CAR-NK, a Splendid Strategy for Cancer, Especially for Gynecologic Tumor
ABSTRACT
Background
NK cells are a class of innate lymphocytes capable of nonspecifically killing tumor cells without MHC restriction or prior sensitization. Recent advancements in biotechnology, particularly the development of chimeric antigen receptors (CAR) and related technologies, have enabled targeted tumor cell elimination. CAR endows NK cells with enhanced functionality, with the extracellular domains typically consisting of single-chain variable fragments (scFv) for targeting specific antigens. CAR-NK cells have shown excellent results in several preclinical studies and clinical trials for hematologic malignancies. However, their clinical application in the treatment of solid tumors is still insufficient. Current treatments for gynecological cancers primarily involve surgery, chemotherapy, and radiotherapy, all of which often present substantial side effects and variable efficacy. While CAR-T cell therapy has shown effectiveness in certain gynecological tumors, its clinical application is hindered by severe side effects, such as Cytokine Release Syndrome (CRS) and Graft-Versus-Host Disease (GVHD). CAR-NK cell therapy offers improved safety profiles in clinical applications.
Objective
This review aims to systematically evaluate recent methodological innovations in CAR-NK engineering and their translational potential in tumor-targeted treatment, providing valuable insights for clinical trials and studies.
Methods
Electronic databases, including PubMed and Web of Science were searched for relevant literature. Keywords are as follows: CAR-NK cell; Chimeric antigen receptor; Solid tumor; cell therapy; gynecological cancers.
Results
CAR-NK engineering has innovations such as multi-targeted CAR design, gene editing for enhanced persistence, and “off-the-shelf” CAR-NK cells compared to CAR-T cells.
Conclusion
CAR-NK cell therapy combines safety and anti-tumor efficacy, particularly for gynecological cancers.
1 Introduction
Natural killer (NK) cells, constituting 10%–15% of peripheral blood lymphocytes and defined by the surface markers CD3 − CD56 + , mediate innate immunity through their ability to perform effective immune surveillance and eliminate cancer cells without antigen-specific MHC restriction or prior sensitization. Approximately 90% of circulating NK cells are mature CD56dimCD16bright cells, primarily responsible for mediating cytotoxic immune responses. The remaining 10% are immature CD56brightCD16dim NK cells, primarily as cytokine producers (particularly IFN-γ) mediating immunomodulatory functions [1]. NK cell activation is not a binary process regulated by a single molecular switch or signaling pathway. The equilibrium between activating and inhibitory receptor signaling determines NK cells' cytotoxic potential.aluated in clinical trials for cervical. The tumor microenvironment contains multiple inhibitory mechanisms that impair NK cell function, including immunosuppressive cellular components (dendritic cells, regulatory macrophages, Treg cells, myeloid-derived suppressor cells (MDSCs), and cancer-associated fibroblasts); and soluble factors such as indoleamine 2,3-dioxygenase (IDO), transforming growth factor-β (TGF-β), and prostaglandin E2 (PGE2) [2-4].
CAR-NK cells are generated by introducing a chimeric antigen receptor (CAR) gene into NK cells. The CAR structure comprises an extracellular recognition domain and an intracellular signaling region. The extracellular domain typically consists of a single-chain variable fragment (scFv), enabling CAR-NK cells to specifically recognize target cells. The intracellular region primarily contains multiple signaling domains. The first-generation CAR molecules relied on a single signaling domain [5], but their limited functionality and poor persistence [6] prompted the development of second- and third-generation CARs. These advanced generations incorporate one or more costimulatory domains, enhancing both the viability and intracellular effector signaling of CAR-NK cells [7, 8]. Recently, fourth-generation CAR molecules, which incorporate cytokine payloads, have been developed [9, 10], potentially paving the way for more effective clinical applications.
While early-stage gynecological cancers are mainly managed through surgery, treatment options for advanced gynecological cancers—whether surgical, radiotherapeutic, chemotherapeutic, targeted, or immunotherapeutic—remain limited [11-13]. Given the promising therapeutic outcomes of CAR-based therapies in hematological malignancies, there is strong potential for CAR technology to be adapted for gynecological cancers.
CAR-T cell therapy has received approval from the U.S. Food and Drug Administration (FDA) for the treatment of hematologic malignancies, demonstrating significant clinical efficacy [14]. However, it is associated with severe side effects, including graft-versus-host disease (GVHD) and cytokine release syndrome (CRS) [15-17]. The release of high levels of inflammatory cytokines, such as IL-6 and IL-10, during CAR-T cell activation can trigger systemic inflammation, enhance immune cell activation, and increase vascular permeability, leading to symptoms like fever and organ damage. Given the tumor contamination in the blood of patients requiring CAR-T cell therapy, allogeneic CAR-T cells are often preferred. However, the mismatch between the T-cell surface receptors and the recipient's MHC molecules can lead to cytotoxicity against the host's cells, initiating GVHD.
In recent years, CAR-NK cell therapy for solid tumors has garnered increasing attention. While CAR-NK cells remain underutilized in solid tumor applications, promising preclinical studies and clinical trials suggest their considerable potential. As an alternative to CAR-T therapy, CAR-NK cell therapy has shown favorable safety profiles in previous research [18] and comparable efficacy against malignant tumors. This review explores the fundamental role of NK cells, introduces the design and optimization of CAR constructs, and examines both completed and ongoing clinical and preclinical studies on CAR-NK cells for malignant tumor treatment. A comparative analysis of CAR-T and CAR-NK therapies will highlight the potential value of advancing CAR-NK cell therapies, particularly for the treatment of solid tumors such as gynecologic cancers.
2 The Basement Role of NK Cells Against Cancer in the Normal Immune Mechanism
NK cells are innate lymphocytes capable of recognizing and lysing transformed or virally infected cells without prior sensitization. Their activation status is determined by the balance between inhibitory and activating receptors. The functional model of NK cells is illustrated in Figure 1. Under normal conditions, inhibitory killer immunoglobulin (Ig)-like receptors (KIRs) on NK cells interact with major histocompatibility complex class I (MHC I) molecules on healthy cells, preventing NK cell activation [1]. In contrast, virus-infected and malignant cells often downregulate MHC I, leading to disengagement of KIRs and the subsequent activation of NK cell effector functions against “non-self” cells. This mechanism of NK cell recognition contrasts with the T-cell-mediated response to target cells. Activated NK cells secrete perforin and granzyme; perforin disrupts the target cell membrane, facilitating granzyme entry to induce apoptosis [19]. Additionally, NK cells can trigger apoptosis by binding “death receptors” on target cells with death ligands such as FasL and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [19], resulting in direct target cell lysis. Although NK cell cytotoxicity is non-antigen-specific, it can be directed against abnormal cells via antibody-dependent cellular cytotoxicity (ADCC). In this process, antibodies bind to antigens on target cells, exposing the Fc region of IgG, which interacts with Fc receptors on NK cells, specifically CD16 and CD32. Activation of these receptors leads to degranulation and the release of cytotoxic granules, inducing apoptosis in target cells [19]. TNF not only promotes tumor cell apoptosis but also stimulates IFNγ secretion from NK cells, enhancing their cytotoxic activity by augmenting the release of granzyme and perforin. In addition to direct cytotoxicity, NK cells recruit T cells and macrophages to infection sites through IFN and TNF production [19]. However, TGF-β inhibits NK cell function, suggesting that targeting TGF-β with agents such as YM101 or M7824 may enhance NK cell activity [20, 21]. NK cells are pivotal effector cells of the innate immune system, capable of lysing tumor cells without the need for tumor-specific antigen presentation.
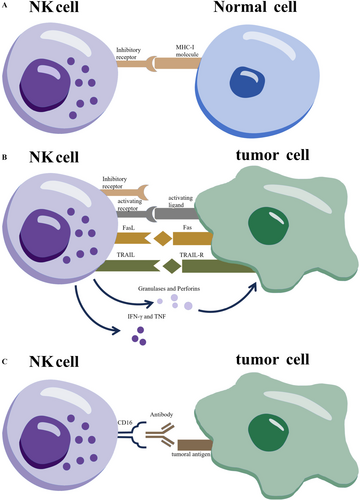
3 The Construct of CAR-NK Cells and the Differences Between CAR-NK and CAR-T Cells
Cell sources for CAR-T cell therapy include autologous peripheral blood, allogeneic sources, and umbilical cord blood or induced pluripotent stem cell (iPSC) differentiation sources. However, due to the limited availability and complexity of preparing umbilical cord blood and iPSC-derived sources, these are not considered in this review. The quality of peripheral blood from patients requiring CAR-T treatment varies, with potential tumor contamination, making allogeneic T cells the preferred choice. However, T cells from allogeneic sources may present a risk of graft-versus-host disease (GVHD) due to the mismatch between the TCR of donor T cells and the recipient's MHC molecules, leading to unwanted cytotoxicity against normal tissue. In contrast, NK cell activation is independent of MHC class I molecules, making allogeneic NK cell transplantation a safer alternative, as it does not induce GVHD or other alloimmune or autoimmune toxicities. Additionally, when autologous NK cells are used for overdose therapy, their interaction with MHC molecules on autologous cells produces inhibitory signals, preventing damage to normal tissue [15]. This characteristic enables the use of “off-the-shelf” allogeneic NK cells from healthy donors [16].
While the advent of CAR-T cells has revolutionized tumor immunotherapy, a major side effect to consider is cytokine release syndrome (CRS), where T cells release high levels of pro-inflammatory cytokines, such as IL-6 and IL-10, which can lead to nontumor tissue damage. Due to differences in NK cell activation and signaling pathways, the risk of CRS is significantly lower in NK cell-based therapies [22].
T cells exhibit a relatively long lifespan, and in the context of the severe toxicities discussed earlier, this prolonged lifespan complicates the management of off-target effects. To mitigate these risks, suicide genes may need to be incorporated to regulate excessive immune responses. In contrast, NK cells have a shorter lifespan, which naturally reduces the risk of persistent “on-target, off-tumor” effects, even without the use of suicide genes [23]. However, the limited survival period of NK cells necessitates further evaluation and enhancement of the durability and overall efficacy of CAR-NK cell therapy. Potential strategies for improving this are outlined below.
Regarding their cytotoxic mechanisms, CAR-T cells rely on the CAR to recognize tumor antigens and activate their killing function through the T-cell receptor (TCR). However, tumor cells often downregulate MHC I molecules on their surfaces, limiting the effectiveness of the TCR in such cases. In contrast, CAR-NK cells can bypass this limitation. Even when tumor cells downregulate MHC I-like molecules, CAR-NK cells can still exert their cytotoxic effects via the “missing self” pathway, leveraging their intrinsic immunity to eliminate tumor cells.
Furthermore, unlike CAR-T cells, CAR-NK cells employ intracellular costimulatory domains that are not solely dependent on the CD3ζ pathway. These domains contain three types of immunoreceptor tyrosine-based activation motifs (ITAMs). DAP12, a specialized junction molecule associated with NK cell receptors, contains an ITAM that interacts with Syk and ZAP70 kinases, enhancing NK cell activation and cytokine release [24], in contrast to the CD3ζ-based signaling pathway in T cells. Notably, fusing NKG2D as the extracellular recognition domain of CAR with DAP12 as the intracellular signaling domain enhances CAR-NK cell function, as DAP12 is an endogenous signal that activates the NKG2D receptor [16]. A comparative summary of CAR-NK cells and CAR-T cells is presented in Table 1.
| Perspective | CAR-T cells | CAR-NK cells | |
|---|---|---|---|
| Manufacturing | Time-consuming, complex, and requires personalization | Potential for “off-the-shelf” use, easier to manufacture | |
| Basic immunological characteristics | Restricted by MHC I | MHC I independency | [23] |
| GVHD | Cause GVHD | Not cause GVHD | [15, 16] |
| CRS | High incidence of severe CRS | The incidence and severity of CRS is low | [17] |
| Lifespan | longer lifespan, usually months to years | Shorter lifespan, usually 2 weeks | [25, 26] |
| Design of intracellular structural domains | CD3z with or without other costimulatory molecular | CD3z with or without other costimulatory molecular DAP12 is more cytotoxic than CD3z |
[19] |
4 The Current Application of CAR-NK Cells in Cancer
In recent years, CAR-NK cell therapy has gained increasing attention as a promising treatment for cancer, with a growing number of clinical trials enrolling patients [18]. This trend facilitates a more comprehensive evaluation of the efficacy and safety of CAR-NK cell therapy. A partial summary of the application of CAR-NK cell therapy in cancer treatment is provided in Table 2.
| Row | Gov Identifier | Conditions | Status |
|---|---|---|---|
| 1 | NCT05574608 | CD123-positive acute myeloid leukemia (AML) | Recruiting |
| 2 | NCT06045091 | relapsed/refractory multiple myeloma or plasma cell leukemia | Recruiting |
| 3 | NCT05673447 | CD19-positive diffuse large B cell lymphoma | Recruiting |
| 4 | NCT06696846 | relapsed/refractory T-lymphoma and acute myeloid leukemia; CD70 positive | Recruiting |
| 5 | NCT06307054 | acute myeloid leukemia; CLL1-positive | Recruiting |
| 6 | NCT03692767 | Relapsed and Refractory B Cell Lymphoma; CD22-positive | Recruiting |
| 7 | NCT03383978 | HER2-positive glioblastoma or its variant gliosarcoma | Active, not recruiting |
| 8 | NCT06464965 | the positive expression of CLDN18.2 ≥ 10%;pancreatic cancer and gastric cancer | Recruiting |
| 9 | NCT06652243 | Glypican-3 (GPC3)-Positive Advanced Hepatocellular Carcinoma | Not yet recruiting |
| 10 | NCT05922930 | at least 1 + TROP2 expression; Ovarian Cancer | Recruiting |
| 11 | NCT05703854 | CD70-positive osteosarcoma or mesothelioma | Recruiting |
4.1 CAR-NK Cells in Solid Tumors
Several reviews have addressed the use of CAR-NK cells for cancer treatment [3, 27-29]. In the case of solid tumors, the diversity of antigens and the increasing variety of cancer types pose challenges. Anti-HER2-engineered NK cells have been developed to target breast cancer [30]. For glioblastoma, anti-HER2-NK92 cells are being investigated, with a clinical trial currently underway (NCT03383978) [31, 32]. In gastric cancer, anti-HER2-NK92 cells have demonstrated synergy with apatinib in preclinical studies [33]. Additionally, clinical trials are recruiting patients for CAR-NK cells targeting Claudin 18.2 (CLDN18.2) in gastric and pancreatic cancers (NCT06464965). Anti-Robo1-CAR-NK-92 cells, combined with brachytherapy, have been shown to inhibit pancreatic carcinoma in mouse models [34]. Under the synergistic effect of radiotherapy, anti-CPG3-NK92 cells engineered with CXCR2 displayed potent cytotoxicity against hepatocellular carcinoma (HCC), though the antitumor effect varied with different radiation doses [35]. Anti-GPC3-NK cells are set to be tested in volunteers with primary HCC in a clinical trial (NCT06652243). In colorectal cancer, NKG2D-CAR-NK cells, administered via local infusion to three advanced patients, resulted in tumor regression targeting NKG2D ligands [16]. Anti-HER1-CAR-NK cells showed strong tumor-killing activity against head and neck squamous cell carcinoma, but upregulation of CD44v6 expression may necessitate a multi-target combination therapy approach [36].
4.2 CAR-NK Cells in Hematologic Tumors
In hematologic malignancies, CAR-NK cell immunotherapy has shown promising results. In multiple myeloma (MM) [37, 38], CS1-CAR-NK cells were found to effectively inhibit tumor cell proliferation and significantly improve survival in tumor-bearing mice in an allogeneic transplant model [37]. Additionally, three types of CAR-NK cells targeting CD19 have demonstrated selective cytotoxicity against tumor cells [38]. GPRC5D and BCMA (NCT06045091) are also being explored as alternative targets [39, 40]. A recent study by Yang et al. [41] revealed that CAR-NK cells targeting CD19/CD20 exhibited enhanced cytotoxicity against acute lymphoblastic leukemia (ALL), even in the absence of target antigens.
While data from preclinical and clinical trials indicate that CAR-NK cells show significant efficacy and safety in treating solid tumors [18], several challenges remain to be addressed, which are discussed below.
5 The Current Strategies to Improve and Optimize CAR-NK Cells
CAR-NK cells offer the advantage of avoiding the common issues associated with CAR-T cells, such as GVHD and CRS. However, enhancing the targeted killing of tumor cells by CAR-NK cells remains a significant research challenge. This review discusses current strategies aimed at improving CAR-NK cell efficacy, providing potential directions for future technological advancements. These methods are summarized in Figure 2.
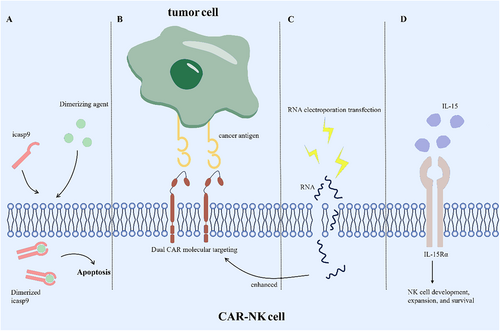
5.1 Enhancing Sustainability of CAR-NK Cells
The relatively short lifespan of CAR-NK cells, typically around 2 weeks, presents a challenge for their ability to fully accomplish tumor cell elimination. Extending the persistence of CAR-NK cells in vivo is essential for enhancing their effectiveness in targeting tumor tissue. A recent study on fourth-generation CAR molecules addressed this by constructing NK cells expressing CAR.19-IL-15/IL-15Rα, which demonstrated enhanced proliferation and strong cytotoxicity against the CD19-positive Raji cell line [42]. However, further evaluation is necessary to balance prolonged NK cell persistence with the potential risks of nontumor tissue toxicity, as discussed in detail later.
5.2 Addressing Low Cancer Antigen Expression in CAR-NK Cell Therapy
In the absence of overt pathological conditions, tumor cells may shed their specific targeting molecules due to the action of metal shearing enzymes on their surface. This process not only reduces the presence of specific targeting proteins on the tumor cell surface but also results in the masking of activated receptors on the CAR-NK cell surface. Consequently, the ability of CAR-NK cells to effectively kill tumor cells is compromised, leading to reduced therapeutic efficacy [43, 44].
To overcome these challenges, one approach involves multitargeting tumor cells to enhance their elimination. A recent study by Zhang et al. developed CAR-NK cells targeting both NKG2DL and ErbB2 to address the issue of soluble NKG2DL interference [45]. The bispecific CAR structure offers a solution to the problem of downregulated cancer antigens and insufficient precision [46]. Additionally, RNA electroporation transfection has shown promise in addressing potential immune escape pathways by enabling transient CAR expression. This method has been found to temporarily boost CAR activity, enhancing the cytotoxic capabilities of CAR-NK cells. NKG2D-CAR-NK cells targeting NKG2D ligands have been applied to patients with colorectal cancer [16], with electroporation increasing NKG2D expression and improving tumor cell killing. Furthermore, recent reports suggest that lipid nanoparticle (LNP) transfection of umbilical cord blood-derived NK cells may offer advantages over electroporation, though further evaluation of the receptor function of mRNA-LNP-treated NK cells is required [47].
5.3 Combating Off-Target Effects
The strategy of enhancing CAR-NK cells to overexpress specific ligands in response to low antigen expression in tumor cells introduces a significant risk of off-target effects. The expression of tumor antigens, albeit in small amounts, in nontumor tissues is an inherent characteristic. Specifically, many target proteins that are highly expressed on the surface of tumor cells are also found in normal tissue cells. For instance, macrophages and dendritic cells also express NKG2DL [48]. Both CAR-T and CAR-NK cell therapies are susceptible to off-target effects, highlighting a potential risk for CAR-NK cell treatment [49]. While CAR-NK cells typically have a short lifespan, around 2 weeks [25], limiting potential damage, the possibility of nontumor toxicity remains a concern. Technological advancements, such as making CAR-NK cells tunable, offer a means to mitigate off-target effects [50]. Recent research has demonstrated the feasibility of incorporating a suicide gene, such as the icasp9 suicide switch, within the CAR-NK construct [51]. For patients requiring repeated CAR-NK cell infusions, nontumor toxicity can be managed by administering chemicals that promptly terminate CAR-NK cell activity after they have fulfilled their therapeutic role, thus minimizing their impact on healthy tissues [52]. Furthermore, CAR-NK cells supported by IL-15 to extend their lifespan can be engineered with the icasp9 suicide gene, offering a refined approach to balance effective tumor cell clearance while minimizing nontumor toxicity.
6 The Elusory State of CAR-NK Cells Against Gynecological Cancers
Cervical, endometrial, and ovarian cancers constitute the most gynecologic malignancy with both high morbidity and mortality. Cervical cancer is the most common gynecologic cancer and the second leading cause of cancer death in women behind breast cancer and the mortality of cervical cancer patients in poor counties is twice that of women in affluent counties [53]. Endometrial cancer is the fourth most common cancer and the fifth most common cause of cancer death in women [54]. Ovarian cancer accounts for 3.6% of all cancer cases but 4.3% of all cancer deaths, which is the second most common cause of gynecologic cancer death next to cervical cancer, the seventh most common and the eighth most common cancer death in women [55]. Since the immunotherapy strategy represented by CAR has shown significant progress in hematologic malignancies and other solid tumors, it is more urgent to conceive the application of immunotherapy in gynecological cancers for women's health.
It is very worth considering where we are and where we are going in the realm of CAR therapy strategies against gynecological cancers. Totally, CAR-T is more widely studied for gynecological cancers than CAR-NK cells.
6.1 Prospects for the Development of CAR-NK Cell Therapy in Endometrial Cancer
Endometrial cancer is the leading malignancy of the female reproductive system in developed countries and ranks second in China. The standard first-line chemotherapy for recurrent or metastatic endometrial cancer is carboplatin combined with paclitaxel; however, patient outcomes remain poor, with a median progression-free survival of less than 2 years [56]. Despite improvements in survival across a variety of solid tumors, the prognosis for endometrial cancer has not seen significant progress in recent years [57, 58]. With increasing insights into the unique molecular and genomic features of type II endometrial cancers and the evolving understanding of the immune microenvironment in these tumors, there is a pressing need for molecularly targeted therapies or immunotherapies [59]. While the development of biomarker-driven targeted therapies for endometrial cancer has been slow [60], the advancement of immunotherapies appears even more critical. MISIIR is a promising target due to its specificity for the female reproductive system and could serve as an effective target for endometrial cancer. Recent studies have shown that MISIIR is overexpressed in both ovarian and endometrial cancers but not in normal tissue cells, and CAR-T cells targeting MISIIR have demonstrated significant success in lysing MISIIR-overexpressing cervical cancer model mice and patient-derived tumor cells [61].
6.2 Current Status and Development Prospects of CAR-NK Cell Therapy for Ovarian Cancer
Ovarian cancer, particularly epithelial ovarian cancer, is challenging to diagnose at an early stage, leading to most cases being diagnosed at an advanced stage. As a result, prognosis is often poor due to limited treatment options. Tumor reduction surgery and platinum-based chemotherapy remain the mainstay treatments; however, recurrence after treatment is common [62], highlighting the urgent need for novel and more effective therapeutic approaches.
The antigen and CAR design strategies used to engineer T cells or NK cells for ovarian cancer, particularly epithelial ovarian cancer, are highly versatile [63-68]. Progress in antigen-targeted therapies for ovarian cancer has accelerated due to the extensive identification of specific or associated antigens. For instance, one study developed anti-CD133-CD28-41BB-CD3ζ-CAR-NK92 cells targeting CD133-positive ovarian cancer cells, while another designed anti-CD24-CD28-41BB-CD3ζ-CAR-NK92 cells to selectively eliminate CD24-positive ovarian cancer cells. Both CD133 and CD24 are cancer stem cell markers applicable to other cancers as well [64, 67]. Folate receptor alpha (FRα), overexpressed in 90% of ovarian cancers, has been targeted by second-generation CAR-NK92 cells, which exhibited strong cytotoxicity against FRα-positive ovarian cancer cells [63]. Additionally, anti-mesothelin CAR-NK cells derived from iPSCs have been shown to inhibit cancer growth in an ovarian cancer xenograft model [68].
6.3 Prospects for the Development of CAR-NK Cell Therapy in Cervical Cancer
CAR-NK cell therapy has not yet been applied to cervical cancer. However, other immunotherapeutic approaches are being explored, with a primary focus on targeted therapies for HPV-related antigens, as most cervical cancers are driven by a well-defined high-risk HPV-related pathogenesis. Monoclonal antibodies such as C1P5 (anti-HPV E6) and TVG701Y (anti-HPV E7) have been studied and shown to inhibit tumor growth in cervical cancer mouse models [69]. In recent years, the effectiveness of immune checkpoint inhibitors in treating cervical cancer has improved, and combinations of immune checkpoint inhibitors with HPV therapeutic vaccines, chemotherapy, or radiotherapy are being considered for clinical use [70-72]. Pembrolizumab has received approval for treating PD-L1-positive cervical cancer [73]. Several antigens, including GD2, PSMA, Muc1, and mesothelin, have been targeted in CAR-T cell therapies for cervical cancer (ClinicalTrials.gov identifiers: NCT03356795 and NCT01583686). Additionally, HER2-targeted CAR-T cells have been evaluated in clinical trials for cervical cancer, and preclinical studies have focused on CD47 [74, 75].
The relatively limited use of CAR-T cell therapy in cervical cancer, compared to other solid tumors, can be attributed to the scarcity of specific and exclusive cancer-associated antigens in this type of cancer. Ideal candidates for CAR targeting should be broadly expressed on cancer cells while being absent or minimally present on normal tissues. HER2, with an overexpression rate of 38%–94% in cervical cancer, and mesothelin, with an overexpression rate of approximately 25%, are the primary antigens currently targeted by CAR-T cells in cervical cancer [74].
7 Conclusion
This review examines the critical role of NK cells and compares CAR-NK cell therapy with CAR-T cell therapy. In numerous clinical and preclinical studies, CAR-NK cells have shown tumor-targeting efficacy comparable to CAR-T cells. However, CAR-NK cells offer distinct advantages, such as their ability to avoid many common toxicities, including CRS and GVHD, during treatment. Despite these promising outcomes, several challenges remain in optimizing CAR-NK cell therapy. To address the issue of cancer antigen downregulation, strategies such as multi-target targeting or enhancing CAR expression may be considered. To extend the efficacy of CAR-NK cells, cytokine support could be explored, and to improve their tunability, the introduction of suicide genes may be a viable solution. As various innovative approaches continue to emerge, the application of CAR-NK cell therapy in gynecological oncology is still in its early stages. However, with the advancement of various biotechnologies, CAR-NK therapy, as a prominent form of immunotherapy, holds the potential to herald a new era in the treatment of cancer, offering hope for the cure of a large number of patients.
Author Contributions
Yisen Cao: conceptualization, investigation, methodology, software, writing – original draft, validation, visualization, writing – review and editing, formal analysis, project administration, data curation, supervision, resources. Liying Wang: investigation, funding acquisition, writing – original draft. Liang Wang: conceptualization, investigation, writing – original draft, validation, visualization, writing – review and editing, formal analysis, project administration, supervision, resources.
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript. This study was supported by National Natural Science Foundation of China (NSFC) grant (82303734) and Fujian Medical University Training Program of Innovation and Entrepreneurship for Undergraduates (C2025072).
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.