Opposite effects of systemic and local conditional CD11c+ myeloid cell depletion during bleomycin-induced inflammation and fibrosis in mice
Abstract
Rationale
Elevated levels of CD11c+ myeloid cells are observed in various pulmonary disorders, including Idiopathic Pulmonary Fibrosis (IPF). Dendritic cells (DCs) and macrophages (MΦ) are critical antigen-presenting cells (APCs) that direct adaptive immunity. However, the role of CD11c+ myeloid cells in lung extracellular matrix (ECM) accumulation and pulmonary fibrosis is poorly understood.
Objective
We aimed to investigate the impact of depleting CD11c+ myeloid cells, including DCs and macrophages, during bleomycin-induced pulmonary fibrosis in mice.
Methods
We used a diphtheria toxin (DTx) receptor (DTR) transgenic mouse model (CD11c-DTR-Tg) to deplete CD11c+ myeloid cells through two methods: Systemic Depletion (SD) via intraperitoneal injection (i.p.) and local depletion (LD) via intranasal instillation (i.n.). We then assessed the effects of CD11c+ cell depletion during bleomycin-induced lung inflammation and fibrosis.
Results
Fourteen days after bleomycin instillation, there was a progressive accumulation of myeloid cells, specifically F4/80-MHCII+CD11c+ DCs and F4/80 + MHCII+CD11c+ MΦ, preceding mortality and pulmonary fibrosis. Systemic depletion of CD11c+ DCs and MΦ via i.p. DTx administration in CD11c-DTR-Tg mice protected against bleomycin-induced mortality and pulmonary fibrosis compared to wild-type (WT) mice. Systemic depletion reduced myeloid cells, airway inflammation (total leukocytes, neutrophils, and CD4+ lymphocytes in bronchoalveolar lavage (BAL), inflammatory and fibrogenic mediators, and fibrosis-related mRNAs (Collagen-1α1 and α-SMA). Increased anti-inflammatory cytokine IL-10 and CXCL9 levels were observed, resulting in lower lung hydroxyproline content and Ashcroft fibrosis score. Conversely, local depletion of CD11c+ cells increased mortality by acute leukocyte influx (predominantly neutrophils, DCs, and MΦ in BAL) correlated to IL-1β, with lung hyper-inflammation and early fibrosis development.
Conclusion
Systemic depletion of CD11c+ cells confers protection against inflammation and fibrosis induced by Bleomycin, underscoring the significance of myeloid cells expressing F4/80-MHCII+CD11c+ DCs and F4/80 + MHCII+CD11c+ MΦ orchestrating the inflammatory milieu within the lungs, potentially as a source of cytokines sustaining pulmonary chronic inflammation leading to progressive fibrosis and mortality.
1 INTRODUCTION
Pulmonary fibrosis represents the common end-stage manifestation among a heterogeneous array of lung disorders, termed Interstitial Lung Diseases (ILDs). These ILDs are categorized together owing to shared clinical presentations, pathological classifications, physiological and radiographic changes, albeit with varying degrees of morbidity.1 Among these disorders, Idiopathic Pulmonary Fibrosis (IPF) stands out as a lethal chronic fibro-proliferative disease of obscure etiology and lacking effective therapeutic interventions. It is typified by an aberrant accumulation of collagen within the pulmonary parenchyma. Clinical manifestations of IPF encompass deteriorating dyspnea, progressive decline in lung volume, and impaired gas exchange.2-4 IPF exhibits a prevalence of 30 cases per 100,000 individuals, with an annual incidence of 34,000 new cases in the United States.5 Typically, mortality in IPF stems from the progressive decline in lung function over time, culminating in respiratory failure. On average, individuals diagnosed with IPF have a survival expectancy of 3 to 5 years post-diagnosis.2 IPF is a progressive and fatal interstitial disease with limited therapies. Previously, therapy for IPF predominantly was the chronic administration of corticosteroids, often supplemented with immunosuppressive agents. However, the emergence of adverse effects during the treatment course significantly constrains its efficacy.3, 6-8 Presently, the therapy for IPF is based on anti-fibrotics. Although Pirfenidone and Nintedanib are currently approved for treating IPF, patients still have high mortality rates and a median survival duration of only 3–5 years.9-12 Regarding the origin of IPF, extensive research efforts have delved into elucidating the preceding events that trigger the abnormal scarring of lung tissue. Despite these efforts, the ontogenesis of IPF remains controversial and poorly understood.13, 14 Presently, several hypotheses are proposed regarding the origin of IPF: (i) Fibrosis may arise due to a breakdown in communication or crosstalk between epithelial cells and fibroblasts, leading to collagen deposition independent of prior pulmonary inflammation. (ii) Alternatively, it can develop via an inflammatory pathway, wherein fibrosis results from preceding alveolitis and tissue damage.13-18
The lung exhibits specific characteristics concerning the innate immune organization in health conditions and response upon stimulation. Myeloid cells are extensively distributed throughout the healthy lung and play pivotal roles in various respiratory disorders. They have been unequivocally implicated in the pathogenesis of chronic diseases.19, 20 Myeloid cells such as Macrophages (MΦ) and Dendritic cells (DCs) serve as professional antigen-presenting cells (APCs), possessing a distinct capacity to initiate and regulate specific immune responses. The presence of a range of MΦ and DCs populations in the lungs constitutes an innate and remarkably responsive sentinel network strategically positioned in and around the airways.19-24 Through the continuous conveyance of antigenic information from the airways to the pulmonary lymph nodes, the APCs exert a profound influence on immune responses within the lungs, capable of both positive and negative modulation.19-22, 25 APCs such as DCs and MΦ are primarily distinguished by their abundant expression of MHCII (Major Histocompatibility Complex class II—HLA-DR) and the integrin CD11c as surface markers predominantly expressed by myeloid cells. Additionally, MΦ express monocyte lineage markers (F4/80 and Ly6C), whereas DCs do not.19, 20 The integrin CD11c (integrin α-X subunit, also referred to as leukocyte surface antigen p150, αXβ2, and CR4) is a transmembrane glycoprotein weighing 145–150 kDa, belonging to the β2 integrin family.26 CD11c is predominantly expressed by cells of myeloid origin and serves as markers for defining leukocyte sub-populations, particularly since they are primarily expressed by macrophages and DCs.19, 20, 26 As an integrin, CD11c plays crucial roles in regulating three critical aspects of immune cell function that contribute to and modulate immune responses: (i) orchestrating leukocyte recruitment to sites of inflammation, (ii) mediating the formation of cell–cell contacts, and (iii) triggering downstream effects on cellular signaling pathways of activation.26 In lung sites, the populations of APCs that exhibit high co-expression of MHCII and CD11c primarily consist of Alveolar Macrophages (AMΦ) and Myeloid Dendritic Cells (mDCs), whereas Plasmacytoid Dendritic Cells do not typically display this co-expression pattern.19-22, 25
Myeloid cells present in the airways and lung parenchyma possess the potential to sustain chronic inflammation through continual lymphocytic infiltration while also regulating cytokine and chemokine levels.20, 22, 27-30 The expression of cytokines and chemokines during aberrant chronic inflammation results in the persistent accumulation of myeloid cells in the lungs. This sustained presence of myeloid cells leads to chronic activation of fibroblasts, producing abnormal tissue repair responses.27, 30-36 In the context of ILDs, various subsets of Mϕ and mDCs have been documented in the bronchoalveolar lavage fluid (BALF) and lungs from IPF patients compared to healthy control.27, 37-40 Despite clinical evidence indicating a correlation between elevated numbers of MΦ and mDCs and the extent of pulmonary fibrosis in IPF, the precise role of CD11c+ myeloid DCs and MΦ populations in the context of pulmonary fibrosis remains uncertain,41, 42 and was the objective of this investigation. Here, we investigated the involvement of myeloid CD11c+ DCs and MΦ in advancing experimental pulmonary fibrosis induced by Bleomycin. Our findings revealed an augmentation in the myeloid CD11c+ populations, including MΦ (F4/80 + MHCII+ CD11c+) and DCs (F4/80− MHCII+ CD11c+), within the fibrotic lungs of bleomycin-treated mice, exhibiting a correlation with the progression of pulmonary fibrosis. To further elucidate the role of CD11c+ MΦ and DCs populations in experimental pulmonary fibrosis, conditional depletion of myeloid CD11c+ cells was achieved using a diphtheria toxin (DTx) receptor (DTR) transgenic mouse model (CD11c-DTR-Tg).43 Through two distinct protocols, conditional CD11c+ systemic depletion and conditional CD11c+ local depletion via DTx administration, we investigated the impact on bleomycin-induced pulmonary fibrosis. Conditional systemic depletion of CD11c+ DCs and MΦ using CD11c-DTR-Tg mice conferred protection against bleomycin-induced mortality and pulmonary fibrosis. This protection was associated with reduced influx of myeloid F4/80-MHCII+CD11c+ DCs and F4/80 + MHCII+CD11c+ MΦ into the airways, accompanied by diminished levels of cytokines and chemokines, as well as attenuated airway inflammation and fibrogenesis. However, conditional local depletion of CD11c+ cells proved deleterious, exacerbating lung hyper-inflammation induced by Bleomycin, with early fibrosis development. These findings suggest that CD11c+ DCs and MΦ mediate chronic lung inflammation, sustaining tissue chemokine and cytokine levels that precede pulmonary fibrosis induced by Bleomycin in mice. Consequently, therapeutic strategies targeting CD11c+ DCs and MΦ may present a novel approach to treating lung fibroproliferative diseases such as IPF.
2 MATERIAL AND METHODS
2.1 Animals
C57Bl/6j (WT) and CD11c DTR transgenic mice CD11c-DTR-Tg (B6.FVB-Tg (Itgax-DTR/EGFP)57Lan/J),43, 44 were bred in animal facility from Laboratório de Imunofarmacologia/UFMG at Instituto de Ciências Biológicas/UFMG. Mice were grouped into three to five animals per cage in a ventilated rack and kept under controlled light conditions in the animal facility of the Laboratory of Pulmonary Immunology at Instituto de Ciências Biológicas/UFMG, under pathogen-free conditions with filtered water and food ad libitum.
2.2 Conditional depletion of CD11c+ myeloid cells in vivo
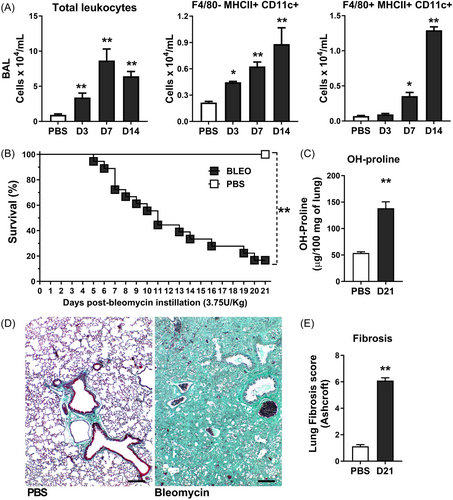
Conditional depletion of CD11c+ myeloid cells in vivo was achieved using DTx (D-0564; Sigma-Aldrich, St. Louis, MO). DTx was reconstituted to a concentration of 2 mg ml−1 in sterile water and subsequently diluted to 2.5 μg ml−1 in sterile saline. CD11c-DTR-Tg mice were subjected to conditional depletion, following previously established protocols,43, 44 through two distinct methods: (i) Systemic depletion (SD) involved intraperitoneal injection (i.p.) of DTx at a dosage of 5 ng/g of body weight (Figure 2A); and (ii) Local depletion entailed intranasal instillation (i.n.) of DTx at a dosage of 1 ng/g of body weight (Figure 6A), diluted in sterile PBS, and both administered 24 h before the challenge with bleomycin or control mice.
2.3 Bleomycin-induced lung injury and fibrosis in mice
Male wild-type (WT) and CD11c-DTR mice aged 8–10 weeks were utilized in a bleomycin-induced lung injury and fibrosis model, conducted in accordance with previously established procedures.45-49 Mice mortality across different groups was monitored daily over 21 days. In brief, a single intranasal instillation of either a 40 μL injection containing 3.75 mg/kg of bleomycin sulfate (Blenoxane; Bristol-Meyers Squibb) per kg of body weight (equivalent to 3.75 Units of body weight) diluted in PBS, or PBS alone (vehicle), was administered. All experiments were conducted in compliance with institutional guidelines and approved by the Brazilian animal ethics committee CETEA/UFMG (Protocol number 232/2012), adhering to national and international laws.
2.4 Assessment of airway and alveolar leukocytes
Bronchoalveolar lavage (BAL) was conducted to retrieve leukocytes from the alveolar space, following established protocols, as described previously.45-49 The trachea was exposed, and a polyethylene catheter (BD Biosciences) with a 1.7-mm outside diameter was inserted. BAL was performed by washing the lungs three times with two separate 1-ml aliquots of PBS. Then, BAL samples were centrifuged at 600 × g for 10 min at 4°C. The resulting cell pellet was resuspended in PBS, and the total number of leukocytes was determined by cell counting after dilution in Türk's solution (Merck Millipore) using a Neubauer chamber (Blaubrand). Differential BAL cell counts were obtained from Cytospin preparations (Shandon III), and the percentage of leukocyte populations stained with May-Grünwald-Giemsa (Laborclin) was evaluated under an optical microscope. Additionally, leukocyte sub-populations were determined by FACS analysis.
2.5 FACS analysis of airway leukocytes
Leukocytes obtained from BAL were stained with fluorochrome-conjugated monoclonal antibodies, including anti-CD3, anti-CD4, anti-CD8, anti-γδTCR, anti-F4/80, anti-MHCII, anti-CD11c, and anti-GR1, or isotype controls (BD Pharmigen™). Subsequently, they were acquired using a FACSCanto II cytometer (BD Biosciences) using BD FACSDiva software (BD Biosciences), following established protocols as described previously.45-47 Before staining with leukocyte antibodies, BAL cells underwent hypotonic lysis to remove residual erythrocytes. Subsequently, the cell suspensions were incubated with Fc Block antibodies (BD Pharmigen™). After staining, cells were fixed with 4% paraformaldehyde, and 100,000 events were acquired in BAL samples. The gating strategy initially involved the Boolean gate of myeloid populations, singlets, and time. Analyses were performed using FlowJo V10.1 software (Tree Star, Ashland, OR, USA). The populations were determined in histograms of the constitutive marker. The leukocyte and phenotypic populations were calculated based on the expression of surface markers: Neutrophils (GR1+F4/80-), T CD4 lymphocytes (CD3+CD4+), T CD8 lymphocytes (CD3+CD8+), γδ lymphocytes (CD3+γδTCR+), Macrophages (F4/80+MHCII+ CD11c+), and Dendritic Cells (F4/80- MHCII+ CD11c+). The frequency, presented as a percentage of the analyzed population compared to the total acquired events, was utilized in the construction of graphs. The number of cells collected by BAL was adjusted based on the total cell counts.
2.6 Lung histopathology
The left lung was removed and fixed in 4% neutral phosphate-buffered formalin (pH 7.4), as we previously described.45-49 The tissues underwent gradual dehydration in ethanol, followed by embedding in paraffin and cutting into 4 µm sections. These sections were then stained with H&E or Gomori's trichrome (Leica Biosystems) and examined under light microscopy by a pathologist in a blind manner. Semiquantitative histopathological analyses of inflammation were conducted as previously described.47, 49 Briefly, ten random images per lung were acquired at 20× magnification, and the pulmonary inflammation score was recorded on a six-grade scale. A score of “0” corresponded to less than 1% of lung tissue area inflamed, while higher scores indicated increasing levels of inflammation. The hemorrhage score was based on a four-degree scale, ranging from the absence of hemorrhage to the presence of intense hemorrhage. For semiquantitative analysis of Gomori's trichrome-stained slices, the Ashcroft fibrosis score was determined according to custom-designed criteria for evaluating lung fibrosis, as previously described.46, 49 Images of lung sections were captured using a digital camera (Optronics DEI-470) connected to a microscope (Olympus IX70) with magnifications of 40× or 100×. Scale bars of 100 μm were utilized for Gomori's trichrome images, while those of 200 μm were used for H&E images.
2.7 Lung hydroxyproline (OH-proline) quantification
Lung fragments were harvested for hydroxyproline quantification to assess collagen content in the lung tissue, following established protocols as previously described.46-49 Fragments weighing 100 mg of lung tissue were homogenized in 0.2% saline, followed by freezing and lyophilization. The assay was conducted using the lyophilized material, which underwent alkaline hydrolysis in 300 μL of H2O supplemented with 75 μL of 10 M NaOH at 120°C for 20 min. Subsequently, an aliquot of 50 μL of the hydrolyzed tissue was combined with 450 μL of Chloramine T (Sigma-Aldrich) oxidizing reagent (comprising 0.056 M Chloramine T and 10% n-propanol (Rauter Química Ltda) in acetate-citrate buffer [pH 6.5]) and allowed to react for 20 min. A hydroxyproline standard curve was prepared using a similar approach. Color development was initiated by adding 500 μL of 1 M p-dimethylaminebenzaldehyde (Sigma-Aldrich) diluted in n-propanol–perchloric acid (2:1 [vol/vol]). The absorbance was measured at 550 nm using a spectrophotometer (Emax; Molecular Devices).
2.8 Cytokine measurement in lung tissue
One hundred milligrams of lung tissue were homogenized in 1 mL of PBS containing protease inhibitors (Complete tablets, Roche Diagnostic; PMSF, Sigma-Aldrich) and 0.05% Tween 20 (Sigma-Aldrich), following previously established protocols.45-49 Samples of lung homogenate or BALF were then centrifuged for 10 min at 3000 × g, and the supernatant was immediately used for assay at a 1:2 dilution in PBS. The chemokines CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES, CCL11/Eotaxin, CXCL1/KC, and CXCL9/MIG, as well as the cytokines IL-1β, IL-6, IL-10, and active TGF-β1 levels, were quantified in lung homogenate and BALF samples using DuoSet Enzyme-Linked Immunosorbent Assay (ELISA) kits (R&D Systems), following the manufacturer's instructions, as previously described.45-49
2.9 RNA isolation and quantitative real-time PCR
Lungs were harvested for analysis of fibrogenesis markers, precisely the expression of Collagen1α1 (Col1a1) and α-Smooth Muscle Actin (Acta2) mRNAs, using quantitative Real-Time PCR, following established protocols as previously described.45, 47, 49 Total lung RNA was extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. First-strand cDNA synthesis was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) with 2 μg of total RNA, following the manufacturer's instructions. Real-time quantitative PCR was carried out on an ABI PRISM Step-One sequence-detection system (Applied Biosystems, Carlsbad, CA) using SYBR Green PCR Master Mix (Applied Biosystems). The amplification protocol consisted of one cycle at 95°C for 10 min, followed by 39 cycles at 95°C for 15 s and 60°C for 1 min. The relative expression level of genes was determined using the 2(-deltaCt) method, and the data were normalized by the expression levels of the 18S ribosomal subunit. All reactions were performed in duplicate. The following primers were used:
| Collagen-1α1 (Col1a1): | Forward: 5’- ATG TTC AGC TTT GTG GAC CTC - 3’ |
| Reverse: 5’- GCA GCT GAC TTC AGG GAT GT - 3’ | |
| α-Smooth Muscle Actin (Acta2): | Forward: 5’- GAC ACC ACC CAC CCA GAG T - 3’ |
| Reverse: 5’- ACA TAG CTG GAG CAG CGT CT - 3’ | |
| 18S: | Forward: 5’- CTC AAC ACG GGA AAC CTC AC - 3’ |
| Reverse: 5’- CGC TCC ACC AAC TAA GAA CG - 3’ |
2.10 Statistical analysis
The graphs were plotted using Prism version 10.1.1 (GraphPad), and values are presented as mean ± standard error of the mean (SEM). One-way ANOVA followed by Tukey post-hoc test was used for parametric data. The nonparametric data were expressed as the mean ± standard error of the mean (SEM) values and analyzed by Kruskal-Wallis followed by Dunn's test. The Mantel-Cox log-rank test was used to determine differences between survival curves. Statistical significance was accepted when p < .05. Statistical significance is indicated as *p < .05, **p < .01, and ***p < .001.
3 RESULTS
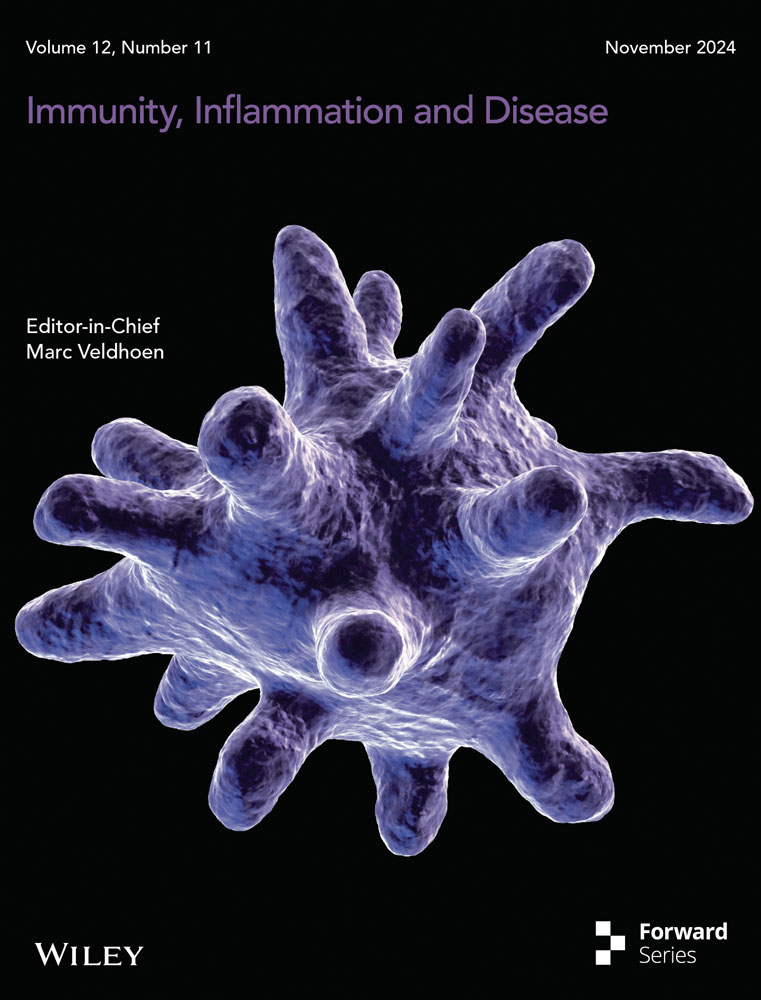
3.1 Bleomycin-induced pulmonary inflammation and mortality in mice were associated with a progressive influx of CD11c+ myeloid dendritic cells (DCs) and macrophages (MΦ), which preceded the development of lung fibrosis
Myeloid DCs and MΦ play pivotal roles in orchestrating inflammation, immunity, and tolerance, making them critical targets for immunotherapy. We further characterized the phenotype of myeloid cell influx into the airways in bleomycin-treated mice. To do this, myeloid DCs (F4/80- MHCII+ CD11c+) and MΦ (F4/80 + MHCII+ CD11c+) were kinetically analyzed by flow cytometry of lung single-cell suspensions prepared from bronchoalveolar lavage fluid after 3, 7, and 14 days following instillation. An increased number of total airway leukocytes was observed starting from day 3, peaking after 7 days, and remaining elevated until day 14 after bleomycin instillation compared to PBS-instilled control WT mice (Figure 1A). Bleomycin instillation progressively induced myeloid cell influx into the airways (Figure 1A), with DCs (F4/80- MHCII+ CD11c+) increasing from day 3 until day 14 and MΦ (F4/80 + MHCII+ CD11c+) increasing from day 7 until day 14 (Figure 1A), compared to PBS-instilled control mice. Therefore, myeloid DCs (F4/80- MHCII+ CD11c+) and MΦ (F4/80 + MHCII+ CD11c+) concomitantly increased after 7- and 14-days post-bleomycin instillation (Figure 1A). Bleomycin induced pronounced mice mortality after 7 days of instillation (Figure 1B), occurring in parallel with concomitant airway influx of DCs (F4/80- MHCII+ CD11c+) and MΦ (F4/80 + MHCII+ CD11c+) compared to PBS-instilled control mice (Figure 1A). After 21 days of bleomycin instillation, WT mice developed extended pulmonary fibrosis compared to PBS-instilled control mice, with increased hydroxyproline lung tissue content (Figure 1C), marked by a diffuse and dense pattern of fibrosis with airway collapse as visualized by Gomori's Trichrome staining (green) (Figure 1D), also exhibiting a high grade of pulmonary fibrosis as depicted by Ashcroft score (Figure 1E).
Collectively, Bleomycin induced pulmonary inflammation and mortality related to progressive and concomitant airway influx of CD11c+ myeloid DCs and MΦ starting 7 days after instillation, preceding chronic lung fibrosis in mice.
3.2 Conditional systemic depletion of CD11c+ myeloid cells attenuated lung inflammation due to reduced CD11c+ dendritic cells (DCs) and macrophages (MΦ) after 7 days of bleomycin instillation in CD11c-DTR-Tg mice
Defining that CD11c+ myeloid DCs and MΦ airway influx are related to inflammation that precedes lung fibrosis, we sought to determine the role of CD11c+ myeloid cells in sterile innate immune response, wherein chronic lung inflammation causes pulmonary fibrosis. Using CD11c-DTR-Tg mice, we systemically depleted CD11c+ myeloid cells via DTx i.p. injection 24 h before intranasal bleomycin instillation to evaluate the impact on leukocyte influx in airways after 7 days (Figure 2A), at the inflammatory peak. Bleomycin instillation induced increased airway leukocyte influx after 7 days in WT mice (Figure 2B), with elevated lymphocytes and neutrophil numbers in BAL, as shown by cytospin preparations (Figure 2C) compared to PBS-instilled mice. FACS analysis confirmed increased counts of CD3 + CD4+ and CD3+γδ lymphocytes (Figure 2D), as well as GR1 + F4/80- neutrophils (Figure 2E) in BAL of WT mice challenged with Bleomycin. Conditional systemic depletion using CD11c-DTR-Tg mice reduced total leukocyte numbers (Figure 2B), marked by a low influx of lymphocytes and neutrophils by cytospin (Figure 2C), primarily decreasing CD3 + CD4+ lymphocytes (Figure 2D) and GR1 + F4/80- neutrophils (Figure 2E) in BAL after 7 days of bleomycin instillation. Bleomycin induced CD11c+ myeloid cell influx into the airways, with myeloid DCs (F4/80- MHCII+ CD11c+) and MΦ (F4/80 + MHCII+ CD11c+) concomitantly increased after 7 days of instillation (Figure 2E), as previously shown (Figure 1A). Conditional systemic depletion of CD11c+ cells using CD11c-DTR-Tg mice abrogated the DCs (F4/80- MHCII+ CD11c+) and MΦ (F4/80 + MHCII+ CD11c+) BAL numbers induced by bleomycin instillation after 7 days (Figure 2E), confirming that CD11c+ depletion by DTx impacts the influx of DCs and MΦ populations in airways.
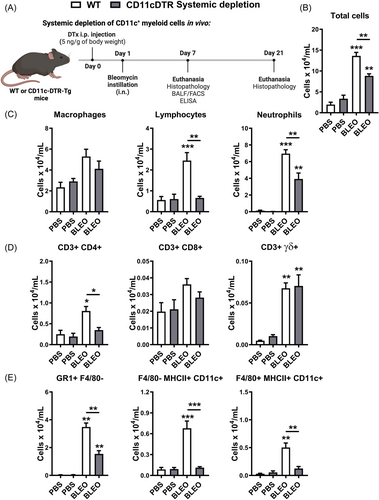
We further investigated the impact of bleomycin-induced tissue injury and inflammation in the context of conditional systemic depletion of CD11c+ myeloid cells. We evaluated lung histopathology using H&E stained slices, and inflammation was analyzed in all lung compartments (vascular, parenchymal, and airways) after 7 days of bleomycin instillation (Figure 3). Histopathological analysis revealed that Bleomycin induced lung injury 7 days after instillation in WT mice, with marked lung vascular inflammation, parenchymal leukocyte infiltration, and airway inflammation (Figure 3A), resulting in a multifocal pattern of lung inflammation (Figure 3B) and elevated overall score (Figure 3A) compared with PBS-instilled mice. However, conditional systemic depletion of CD11c+ cells using CD11c-DTR-Tg mice attenuated tissue injury and inflammation induced by Bleomycin compared to WT mice, with reduced inflammatory aspects of all lung compartments evaluated (Figure 3A) and increased preserved lung areas (Figure 3B).
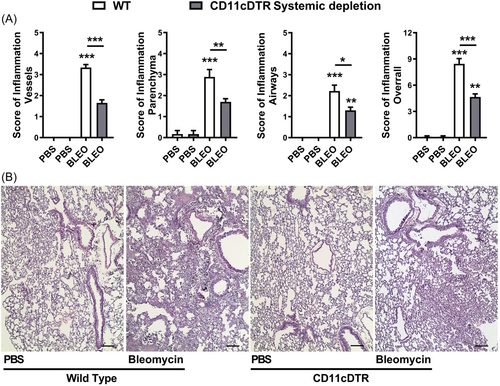
Thus, this suggests that CD11c+ DCs and MΦ may contribute to the development of an inflammatory milieu in the lungs, with leukocyte influx and tissue injury induced by Bleomycin, and systemic depletion of CD11c+ cells was able to restrict these pathological manifestations in CD11c-DTR-Tg mice.
3.3 Conditional systemic depletion of CD11c+ myeloid cells changes the chemokine and cytokine profile, impacting the low expression of fibrogenic markers induced by bleomycin after 7 days in CD11c-DTR-Tg mice
Myeloid cells, such as DCs and MΦ, are distributed throughout the airways and lung parenchyma. They can perpetuate chronic inflammation through sustained expression of cytokines and chemokines. This perpetuation contributes to aberrations in chronic inflammation, thereby fostering a persistent activation of fibroblasts. Consequently, this sustained activation results in an abnormal tissue repair process.27, 30-36 Utilizing CD11c-DTR-Tg mice, we conducted a comprehensive investigation into the effects resulting from the conditional systemic depletion of CD11c+ myeloid cells on the chemokine and cytokine profile induced by bleomycin instillation over a 7-day period. Employing ELISA, we quantified the levels of CCL2, CCL3, CCL4, CCL5, CCL11, CXCL1, CXCL9, IL-1β, IL-6, IL-10, and TGF-β1 in BAL and lung tissue samples. Bleomycin instillation in WT mice elicited significantly higher levels of CC and CXC chemokines in BAL fluid compared to phosphate-buffered saline (PBS)-instilled mice, as demonstrated by previous studies,30, 50 compared to PBS-instilled mice (Figure 4A). However, in the context of conditional systemic depletion of CD11c+ myeloid cells, there was a notable decrease in CC chemokines (CCL2, CCL3, CCL4, CCL5), along with comparable levels of CCL11, reduced levels of CXCL1, and elevated levels of CXCL9 in BAL fluid of CD11c-DTR-Tg mice compared to WT mice, 7 days post-bleomycin instillation (Figure 4A). Analyzing the inflammatory and fibrogenic cytokines in lung homogenates, we observed that bleomycin-induced increased levels of pro-inflammatory IL-1β and IL-6, alongside elevated levels of pro-resolutive IL-10 and pro-fibrogenic TGF-β1 at day 7 in WT mice compared to PBS-instilled mice (Figure 4B). However, the conditional systemic depletion of CD11c+ cells resulted in decreased levels of pro-inflammatory cytokine IL-6, reduced levels of pro-fibrogenic cytokines IL-1β and TGF-β1, but increased levels of pro-resolutive IL-10 in the lungs of bleomycin-challenged CD11c-DTR-Tg mice compared to WT mice (Figure 4B).
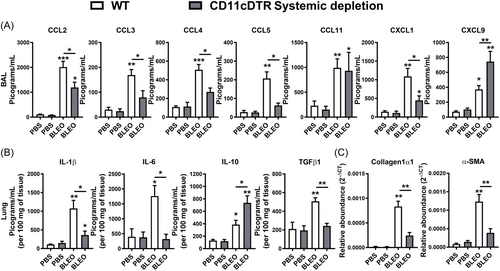
Given the observed differential expression of chemokines and cytokines in the lungs of CD11c-DTR-Tg mice compared to WT mice after bleomycin challenge (Figure 4A, B), particularly those associated with pro-fibrogenic actions (CCL2, CXCL1, IL-1β, and TGF-β1), we proceeded to investigate the expression of mRNA transcripts related to fibrogenesis (Figure 4C) via real-time PCR from lung tissue. We evaluated the expression of Collagen-1α1 (col1a1) and α-Smooth Muscle Actin (Acta2). Bleomycin-induced mRNA expression of Collagen-1α1 and α-Smooth Muscle Actin in WT mice after 7 days of instillation, indicative of fibroblast activity and extracellular matrix (ECM) production, was abrogated by the conditional systemic depletion of CD11c+ myeloid cells using CD11c-DTR-Tg mice (Figure 4C).
Hence, it appears that CD11c+ DCs and MΦ may foster an inflammatory milieu in the lungs through chemokine and cytokine production induced by Bleomycin, sustaining leukocyte influx and fibroblast activity, phenomena attenuated by the systemic depletion of CD11c+ DCs and MΦ in CD11c-DTR-Tg mice.
3.4 The conditional systemic depletion of CD11c+ myeloid cells confers protection to CD11c-DTR-Tg mice against bleomycin-induced pulmonary fibrosis and mortality
Bleomycin administration in WT mice elicited pronounced lung inflammation, along with concurrent expression of ECM remodeling markers after a 7-day, and this inflammatory response was mitigated upon conditional systemic depletion of CD11c+ DCs and MΦ in CD11c-DTR-Tg mice. Therefore, we endeavored to ascertain the influence of CD11c+ myeloid cell depletion on initiating and preceding pulmonary fibrosis 21 days post-bleomycin instillation in mice. By analyzing lung tissue samples for hydroxyproline content, indicative of collagen deposition, we observed an increase in bleomycin-induced collagen accumulation 21 days post-instillation in WT compared to PBS-instilled mice, which was attenuated in CD11c-DTR-Tg mice upon early conditional systemic depletion of CD11c+ myeloid cells using DTx administered 24 h before bleomycin challenge (Figure 5A). Furthermore, pulmonary fibrosis was quantified via assessment of the Ashcroft fibrosis score using Gomori's Trichrome stained lung sections. WT mice exhibited dense and diffuse interstitial lung fibrosis after 21 days of bleomycin instillation, characterized by elevated Ashcroft scores and marked alterations in pulmonary architecture, including alveolar septal collapse and large contiguous fibrotic masses, in contrast to PBS-instilled mice. However, these fibrotic changes were markedly reduced in CD11c-DTR-Tg mice subjected to early conditional systemic depletion of CD11c+ myeloid cells and challenged with Bleomycin, presenting with lower-grade Ashcroft scores and focal, smaller fibrotic masses interspersed with preserved lung parenchyma (Figure 5B, C). Progressive bleomycin-induced pulmonary fibrosis was associated with aberrant collagen accumulation within the lung parenchyma (Figure 5A, C). While bleomycin administration resulted in 90% mortality 21 days post-instillation, a 60% survival rate was achieved when CD11c+ myeloid cells were conditionally systemically depleted in CD11c-DTR-Tg mice (Figure 5D).
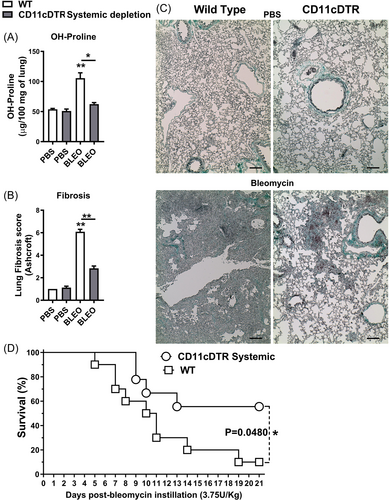
Taken together, these findings suggest that CD11c+ DCs and MΦ may contribute to chronic tissue remodeling, and early conditional systemic ablation of CD11c+ myeloid cells confers protection against bleomycin-induced fibrotic disease, thereby enhancing survival in CD11c-DTR-Tg mice.
3.5 The conditional local depletion of CD11c+ myeloid cells exacerbates pulmonary hyperinflammation and increases mortality induced by bleomycin in CD11c-DTR-Tg mice
CD11c+ DCs and MΦ systemic depletion in CD11c-DTR-Tg mice protect mi e from inflammation and fibrosis, thereby enhancing survival. In light of these findings, we further explored the impact of conditional local depletion of CD11c+ myeloid cells on bleomycin-induced tissue injury and inflammation. To achieve this, we locally depleted CD11c+ myeloid cells using intranasal instillation of DTx in CD11c-DTR-Tg mice 24 h before intranasal bleomycin instillation (Figure 6A), aiming to evaluate survival and leukocyte influx into the airways at the peak of inflammation after 7 days.
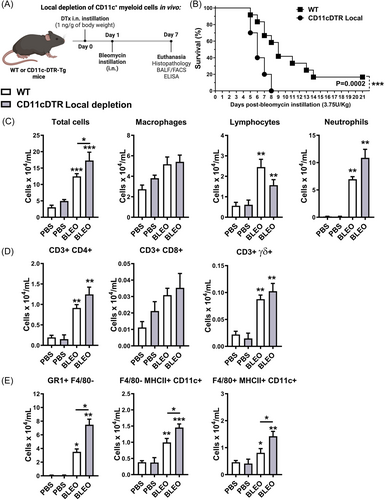
Conditional local depletion of CD11c+ myeloid cells using DTx in CD11c-DTR-Tg mice led to increased mortality, with Bleomycin inducing 100% mortality compared to a 60% survival rate observed in WT mice 8 days post-instillation (Figure 6B). Furthermore, conditional local depletion of CD11c+ myeloid cells resulted in increased total leukocyte numbers in BAL after 7 days of bleomycin instillation (Figure 6C), primarily driven by an influx of GR1 + F4/80- neutrophils (Figure 6E), but not lymphocytes (Figure 6D). Surprisingly, Bleomycin induced increased recruitment of CD11c+ myeloid DCs (F4/80- MHCII+ CD11c + ) and MΦ (F4/80 + MHCII+ CD11c + ) into the airways after 7 days of instillation in CD11c-DTR-Tg mice subjected to conditional local CD11c+ depletion, contrasting with WT mice (Figure 6E), suggesting that local CD11c+ depletion by DTx exacerbates leukocyte influx, particularly neutrophils, DCs, and MΦ populations in the airways.
Examining the chemokine and cytokine profile induced by Bleomycin after 7 days of instillation, we found that while WT mice exhibited significantly elevated levels of CC and CXC chemokines in BAL fluid, only CCL4 and CCL11 were increased in CD11c-DTR-Tg mice subjected to conditional local CD11c+ depletion (Figure 7A). Analysis of inflammatory and fibrogenic cytokines in lung homogenates revealed increased levels of IL-1β and low levels of IL-6 in CD11c-DTR-Tg mice, alongside similar levels of pro-resolutive IL-10 and pro-fibrogenic TGF-β1 compared to WT mice (Figure 7B). Additionally, bleomycin-induced mRNA expression of Collagen-1α1 and α-Smooth Muscle Actin, indicative of fibroblast activity and ECM production, was observed in WT mice similarly found in CD11c-DTR-Tg mice subjected to conditional local CD11c+ depletion (Figure 7C).

Histopathological analysis demonstrated marked lung injury induced by Bleomycin in WT mice after 7 days, characterized by lung vascular inflammation, parenchymal leukocyte infiltration, and airway inflammation, resulting in a multifocal pattern of lung inflammation and elevated overall score (Figure 8A, C). However, conditional local depletion of CD11c+ cells in CD11c-DTR-Tg mice exacerbated tissue injury and inflammation induced by Bleomycin compared to WT mice, with increased inflammatory scores of lung parenchyma and airways. diffuse lung inflammation and edema observed (Figure 8A, C). The pulmonary fibrosis was quantified at day 7 post-bleomycin instillation via assessment of the Ashcroft fibrosis score using Gomori's Trichrome stained lung sections (Figure 8B, D), as we previously described.48, 51 WT mice exhibited lower-grade Ashcroft scores and focal fibrosis, with smaller airway thickness interspersed with preserved lung parenchyma at day 7. However, upon local CD11c depletion, there is a discrete increase in collagen accumulation and airway thickness with alveolar septal collapse, compared to WT, as depicted by the Ashcroft score (Figure 8B, D).
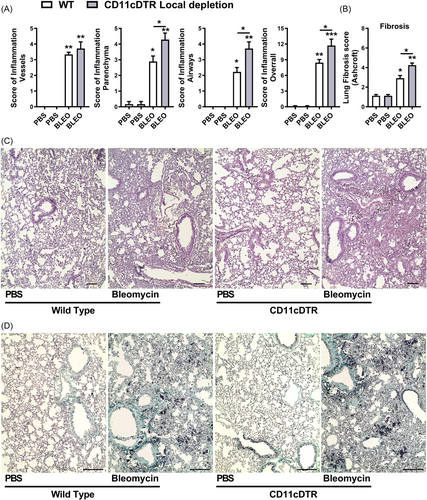
In summary, conditional local depletion of CD11c+ myeloid cells in CD11c-DTR-Tg mice exacerbates bleomycin-induced lung inflammation and tissue injury, contributing to the development of a hyperinflammatory lung milieu and early fibrosis, characterized by sustained production of chemokines and cytokines.
4 DISCUSSION
Idiopathic pulmonary fibrosis (IPF) stands as one of the most devastating progressive interstitial lung diseases. Its pathogenesis is characterized by an aberrant interplay among injured alveolar cells, leukocytes, and myofibroblasts, culminating in excessive fibroproliferation.52 In various clinical studies, diverse clinical evidence has been documented delineating the extent of pulmonary fibrosis and the presence of distinct subsets of MΦ and mDCs in BALF and lung tissues from patients with ILDs, including IPF.27, 37-40 Indeed, the precise role of CD11c+ DCs and MΦ in the context of pulmonary fibrosis remains elusive and subject to ongoing investigation.41, 42
In our study, we delved into the role of myeloid CD11c+ DCs and MΦ in the progression of experimental pulmonary fibrosis induced by Bleomycin. Our findings revealed several key insights: (i) We observed that the pulmonary inflammation and mortality induced by bleomycin correlate with a progressive influx of CD11c+ myeloid DCs and MΦ into the airways, preceding the development of lung fibrosis; (ii) Conditional systemic depletion of CD11c+ myeloid cells resulted in the attenuation of lung inflammation, accompanied by a reduction in the numbers of CD11c+ DCs and MΦ following bleomycin instillation; (iii) This systemic depletion altered the chemokine and cytokine profile, leading to decreased expression of fibrogenic markers, thereby protecting against bleomycin-induced pulmonary fibrosis and mortality in CD11c-DTR-Tg mice; (iv) Conversely, conditional local depletion of CD11c+ myeloid cells exacerbated pulmonary hyper-inflammation, enhancing mortality in CD11c-DTR-Tg mice following bleomycin instillation. These findings collectively support the notion that myeloid cells expressing CD11c, including DCs (F4/80-MHCII+CD11c + ) and MΦ (F4/80 + MHCII+CD11c + ), play critical roles in lung inflammation. These myeloid cells may serve as a source of cytokines and chemokines,30, 53, 54 thereby sustaining chronic inflammation that ultimately leads to progressive pulmonary fibrosis and mortality.
Myeloid cells such as MΦ and mDCs are naturally present in the airways and lung parenchyma during periods of health. However, they can initiate and perpetuate chronic inflammation through continuous lymphocytic infiltration and maintenance of cytokine and chemokine levels. This sustained inflammatory milieu contributes to the formation of fibroblastic foci and eventual tissue fibrosis.20, 22, 27, 28, 30 Indeed, studies have consistently shown an abundance of MΦ and mDCs in BALF and lung tissues obtained from patients diagnosed with IPF.27, 37-40 These findings underscore the significant role of myeloid cells in the pathogenesis of IPF and other ILDs. Our results indicate a progressive increase in the influx of CD11c+ myeloid cells, particularly DCs (F4/80-MHCII+CD11c+) and MΦ (F4/80 + MHCII+CD11c+), following bleomycin instillation and preceding lung fibrosis in mice, are consistent with existing literature.37-39, 55, 56 This parallels the observed manifestation in humans with IPF, where there is an influx of macrophages and myeloid dendritic cells into the airways. These findings suggest a potential translational relevance of your experimental model to the human condition of IPF.27, 37-40 Based on these results, we formulated a hypothesis to investigate whether conditional depletion of CD11c-expressing myeloid cells would influence the progression of inflammation preceding pulmonary fibrosis induced by Bleomycin in mice.
To gain deeper insights into the role of CD11c+ MΦ and mDCs populations during experimental pulmonary fibrosis, we opted to conditionally deplete myeloid CD11c+ cells using CD11c-DTR-Tg mice.43 We aimed to evaluate the impact of conditional systemic (i.p.) and local (i.n.) depletion of CD11c-expressing myeloid cells on inflammation and fibrosis induced by Bleomycin in mice. Exploring the functions exhibited by various MΦ and DC subsets in vivo has encountered limitations due to difficulties depleting specific DC populations in murine models.43, 57 However, in myeloid cell research emerges with the utilization of inducible cell ablation facilitated by transgenic expression of a high-affinity DTR.43, 57 However, some limitations of this model lead to caution in their interpretation, in which CD11c-DTR mice develop fatal fulminant myocarditis after local or systemic treatment with DTx.58 Nevertheless, we did not detect any adverse reactions induced by systemic DTx administration in mice, such as heightened mortality. Instead, we observed protection against bleomycin-induced inflammation and fibrosis with CD11c systemic depletion, consistently with literature.53, 59, 60 CD11c depletion using clodronate or CD11c-DTR local or systemically protects against lung fibrosis in preventive or therapeutic protocols of depletion.53, 59, 60 Collectively, these results underscore the impact on the fibrogenesis process.
In the lungs, CD11c is predominantly expressed by myeloid cells such as AMΦ and mDCs. These cells play crucial roles in maintaining tissue homeostasis, orchestrating immune responses, and contributing to the pathogenesis of various diseases.19-22, 25 Several studies have aimed to elucidate the role of CD11c-expressing MΦ and mDCs in the context of pulmonary diseases. Mouse lung infection models have highlighted the importance of CD11c+ myeloid subsets in balancing immunity and inflammation. Depletion of CD11c+ MΦ or mDCs has been associated with compromised pathogen control efficiency and secondary tissue damage, underscoring their central role in immune response and pathology. In particular, the depletion of CD11c+ myeloid cells has been shown to exacerbate infection outcomes significantly. This is evidenced by increased bacterial loads and more severe pathology, ultimately leading to accelerated mortality during Staphylococcus aureus bacteremia.61 Indeed, depletion of CD11c+ myeloid cells exacerbates acute lung injury (ALI), which is characterized by multiorgan damage. This suggests that CD11c+ myeloid cells play a crucial role in mitigating the severity of ALI and in maintaining tissue homeostasis during acute inflammatory responses.62 The presence of CD11c+ cells is essential for an effective immune response against influenza virus infection. Depletion of CD11c+ cells exacerbates aspects of infection, hindering the development of virus-specific CD8 + T cells. This impairment correlates with heightened clinical severity and delayed viral clearance.63 In contrast to infectious models, in chronic noninfectious models such as asthma, the depletion of CD11c+ cells abolishes disease features in mice during chronic allergic lung disease. This suggests a distinct role for CD11c+ cells in the pathogenesis of chronic airway allergy compared to infectious models.64 Similarly, in the context of sterile chronic airway inflammation, our findings demonstrated that conditional systemic depletion of CD11c+ myeloid cells attenuated lung injury and inflammation but not conditional local depletion. This attenuation was attributed to the impaired influx of CD11c+ DCs and MΦ after bleomycin instillation in CD11c-DTR-Tg mice. Consequently, there was a reduction in the degree of fibrosis and increased survival rates.
The persistent accumulation of myeloid cells in the lungs contributes to the activation of fibroblasts and abnormal tissue repair, a process sustained by the continued cytokine production.27, 30-36, 53, 54 Tissue-related factors play a pivotal role in shaping the fate and function of immune cells, and the plasticity of leukocytes enables them to adapt to the tissue environment. This phenomenon, known as leukocyte tissue adaptation, involves the trafficking and residence of immune cells in specific tissue microenvironments,65 such as gut and lung mucosa. Indeed, myeloid cells in the lungs play a crucial role in discerning between natural stimulation from harmless microbiota or infections and exposure to noxious agents. They influence both leukocyte and tissue cells by serving as a continuous source of cytokines and chemokines. Additionally, myeloid cells promote the recruitment of specific leukocyte sub-populations, thereby maintaining chronic lymphocytic infiltration in the lung tissue.20, 22, 27, 28, 30 In our hands, using the local depletion of CD11c+ myeloid cells previous to Bleomycin-induced hyper-inflammation with signs of accelerated fibrogenesis, contrasting our systemic depletion finds in vivo. In contrast, Gibbons et al. demonstrated that local CD11c+ depletion using DTx or clodronate administered therapeutically in the fibrogenesis phase ameliorates bleomycin-induced lung fibrosis in mice.59 However, we aimed to deplete CD11c+ cells with just one hit in the initial phase preceding the Bleomycin and evaluate the impact in the course of disease development. CD11c + CD103+ DCs might have an anti-inflammatory as well as anti-fibrotic nature in pulmonary fibrosis development,56 and CD11c+ local depletion may accelerate the disease progression. Thus, the local CD11C+ depletion in the early phase may lead to lung leukocyte maladaptation, favoring the recruitment of circulating myeloid cells and neutrophils, exacerbating the inflammatory response and greater lung injury, and consequently, mortality induced by bleomycin challenge.
Our findings demonstrate that conditional systemic depletion of CD11c+ myeloid cells effectively mitigated lung injury and inflammation following bleomycin instillation in CD11c-DTR-Tg mice. This intervention led to reductions in both leukocyte infiltration and cytokine levels. Specifically, the systemic depletion of CD11c+ myeloid cells resulted in decreased levels of CC chemokines (CCL2, CCL3, CCL4, CCL5) and CXC chemokine (CXCL1). However, local depletion of CD11c+ myeloid cells increases CCL4 and CCL11 in the lungs, which are critical for leukocyte chemoattraction and lung fibrosis.30, 50, 66 CCL4 can interact with multiple receptors (CCR1, CCR3, and CCR5) meanwhile, CCL11 binds to CCR3, and those receptors are distributed in a range of leukocytes, including myeloid cells (DCs and MΦ) and granulocytes (neutrophils and eosinophils).66 Thus, supporting an inflammatory milieu with neutrophils, DCs, and MΦ recruitment induced by local CD11c depletion. Lung-resident macrophages, which include alveolar macrophages and interstitial macrophages, exhibit a high degree of diversity but coordinated chemokine signatures, highlighting the specialized roles of lung-resident macrophages, defined by their coordinated chemokine production and regulating immune cell influx,29 and contribute to lung fibrosis.30 Concurrently, we observed lower counts of T CD4+ lymphocytes and neutrophils in BALF from bleomycin-challenged mice following conditional systemic depletion of CD11c+ myeloid cells but increased neutrophils in local depletion of CD11c+ myeloid cells. Myeloid cells serve as a significant source of chemokines,27, 30, 66 regulating the influx of leukocytes into the lungs. This process, if dysregulated, abnormal chemokine expression can lead to increased tissue damage and contribute to the development of fibrosis.30 Indeed, myeloid cells not only regulate leukocyte influx but also act as producers of cytokines.29 These cytokines play critical roles in activating both leukocytes and resident cells, thereby shaping tissue adaptation in response to tissue injury.
In the context of pulmonary fibrosis, myeloid cells, such as DCs and AMΦ, play a crucial role in producing profibrogenic cytokines, including IL-1β, IL-6, and TGF-β1, while also capable of producing the anti-inflammatory cytokine IL-10.35, 40, 42, 67, 68 IL-1β,69, 70 IL-6,71 and TGF-β172 are potent inducers of fibrosis as they not only affect leukocytes but also activate resident cells such as fibroblasts and myofibroblasts, leading to proliferation, differentiation, and collagen production.73 Our study demonstrated that conditional systemic depletion of CD11c+ myeloid cells effectively impacted cytokine levels following bleomycin instillation in CD11c-DTR-Tg mice. Specifically, there was a decrease in IL-1β, IL-6, and TGF-β1 levels in the lungs. However, local depletion of CD11c+ myeloid cells enhanced IL-1β levels correlated with increased GR1+ influx, early fibrosis, and mortality. Neutrophils are fundamental in the development of pulmonary fibrosis, contributing to the exacerbation and duration of inflammation, and fibrotic lung disease alters neutrophil trafficking and promotes neutrophil elastase and NETs release.48, 74, 75 IL-1β is produced by neutrophils and AMΦ,76, 77 and IL-1β-producing leukocyte traffic to the lungs in response to Bleomycin supports the concept that pro-inflammatory cytokine production by lung neutrophils and MΦ may contribute to the development of lung inflammation and fibrosis. Infact, bleomycin-induced chronic lung inflammation requires the inflammasome pathway and IL-1R1/MyD88 signaling, and IL-1β represents a critical effector of pathology and therapeutic target of inflammation and fibrosis.69 Interestingly, neither systemic nor local depletion of CD11c+ myeloid cells altered the levels of the pro-resolving and anti-inflammatory cytokine IL-10,68, 78 following bleomycin instillation in CD11c-DTR-Tg mice. In addition to the observed decrease in profibrogenic cytokine levels, systemic depletion of CD11c+ myeloid cells was associated with reduced expression of fibrogenesis markers in the lungs.79 Specifically, mRNA transcriptions of Collagen1α1 and α-Smooth Muscle Actin were downregulated in systemic depletion of CD11c+ myeloid cells, indicating decreased fibroblast activity,79 but not observed by local depletion of CD11c+ myeloid cells. This suggests that the systemic depletion of CD11c+ myeloid cells may mitigate fibrogenesis by suppressing fibroblast activation and subsequent collagen production in the lung tissue. Taken together, these findings support that conditional systemic depletion of CD11c+ myeloid cells effectively reduced the levels of profibrogenic cytokines, potentially contributing to the attenuation of fibrogenesis progression and mortality.
Myeloid cells such as MΦ and mDCs are naturally present in the airways and lung parenchyma during homeostasis. However, under certain conditions, they have the potential to initiate and perpetuate chronic inflammation, contributing to the formation of fibroblastic foci and tissue fibrosis.20, 22, 27, 28 Tissue repair is an essential protective response following injury, but it becomes problematic when injuries are repetitive or prolonged, potentially leading to tissue fibrosis. In this context, the myofibroblast-macrophage cell circuit plays a crucial role in either tissue healing or fibrosis. In “hot fibrosis,” there is an excessive accumulation of extracellular matrix and scar formation, characterized by a significant presence of macrophages supporting fibroblast activity.80 Our findings demonstrate that conditional systemic depletion of CD11c+ myeloid cells effectively mitigated the pathological state of excessive scarring following bleomycin instillation in CD11c-DTR-Tg mice, primarily due to impaired activity of CD11c+ DCs and MΦ. Moreover, this depletion disrupted the myofibroblast-macrophage cell circuit, potentially switching off the chronic activation of myofibroblasts by myeloid cells. This intervention may lead to a transition from “hot fibrosis,” characterized by macrophage-supported fibroblast activity, to “cold fibrosis,” which lacks macrophages and exhibits impaired wound healing.80
Despite clinical evidence demonstrating a correlation between increased numbers of MΦ and mDCs and the degree of pulmonary fibrosis in IPF,27, 37-40 the specific role of CD11c+ myeloid populations in the context of pulmonary fibrosis remains unclear,41, 42 and was the objective of this investigation. Here, we showed that systemic depletion of CD11c+ cells, not local depletion, confers protection against inflammation and fibrosis induced by Bleomycin, underscoring the significant role of myeloid cells expressing F4/80-MHCII+CD11c+ DCs and F4/80 + MHCII+CD11c+ MΦ contributing with the initial phase of disease and progression. The single-dose model of Bleomycin has been instrumental in understanding fibrotic lung remodeling but fails to recapitulate several features of IPF and is self-resolutive after 2 months.81, 82 However, repetitive intratracheal bleomycin results in marked lung fibrosis with prominent alveolar epithelial cell hyperplasia, resembling the IPF features.81 Thus, future experiments on the depletion of CD11c+ myeloid cells locally and systemically in the model of fibrosis induced by bleomycin repetitions, or even in the case of non-resolving fibrosis such as silicosis, will be essential to dissect more precisely the role of these cells during the fibrogenesis process, and we are interested in exploring this further. In conclusion, the systemic depletion of CD11c+ cells during bleomycin administration confers protection against inflammation and fibrosis, underscoring the significance of myeloid cells expressing F4/80-MHCII+CD11c+ DCs and F4/80 + MHCII+CD11c+ MΦ orchestrating the inflammatory milieu within the lungs, potentially as a source of cytokines sustaining pulmonary chronic inflammation leading to progressive fibrosis and mortality.
AUTHOR CONTRIBUTIONS
Gabriel Augusto Oliveira Lopes: Formal analysis; Investigation; Writing—original draft; Writing—review and editing. Braulio Henrique Freire Lima: Formal analysis; Investigation; Writing—original draft; Writing—review and editing. Camila Simões Freitas: Formal analysis; Investigation; Writing—original draft; Writing—review and editing. Andiara Cardoso Peixoto: Formal analysis; Investigation; Writing—review and editing. Frederico Marianetti Soriani: Formal analysis; Investigation; Writing—review and editing. Geovanni Dantas Cassali: Formal analysis; Investigation; Writing—review and editing. Bernhard Ryffel: Writing—original draft; Writing—review and editing. Mauro Martins Teixeira: Writing—review and editing. Fabiana Simão Machado: Conceptualization; Writing—original draft; Writing—review and editing. Remo Castro Russo: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing—original draft; Writing—review and editing.
ACKNOWLEDGMENTS
RCR is grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) under Bolsa PQ (Grant Agreement No. 309810/2017-5, No. 312839/2020-0, No. 313839/2023-9), and edital Universal 01/2016 – Faixa A/CNPq Grant Agreement No. 422260/2016-9; the Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG) Edital Universal under Grant Agreement No. APQ-02571-21 and Rede Mineira de Pesquisa Translacional em Imunobiológicos e Biofármacos no Câncer – REMITRIBIC under Grant Agreement No. RED-00031-21; the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Programa CAPES-PrInt/UFMG edital 05/2022, and Le Studium Loire Valley – Institute for Advanced Studies (LE STUDIUM Visiting Researcher/ARD CVL Biopharmaceuticals 2023). This work was supported by Centre National de Recherche Scientific (CNRS) and the European Regional Development Fund (FEDER ExposomeInflam). Illustrations were created with BioRender. com.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.