Hotspots of zoonotic disease risk from wildlife hunting and trade in the tropics
野生动物狩猎和贸易引发人畜共患病风险的热带热点地区
Editor-in-Chief & Handling Editor: Prof. Ahimsa Campos-Arceiz
Abstract
enWildlife hunting and trade creates numerous direct human–animal contact opportunities that can facilitate transmission of zoonotic diseases pantropically. Yet, there is a lack of knowledge of which species and areas have a high risk of zoonotic disease spillover due to wildlife hunting and trade. We combined maps of hunting pressure, recent data sets on human-shared pathogens, and trade volumes for 614 mammalian host species to map zoonotic disease risk from wildlife hunting and trade pantropically. We also assessed the relationship between zoonotic disease risk from hunting and probability of emerging zoonotic diseases using generalised least square regression. We identified hotspots of zoonotic disease risk from hunting in central America, China, and central Africa. When considering wildlife trade frequency, high risk occurs in southern Africa and China. Zoonotic disease risk from wildlife hunting aligns significantly with the risk of zoonotic emerging infectious diseases, suggesting that hunting is a key driver of zoonotic disease outbreaks of pandemic potential. Interventions that regulate the hunting of host species and considers the socioeconomic motivations behind hunting could reduce the risk of zoonotic disease spillover.
摘要
zh野生动物狩猎和贸易创造了大量人与动物直接接触的机会,可促进泛热带人畜共患病的传播。然而,对于哪些物种和地区因野生动物狩猎和贸易而具有人畜共患病外溢的高风险尚未明确。我们结合狩猎压力地图、人类共享病原体的最新数据集以及614种哺乳动物宿主物种的贸易量,绘制了泛热带野生动物狩猎和贸易导致人畜共患病风险的地图。我们还利用广义最小二乘法回归评估了狩猎造成的人畜共患病风险与新出现的人畜共患病概率之间的关系。我们在中美洲、中国和非洲中部发现了狩猎导致人畜共患病风险的热点地区。考虑到野生动物贸易频率,南部非洲和中国的风险较高。狩猎野生动物导致人畜共患病的风险与人畜共患新发传染病的风险显著一致,这表明狩猎是导致可能大流行的人畜共患疾病爆发的关键因素。对狩猎宿主物种进行监管并考虑狩猎背后的社会经济动机的干预措施可以降低人畜共患病外溢的风险。【审阅:王易孚】
Plain language summary
enWildlife hunting and trade results in more interactions between humans and animals which can lead to the spread of zoonotic diseases—diseases that can spread between humans and animals. We combined data on wildlife hunting, trade, and diseases that affects both humans and animals to generate a map of zoonotic disease risk from wildlife hunting and trade. We also assessed the spatial correlation between zoonotic disease risk from hunting and probability of emerging zoonotic diseases. High zoonotic disease risk from hunting was observed in central America, China and central Africa and high risk from trade was observed in southern Africa and China. The spatial distribution of zoonotic disease risk from hunting correlates significantly with the probability of emerging zoonotic diseases. This suggests that hunting is one of the key drivers of emerging zoonotic diseases. Hence, regulating wildlife hunting could reduce the risk of emerging zoonotic diseases. However, care should be taken when implementing these hunting regulations as we need to consider the livelihoods and cultural aspects of hunting by local and Indigenous People.
通俗语言摘要
zh野生动物狩猎和贸易会导致人类与动物之间更多的接触,从而导致人畜共患病的传播——人畜共患病可以在人类和动物之间传播。我们将野生动物狩猎、贸易以及影响人类和动物的疾病的数据结合起来,行成了一张野生动物狩猎和贸易导致的人畜共患病风险地图。我们还评估了狩猎带来的人畜共患病风险与新出现的人畜共患病概率之间的空间相关性。在美洲中部、中国和非洲中部观察到狩猎导致人畜共患病的高风险,在非洲南部和中国观察到贸易导致人畜共患病的高风险。狩猎造成的人畜共患病风险的空间分布与新出现的人畜共患病的概率显著相关。这表明,狩猎是新出现的人畜共患病的主要驱动因素之一。因此,规范野生动物狩猎可以降低新出现的人畜共患病的风险。不过,在实施这些狩猎法规时应小心谨慎,因为我们需要考虑当地人和土著人狩猎的生计和文化方面的问题。
Practitioner points
en
-
We find evidence that wildlife hunting is potentially one of the main drivers of emerging zoonotic diseases.
-
Hunting regulations that consider the hunters' socioeconomic motivations are needed in central America, China and central Africa as they are hotspots of high zoonotic disease risk from hunting.
-
China and southern Africa were identified to export high zoonotic disease risk from hunted species.
实践者要点
zh
-
我们的研究表明,野生动物狩猎可能是导致新出现人畜共患病的主要原因之一。
-
中美洲、中国和非洲中部是狩猎导致人畜共患病的高风险热点地区,因此需要制定考虑狩猎者社会经济动机的狩猎法规。
-
中国和南部非洲被认定为狩猎物种导致人畜共患病风险较高的地区。
1 INTRODUCTION
Wildlife hunting is deeply embedded in the traditional culture of rural communities in the tropics (Ingram et al., 2021). It provides human health benefits in rural communities in low- to middle-income tropical nations via the acquisition of nutrients and protein from wild meat (Ingram et al., 2021). For instance, the loss of access to wild meat in rural communities can lead to micronutrient deficiencies such as iron-deficiency anaemia (Golden et al., 2011). Yet, increasing human affluence coupled with enhanced accessibility to forests are driving a sharp rise in consumption and trade of wildlife products (Benítez-López et al., 2017; Ingram et al., 2021). As a result, many species are overhunted and depleted across their range (Ripple et al., 2016; Ziegler et al., 2016). Hunting can also increase the risk of zoonotic disease transmission (Ingram et al., 2021) through close human–animal contact during hunting, handling, and butchering (Dell et al., 2020; Wolfe et al., 2005).
Besides facilitating the transmission of known zoonotic diseases, hunting could also contribute to the emergence of new human diseases through spillover effects. Disease spillover from wildlife is a tangible risk as over 70% of zoonotic emerging infectious diseases (EID) originate from wildlife (Ingram et al., 2021; Jones et al., 2008). For instance, MERS-CoV, SARS-CoV-1, and the Nipah virus have reported zoonotic origins, with its pathogen spillover potentially because of close contact with wildlife (Al-Tawfiq et al., 2014; Williams et al., 2021). The recent SARS-CoV-2, although still debated, was identified to have resulted from at least two zoonotic transmission events; a spillover event from bat coronaviruses and an unidentified mammal species that was traded in the wildlife market (Pekar et al., 2022).
Research efforts to identify correlates of zoonotic disease risk across space have unveiled certain patterns. The risk of zoonotic disease spillover was found to be higher in tropical regions and associated with areas with high richness of wildlife host species (Allen et al., 2017). Additionally, high zoonotic disease risk was found to be associated with forested areas undergoing agricultural land use change (Allen et al., 2017). Furthermore, some species might be more likely to host zoonotic pathogens such as those phylogenetically related to humans (Olival et al., 2017) or those that are domesticated (Johnson et al., 1924).
Wildlife trade, of both live and dead animals and animal parts, can further spread zoonotic diseases outside of the host species native range (Guarner et al., 2004; Shivaprakash et al., 2021). The trade increases contact between host species and other species such as domesticated species, nonnative wildlife species, and humans (Shivaprakash et al., 2021; Smith et al., 2012). This creates more opportunities for transmission and increases the risk of zoonotic disease spillover events (Shivaprakash et al., 2021). For instance, monkeypox was introduced to the United States from Ghana through the pet trade of exotic African rodents (Guarner et al., 2004). Additionally, wildlife trade also drives hunting in rural areas (Ingram et al., 2021) with a substantial portion of hunted prey items usually sold to local markets (Kümpel et al., 2010).
Research has focused on understanding environmental factors and biological traits that may drive the global spatial distribution of zoonotic disease risk (Allen et al., 2017; Jones et al., 2008). However, there is limited information on which species have high zoonotic disease risk from hunting and trade. To fill this gap, here we: (i) identify the level of zoonotic disease risk from wildlife hunting and trade (thereafter referred to as zoonotic disease risk from hunting and zoonotic disease risk from trade, respectively) in mammal species and taxonomic orders and (ii) develop pantropical hunting and trade zoonotic disease risk maps based on the distribution of host species, as well as their hunting pressure and trade volumes. Compared to other pathways of zoonotic disease risk, namely land-use change (e.g., increasing livestock-wildlife interactions, agricultural expansion, deforestation, altering disease vectors prevalence), medical industry change, climate, human demography, and breakdown of public health (Allen et al., 2017; Durrance-Bagale et al., 2021), wildlife hunting and trade represent a glaring research gap (Cantlay et al., 2017; Coker et al., 2011). Our study is novel in its focus on these two poorly known pathways of zoonotic disease risk at broad scales. Our results could help inform future hunting regulations and research to prevent the emergence of zoonotic diseases from hunting activities and wildlife trade.
2 MATERIALS AND METHODS
2.1 Overview and data sources
This study is limited to the tropics (42.6°N to 37.5°S) as there is a higher risk of zoonotic disease in tropical regions (Allen et al., 2017; Jones et al., 2008). First, we calculated the zoonotic disease risk from hunting activities using two variables (number of human-shared pathogens and hunting pressure). The number of human-shared pathogens—pathogens (i.e., bacteria, viruses and parasites) with documented evidence of capacity to infect both humans and animals—of 780 mammalian host species was extracted from Gibb et al.'s data set (Gibb et al., 2020). The extent of defaunation, following Benítez-López et al. (2019) was used as a proxy to hunting pressure. Next, we analysed the spatial correlation between zoonotic disease risk from hunting activities and the risk of zoonotic EID to ascertain if hunting is one of the drivers of zoonotic EID events. Lastly, data on legal and illegal wildlife trade between countries at the species level were collected from Scheffers et al. (2019), the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) (Convention on International Trade in Endangered Species of Wild Fauna and Flora, 2021), and the Wildlife Trade Portal (WTP) (TRAFFIC, 2022) (Figure 1).
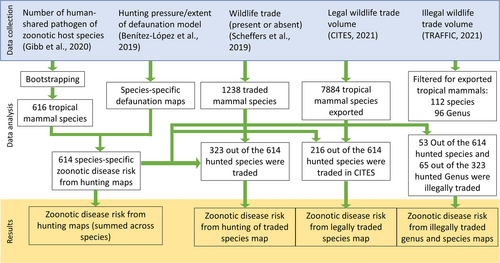
2.2 Accounting for research effort
The number of identified pathogens in a host species is expected to increase as a function of the number of published studies, that is, the research effort in the study of the pathogens of a species could bias its perceived pathogen richness. To account for research effort and reduce its potential bias, we adopted Gibb et al.'s (2020) bootstrapping method whereby a proportion of nonhost species was converted to host species based on a false classification rate (see Supporting Information S1: Appendix). The bootstrapping method converted 34 nonhost species to host species, resulting in a total of 814 mammalian host species, with 614 terrestrial tropical species.
2.3 Calculating the zoonotic disease risk from hunting activities
We applied the hunting-induced defaunation model developed by Benítez-López et al. (2019). to project the impact of hunting pressure on the abundance of each tropical species (i.e., defaunation). We included the distance to hunter's access points, human population density, protection status (whether hunting occurred in a protected area), and poverty levels as spatial predictors of hunting pressure, and species' body mass as a predictor of species-specific sensitivity to population decline due to hunting, The model projections range from 0 (no defaunation/no hunting pressure) to 1 (fully defaunated/high hunting pressure) (Benítez-López et al., 2019). Data on species range was obtained by overlapping species' range data from the International Union for Conservation of Nature (IUCN) with forest canopy cover of more than 20% (Hansen et al., 2013) to exclude human-dominated areas. The defaunation index of each host species was then predicted throughout its range at 1 × 1 km resolution using Benítez-López et al.'s defaunation model (see Supporting Information S1: Appendix for more details). Then we stacked model predictions across species to depict areas that are potentially under high hunting pressure due to their accessibility and current faunal composition.
2.4 Correlation between zoonotic disease risk from hunting and zoonotic EID risk
The probability of a zoonotic EID event was predicted by Allen et al.'s (2017) boosted regression trees model using human activity, animal host data, and environmental factors while accounting for reporting effort. The correlation between zoonotic disease risk from hunting (summed across species) and the probability of a zoonotic EID event was analysed using generalised least squares regression (Pinheiro et al., 2022). The variables were log-transformed to reduce data skewness. To account for spatial autocorrelation, five models with different spatial correlation structures were proposed (Supporting Information S1: Appendix Table S1). The model with exponential spatial correlation structure had the lowest Akaike information criterion (AIC) and was selected as the final model. It should be noted that population density is a factor in our calculation of defaunation index and also a predictor in Allan et al. (2017). Although using both predictors could help both models agree, both analyses present different sets of predictors and research questions.
2.5 Calculating the zoonotic disease risk from trading hunted species
The WTP is maintained by TRAFFIC (2022) which compiles open-source illegal wildlife trade data from various official and online sources. Scheffers et al. (2019). only have presence and absence data (0 or 1) for each species while CITES and TRAFFIC have the volume of trade data. Scheffers et al. (2019) compiled traded species data from CITES and the International Union for the Conservation of Nature Red list of Threatened Species (IUCN Red List). This data set gave us an overview of all traded mammal species for both legal and illegal trade. A total of 323 out of the 614 species in the hunting data set were reported to be traded. The zoonotic disease risk from hunting of these 323 species were then summed up for each cell, generating a map of zoonotic disease risk from hunting of traded species obtained from Scheffers et al. (2019).
The CITES trade data were cleaned and filtered to only include records with tropical countries as exporters (see Supporting Information S1: Appendix). Out of the 614 species in the hunting data set, only 216 were recorded in the CITES trade. The number of CITES exported transactions for each species and country were then scaled from 0 to 1. The number of transactions for each species were combined with country polygons obtained from the Word Bank database (The World Bank, 2020) to create species-specific raster maps of the number of transactions in each country. These raster maps have the assumption that the number of transactions is the same within the country. These species-specific CITES raster maps were then multiplied by the species-specific zoonotic disease risk from hunting raster maps for all 216 species. The 216 combined maps (CITES ∙ zoonotic disease risk from hunting) were summed up for each raster cell, generating the zoonotic disease risk from hunting from the legal trade.
The WTP data were first cleaned and filtered (see Supporting Information S1: Appendix for details). Two levels of identification were calculated for the illegal trade: genus- and species-level. The WTP data only represent an unknown portion of all illegal wildlife trade and may thus be incomplete and biased. Biases arise from varying enforcement and reporting effort by countries 't Sas-Rolfes et al., 2019) and the fact that not all commodities seized are reported to species- or genus-level. We developed a gravity model coupled with an underreporting submodel to correct these biases (Symes et al., 2018). We include country-level predictors such as gross domestic product, rule of law, and reporting effort as predictors for the gravity model (see Supporting Information S1: Appendix). To calculate the zoonotic disease risk from hunting for illegal trade, we used the same approach as the legal trade and averaged the zoonotic disease risk from hunting at genus-level per raster cell. Out of the 614 species, only 53 species and 65 genera were recorded in the WTP data.
All maps were generated using tmap (Tennekes, 2018) and legend breaks were generated using Jenks natural breaks. All analyses were performed using R statistical software (version 4.1.0) (RStudio Team, 2022). Figures were generated with ggplot2 (Wickham, 2016).
3 RESULTS
3.1 Pantropical spatial distribution of hotspots of zoonotic disease risk from hunting
Our results reveal that the zoonotic disease risk from hunting is high in central America (Guatemala, Honduras, Costa Rica, Panama and Colombia) and China, followed by central Africa (Cameroon, Northern Albertine Rift, Guinea and Côte d'Ivoire) (Figure 2). The contribution of hunting pressure and pathogen richness to zoonotic disease risk from hunting differs between the identified hotspots. The high zoonotic disease risk from hunting in central Africa was driven by its high hunting pressure, while a combination of high hunting pressure and high pathogen richness contributed to the high zoonotic disease risk from hunting seen in China (Supporting Information S1: Appendix Figure S1).
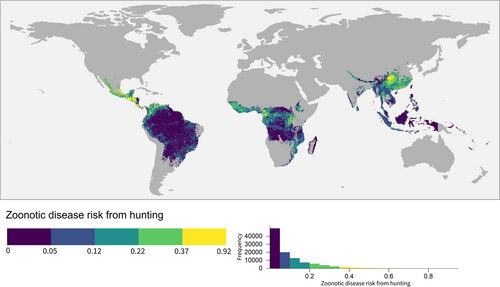
To ascertain whether the high zoonotic disease risk from hunting was an artifact of summing over a large number of species (Mollentze & Streicker, 2020), we also mapped the average risk across host species richness. Our maps of averaged zoonotic disease risk from hunting still showed a similar spatial pattern (Supporting Information S1: Appendix Figure S2). Our alternative scenario exploring a nonlinear relationship between hunting pressure and zoonotic disease risk also yielded similar geographical trends (Supporting Information S1: Appendix Figure S3).
Our generalised least squares model showed that zoonotic disease risk from hunting was significantly correlated to the probability of a zoonotic EID event from Allen et al. (2017) (p < 0.01) (Supporting Information S1: Appendix Table S2).
3.2 Pantropical spatial distribution of hotspots of zoonotic disease risk from international trade
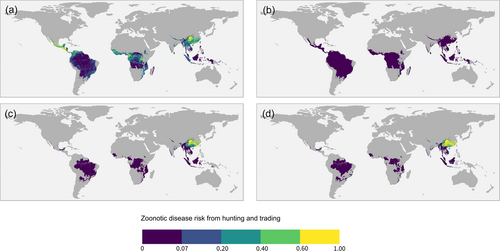
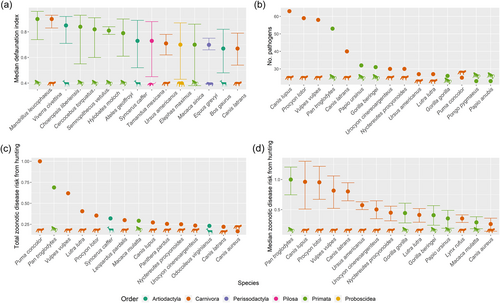
Hotspots of zoonotic disease risk from trade based on Scheffers et al. (2019) data set closely followed the distribution of zoonotic disease risk from hunting alone (Figures 2 and 3b). Southern Africa (South Africa, Zimbabwe and Tanzania) was identified as a hotspot of zoonotic disease risk from legal trade (Figure 3b). Conversely, the main hotspot of zoonotic disease risk from illegal trade was China (Figure 3c,d). Orders Primata and Proboscidea had the highest mean zoonotic disease risk from legal trade while Carnivora had the highest risk for illegal trade. The species with the highest zoonotic disease risk from legal trade was the Cape baboon (Papio ursinus), and for the illegal trade, the leopard (Panthera pardus).
3.3 Zoonotic disease risk in orders
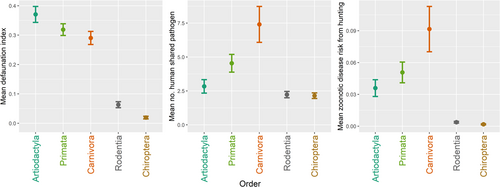
Orders Carnivora and Primata consistently showed high zoonotic disease risk as they had the highest average number of human-shared pathogens and the highest mean zoonotic disease risk from hunting (Figure 4). In contrast, orders Chiroptera and Rodentia had a relatively lower zoonotic disease risk from hunting due to their lower DI (Figure 4). The top 15 species with high zoonotic disease risk from hunting consisted mostly of carnivores and primates (Figure 5). Species with the highest total zoonotic disease risk from hunting summed across their range were Puma concolor (cougar), Pan troglodytes (chimpanzee), and Vulpes vulpes (red fox). Conversely, species with the greatest median zoonotic disease risk from hunting per raster cell were Pan troglodytes (chimpanzee) (1, interquartile range [IQR] = 0.46), Canis lupus (grey wolf) (0.96, IQR = 0.79), and Procyon lotor (common raccoon) (0.95, IQR = 0.55) (Figure 5). Our nonlinear disease risk-hunting pressure scenario resulted in shifts in species rankings, but the same few species remained on the top (Supporting Information S1: Figure S4).
4 DISCUSSION
In this study, we leveraged data from comprehensive data sets on human-shared pathogens, spatial information on hunting pressure, and wildlife trade to derive maps of zoonotic disease risk from hunting and trade. Our map of zoonotic disease risk from hunting was significantly correlated (p < 0.01) to previous map of overall zoonotic EID risks (Allen et al., 2017), suggesting that hunting is one of the main drivers of zoonotic EID risk (Allen et al., 2017; Jones et al., 2008). Previous studies on drivers of zoonotic EID risk singled out wildlife host species diversity as one of the main predictors (Allen et al., 2017; Jones et al., 2008). Jones et al. (2008) found that the number of EID events of wildlife origin is positively correlated with host species richness, whereas Allen et al. (2017). reported a relationship in which EID risk was higher at low and very high species richness. Our results show that the differing relationship between species richness and zoonotic EID risk reported by Jones et al. (2008) and Allen et al. (2017) may be explained by spatial factors. Central America was identified as a hotspot of zoonotic disease risk from hunting due to its high host species richness, moderate hunting pressure and intermediate pathogen numbers compared to other regions. Conversely, host species richness played a limited role in Africa and China. Africa's high zoonotic disease risk from hunting were due to high hunting pressure, while in China it was due to a combination of high hunting pressure and high pathogen richness per hunted host species. Although the maps were correlated, there were still areas of disagreement. This disagreement may be attributed to the other pathways of zoonotic disease spillover (Allen et al., 2017; Durrance-Bagale et al., 2021). Hence, further research considering other pathways could shed light on the differences observed.
Hunting regulations could reduce the risk of zoonotic disease spillover. However, subsistence hunting is an important cultural activity and protein source to local communities and Indigenous Peoples (Ingram et al., 2021). The recognition of land rights, and with them hunting rights, of Indigenous Peoples is fundamental to prevent tropical deforestation and forest degradation (Sze et al., 2021). Reduced deforestation and forest degradation would ultimately contribute to reducing human exposure to zoonotic disease risk brought about by land clearing and unsustainable hunting. Conversely, stricter regulations may be applied to recreational hunting (e.g., China: Xishuangbanna; Chang et al., 2017) and educating hunters on the risk of zoonosis infections from hunting (Friant et al., 2015). Local wild meat markets are another point of intervention to reduce disease spillover risk. Regulating the sale of live animals and fresh meat of specific high-risk species and implementing better sanitation practices can help to reduce zoonotic disease spillover risk while still maintaining the livelihoods of local communities (Mayor et al., 2022).
Our results indicate a close relationship between zoonotic disease risk from trade and zoonotic disease risk from hunting. The hotspots of zoonotic disease risk from trade based on Scheffers et al. (2019). closely followed the distribution of zoonotic disease risk from hunting. This suggests that areas with high zoonotic disease risk from hunting might contribute to the spread of zoonosis to other areas through trade (Smith et al., 2012; Wolfe et al., 2005). This is especially true for Africa, as more hunted species were traded as compared to Asia and south America (Supporting Information S1: Appendix Figure S5).
Other related studies on zoonotic disease risk from wildlife trade also identified orders Primata and Carnivora as major zoonotic disease reservoirs (Shivaprakash et al., 2021), with Primata contributing to 99% of the zoonosis potential of the CITES trade (Borsky et al., 2020). Some of the zoonotic pathogens identified in traded wild meat and live primates include Staphylococcus sp (Chaber & Cunningham, 2016), Leptospira spp (Almeida et al., 2016), and Simian foamy virus (Smith et al., 2012). The presence of pathogen in traded wild meat indicates that hunted host species can increase the zoonotic disease risk in other areas because of wildlife trade (Bezerra-Santos et al., 2021).
Although much surveillance effort has been placed on orders Primata, Chiroptera, and Rodentia, our results suggested that Carnivora had high zoonotic disease risk as well. Among the mammalian orders, Carnivora had the highest proportion of zoonotic disease host species (49%), as compared to Primata (21%), Chiroptera (9.8%), and Rodentia (10.7%) (Han et al., 2016). Zoonotic diseases that spilled over from carnivores to humans include rabies from skunks and domesticated dogs (Velasco-Villa et al., 2017), and severe acute respiratory syndrome from palm civets (Wang et al., 2005).
The high zoonotic disease risk for carnivores might be because of their functional ecological roles (Han et al., 2021). The majority of zoonotic pathogens found in carnivores are parasitic helminths with complex life cycles that require trophic transmission (Han et al., 2021), hence, the high risk in carnivores could be due to the bioaccumulation of zoonotic diseases from their prey (Han et al., 2016, 2021; Wang et al., 2005). Furthermore, carnivorans with a more generalist diet tend to harbour more zoonotic parasites because of their diet and foraging behaviour (Han et al., 2021). Additionally, the evolutionary loss of inflammasome activation—activation of protein complexes that contain pathogen-recognition receptors to drive inflammation to control infections—in carnivores exposes them to infection. This allows them to be asymptomatic carriers of diseases (Digby et al., 2021) and acquire zoonotic pathogens through their diet (Digby et al., 2021; Han et al., 2021).
Although orders Chiroptera and Rodentia had a relatively lower zoonotic disease risk from hunting, they are the largest mammalian orders with high species richness (Han et al., 2016; Mollentze & Streicker, 2020). As pathogen richness increases with host species richness (Mollentze & Streicker, 2020), orders Chiroptera and Rodentia are important reservoirs and could transmit zoonotic diseases through intermediate host species and contact with humans even if not hunted (Williams et al., 2021). For instance, bats were postulated as the reservoir for Nipah virus and the virus was transmitted to humans via pigs as the intermediate host species (Mhod Nor et al., 2000; Williams et al., 2021). Rodents may also transmit arenaviruses to humans through indirect contact via inhalation of aerosolized excrement (Williams et al., 2021). Hunting can also indirectly increase the zoonotic disease risk of bats and rats. For instance, hunting-induced population declines of predators and competitors may result in overabundant populations of nonhunted host species with a fast-paced life history, such as rodents and bats, and this may compromise the dilution effect provided by high species diversity (Civitello et al., 2015). Hence, under high hunting pressure, Chiroptera and Rodentia would remain important reservoirs of zoonotic pathogens, with the potential of spreading these pathogens through close contact with domestic animals and contamination of the environment even if they are not the target of hunters.
The zoonotic disease risk from hunting was calculated based on the assumption that pathogens found in both humans and animals have a higher risk of zoonotic disease spillover. However, some pathogen strains might be species-specific, resulting in a lower probability of zoonotic disease transmission for these pathogens (Anoh et al., 2015). Nevertheless, we argue that wildlife with more pathogens shared with humans will have more spillover potential (Gortázar et al., 2016; Singh et al., 2023). This wider scope also allows inclusion of probable future spillover. In any case, future studies including variables on the likelihood of pathogens crossing the species barrier (e.g., length of exposure and multi-host pathogenicity) (Gortazar et al., 2014; Singh et al., 2023) is needed.
Our study was conducted at a broad scale inherently presenting limitations that make the results unable to capture variations at the local level. For example, due to data paucity, our analyses did not consider differences in hunting practices, hunting taboos, butchering, transport, and consumption at different locations. For instance, hunting rodents is a taboo for certain Shona groups in Zimbabwe as their totems are rodent species (Mazarire, 2016).
Model-based DI was used as a proxy for hunting pressure because of the lack of species-specific hunting pressure maps based on observational data. Hunting pressure varies over time while the DI values only show a snapshot in time. The model also did not account for synergies between accessibility, deforestation, fragmentation, and hunting pressure (Benítez-López et al., 2019). We considered two scenarios (linear and nonlinear) for the relationship between DI and zoonotic disease risk. However, due to data limitations, these scenarios are conjectures, and the true relationship is probably species-dependent.
5 CONCLUSIONS
Locations with high zoonotic disease risk from hunting were found in all three tropical continents. The spatial distribution of zoonotic disease risk from hunting aligns spatially with zoonotic EID risk maps (Allen et al., 2017; Jones et al., 2008), further supporting hunting as an important driver of zoonotic EID events. As a portion of hunted wildlife are usually traded for income, the zoonotic disease risk from hunting can be further spread to urban areas and other continents. Southern Africa and China were identified to be major actors in the legal and illegal wildlife trade, and potential hotspots of zoonotic disease risk because of these activities. Orders Carnivora and Primata were found to have the highest zoonotic disease risk from hunting and trade. Conversely, species-rich mammalian orders like Chiroptera and Rodentia have high host species richness and could transmit zoonotic diseases through intermediate host species and increased contact with humans. We call for a regulation of wildlife hunting and trade of high disease-risk species, instead of a complete ban, to reduce the likelihood of zoonotic disease spillover and maintain livelihoods.
AUTHOR CONTRIBUTIONS
Jacqueline Choo: Formal analysis; writing—original draft; writing—review and editing. Le T. P. Nghiem: Formal analysis; writing—review and editing. Serene Chng: Writing—review and editing. Luis R. Carrasco: Conceptualization; supervision; writing—review and editing. Ana Benítez-López: Methodology; resources; supervision; writing—review and editing.
ACKNOWLEDGEMENTS
We thank funding from the Singapore Ministry of Education of Singapore Tier 1 grant A-0004770-00-00. A.B.-L. was supported by an EMERGIA grant (EMERGIA20_00252) from the Junta de Andalucía (Spain) and a Ramón y Cajal grant (RYC2021-031737-I) funded by MCIN/AEI/10.13039/501100011033 and the EU (‘NextGenerationEU’/PRTR). The funding sources did not have a role in the writing of the manuscript or the decision to submit for publication or involved in any other way. The authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data and codes are available at the figshare repository at https://figshare.com/s/7752545038f8c3ed19d7.




