Construction of Reporter Phage T4::Nluc and Its Application in the Detection of Escherichia coli in Urinary Tract Infections
Funding: This study was funded by the National Natural Science Foundation of China (grant number 82172364) and the Natural Science Foundation of Beijing Municipality (grant number L244076).
ABSTRACT
Background
Urinary tract infections (UTIs) are one of the most common infectious diseases worldwide, predominantly caused by Escherichia coli. We constructed a reporter phage T4::Nluc to achieve rapid, sensitive, and specific detection of Escherichia coli in UTIs.
Methods
T4::Nluc was constructed using the CRISPR/Cas9 system combined with homologous recombination and was confirmed through Sanger sequencing. The biological properties of T4 and T4::Nluc were compared. Time-luminescence curves were detected to investigate the limit of detection (LOD) and the influence of urine. Additionally, the specificity of T4::Nluc was examined by co-culturing it with other pathogens. In total, 104 urinary Escherichia coli isolates were collected to assess detection coverage. Finally, 698 urine samples were collected for clinical validation.
Results
T4::Nluc was confirmed to be correct. The one-step growth curves of T4 and T4::Nluc were similar, but the optimal multiplicity of infection for T4 was 1, and that for T4::Nluc was 0.1, indicating that genetic modification had some effect. The LOD was 104 colony-forming unit/mL detected at 220 min. Urine did not affect detection and T4::Nluc did not cross-react with other pathogens. T4::Nluc could detect 38.46% of clinical strains, demonstrating higher sensitivity than the double-layer overlay assay (25.96%). In clinical urine samples, its detection sensitivity was 36.59%, and the specificity was 100%.
Conclusion
T4::Nluc was successfully constructed and could detect Escherichia coli with superior sensitivity and specificity compared with traditional diagnostics, fulfilling the diagnostic criteria for UTIs while significantly reducing the detection time. This presented a novel approach for rapid and accurate detection of E. coli in UTIs.
Abbreviations
-
- AST
-
- antibiotic sensitivity testing
-
- BSA
-
- bovine serum albumin
-
- CBM
-
- carbohydrate binding module
-
- CFU
-
- colony-forming unit
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- ER
-
- emergency room
-
- LB
-
- Luria-Bertani broth
-
- LOD
-
- limit of detection
-
- MOI
-
- multiplicity of infection
-
- Nluc
-
- Nanoluciferase
-
- PCR
-
- polymerase chain reaction
-
- PFU
-
- plaque-forming unit
-
- UTI
-
- urinary tract infection
-
- β-gal
-
- β-galactosidase
1 Introduction
Urinary tract infections (UTIs) are one of the most common infectious diseases worldwide [1], with a multifactorial etiology involving a variety of bacteria and certain fungi [2], including Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis, and Staphylococcus saprophyticus [3]. Among them, E. coli is the most common Gram-negative bacterium in patients with UTIs [3], accounting for 70%–95% of all UTIs [4]. UTIs can clinically manifest as mildly irritative voiding, urethritis, cystitis, pyelonephritis, bacteremia, sepsis, and even death [1, 5]. Given the high recurrence rate of UTIs and the increasing antibiotic resistance of pathogens [3], these infections impose a significant economic and public health burden, with a global prevalence of approximately 150 million cases per year, an estimated direct healthcare expenditure of more than 6 billion dollars, and a severe impact on the quality of life of patients [2].
Currently, the gold standard for diagnosing UTIs is the presence of symptoms accompanied by positive urine culture [2]. The diagnostic threshold is a single-type bacterial colony count ≥ 105 colony-forming unit (CFU)/mL in quantitative culture of clean midstream urine [6]. Traditional detection methods based on bacterial culture are time-consuming, labor-intensive, and have a high rate of missed detection. Instant screening methods such as lateral flow chromatographic assay and flow cytometry encounter challenges in terms of strain identification accuracy [7]. Molecular biology techniques include matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, fluorescence in situ hybridization, polymerase chain reaction (PCR), gene chip technology, and metagenomic next-generation sequencing fail to differentiate between viable and dead bacteria while also requiring technical expertise and incurring significant costs [7, 8]. The development of an economical, convenient, rapid, sensitive, accurate, and specific method for the detection of pathogenic bacteria in UTIs is of great significance for the early intervention and precise treatment of patients as well as the prevention of antibiotic resistance.
Phages are a type of virus that specifically infects bacteria [9]. They attach to and invade viable host bacteria, resulting in the production of numerous progeny phages [10], exhibiting specificity at the genus, species, or strain level [2]. Phages are easy to produce, low-cost, and stable under extreme environments characterized by significant fluctuations in temperature, pH, or ionic strength; they also possess the ability to discriminate between viable and dead bacteria [11, 12], rendering them valuable for identifying diverse bacterial populations [10]. Genetically engineered reporter phages exhibit even greater potential for the detection of clinical pathogens, where a heterologous reporter gene is introduced into the phage genome. Upon infection, the reporter gene is expressed, leading to the production of a detectable signal that indicates the presence of viable host bacteria [2, 10]. Nanoluciferase (Nluc) is a compact and exceptionally stable enzyme that rapidly produces robust bioluminescent signals upon the addition of a substrate, independent of ATP, and stands out as one of the most widely employed reporter genes [2, 13, 14].
A variety of techniques for genetically engineering phages have been developed. Traditional homologous recombination, λ-Red recombination, bacteriophage recombineering of electroporated DNA, and in vitro synthesis and rebooting technology have low recombination efficiency, rely on the successful transformation of host bacteria, and are difficult to screen because of the lack of recombinant markers [15-17]. Additionally, engineering phages with large genome poses challenges. The emergence of the CRISPR/Cas9 system has significantly accelerated the development of gene editing technology and greatly improved its efficiency and accuracy, making it a powerful tool for engineering prokaryotic and eukaryotic systems [18]. The CRISPR/Cas9 system was employed by Shen et al. to successfully integrate the red fluorescent protein gene into the phage of Klebsiella, achieving an editing efficiency of 87.5% [19]. Duong et al. achieved a > 99% recombination efficiency in T4 phage using the CRISPR/Cas9 system [18].
In this study, the CRISPR/Cas9 system combined with homologous recombination was employed to integrate the Nluc gene block into E. coli phage T4 to construct a reporter phage (T4::Nluc) for rapid, sensitive, specific, and accurate detection of E. coli in clinical UTIs, providing a new approach for timely diagnosis and effective treatment.
2 Materials and Methods
2.1 Strains, Phages, and Plasmids
E. coli BL21 (DE3) and phage T4 were purchased from the American Type Culture Collection. The 104 clinical urine isolates of E. coli and other common clinical pathogens were obtained from Department of Clinical Laboratory Medicine, The First Medical Center of PLA General Hospital (Table S1). All clinical strains were identified by mass spectrometry. The pCRISPR and pCas9 plasmids were both purchased from Hunan Fenghui Biotechnology Co. Ltd (Changsha, China).
2.2 Reagents and Instruments
Luria-Bertani broth (LB) (Sangon, Shanghai, China); agar, bovine serum albumin, creatinine, and urea (Solarbio, Beijing, China); agarose G-10 (Biowest, Spain); BsaI, T4 Polynucleotide Kinase, and T4 DNA Ligase (New England Biolabs, United States); Phanta Max Super-Fidelity DNA Polymerase, FastPure Plasmid Mini Kit, FastPure Gel DNA Extraction Mini Kit, and ClonExpress Ultra One Step Cloning Kit (Vazyme, Nanjing, China); Amicon Ultra-15 100K (Millipore, United States); Nano-Glo Luciferase Assay (Promega, Fitchburg, United States).
PCR amplification instrument (Applied Biosystems, United States); Electrophoresis apparatus, Gel Imager System (BIO-RAD, United States); Ultracentrifuge (Thermo Scientific, WX100 + AH-629, United States); Infinite M200 PRO and Tecan i-control, 2.0.10.0 software (Tecan, Switzerland).
2.3 Preparation of Competent Cells
Using LB broth, BL21 or BL21 containing the target plasmid was cultured to the logarithmic phase at 37°C and 200 r/min. The bacterial solution was bathed in ice for 10 min before being centrifuged at 6000 × g and 4°C for 10 min. An equal volume of 0.1 mol/L calcium chloride solution stored at 4°C was added to the pellet. After thorough mixing and further incubation on ice for 30 min, it was centrifuged. The resulting pellet was mixed with calcium chloride solution and glycerin, and stored at −80°C.
2.4 Construction of Engineered Plasmids
To construct pCRISPR-cr6, the cleavage site of BsaI was added to both ends of the single guide RNA soc-cr6 (Table S2), which was synthesized by Sangon Biotech (Shanghai, China). The oligonucleotides were phosphorylated by T4 Polynucleotide Kinase and annealed to double-stranded DNA. The circular pCRISPR plasmid (containing the kanamycin resistance gene) was digested overnight at 37°C by BsaI and ligated to annealed soc-cr6 at 4°C overnight with T4 DNA Ligase. Next, the pCRISPR-cr6 plasmid was transformed into BL21 and plated on LB solid agar plates containing kanamycin for overnight cultivation. The pCRISPR-cr6 plasmid was verified by colony PCR (Table S3 and S4) and Sanger sequencing, then extracted by FastPure Plasmid Mini Kit and stored at −20°C.
To construct the homologous recombinant plasmid, the Nluc gene block was obtained from our previous research (Table S5). The upstream and downstream homologous arms (Table S5), as well as primers UHA-F, UHA-R, Nluc-F, Nluc-R, DHA-F, and DHA-R containing 20 bp sequence overlaps at both ends (Table S2), were synthesized by Sangon Biotech (Shanghai, China). The homologous arms and Nluc gene block were amplified by PCR and purified with the FastPure Gel DNA Extraction Mini Kit. The extracted products served as templates for PCR to ensure that the recovered DNA fragments were correct. The ClonExpress Ultra One Step Cloning Kit was used to construct the homologous recombinant plasmid using Gibson assembly. The homologous recombinant plasmid (containing ampicillin and kanamycin resistance genes) was transformed into BL21 and verified by colony PCR with seven pairs of different primers.
2.5 Screening of T4::Nluc by PCR
The triplasmid BL21 was cultured to the logarithmic stage and infected with T4 by a double-layer overlay assay [14]. Specifically, 200 μL of log-phase triplasmid BL21 was mixed with 100 μL of T4 with an appropriate titer in molten soft agar (LB + 0.625% agar [w/v]) at 55°C, and then immediately poured onto an LB solid plate. After overnight incubation at 37°C, 21 phage plaques were selected each time; approximately 0.5 μL phage solution was collected from the center of the plaque and mixed with 10 μL of 10 mM EDTA, then incubated at 65°C for 10 min and cooled to 4°C. The resulting mixture served as a template for PCR to screen for T4::Nluc.
2.6 Sucrose Density Gradient Centrifugation
2.7 Comparison of Biological Properties Between T4 and T4::Nluc
For the one-step growth curve [20, 21], 5 mL of T4 or T4::Nluc with a titer of 1 × 107 plaque-forming unit (PFU)/mL was mixed with 5 mL of BL21 at a concentration of 1 × 108 CFU/mL. This was incubated in a water bath at 37°C for 10 min and centrifuged at 5000 × g for 10 min. The sediment was washed with PBS buffer. After centrifugation, the sediment was resuspended in 10 mL of LB broth and incubated at 37°C and 200 r/min for 120 min. The culture was taken every 10 min and filtered by a 0.22 μm sterilization filter, and the phage titers were determined by the double-layer overlay assay. The burst size was the count of phages released at the end of the outbreak period divided by the count of host bacterial cells infected during the latent period [22].
To determine the optimal multiplicity of infection (MOI), T4 or T4::Nluc was mixed with BL21 (1.87 × 108 CFU/mL) at MOIs of 10, 1, 0.1, 0.01, 0.001, 0.0001, and 0.00001. This was then incubated at 37°C and 200 r/min for 6 h. After filtration by 0.22 μm sterilization filters, the titers of T4 and T4::Nluc after incubation were determined.
2.8 The Limit of Detection for T4::Nluc
A volume of 5 mL of serially diluted BL21 (1 × 106–101 CFU/mL) was co-cultured with T4::Nluc at a final concentration of 3 × 106 PFU/mL for 7 h. The group consisting only of T4::Nluc served as a negative control. The culture (50 μL) was sampled every 10 min, and 20 μL of substrate was added before measuring luminescence signals. The threshold was defined as the average background luminescence plus three standard deviations; the LOD was the minimum bacterial concentration required to produce a luminescence signal above the threshold [2].
2.9 Influence of Urine on Detection
Bovine serum albumin (BSA) solutions of 0.5, 0.1, and 0.01 g/L, creatinine solutions of 100, 10, and 0.5 mmol/L, and urea solutions of 1000, 333, and 10 mmol/L were prepared with LB broth. The final concentration of BL21 in each group was 106 CFU/mL. At the same time, BL21 (106 CFU/mL) with only LB broth was set as a positive control, and only LB broth was set as a negative control. The same amount of T4::Nluc was added to the groups and co-cultured at 37°C and 200 r/min for 3 h, during which luminescence was measured every 10 min.
Clean midstream urine was collected from the clinic and filtered using a 0.22 μm sterilization filter. The filtered urine was mixed with LB so that the proportion of urine in each group was 0%, 20%, 40%, 60%, 80%, and 100% while maintaining a consistent concentration of BL21 (2.72 × 107 CFU/mL) across all groups. In addition, groups consisting of only LB broth or urine were established. An appropriate amount of T4::Nluc was added to the above groups and co-cultured at 37°C and 200 r/min for 5 h, during which luminescence was measured every 10 min.
2.10 The Detection Specificity of T4::Nluc
BL21, E. faecalis, Enterococcus faecium, K. pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, and Staphylococcus hominis were cultured overnight at 37°C and 200 r/min. They were then co-cultured with T4::Nluc, respectively, for 5 h, during which the luminescence signals were measured every 10 min. The group with only T4::Nluc served as a negative control.
2.11 The Detection Coverage of T4::Nluc in Clinical Strains
The double-layer overlay assay was performed with 200 μL of clinical E. coli cultured overnight mixed with molten soft agar (LB + 0.625% agar [w/v]) at 55°C and immediately poured onto an LB solid agar plate. After solidification, 3 μL of T4::Nluc was taken for spotting, which was incubated at 37°C overnight. The appearance of phage plaques indicated that T4::Nluc was sensitive to this strain and could lyse it, otherwise it was considered insensitive.
The luminescence assay was performed by incubating the 104 clinical strains at 37°C and 200 r/min for 5 h, followed by the addition of T4::Nluc before co-culture for another 3.5 h; 50 μL of the co-cultured solution was taken out for luminescence signal determination.
2.12 Validation of Clinical Samples
The 698 fresh urine samples were collected from Department of Clinical Laboratory Medicine, The First Medical Center of PLA General Hospital from December 16, 2024, to January 8, 2025, and were identified by urine culture and mass spectrometry (Table S6). T4::Nluc with a final concentration of 109 PFU/mL was added to the urine, and the luminescence signal was determined after co-culture at 37°C and 200 r/min for 4 h. The luminescence assay was carried out simultaneously with urine culture, and the identification results were compared to analyze the diagnostic performance indicators such as detection sensitivity and specificity.
2.13 Statistical Analysis
SPSS 27.0.1, GraphPad Prism 8.0.2, and OriginPro 2024b were used for statistical analysis and plotting. Changes in the luminescence values of T4::Nluc before and after sucrose density gradient ultracentrifugation were analyzed by multiple t-tests. The Mann-Whitney U test was used to analyze whether the one-step growth curves of T4 and T4::Nluc were statistically different. The Kruskal-Wallis H test was used to compare the time-luminescence curves of different concentrations of different urine components and different proportions of urine. Statistical differences were set at p < 0.05.
3 Results
3.1 Construction and Purification of Reporter Phage T4::Nluc
We constructed a CRISPR/Cas9 system for targeted cleavage of the soc gene of T4, resulting in a large number of broken double-stranded phage DNA fragments for subsequent homologous recombination. The single guide RNA used in this study was soc-cr6, which was selected from the crRNA library established by Duong et al. with high cleavage efficiency [18]. We successfully constructed the pCRISPR-cr6 plasmid through enzyme digestion and ligation (Figure 1a), as confirmed by PCR and Sanger sequencing (Figure 1b and Table S5). Next, the CRISPR/Cas9 system was established by sequentially transforming the pCas9 and pCRISPR-cr6 plasmid into E. coli BL21 (Figure 1c).
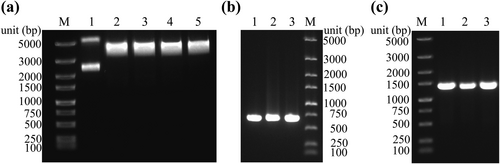
Construction of the CRISPR/Cas9 system. (a) The results of enzyme digestion of the pCRISPR plasmid. Lane 1. pCRISPR circular plasmid; lanes 2 3, 4, and 5 are the results of pCRISPR enzyme digestion. (b) PCR validation of pCRISPR-cr6 plasmid (primers: soc-cr6-F, pCRISPR-R, 646 bp), three replicates. (c) Plasmid transformation validation of pCas9-BL21 (primers: pCas9-F, pCas9-R, 1335 bp), three replicates. M. Marker.
To integrate the Nluc gene block into the T4 genome through homologous recombination, we constructed a homologous recombinant plasmid utilizing Gibson assembly (Figure 2a). We obtained the upstream and downstream homologous arms as well as the Nluc gene block by PCR (Figure 2b). Subsequently, DNA gel extraction and PCR were performed again to ensure the products were correct (Figure 2c). Colony PCR demonstrated successful construction of the homologous recombinant plasmid after Gibson assembly (Figure 2d).
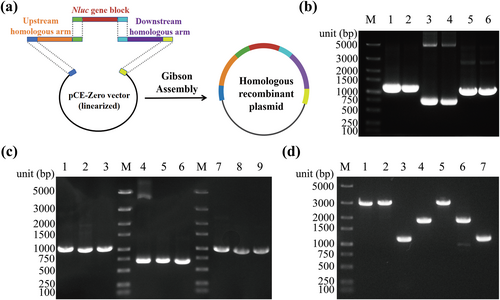
Construction of a homologous recombinant plasmid. (a) Schematic diagram of Gibson Assembly. (b) PCR amplification of the upstream 1041 bp homologous arm, the Nluc gene block (702 bp), and downstream 1000 bp homologous arm, two replicates. (c) Verification of DNA gel extraction. Lanes 1, 4, and 7 were PCR amplification of the upstream 1041 bp homologous arm, the Nluc gene block (702 bp), and downstream 1000 bp homologous arm; lanes 2, 5, and 8 were the gel extraction products; lanes 3, 6, and 9 were the results of PCR using gel extraction products as templates. (d) PCR validation of homologous recombinant plasmid. Lane 1. Primers: UHA-F, DHA-R, 2761 bp; lane 2. Primers: UHA-F, M13R, 2840 bp; lane 3. Primers: M13F, UHA-R, 1119 bp; lane 4. Primers: Nluc-F, M13R, 1788 bp; lane 5. Primers: M13F, DHA-R, 2808 bp; lane 6. Primers: M13F, Nluc-R, 1801 bp; lane 7. Primers: DHA-F, M13R, 1106 bp. M. Marker.
To improve the integration efficiency of the Nluc gene block, we combined the CRISPR/Cas9 system with classical homologous recombination and adopted a one-step method to achieve efficient construction and screening of reporter phage T4::Nluc. We transformed the homologous recombinant plasmid into pCas9-pCRISPR-cr6-BL21 to construct the triplasmid BL21 (Figure 3a), which provided the environment for recombination. Upon injection of the T4 genome into triplasmid BL21 and subsequent synthesis of phage nucleic acid, recombination occurred between the T4 genome and the homologous recombinant plasmid, leading to integration of the Nluc gene block into T4, resulting in the production of T4::Nluc (Figure 4a).
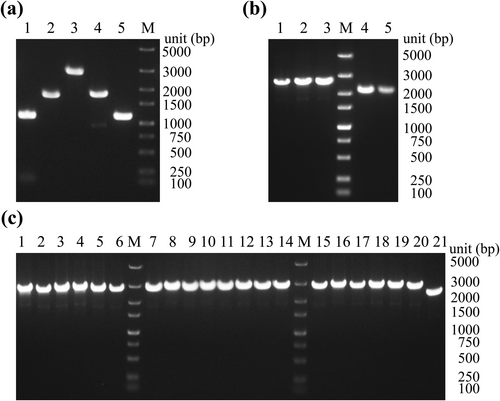
Homologous recombination to construct T4::Nluc. (a) Successful transformation of the homologous recombinant plasmid into pCas9-pCRISPR-cr6-BL21. Lane 1. Primers: M13F, UHA-R, 1119 bp; lane 2. Primers: Nluc-F, M13R, 1788 bp; lane 3. Primers: M13F, DHA-R, 2808 bp; lane 4. Primers: M13F, Nluc-R, 1801 bp; lane 5. Primers: DHA -F, M13R, 1106 bp. (b) The preliminary screening of T4::Nluc by PCR. Templates for lanes 1, 2, and 3 were T4::Nluc (primers: T4 Recombination Validation-F, T4 Recombination Validation-R, 2837 bp), and templates for lanes 4 and 5 were T4 (primers: T4 Recombination Validation-F, T4 Recombination Validation-R, 2358 bp). (c) Results of T4::Nluc purity confirmation. Lanes 1 to 20 were the PCR results using the co-cultured T4::Nluc as the template, and the template of lane 21 was T4. M. Marker.
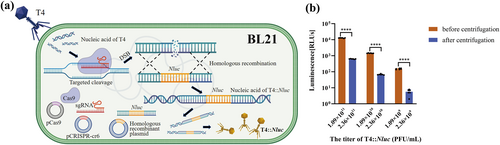
Schematic diagram of T4::Nluc construction and reduction of background protein. (a) Schematic diagram of the CRISPR/Cas9 system combined with homologous recombination technology to construct T4::Nluc. (b) Comparison of luminescence values of T4::Nluc before and after centrifugation. The analysis was performed using multiple t-tests (****p ≤ 0.0001).
In the process, the CRISPR/Cas9 system induced targeted cleavage of the soc sequence of the T4 genome, resulting in a substantial increase in DNA double-strand breaks, greatly enhancing homologous recombination efficiency. Simultaneously, the CRISPR/Cas9 system exerted an anti-selection effect by specifically cleaving the nucleic acid of wild-type T4 [2, 18], leading to an elevated proportion of T4::Nluc in progeny phages and facilitating screening (Figure 4a), though some wild-type T4 remained. To obtain purified T4::Nluc, we performed a double-layer overlay assay for screening from plaques through PCR (Figure 3b), and the screened product was further co-cultured with pCas9-pCRISPR-cr6-BL21, leveraging the CRISPR/Cas9 system's ability to cleave the T4 genome. Ultimately, PCR and Sanger sequencing confirmed that the T4::Nluc was pure and correct (Figure 3c and Table S5).
As the Nluc protein produced during biosynthesis was free and was not assembled in T4::Nluc, direct utilization of high-titer T4::Nluc culture would produce significantly elevated background luminescence signal, resulting in false positives. Therefore, the T4::Nluc was propagated and physically concentrated, followed by sucrose density gradient centrifugation to reduce Nluc protein in the culture. With the increase of titer, the luminescence signal of T4::Nluc after centrifugation decreased significantly compared with that before centrifugation (Figure 4b, p < 0.01), which would significantly improve the sensitivity of detection and avoid false positives.
3.2 Comparison of Biological Properties Between T4 and T4::Nluc
Considering that partial deletion of the soc gene and integration of the Nluc gene block may adversely affect the phage, we compared the biological properties of T4 and T4::Nluc. The one-step growth curves showed that the latent period of both T4 and T4::Nluc was 10 min, and the bursting phase occurred between 10 and 50 min (Figure 5a and Table S7). The burst size was determined to be 21.04 PFU/cell for T4 and 17.50 PFU/cell for T4::Nluc. However, statistical analysis revealed no significant difference in the one-step growth curves between T4 and T4::Nluc (p > 0.05). The efficiency of phage proliferation varied with different MOI, and that corresponding to the group with the highest phage titer was defined as the optimal MOI [20]. Within the range of MOI from 10 to 0.00001, T4 achieved the highest titer (4.07 × 1011 PFU/mL) at MOI = 1, while T4::Nluc reached its peak titer (4.54 × 1011 PFU/mL) at MOI = 0.1 (Figure 5b and Table S8). This was likely because the soc gene still had some biological function despite being non-essential to T4 [23] and/or the expression of Nluc negatively affected phage performance.
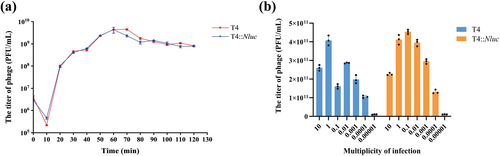
Comparison of biological properties between T4 and T4::Nluc. (a) One-step growth curves of T4 and T4::Nluc. Mann-Whitney U test was used for analysis, and the result showed that p = 0.840, indicating no significant difference. (b) The optimal MOI determination of T4 and T4::Nluc. Within the range from 10 to 0.00001, the optimal MOI for T4 was 1 and that for T4::Nluc was 0.1.
3.3 The Detection Performance of T4::Nluc
To use T4::Nluc for the detection of UTI-related E. coli, it was necessary to clarify its LOD. T4::Nluc was co-cultured with BL21 in a series concentration ranging from 1 × 106 to 1 × 101 CFU/mL for 7 h. The time-luminescence curves showed that T4::Nluc could detect ≥ 1 × 104 CFU/mL of BL21 within 220 min (Figure 6a), which satisfied the diagnostic criteria for clinical UTIs (≥ 105 CFU/mL) while significantly reducing detection time [6].
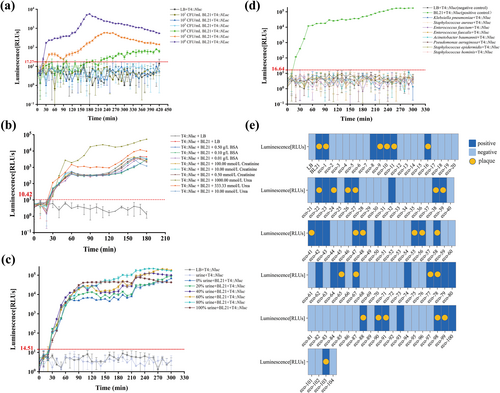
The detection performance of T4::Nluc. (a) The limit of detection for T4::Nluc. The “LB + T4::Nluc” group was the negative control (background luminescence), the positive threshold = the average background luminescence value plus three standard deviations, and the LOD was the minimum bacterial concentration required to produce a luminescence signal above the threshold. The threshold was calculated as RLUs ≥ 17.27. After co-culture with different concentrations of BL21 and an equivalent amount of T4::Nluc, BL21 at a concentration of ≥ 1 × 104 CFU/mL could be detected within 220 min. (b) Influence of BSA, creatinine, and urea on detection. The positive threshold was RLUs ≥ 10.42. Except for the negative control (LB + T4::Nluc), positive signals were detected in all groups at 30 min, and Kruskal-Wallis H test analysis showed that there was no significant difference in comparison among BSA, creatinine, and urea groups with different concentrations (p > 0.05). (c) Influence of urine on detection. The positive threshold was RLUs ≥ 14.51. Using the Kruskal-Wallis H test, pairwise comparisons were made between the signals of 0%, 20%, 40%, 60%, 80%, and 100% urine groups, p > 0.05. The comparison between “urine + T4::Nluc” and “LB + T4::Nluc” showed p > 0.05, indicating that urine did not affect the detection performance of T4::Nluc. (d) The detection specificity of T4::Nluc. The positive threshold was RLUs ≥ 16.64. After co-culture with different common UTI pathogens, T4::Nluc only produced a positive luminescence signal in the E. coli group. (e) The detection coverage of T4::Nluc in clinical E. coli. The double-layer overlay assay (gold standard) could detect 27/104 (25.96%) of clinical E. coli isolates from urine, while the luminescence assay could detect 40/104 (38.46%), indicating higher sensitivity.
Urine is a complex biological fluid, and its composition and ionic strength can vary widely among patients [2, 24]. To investigate the potential impact of the clinical urine on phage infection efficiency and luminescence values, we set up groups of BSA (simulating urinary albumin), creatinine, and urea at different concentrations (low, normal, and high) to study the effects of common urine components. Except for the negative control, all groups were detected as positive at 30 min (Figure 6b), and there was no significant difference in the luminescence signal between groups of different metabolites and concentrations (p > 0.05), indicating that they did not affect luminescence detection. Additionally, the total urine was further set at different proportions containing the same amount of E. coli for comprehensive analysis. All groups were positive at 30 min (Figure 6c), and there was no significant difference in signals (p > 0.05), indicating that urine did not cause any additional or reduction in luminescence. These results indicated that urine did not affect the performance of T4::Nluc and no sample pretreatment was required.
UTIs are usually caused by a variety of pathogens, and therefore several common clinical pathogens causing UTIs (E. faecalis, E. faecium, K. pneumoniae, A. baumannii, P. aeruginosa, S. aureus, S. epidermidis, and S. hominis) were selected for co-culture. T4::Nluc only produced detectable luminescence signals when co-cultured with BL21 (Figure 6d), showing excellent detection specificity.
E.coli in clinical UTIs shows clonal diversity, but phages often had great specificity at the genus, species, or strain level [2], resulting in a narrow host range. We investigated the detection coverage of T4::Nluc in 104 strains of clinical urine-isolated E. coli using the classic double-layer overlay assay (spot method), the gold standard for confirming bacterial susceptibility to a particular phage, as well as a luminescence assay [13]. The double-layer overlay assay identified 27/104 (25.96%) of clinical urine E. coli isolates, while the detection coverage of the luminescence assay was 40/104 (38.46%) (Figure 6e and Table S9). This finding indicated that the latter was more sensitive and exhibited greater clinical application potential. This could be attributed to the stringent requirements imposed by the double-layer overlay assay on both the bacterial host's condition and phage proliferation cycle integrity [2, 25]. Additionally, relying solely on macroscopic observation of plaques may lead to omission or misjudgment. By contrast, the luminescence assay only requires phages to infect the host and synthesize Nluc protein after injection of nucleic acid, which enables the detection of low-level infection, exhibiting broader detection coverage.
3.4 Validation With Clinical Samples
To further clarify the detection performance of T4::Nluc in real complex clinical samples, we collected 698 fresh urine samples for large-scale clinical sample validation (Table S6). Among them, 82 cases of E. coli were identified by urine culture and mass spectrometry (gold standard), while 30 cases were detected by luminescence assay (sensitivity = 36.59%), which may be because of the narrow host spectrum of T4::Nluc in clinical clonally diverse E. coli (Table 1). However, the detection specificity was 100%, which was related to the high host specificity of the phage itself, indicating that it did not have cross-reaction and false positive (misdiagnosis) results, revealing great advantages in pathogen identification accuracy.
| Luminescence assay | Urine culture and mass spectrometry (gold standard) | Total | |
|---|---|---|---|
| Positivea | Negativeb | ||
| Positive | 30 | 0 | 30 |
| Negative | 52 | 616 | 668 |
| Total | 82 | 616 | 698 |
- a Positive (gold standard), among the 82 samples, six were mixed infections containing E. coli.
- b Negative (gold standard), of the 616 samples, 408 were sterile urine and the rest were non-E. coli-infected samples.
4 Discussion
UTIs are very common, and can easily become recurring and chronic [26, 27]. The inappropriate utilization of antibiotics can result in adverse drug reactions, worsening clinical symptoms, increased mortality rates, and the emergence of multidrug-resistant pathogens [28-31]. Rapid and accurate identification of pathogens and drug resistance screening hold immense significance in reducing non-empirical treatments, decreasing the prevalence of drug-resistant bacteria, and even potentially saving patient lives [32], especially in emergency and primary care settings [33].
The CRISPR/Cas9 system combined with homologous recombination technology was employed to integrate the Nluc gene block into the genome of T4, resulting in the construction of a reporter phage T4::Nluc. On the basis of T4::Nluc, an innovative method for rapid and accurate detection of E. coli in UTIs was proposed. After co-culture of T4::Nluc with urine, the presence of E. coli was determined by analyzing the luminescence signal.
T4 phage was selected for genetic modification because it is a model phage that has been fully studied [34], is relatively easy to source, and has complete and clear genetic information, multiple modification sites to choose from, and low modification risk. The integration of the Nluc gene block into the T4 genome can be performed by direct insertion or substitution of non-coding regions or non-essential genes. However, direct insertion without removing any sequence often causes the phage genome to exceed the capsid size limit, resulting in packaging problems or the loss of other genes, affecting phage activity [14, 35]. The substitution of non-coding regions may also affect structural or regulatory functions [36]. In this study, we selected the non-essential gene soc as the modification site of the Nluc gene block [37] to minimize the influence of genetic engineering on the phage. The reporter protein Nluc has a 150-fold increase in specific activity and a smaller size (19 kD) compared with other conventional luciferases [38], allowing it to maintain superior reporter function while minimizing the change in phage genome size, ensuring efficient packaging of phage capsids [39].
T4::Nluc demonstrated superior performance in the detection of E. coli in UTIs. It could detect viable BL21 at ≥ 1 × 104 CFU/mL within 220 min, meeting the diagnostic criteria for UTIs (1 × 105 CFU/mL) [6, 14], with higher concentrations of viable bacteria. Compared with traditional urine culture, mass spectrometry, or other techniques (18–30 h) [7], T4::Nluc testing could significantly reduce the result turnaround time.
In recent years, many new detection methods have been developed, although they have shortcomings (Table 2), particularly in their inability to distinguish between viable and dead bacteria. By contrast, the approach based on T4::Nluc for the detection of E. coli in urine can specifically identify viable E. coli, accurately assess patient status and the effectiveness of antibiotic treatment. In addition, this method is not influenced by urine, meaning that no sample pretreatment is required and the detection process is simple to operate. The associated equipment and technical demands are minimal and low-cost, and the results are clear and easy to analyze. During the detection process, T4::Nluc exhibits an exceptionally high level of host-specificity towards E. coli, and its detection specificity remains 100% in complex clinical urine tests. This technology demonstrates significant advantages in the rapid and precise detection of E. coli in clinical UTIs.
| Detection mechanism | Detection time | The limit of detection (CFU/mL) | Sample type | Detection device | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|
| Combine bifunctional magnetic-fluorescent microparticles with T4 and use them as probes to capture E. coli and perform flow cytometry | 15 min | 104 | E. coli MG1655 culture broth | Flow cytometer | Rapid, specific, efficient, inexpensive | Complex bioconjugation and high demand for technical proficiency | [40] |
| Employ T7 phage to instigate the lysis of the bacteria and the release of β-gal, which was detected by the nanophotonic detection chip subsequently | 8 h | 10 | E. coli BL21 in simulated spinach wash water | The nanophotonic device | Without the need for culturing or nucleic acid extraction, specific and sensitive | Challenging and expensive chip design, complicated operational procedures | [41] |
| Perform loop-mediated isothermal amplification (LAMP) and detect the DNA amplification by Hoechst 33,258 redox molecule and linear sweep voltametry | 1 h | 24 | E. coli in LB broth or in lower than 20% of filtered urine | Microfluidic electrochemical biosensor | No need for fixed probes, no need for DNA extraction and purification, rapid and sensitive | High demand for technical proficiency and equipment, LAMP amplification might be affected by urine, inability to distinguish between viable and dead bacteria | [42] |
| Pre-load the submicron particles conjugated with E. coli antibodies in paper microfluidic channel and immunoagglutination was quantified by angle-specific Mie scatter | < 30 s | 10 | E. coli K12 in human urine | The microfluidic paper analytical device | Low-cost, point-of-care, specific and sensitive | Inconsistency of results caused by the performance differences of smartphones, inability to distinguish between viable and dead bacteria | [27] |
| Create T7 Nanoluc reporter phages by in vitro assembly, incubate them with E. coli BL21, and then add the substrate to measure the luminescence signal | 2 h | 5 × 102 | E. coli BL21 culture broth | A BioTek synergy plate reader | Simple operation, concise and understandable results, rapid and specific | Challenge in large-genome reporter phage construction and risk of genetic mutation in phages | [43] |
| Paper-based ELISA for E. coli and colorimetric reaction | 5 h | 105 | E. coli DH5α culture broth | A portable scanner or smartphone camera | Low cost, less medical waste, exam in ER or home, no complex preprocessing | Complex operation, readout required and the clinical performance remained to be verified | [4] |
| Create reporter phages T7NLC and incubate them with E. coli BL21, concentrate NanoLuc-CBM fusion reporter protein by microcrystalline cellulose, and then add the substrate to measure the luminescence signal | 3 h | < 10 | E. coli BL21 in drinking water | A luminescent plate reader | Low cost, rapid and sensitive, great potential for application | Inefficient infection of E. coli in the larger samples, the possibility of signal interference and cumbersome operation | [44] |
- Abbreviations: β-gal, β-galactosidase; CBM, carbohydrate binding module; ELISA, enzyme-linked immunosorbent assay; ER, emergency room.
However, the T4::Nluc-based method for the detection of E. coli also has some limitations. The LOD is considered less than ideal, which may be because of the larger size of the T4::Nluc genome (approximately 169,382 bp) and lower burst size (17.50 PFU/cell) compared with other phages. Consequently, it becomes challenging to produce detectable luminescence signals in bacterial fluids with lower concentrations. The LOD could be effectively lowered through appropriate preculture [37, 44, 45], which allows the bacteria to proliferate to a higher concentration before detection. Alternatively, the reporter protein can be concentrated before detection by using a filter of an appropriate size or fusing the reporter gene with a carbohydrate-binding module, followed by utilization of microcrystalline cellulose for concentration [41, 44]. It can also be combined with immunomagnetic separation, where the E. coli is captured and enriched by immunomagnetic beads and then co-cultured with the reporter phage for detection [46]. Additionally, the phage can be directly immobilized onto magnetic nanoparticles, enabling the isolation, enrichment, and detection of E. coli [47]. Microfluidic chip could also be employed to decrease the sample volume, concentrate pathogens, and subsequently utilize reporter phage for detection. The aforementioned methods can not only lower the LOD but also shorten the detection time.
Another limitation is the relatively low clinical detection coverage (38.46%) and sensitivity (36.59%) of T4::Nluc. This was primarily attributed to the narrow host spectrum of the phage and the polyclonality of clinical E. coli, which led to incomplete identification. Because the gp37 gene of the T4 phage plays a crucial role in determining its host specificity [34], it can be further genetically engineered to broaden the host spectrum, such as through selective exchange or chimerism with receptor-binding protein genes of other phages with complementary host range. For example, Chen et al. replaced the host determinant gene region between two T4-like phages so that the modified phage obtained the host range of the two original phages, allowing it to identify another 8 E. coli strains [48]. Ando et al. used a yeast-based high-throughput phage genetic engineering platform to overcome the species barrier by exchanging phage tail fiber genes, which are closely related to host specificity so that the E. coli phage T3 could simultaneously infect E. coli and the pathogenic Yersinia pseudotuberculosis [16]. Mutations could also be made to the gp37 gene. As reported in Yehl et al.’s study, a library comprising millions of phage tail fiber sequence mutants was constructed on a common phage structural scaffold, from which infectious phages with an expanded or modified host range were generated [49]. T4::Nluc can also be combined with other reporter phages possessing complementary host ranges to form cocktails,to further broaden the host spectrum. In addition to T4::Nluc, we have successfully constructed T6::Nluc and T7::Nluc. Employing the double-layer overlay assay, T4::Nluc could identify 27/104 (25.96%) clinical urinary E. coli. When T4::Nluc was combined with T7::Nluc, 31/104 (29.81%) E. coli could be recognized, and the combination of T4::Nluc with T6::Nluc enabled the identification of 89/104 (85.58%) E. coli (data not yet published). Similar to Haines et al.’s study, the three different E. coli phages UP17, JK08, and 113 were combined as a cocktail to increase the coverage of clinical E. coli from about 70% coverage by each phage to 95% [50].
In addition to diagnosing UTIs, the detection method based on T4::Nluc will have broad application in the timely and accurate diagnosis of many other types of clinically important infectious diseases caused by E. coli, such as enteritis, sepsis, and neonatal meningitis. Additionally, T4::Nluc can be combined with other phages such as S. aureus reporter phages ISP and MP11, K. pneumoniae phage rTUN1::nLuc or E. faecalis phages EfS3::nluc and EfS7::nluc for the detection of pathogens in samples of mixed infections [2, 14, 51]. T4::Nluc can also be utilized for rapid antibiotic sensitivity testing (AST) of E. coli to facilitate the development of efficient antibiotics. The change of luminescence signal after co-culture of T4::Nluc with E. coli and antibiotics is related to drug sensitivity. Jain et al. used phage TM4-nluc to identify drug-resistant Mycobacterium tuberculosis [52], and Braun et al. employed phage rTUN1::nLuc to directly perform rapid and real-time AST of K. pneumoniae K64 strains in clinical samples [14]. Compared with traditional ASTs (16–24 h) [53], such as broth dilution, epsilometer test, and disk diffusion, reporter phages can significantly shorten the detection time and facilitate the formulation of timely and efficient antibiotic treatment strategies. In the personalized treatment of infectious diseases, T4::Nluc can serve as a therapeutic phage and assist in efficacy monitoring and dose optimization guidance through the change of luminescence signal. When used as an indicator and combined with gene editing technology and proteomic analysis, this phage reporter can be helpful in further elucidating the bacteria-phage interaction process and the phage resistance mechanism of bacteria. Aside from its application in medicine, T4::Nluc can play a prominent role in environmental monitoring, food safety testing, animal husbandry, and aquaculture, among other fields.
5 Conclusions
This study utilized the CRISPR/Cas9 system combined with homologous recombination to successfully construct a reporter phage T4::Nluc, which can be employed for the detection of E. coli in urine samples without any pretreatment with excellent detection speed and specificity. These findings provide a new technical approach for timely and accurate diagnosis as well as effective treatment of clinical UTIs. Additionally, T4::Nluc could become a powerful tool for rapid drug susceptibility testing, phage therapy, environmental and food safety monitoring, as well as other fields.
Author Contributions
Zhiyun Hao: data curation (lead), methodology (lead), validation (lead), visualization (lead), writing – original draft (lead), writing – review and editing (equal). Minwei Li: investigation (equal), methodology (equal), software (equal), writing – review and editing (equal). Qiang Zhao: data curation (equal), resources (equal), validation (equal). Liye Wang: formal analysis (equal), software (equal). Ting Liu: investigation (equal), visualization (equal). Chi Wang: project administration (equal), funding acquisition (equal), supervision (equal), writing – review and editing (equal). Chengbin Wang: conceptualization (equal), funding acquisition (equal), project administration (equal), resources (equal), supervision (equal), writing – review and editing (equal).
Acknowledgments
The authors have nothing to report.
Ethics Statement
This study was approved by the Ethics Committee of PLA General Hospital with approval number S2023-580-02. During the conduct of the research and the writing of the article, we strictly adhered to relevant regulations regarding research ethics.
Consent
This study did not involve any direct participation of individuals throughout the entire process; therefore, it was not necessary to obtain informed consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




