inflammation [52]. Using a two-photon excitation microscope, Honda et al. investigated the number, movement velocity, and morphological changes of EGFP-labeled neutrophils recruited during inflammatory reactions in LysM-EGFP mouse liver following laser irradiation or ischemia reperfusion (I/R) of the liver [53]. After induction of regional necrosis by laser irradiation of the LysM-EGFP mouse livers, time-lapse videos demonstrated that neutrophils treated with FPR antagonist carried out nondirectional random migration around the injury area at 2 h post laser irradiation, with a lower meandering index compared with the untreated control group. At 3 h post laser irradiation, the accumulation of neutrophils in the necrotic area in the control group was significantly inhibited in the FPR antagonist-treated group. In I/R model mice treated with an FPR antagonist, the crawl velocity of neutrophils decreased (1.76 ± 0.28 vs. 2.70 ± 0.25 μm/min) and the ischemic necrotic area of the liver was significantly reduced, suggesting that inhibiting the inflammatory activation caused by neutrophil aggregation can alleviate acute liver injury.
3.2 Application of intravital imaging in liver disease research
Intravital optical microscopy has been widely used to study the immunological mechanisms related to liver diseases. Immuno-cytokinetic activities during bacterial liver infection and acute liver injury has been studied extensively in the last decade and reviewed in other articles [54, 55]. Below, we introduce the application of optical imaging techniques in NAFLD, acute liver infection, and liver tumors from the perspective of in vivo visualization studies.
3.2.1 NAFLD
NAFLD is a chronic liver disease that is rapidly increasing worldwide, along with hepatic steatosis, inflammation, fibrosis, and severe liver failure [56, 57]. However, due to the limited understanding of the pathophysiological mechanism of NAFLD, the assessment and diagnosis of NAFLD are insufficient and effective treatment strategies for NAFLD have not yet been established. An ideal research approach to clarify the cellular and molecular mechanisms of NAFLD pathogenesis in the liver microenvironment would be to perform repeat and long-term intravital imaging monitoring of the liver at the steatosis stage in a mouse model of NAFLD. Seoul-Fluor 44 (SF44), a newly developed fluorescent dye, can selectively label lipid droplet adipocytes in vivo within minutes, with a very low background [58, 59]. Peter Kim's research team successfully recorded the process of lipid droplet accumulation in the liver of NAFLD mice using a customized frame rate laser scanning confocal microscopy imaging system [60] developed by their research group and combining it with SF44 [61]. Over 30% of lipid droplets in NAFLD model mice that were fed an MCD diet had a diameter >3 μm at day 2, and up to 22% had a diameter >9 μm at day 21, whereas all of the observed lipid droplets in the control group, which was fed a conventional diet, had a diameter <3 μm (Figure 5a). Subcellular changes in the position of nuclei due to the accumulation of large lipid droplets in individual hepatocytes were also clearly observed. During the initial phase of the MCD diet, the positions of two nuclei observed in a single hepatocyte remained centered, and there were multiple micro-vesicles in the cytoplasm. After 21 days of the MCD diet, very large lipid droplets formed in the cytoplasm and shifted the position of the nuclei to the periphery (Figure 5b) [61]. Similar imaging observations of the progression of NASH using fluorescent dyes have contributed to the understanding of its pathological mechanisms and improved prognosis.
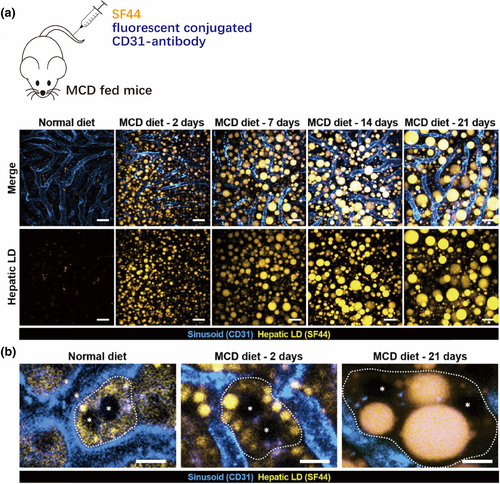
Intravital imaging of hepatic lipid droplet (LD) accumulation in the MCD diet-induced NAFLD mouse model [61]. (a) All LD diameters were <3 μm in the normal-diet group, while those in the MCD-diet group were >3 μm on day 2 and up to 22% were >9 μm on day 21. LDs (yellow), hepatic sinuses (cyan); scale bars, 20 μm. (b) Enlarged view of a single hepatocyte in which the nuclei (asterisks) are surrounded by a growing LD approaching the boundary (dotted line). Scale bars, 10 μm. The images in this figure are reproduced with permission from reference [61].
3.2.2 Liver infection
The liver is the main organ for removing infected bacteria from the blood, and KCs play a major role in this process. Unlike bacteria, parasites are not immediately captured by KCs. Ute Frevert's group used mice expressing Tie2-GFP and lys-EGFP-ki mice (expressing GFP in LSECs and KCs, respectively) to generate Plasmodium sporophyte (GFP labeling) infection models and then performed intravital imaging to study sporophyte behavior. They found that some Plasmodium sporozoites in circulating blood suddenly adhered to the endothelium of the hepatic sinuses and then slid downstream or upstream to the KCs; however, instead of being phagocytic, they passed over the KCs and moved into the hepatic parenchyma, where they eventually invaded hepatocytes (Figure 6) [62]. The movement of this parasite can permanently damage the membrane integrity of the transfected cell, so the ability to cross KCs may be one of the immune-escape strategies that it uses to improve survival during liver infection [63]. In addition to parasitic infections, visual imaging studies of oncolytic viruses have been conducted. Naumenko et al. performed dynamic imaging tracing of the interactions between Alexa Fluor dye-labeled oncolytic viruses and host cells in vivo to explore their systemic distribution. Their results showed that, in the liver, AF647-labeled viruses were preferentially ingested by GFP-labeled KCs over other hepatocyte populations [64]. Notably, current research has focused on infection caused by a single type of pathogen, such as a bacterial, fungal, or HBV infection [65]; however, clinical disease outcomes can be highly complicated and exacerbated by simultaneous or consecutive infection with two or more different microorganisms [66]. The ability to image different microbes during multimicrobial infections to determine how they affect colonization and clearance of each other in the body could inspire new clinical treatments [67].
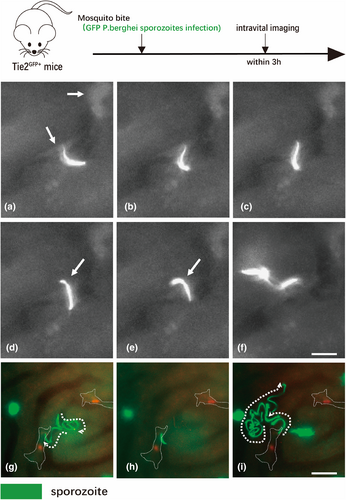
The process of sporozoite invasion of liver cells. (a) After gliding along the liver sinusoid, a sporozoite is shown encountering a KC [62]. (b and c) After a pause, the parasite slowly enters the KC. (d and e) The sporozoite enters the liver parenchyma at a slower rate and with a contractile pattern (arrow). (f) Once in the liver tissue, the sporozoite speeds up and migrates through hepatocytes. (g) A GFP-labeled sporozoite (green) follows the liver sinusoid in a lys-EGFP-ki mouse, eventually encountering a KC (red). (h) The sporozoite stops, facing the phagocyte with its apical cell pole. Outlines of the two KCs are indicated by dotted lines. (i) After slowly passing through KCs, sporozoites enter the liver parenchyma and migrate through hepatocytes. Scale bars = 10 μm. The images in this figure are reproduced with permission from reference [62].
3.2.3 Liver tumors
The rich blood flow of the liver facilitates the metastasis of tumor cells in circulating blood through the hyperpermeable hepatic sinuses, making the liver an organ prone to metastasis [68]. Intravital imaging is a widely used and necessary tool to study the mechanisms of liver metastasis and regression of liver tumors [69]. In the process of tumor development, genetically programmed changes occur in tumor cells and surrounding cells [70]. Bolei Dai et al. established a liver metastasis mouse model with a customized triple-fixed liver window imaging model and tetramer far-red fluorescent protein (tfRFP)-labeled B16 cells expressing apoptosis-related probes, including reactive oxygen species (ROS), caspase-3, and calcium probes. Employing this model, they observed the immune response in liver metastasis using intravital optical molecular imaging [71]. They found that, at the early stage, B16 tumor cells injected into hepatic sinuses via the spleen were influenced by blood flow shear forces and metabolic changes and that the ROS level was gradually increased. This upregulation of ROS induced the activation of intracellular caspase-3 (Figure 7), resulting in cell membrane perforation and the release of intracellular antigen tfRFP, which combined with an extracellular antibody to form an antigen–antibody complex. Further activation of the complement system accelerated and amplified the cell membrane perforation effect, while recruiting neutrophils and F4/80+ macrophages to the liver for synergistic antitumor effects [71].
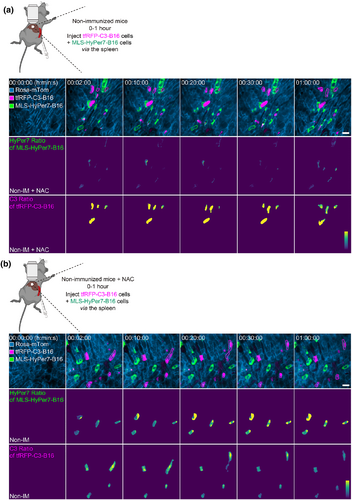
Reactive oxygen species (ROS) elevation and caspase-3 activation in tumor cells circulating in the liver [71]. (a) Representative intravital images of the liver in nonimmunized mice: top row, fluorescence images; middle row, HyPer7 ratio (F488/F405) images of MLS-HyPer7-B16 cells (stably expressing the mitochondria localized-ROS probe); bottom row, C3 ratio (FRET/Cyan Fluorescent Protein) images of tfRFP-C3-B16 cells (stably expressing the caspase-3 probe). Scale bar, 20 μm. (b) Representative intravital images of the liver in nonimmunized mice injected with ROS inhibitor. Scale bar, 20 μm. Figure 7 is reproduced with permission from reference [71].
Anti-CD20 therapy for tumor progression can lead to the depletion of B cells and thus tumor regression, but the exact mechanism remains unclear. Using in vivo liver imaging of mice receiving anti-CD20 treatment, Montalvao et al. revealed that KCs effectively consumed malignant B cells by mediating a sudden arrest and subsequent phagocytosis of circulating B cells in the sinuses of the liver [72]. Dynamic imaging of mice with RFP-labeled B cells after the injection of anti-CD20 antibody showed that circulating B cells begin to stop moving within minutes after injection of the antibody. After 10–30 min, the rounded morphology of the B cells is lost, and the RFP signal appears more diffuse, possibly indicating B-cell death and/or degradation. Subsequently, anti-CD20 therapy was performed on macrophage Fas-induced apoptosis mice with GFP-labeled KCs following adoptive transfer of yellow fluorescent protein-labeled B cells. Almost all of the B cells within the imaging horizon in the antibody-injection group were stagnated on KCs specifically and were phagocytosed by contact KCs a few minutes later, as was also demonstrated by the detection of occasional B-cell fragments in KCs. The results of this study identified the specific antibody-mediated phagocytosis of B cells by KCs as the main mechanism of action in anti-CD20 therapy [72].
Intravital imaging is also a productive method to visually observe the distribution of drugs (e.g., immune checkpoint-blocking drugs) or adoptive immune cells (e.g., chimeric antigen receptor T-cell immunotherapy) and their interactions with target cells in vivo providing valuable dynamic spatiotemporal information for the optimization of liver cancer immunotherapy regimens [73].
4 CONCLUSIONS
The development of intravital optical imaging has opened a new window in the field of liver immunology. As summarized above, high-resolution intravital imaging is widely used to study the dynamic behavior and spatiotemporal interactions of liver immune cells and has provided new insights into the movement behaviors and functions of liver immune cells. Intravital imaging, with its ability to dynamically trace mutual contact and regulatory effects between cells, provides a powerful complement to immunohistochemistry and enables the study of physiological mechanisms within organs and tissues without damaging nearby structures. Nevertheless, we await further advances in the field of optical immunoimaging of the liver. Currently, multiphoton excitation microscopy and spinning disk confocal microscopy are limited in imaging depth. Therefore, photoacoustic imaging techniques with multiscale adjustable imaging depth and large field imaging range are expected to play a crucial role in the intravital imaging of liver immunity. New integrations between intravital imaging and other techniques, designed to achieve specific research aims and create new methods that combine multiple properties, are inspiring. For example, a fast, high-resolution, miniaturized two-photon microscope developed by Heping Cheng's group can be fixed on the head of a mouse to observe neuronal activity in the wake state [74]. According to the latest research results of Qionghai Dai's group, a new framework called digital adaptive optics scanning light-field mutual iterative tomography can achieve high spatiotemporal resolution in 3D volume space, with a large-field 3D imaging capability at the millisecond level [75]. As these techniques are refined and advanced, they are expected to shine in the field of intravital imaging of liver immunity.
Abbreviations
-
- C3
-
- caspase-3
-
- CRIg
-
- complement receptor of the immunoglobulin superfamily
-
- CTLs
-
- cytotoxic T cells
-
- EGFP
-
- enhanced green fluorescent protein
-
- FPR
-
- formyl peptide receptor
-
- GFP
-
- green fluorescent protein
-
- IFN
-
- interferon
-
- IL
-
- interleukin
-
- iNKT cells
-
- invariant natural killer T cells
-
- KCs
-
- Kupffer cells
-
- LD
-
- Lipid droplet
-
- LSECs
-
- liver sinusoidal endothelial cells
-
- MCD
-
- methionine- and choline-deficient
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NKT cells
-
- natural killer T cells
-
- PE
-
- phycoerythrin
-
- RFP
-
- red fluorescent protein
AUTHOR CONTRIBUTIONS
Xuenan Yuan: Investigation (lead); Writing – original draft (lead); Writing – review & editing (equal). Xiang Yu: Writing – review & editing (equal). Bolei Dai: Writing – review & editing (equal). Zhihong Zhang: Supervision (lead); Writing – review & editing (lead).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
This review article does not contain any studies with humans or animals performed by any of the authors.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




