Rapid development of neurological infection in a patient with human immunodeficiency virus following schistosomiasis: A case report
Abstract
HIV-1 and schistosomal infections present significant global health challenges, and neurological manifestations of these pathogens are easily misdiagnosed due to their rarity. Here, we report the case of a 36-year-old patient with acquired immunodeficiency syndrome who was initially diagnosed with human immunodeficiency virus-1 (HIV-1) infection 4 years earlier, although untreated for approximately 3 years until he began antiretroviral therapy (ART) following a tuberculosis diagnosis and hospitalization. Despite achieving virological suppression of HIV-1 1 year after ART, he was readmitted with high fever and headache. Initial therapy for suspected tuberculosis based on clinical performance and brain imaging features failed, and further investigation confirmed an intracranial infection caused by schistosomiasis. Following anti-schistosomal treatment and optimized ART, the patient recovered fully and was discharged. This case of a patient in Asia infected with human immunodeficiency virus (HIV) who rapidly developed a neurological infection subsequent to acquiring schistosomiasis highlights the need for awareness of such coinfections in patients with HIV.
Abbreviations
-
- AIDS
-
- acquired immunodeficiency syndrome
-
- ART
-
- antiretroviral therapy
-
- HAART
-
- highly active antiretroviral therapy
-
- HIV
-
- human immunodeficiency virus
1 BACKGROUND
Due to weakened immune function, people infected with the human immunodeficiency virus-1 (HIV-1) or who have acquired immunodeficiency syndrome (AIDS) have a significantly greater risk of opportunistic infections than individuals without these conditions [1]. Despite the marked reduction in the incidence of AIDS-associated opportunistic infections attributed to the extensive adoption of highly active antiretroviral therapy (HAART) since 1996, the prevalence of such infections continues to be disproportionately elevated in individuals diagnosed with AIDS when juxtaposed against the general population [2]. In the era of HAART, opportunistic infections continue to be a predominant complication, accounting for a considerable disease burden and mortality rate within the HIV/AIDS patient cohort. In 2020, China had 29,517 patients living with advanced schistosomiasis. Analysis of epidemic data indicates that China has a low prevalence of schistosomiasis [3]. Concurrently, as of 30 June 2023, China had reported 1,260,928 patients living with HIV/AIDS and 437,307 deaths due to the disease [4].
Schistosomiasis remains a significant global health challenge, ranking second only to malaria in terms of its prevalence as an infectious disease. This parasitic infection is distributed across 78 countries worldwide and impacts the health and well-being of an estimated 250 million individuals and is responsible for an approximate annual mortality of 280,000 [5]. The principal schistosome species implicated in human infections are Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium. In China, S. japonicum predominates and is designated as a Category B notifiable infectious disease. Globally, instances of co-infection involving HIV and schistosomiasis have been recognized and reported, with a pronounced prevalence in the African region [3]. Nevertheless, most of these coinfections predominantly exhibit gastrointestinal manifestations, and central nervous system involvement is uncommon. This case report elucidates a rare instance where a patient diagnosed with AIDS presented with acute schistosomiasis affecting the central nervous system. This case of neuroschistosomiasis serves as a critical guide for the diagnosis and treatment of ectopic parasitic infections in the setting of HIV/AIDS. To our knowledge, this is the first report of a case of central nervous system schistosomiasis in a patient with HIV/AIDS in Asia.
2 CASE PRESENTATION
A 36-year-old man presented with a one-week history of headache, vomiting, and fever. He had no seizures, altered states of consciousness, or urinary or fecal incontinence. The patient had a history of HIV-1 infection diagnosed 4 years earlier and was currently on a HAART regimen that included lamivudine, tenofovir disoproxil fumarate, and efavirenz. He had been treated with an isoniazid, rifampicin, pyrazinamide, ethambutol (HRZE) regimen for pulmonary tuberculosis for 9 months and discontinued this anti-tubercular treatment upon complete resolution of his pulmonary lesions 6 months earlier. An examination 2 months before the current presentation found an undetectable level of HIV-1 and a CD4 lymphocyte count of 100 cells/μL (reference range: 500–1600 cells/μL).
A physical examination upon admission revealed the following: a body temperature 40.5°C, mild jaundice, noticeable neck stiffness, and a heart rate of 130 beats per minute. Auscultation of the lungs detected clear breath sounds bilaterally. Abdominal examination revealed a soft abdomen with an absence of any overt or rebound tenderness, and his bowel sounds were normal. No edema was observed in the lower extremities, and pathological signs were bilaterally positive on neurological examination.
A complete blood count revealed a white blood cell count of 7.74 × 109/L, with a high eosinophil count constituting 7.9% (reference range: 0.5%–5%). His procalcitonin level was high at 4.67 ng/mL (reference range: <0.05 ng/mL). Hepatic function tests revealed significantly elevated levels of alanine aminotransferase (191 U/L), aspartate transaminase (219 U/L), total bilirubin (71 μmol/L), direct bilirubin (54 μmol/L), and indirect bilirubin (43 μmol/L). The CD4+ cell count was 135 cells/μL, with a CD4+/CD8+ T-cell ratio of 0.7. Human immunodeficiency virus viral load was under the detection limit using a fluorescence immunoassay method. Lumbar puncture yielded clear and colorless cerebrospinal fluid (CSF) with markedly elevated intracranial pressure (26 cmH2O). Cytological evaluation of the CSF revealed a red blood cell count of 2 × 106/L, a white blood cell count of 85 × 106/L, and a differential count revealing 20% polynuclear and 80% mononuclear cells was negative for neoplastic cells. CSF biochemistry revealed levels of chloride at 121.1 mmol/L, glucose at 2.81 mmol/L, and protein at 753.7 mg/L. Specialized staining methods such as India ink, Gram, and acid-fast bacilli yielded negative results. Abdominal ultrasonography was unremarkable.
MRI of the head (Figure 1) revealed elongated T1 and T2 signal patterns in the brainstem, bilateral frontal, temporal, parietal, and occipital lobes, and basal ganglia, manifesting as patches, spots, and nodules. In the FLAIR sequence, high signals were evident, corresponding to narrowed brain sulci and a swollen brainstem with pronounced edematous areas. Notably, while there was significant enhancement of diffuse intracranial nodules, the adjacent edematous regions lacked such enhancement. Additionally, there was a noticeable thickening of the leptomeninges accompanied by linear enhancement.
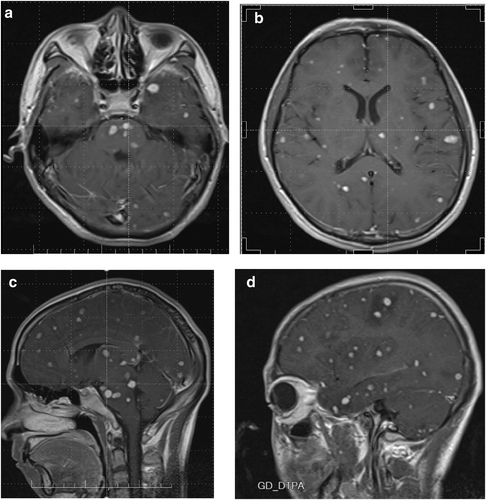
MRI of the head at the time of admission. The brainstem, bilateral frontal temporal parietal occipital lobes, and basal ganglia are covered with patchy and nodular long T1 and T2 signal shadows. The FLAIR sequence shows high signal intensity, corresponding to narrowing of the cerebral sulcus, swelling of the brainstem, and visible areas of edema; intracranial diffuse nodules are significantly enhanced, with no enhancement in the surrounding edema area, and the pia mater is thickened, showing linear and obvious enhancement. (a) Coronal plane. (b) Coronal plane. (c) Sagittal plane. (d) Sagittal plane.
On the basis of a preliminary diagnosis of intracranial dissemination of tuberculosis, an anti-tubercular HRZE regimen was initiated along with moxifloxacin. The fever persisted despite the treatment. CSF pressure remained high at approximately 30 cmH2O upon multiple testing, and CSF protein level increased to as high as 2650 mg/L within the next 2 weeks, when the patient developed sporadic seizures, diminished consciousness, and loss of urinary and fecal control. A blood panel white blood cell count of 15.9 × 109/L revealed 75.1% neutrophils and 11.4% eosinophils. We found that the blood culture result was negative for tubercular bacilli and that there was no significant increase in chest X-ray observations. Comprehensive lumbar puncture results from multiple re-evaluations are provided in Table 1.
| Date (mm-dd) | Pressure (mmH2O) | Staining results | Cell count (×106/L) | Glucose (mmol/L) | Chloride (mmol/L) | Protein (mg/L) |
|---|---|---|---|---|---|---|
| 09–17 | 260 | Negative | 12 | 2.81 | 121.1 | 753.7 |
| 09–19 | 280 | Negative | 110 | 2.63 | 126.8 | 805.1 |
| 09–20 | 290 | Negative | 136 | 2.50 | 122.0 | 905.0 |
| 09–25 | 300 | Negative | 150 | 2.80 | 127.0 | 1008.0 |
| 09–27 | 290 (Dehydrated) | Negative | 350 | 2.60 | 123.8 | 1100.6 |
| 09–28 | 320 | Negative | 260 | 2.67 | 120.6 | 1160.0 |
| 09–29 | 290 (Dehydrated) | Negative | 190 | 2.58 | 124.9 | 1207.0 |
| 09–30 | 300 | Negative | 220 | 2.67 | 123.3 | 1200.0 |
| 10–01 | 320 (Dehydrated) | Negative | 280 | 2.82 | 118.9 | 1100.0 |
| 10–02 | 330 (Dehydrated) | Negative | 260 | 2.70 | 120.5 | 1280.0 |
| 10–07 | 140 | Negative | 8 | 2.62 | 121.0 | 320.0 |
| 10–12 | 140 | Negative | 7 | 2.71 | 125.0 | 350.0 |
| 10–17 | 130 | Negative | 7 | 2.82 | 127.0 | 290.0 |
| 10–22 | 120 | Negative | 8 | 2.91 | 121.0 | 360.0 |
Treatment was initiated with meropenem (1.0 q8h) and vancomycin (0.5 q6h), but fever, sporadic seizures, diminished consciousness, and loss of urinary and fecal control persisted. The patient's condition deteriorated and his consciousness degenerated to a coma. A multidisciplinary meeting raised the possibility of intracranial schistosomiasis due to a high percentage of eosinophils and a family history of schistosomiasis. The patient's wife disclosed that he had contact with water in an area endemic for schistosomiasis. CSF testing revealed IgG against schistosomes or schistosomula, and PMseq revealed the presence of S. japonicum infection in the CSF. An indirect hemagglutination result was positive (1:20). However, schistosome ova were not detected in his stool.
Treatment with praziquantel (administered at 20 mg/kg/day for a continuous span of 6 days) and dexamethasone (dosed at 10 mg/day, sustained over 14 days) was initiated. The patient improved significantly starting from the second day and was discharged 4 weeks later. AIDS treatment resumed with the bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) regimen.
The patient's condition was unremarkable at the most recent follow-up (3 months later). His CD4 count had improved to 200 cells/μL. MRI of the head showed significant absorption of the previously identified lesions (Figure 2).
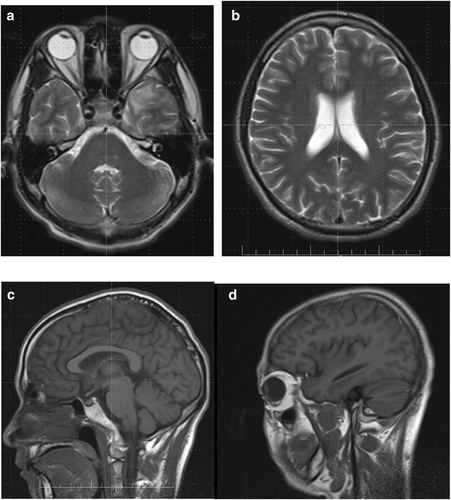
MRI of the head at discharge. Multiple nodular lesions in the brainstem, bilateral frontal, temporal, parietal, occipital lobes, basal ganglia, and limbic system were essentially absorbed compared with the previous MRI findings. (a) Coronal plane. (b) Coronal plane. (c) Sagittal plane. (d) Sagittal plane.
3 DISCUSSION AND CONCLUSIONS
We report the case of a patient with HIV-1 infection who was initially misdiagnosed with tuberculosis suspected according to clinical manifestations and imaging features. Ultimately, the correct diagnosis of neuroschistosomiasis was ultimately made as the disease progressed. Schistosomiasis results from parasitic trematode infections, impacting more than 230 million individuals globally, with Africa home to 90% of recognized infections. The disease primarily stems from infections by Schistosoma mansoni), S. haematobium, and Schistosoma japonicum. S. japonicum is found predominantly in specific regions of China, the Philippines, and Indonesia. The most common complications of schistosomal monoinfections include hepatosplenic inflammation, liver fibrosis, cirrhosis, bladder cancer, and kidney damage. The main damage occurs in the liver, spleen, intestines, and urinary system [6]. Neuroschistosomiasis is a serious form of schistosomiasis that causes neurological issues due to the body's reaction to schistosome eggs in the brain and spinal cord. Symptoms include delirium, unconsciousness, seizures, speech and visual impairments, and motor and coordination problems. People with a tumor-like form of this disease often suffer from increased intracranial pressure, headaches, nausea, vomiting, and further seizures [7].
To our knowledge, there are no reports of neurological complications in patients with both schistosomiasis and HIV. Initially, our patient did not exhibit common symptoms such as seizures or altered states of consciousness and had no issues with urinary or fecal incontinence. However, they struggled with calculations and orientation. A clinical examination highlighted notable neck stiffness. MRI of the head revealed elongated T1 and T2 signal patterns across various brain regions, indicating a nervous system infection.
The patient had been exposed to contaminated water 2 weeks before presentation. Given the rapid progression of the disease following a brief infection period, it is plausible that this accelerated course contributed to the absence of detectable eggs. PMseq has emerged as a vital tool for infectious disease diagnosis. This tool operates by comparing sequencing data to a microbial-specific database and utilizes intelligent algorithms to identify suspected pathogenic microorganisms, thus facilitating rapid and accurate detection [8]. The comprehensive and in-depth reports generated by PMseq have proven valuable for definitive diagnosis of a wide array of microbial infections. In brief, diagnosis can be straightforward when laboratory results are assessed alongside clinical symptoms and medical imaging. However, differential diagnosis remains a crucial step in clinical practice. For instance, the imaging results in this case strongly indicated a potential intracranial tuberculosis infection. Therefore, in light of the severity of the patient's symptoms, known previous infection with, and the inherent difficulty in identifying Mycobacterium tuberculosis bacilli, we initiated anti-tubercular treatment before obtaining definitive etiological results. This decision proved to be essential, at least in excluding the presence of a latent tuberculosis infection.
According to the reported life cycle of S. japonicum, it usually takes at least 3–4 weeks from when cercarial larvae infect the patient to when adult schistosomes release eggs. Infected individuals generally present with symptoms of acute schistosomiasis 5–8 weeks after being infected with cercariae. However, our patient was in contact with contaminated water in an area endemic for schistosomiasis only 2 weeks before symptom onset. We deduced that a patient with an HIV infection might not have sufficient immunity to combat a new infection, and acute schistosomiasis developed more quickly than it would otherwise.
The symptoms of schistosomiasis are generally caused by eggs deposited in the liver, which are released by adult schistosomes in the mesenteric portal vein system. Cerebral schistosomiasis is usually a series of neurological symptoms caused by schistosomal eggs in the blood reaching the patient's brain. Diffuse intracranial nodules were observed throughout MRI of the head, which may indicate schistosomal ova granuloma in the patient's brain. However, schistosome eggs were not detected in the patient's stool and liver ultrasound revealed no indications of schistosomiasis-associated hepatic involvement. Therefore, the location of the adult parasite in our patient may not have been the mesenteric portal system but rather the intracranial venous sinus. The eggs may be concentrated in the parietal lobe by anastomotic branches, which has been observed in animal models and may explain their location in the patient's brain; to our knowledge, eggs have not yet been reported to be found intracranially in human adults. Schistosomulae and adult schistosomes migrate throughout the entire body; however, the apparent location of the eggs does not account for the circulation of the schistosomes. Liver ultrasound suggested that the schistosomal worms might migrate to other systems. However, the underlying mechanism for the presence of eggs in the brain remains unknown and warrants further elucidation.
Numerous studies have validated the reciprocal exacerbation of schistosomiasis and HIV-1 infections, illustrated by two key dynamics: (1) Schistosoma infection amplifies the likelihood of HIV-1 contagion [2, 9]; and (2) the compromised immune defense induced by HIV-1 accelerates schistosomiasis [10]. This interaction was demonstrated by the accelerated progression and intracranial invasion experienced by our patient. Intracranial schistosomal infections are typically rare and are credited to the protective mechanism of the blood-brain barrier. However, the co-existence of HIV-1 and schistosomiasis might undermine this barrier. Even though this patient had a suppressed HIV-1 viral load preceding the schistosomal infection, the potency of the ART regimen was potentially inadequate, which coupled with underlying immune activation could have facilitated a breakdown of the blood-brain barrier. Confronted with this complexity and the adverse reactions induced by efavirenz, we switched the regimen to BIC/FTC/TAF. This novel integrase inhibitor is endorsed by a multitude of international [11]and Chinese guidelines [12], positioning it as the paramount treatment option for both treatment-naive and experienced patients.
Additionally, co-infected patients pose unique treatment challenges. The potential interactions between antiretroviral therapy and praziquantel, drug efficacies, and adverse effects under immunocompromised conditions necessitate comprehensive exploration. Documenting this rare case of co-infection enriches the available epidemiological data, offering insights into potential emerging trends. Our findings underscore the need for heightened surveillance, early detection, and intervention to mitigate public health impact, especially in regions where schistosomiasis is endemic, and HIV prevalence is significant.
In conclusion, we present the case of a patient in Asia with HIV who rapidly developed neurological sequelae subsequent to schistosomal infection. This case augments our clinical insights, highlighting the need for HIV prevention and control strategies in general and the need for awareness of such coinfections within regions characterized by a high prevalence of schistosomiasis.
AUTHOR CONTRIBUTIONS
Yan Liu: Conceptualization (equal); investigation (equal); methodology (equal); resources (equal); software (equal); writing – original draft (equal); writing – review & editing (equal). Guoqiang Zhou: Data curation (equal); formal analysis (equal); funding acquisition (equal); software (equal). Wei Jiang: Project administration (equal); software (equal); visualization (equal). Xianglong Kong: Funding acquisition (equal); resources (equal); supervision (equal); visualization (equal); writing – original draft (equal).
ACKNOWLEDGMENTS
I would like to express my very great appreciation to Gang Xiao, the best coach anyone could have; thank you for your unflagging support, and for encouraging me when I felt overwhelming challenges. I wish to acknowledge the help provided by Changsha Municipal Science and Technology Bureau, special program of key fields in the Changsha Municipal Science and Technology Bureau, Hunan Provincial Natural Science Joint Foundation. I would like to thank the patient for his assistance with the collection of my data.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
This is a real case and patient consent has been obtained.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




