RNA m6A methylation regulates virus–host interaction and EBNA2 expression during Epstein–Barr virus infection
Xiang Zheng and Jia Wang contributed equally to this study.
Abstract
Introduction
N6-methyladenosine (m6A) is the most prevalent modification that occurs in messenger RNA (mRNA), affecting mRNA splicing, translation, and stability. This modification is reversible, and its related biological functions are mediated by “writers,” “erasers,” and “readers.” The field of viral epitranscriptomics and the role of m6A modification in virus–host interaction have attracted much attention recently. When Epstein–Barr virus (EBV) infects a human B lymphocyte, it goes through three phases: the pre-latent phase, latent phase, and lytic phase. Little is known about the viral and cellular m6A epitranscriptomes in EBV infection, especially in the pre-latent phase during de novo infection.
Methods
Methylated RNA immunoprecipitation sequencing (MeRIP-seq) and MeRIP-RT-qPCR were used to determine the m6A-modified transcripts during de novo EBV infection. RIP assay was used to confirm the binding of EBNA2 and m6A readers. Quantitative reverse-transcription polymerase chain reaction (RT-qPCR) and Western blot analysis were performed to test the effect of m6A on the host and viral gene expression.
Results
Here, we provided mechanistic insights by examining the viral and cellular m6A epitranscriptomes during de novo EBV infection, which is in the pre-latent phase. EBV EBNA2 and BHRF1 were highly m6A-modified upon EBV infection. Knockdown of METTL3 (a “writer”) decreased EBNA2 expression levels. The emergent m6A modifications induced by EBV infection preferentially distributed in 3ʹ untranslated regions of cellular transcripts, while the lost m6A modifications induced by EBV infection preferentially distributed in coding sequence regions of mRNAs. EBV infection could influence the host cellular m6A epitranscriptome.
Conclusions
These results reveal the critical role of m6A modification in the process of de novo EBV infection.
1 INTRODUCTION
N6-methyladenosine (m6A) is the most prevalent modification that occurs in mammal messenger RNAs (mRNAs). Its related biological functions are mediated by “writers,” “erasers,” and “readers.” METTL3 and METTL14 serve as the most important catalytic subunits of the RNA methyltransferase complex, which catalytic writing of methyl groups into adenosines.1 The function of m6A is mediated partly by “reader” proteins, mainly identified in members of the YTH domain-containing protein families.2 Besides mammals, m6A modification also occurs in the viral transcript, which is catalyzed by the methyltransferase system of the host cell, and thus to some extent affects the viral life cycle.3 m6A modification promoted replication in Simian virus 404 and influenza A virus5 but attenuated the replication in hepatitis C virus6 and Zika virus.7 The effect of m6A modification on Kaposi's sarcoma-associated herpesvirus (KSHV) replication was cell lines dependent.8 Tan et al.9 found that the cellular m6A/m epitranscriptome was reprogrammed during KSHV latent/lytic infection.
When Epstein–Barr virus (EBV) infects a human B lymphocyte, it goes through three phases: the pre-latent phase, latent phase, and lytic phase (with appropriate stimuli).10, 11 Upon infection, EBV attaches to the receptors on the host cell surface, internalizes, and delivers the viral genome to the cell nucleus, followed by the circularization of the viral DNA and gradual acquisition of an epigenetic signature, including viral DNA nucleosome positioning and repressive chromatin mark introducing. The pre-latent phase lasts for about 10 days, and later comes the latent phase. Upon appropriate stimuli such as 12-O-tetradecanoylphorbol-13-acetate and butyric acid, the lytic phase is induced, leading to virus synthesis.
In the current study, we were particularly concerned about whether m6A modification occurs in viral and cellular transcripts in de novo EBV-infected cells, which is still in the pre-latency phase. We found that EBV infection could influence the m6A methylation pattern of cellular transcripts. EBNA2 and BHRF1 contain m6A modifications in de novo EBV infection. We also found that the m6A machinery could modulate EBNA2 expression.
2 METHODS AND MATERIALS
2.1 Cell culture
BJAB (EBV-negative B lymphoma cell line) and Raji (EBV-positive B lymphoma cell line) cells were maintained in RPMI-1640 (Hyclone) supplemented with 10% fetal bovine serum (FBS). Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood of two health donors by Ficoll centrifugation. Primary B cells were isolated from PBMCs with CD19 microbeads (Miltenyl Biotec). All cell lines were obtained from the ATCC. The cell lines tested negative for mycoplasma contamination. All cell lines were authenticated by short tandem repeat profiling before use. Collections and use of blood samples were approved by the ethical review committees of the appropriate institutions.
2.2 EBV virus preparation and infection
Infectious EBV was produced from the B95.8 cell culture supernatants. Briefly, B95.8 cells were prepared in RPMI-1640 medium containing 10% FBS. Cells were centrifuged and started at a new culture at 2 × 105/ml density in RPMI-1640 medium containing 2% FBS. Cells were cultured at 37°C with 5% CO2 for 2 weeks without changing the medium. Cells were centrifuged at 300g to sediment cells and debris, passed through 0.45-μm Millipore filters, then further centrifuged at 50,000g at 4°C, and resuspended in fresh FBS-free RPMI-1640. To determine the multiplicity of infection (MOI) of EBV, a DNA Quantitative Fluorescence Diagnostic Kit (Sansure Biotech) was used according to the manufacturer's protocol and the published literature.12 In this study, we used 50 MOI EBVs to infect BJAB cells unless otherwise indicated.
2.3 Lentivirus transduction
Short hairpin (shRNA) lentiviruses were obtained from GenePharma. Lentiviral vector plasmids LV3 (H1/GFP&Puro) were used in this study to construct stable cell lines. Lentiviruses were transduced into cells according to the manufacturer's instructions. The shRNA sequences are listed in Table S1.
2.4 Western blot analysis
Protein extracts were resolved by sodium dodecyl sulfate–polyacrylamide gel, transferred to polyvinylidene fluoride membranes, and probed with antibodies against METTL3 (Cat# 15073-1-AP; Proteintech), TLR9 (Cat# 13674; Cell Signaling Technology), FAS (#4233; Cell Signaling Technology), EBNA2 (Cat # MABE8; Millipore), GAPDH (Cat# D110016; Sangon Biotech). Horseradish peroxidase (HRP)-conjugated AffiniPure goat anti-rat IgG (Cat# SA00001-15; Proteintech), anti-rabbit IgG HRP-linked antibody (Cat# 7074; Cell Signaling Technology) was used as the secondary antibody. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal loading control.
2.5 Quantitative reverse-transcription polymerase chain reaction (RT-qPCR) analysis
Total RNAs were extracted using Trizol (Invitrogen). For mRNA reverse transcription, 2 μg of RNA was used to synthesize complementary DNA (cDNA) using a Maxima H Minus First Strand cDNA synthesis kit with dsDNase (Thermo Fisher Scientific) according to the manufacturer's protocol. The levels of gene transcripts were detected by quantitative polymerase chain reaction (qPCR) using specific primers and an SYBR premix Ex TaqII Kit (Takara). The expression levels of mRNA were quantified by measuring cycle threshold (Ct) values and normalized to ACTIN. The data were further normalized to the negative control unless otherwise indicated. The primers used for RT-qPCR are listed in Table S2.
2.6 Methylated RNA immunoprecipitation sequencing (MeRIP-seq) and data analysis
BJAB cells were infected with 50 MOI EBVs for 24 h. The uninfected cells were used as a negative control. Total RNAs were extracted from BJAB cells. Intact mRNA was isolated from total RNAs using the Arraystar Seq-Star™ poly(A) mRNA Isolation Kit according to the manufacturer's protocol, then the isolated mRNA was chemically fragmented to 100-nucleoside-long fragments by incubation in the fragmentation buffer (10 mM Zn2+ and 10 mM Tris-HCl, pH 7.0). The m6A methylated mRNAs were immunoprecipitated with anti-m6A antibody (#202003; Synaptic Systems) and one-tenth of the fragmented mRNAs was kept as input. The major procedures contained immunoprecipitation, washing, and elution. The eluted mRNA fragments were concentrated for RNA-seq library construction. RNA-seq libraries for the m6A antibody-enriched mRNAs and input mRNAs were prepared using the KAPA Stranded mRNA-seq Kit (Illumina). The prepared libraries were diluted to 8 pM and clusters were generated on the Illumina cBot using a HiSeq 3000/4000 PE Cluster Kit. Sequencing was performed using the Illumina HiSeq 4000. Raw data were trimmed using Trimmomatic (v0.32) and aligned to Ensembl reference genome and EBV reference genome (NC_007605.1) using HISAT2 software (v2.1.0). Peak calling and differentially methylated peaks analyzing were performed using the exomePeak (v2.13.2) as described.13 For differential methylated peaks analysis, a fold change of minimally 1.5 and a maximum p value of .05 were considered significantly differential between the two groups to explore as many differentially methylated peaks as possible. The peaks were annotated according to the annotation information in Ensembl database. The EBV reference genome and annotation were downloaded from https://www.ncbi.nlm.nih.gov/nuccore/NC_007605.1. The peaks were visualized in Integrated Genome Viewer (IGV). The raw sequencing data obtained from the MeRIP-seq reported in this study have been deposited in NCBI GEO under accession No. GSE133936.
2.7 MeRIP-RT-qPCR
This procedure was adapted from the published reports.14-16 Briefly, intact poly (A) + RNAs from cells were isolated by using a Magnetic mRNA Isolation Kit (New England Biolabs) according to the manufacturer's protocol, but not randomly fragmented to facilitate reverse transcription with oligo(dT) and PCR amplification. mRNAs were incubated with 5 μg of anti-m6A antibody (#202003; Synaptic Systems) for 2 h at 4°C in IP buffer (150 mM NaCl, 10 mM Tris-HCl, 0.1% NP-40, pH 7.4) containing RNase inhibitor (Promega). IP with normal rabbit IgG were performed in parallel. The mixture was then incubated with protein A/G magnetic beads (Selleck) at 4°C for 2 h. After washing for three times with IP buffer, the bound RNAs were eluted from the beads in IP buffer containing 6.7 mM m6A sodium salt (Santa Cruz Biotechnology) and ethanol precipitated. cDNA synthesis and qPCR analysis were performed as described above.
2.8 RNA immunoprecipitation (RIP) assays
Briefly, Raji cells were transfected with Flag-YTHDF1 or -YTHDF2 or -YTHDF3 plasmids for 48 h. Then, the cells were harvested and washed twice in ice-cold phosphate-buffered saline (PBS). The cell pellet was resuspended in gentle lysis buffer containing RNase inhibitor (Promega) and protease inhibitor (Selleck), and incubated on ice for 20 min. After centrifugation at 12,000 rpm at 4°C for 15 min, the supernatants were incubated with anti-Flag antibody (#F1804; Sigma-Aldrich) or control anti-IgG antibody (#sc-2025; Santa Cruz Biotechnology) conjugated to protein A/G magnetic beads (Selleck) and rotated at 4°C for 4 h. The supernatant was removed, and the beads were washed extensively by wash buffer, followed by adding 0.5 ml of Trizol. cDNA synthesis and qPCR analysis were performed as described above.
2.9 RNase-mediated RNA–protein interaction determining assay
This procedure was adapted from the published reports.17-19 Briefly, BJAB cells were infected with EBV for 24 h. One-tenth of the sample was saved for RNA purification and used as the “before RNase treatment.” Cells were treated with 1% formaldehyde at room temperature with shaking for 10 min. A final concentration of 125 mM glycine was then added dropwise for additional 5-min incubation. Then, cells were washed twice with ice-cold PBS and collected by centrifugation. The pellets were resuspended by sonication in 900 μl gentle lysis buffer containing DNase I (2 U/ml) and a protease inhibitor cocktail, followed by 100 ng/ml RNase incubation at 37°C for 30 min. In total, 100 μl of the sample was saved as the “after RNase treatment.” Then RNAs were extracted. cDNA synthesis was performed using reverse transcriptase and random primers. qPCR analysis was done as described above. The primers are listed in Table S2.
2.10 RNA decay assay
This procedure was adapted from the published reports.20 BJAB cells were plated on 12-well plates with 5 × 105 cells per well. Actinomycin-D (Meilunbio) was added to a final concentration of 5 μg/ml, and cells were collected before or 4 h after adding Actinomycin-D. Then, the cells were processed as described in “RT-qPCR,” except that the data were normalized to before Actinomycin-D treatment.
2.11 Statistical analysis
Statistical significance was calculated using Prism (GraphPad Software) and SPSS17. All experiments were performed in triplicate. Data represent the mean ± SD. Statistical differences were assessed with the unpaired Student t test, and ps < .05 were considered to reflect statistical significance. A one-way analysis of variance test was performed for comparing three or more groups within the same experiment. In all results, NS denotes “not significant,” *p < .05, **p < .01, and ***p < .001 compared with the indicated control group.
3 RESULTS
3.1 EBV infection influences m6A methylation pattern of cellular transcripts
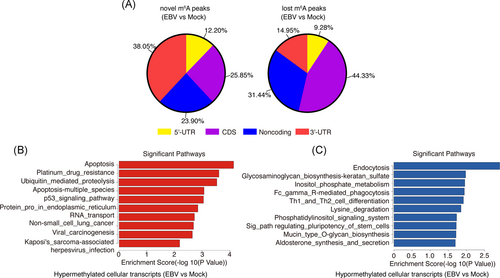
To determine whether EBV infection could modulate the cellular epitranscriptome, we examined the human BJAB cell line at 24 h post-EBV infection by methylated RIP sequencing (MeRIP-seq) experiments. To confirm the successful establishment of EBV infection, we determined the mRNA expression levels of EBNA2 and EBNA-LP, two of the first expressed viral genes during de novo EBV infection.21 EBNA2 and EBNA-LP were detected in EBV-infected samples but not in uninfected samples (shown in Figure S1), which confirmed the success of the virus infection. We analyzed the distribution pattern of cellular m6A peaks and found that the newly “gained” (i.e., novel) m6A peaks were preferentially deposited in 3ʹ untranslated region (UTR) of the cellular transcripts, whereas the “lost” m6A peaks were preferentially distributed in coding sequence (CDS) regions of the cellular transcripts (shown in Figure 1A). There were 918 significantly upregulated m6A peaks (from 416 genes), and 2586 significantly downregulated m6A peaks (from 1046 genes) induced by EBV infection (i.e., EBV infection vs. mock; Figure S2; NCBI GEO data #GSE133936). We then performed a Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of these genes. The apoptosis pathway and endocytosis pathway ranked first in the enriched pathways of hypermethylated genes and hypomethylated genes, respectively (EBV infection vs. mock, shown in Figure 1B,C). These findings suggest that de novo EBV infection could to some extent alter the host cellular N6-methyladenosine epitranscriptome.
3.2 EBV infection modulates FAS and TLR9 m6A methylation levels and expression
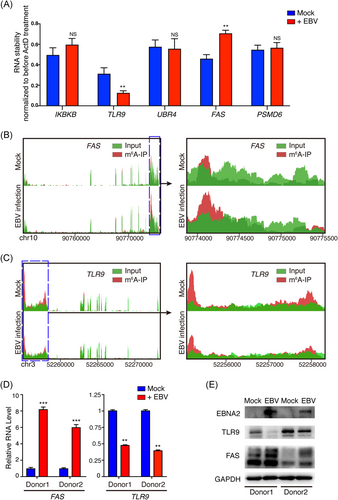
MeRIP-seq showed that EBV infection altered the host N6-methyladenosine epitranscriptome. To determine the effect of EBV infection on host cellular genes, we chose some hypermethylated or hypomethylated genes for further study, which mainly were involved in apoptosis and the immune system. Of these genes, m6A peaks of UBR4, FAS, and PSMD6 exhibited increased abundance upon EBV infection, whereas IKBKB and TLR9 were decreased (shown in Table S3). As m6A modification plays an important role in modulating mRNA stability, we tried to determine the role of EBV infection in regulating these mRNAs' stability. EBV infection enhanced the mRNA stability of FAS, whereas it repressed the mRNA stability of TLR9 (Figure 2A). FAS is related to apoptosis pathway,22 and TLR9 is related to virus infection,23 both of them are involved in pathogenesis of EBV,24, 25 we chose FAS and TLR9 for further study. MeRIP-seq showed that m6A abundance was increased in FAS mRNA transcripts (shown in Figure 2B) whereas decreased in TLR9 mRNA transcripts (shown in Figure 2C) upon EBV infection, suggesting that EBV could modulate their m6A modification levels. We also extracted primary B cells from two healthy donors, and the cells were infected with 10 MOI EBVs. We found that the mRNA and protein levels of FAS were upregulated, whereas TLR9 were downregulated after EBV infection (shown in Figure 2D,E).
3.3 EBV EBNA2 and BHRF1 are m6A-modified during de novo EBV infection
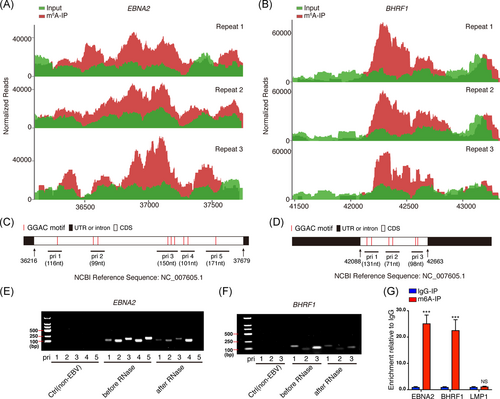
To determine whether viral transcripts are m6A-modified during the course of de novo EBV infection, the MeRIP-seq reads in the EBV-infected BJAB cells were aligned to EBV reference genome (https://www.ncbi.nlm.nih.gov/nuccore/NC_007605.1). Two EBV transcripts, EBNA2 and BHRF1, were found containing m6A modifications, which are located in their CDS regions. The results of the three repeats were highly consistent (shown in Figure 3A,B). The EBNA2 CDS region contained nine “GGAC” motifs (shown in Figure 3C), while BHRF1 CDS contained six “GGAC” motifs (shown in Figure 3D), the canonical m6A motif. According to the distribution of these “GGAC” motifs in EBNA2 and BHRF1 CDS, we designed several pairs of primers (shown in Figure 3C,D and Table S2), and performed formaldehyde cross-linking followed by RNase-mediated experiments to evaluate whether these mRNA regions are protected by binding proteins (e.g., m6A “readers”). The amplified regions from primers 1–4 but not 5 in EBNA2 and 1–3 in BHRF1 were protected in varying degrees against the degradation of RNase (shown in Figure 3E,F). Considering the m6A motifs' location, the results indicated these regions (i.e., primers 1–4 for EBNA2, and primers 1–3 for BHRF1) were probably bound by m6A binding proteins. To further validate m6A modification in the two viral mRNAs, we performed MeRIP-RT-qPCR to detect enriched RNAs after anti-m6A immunoprecipitation. EBNA2 and BHRF1, but not LMP1 RNAs, were specifically enriched by the anti-m6A antibody (immunoglobulin G [IgG], was served as a negative control for anti-m6A antibody). LMP1 is an EBV-encoded oncogene. No m6A-modified LMP1 transcript was detected from BJAB cells at 24-h post-EBV infection (shown in Figure 3G). These results suggested that viral genes EBNA2 and BHRF1 are m6A-modified during de novo EBV infection.
3.4 METTL3 and YTHDFs regulate the expression of EBNA2
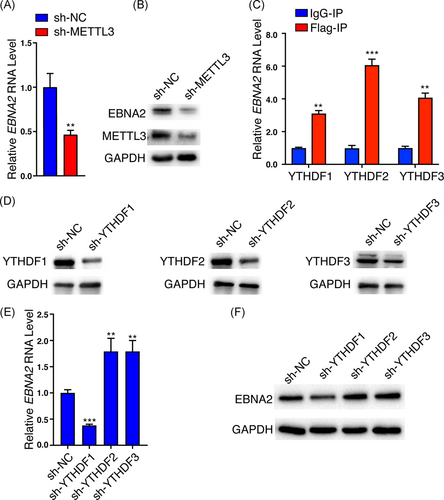
Given the significant deposition of m6A peaks in EBNA2 mRNA, we asked whether m6A modification can regulate EBNA2 expression. The reversible addition and removal of m6A from mRNAs are thought to be dynamically regulated. The m6A “writer” METTL3 is a major component of the methyltransferase complex required for m6A modification, and we determined the effect of METTL3 on EBNA2 expression. We constructed the Raji cell (an EBV-positive B lymphoma cell line) with METTL3 knockdown by means of lentivirus carrying shRNA and found that knockdown of METTL3 inhibited endogenous EBNA2 mRNA and protein expression (shown in Figure 4A,B). Meanwhile, knockdown of FTO, an m6A “eraser,” significantly increased the EBNA2 protein levels (Figure S3). The fate of m6A-modified mRNA is mainly mediated by m6A “readers,” which recognize the site of m6A modification. Several proteins with the YTH domain were shown to bind m6A-modified RNAs. We thus evaluated the effects of “readers” YTHDF1, YTHDF2, and YTHDF3 on EBNA2 expression. We determined the binding ability of the three YTHDFs to EBNA2. As the RIP grade antibodies for YTHDF1, YTHDF2, and YTHDF3 are not commercially available, pulling down the endogenous YTHDFs is not feasible. We transfected the Raji cells with Flag-tagged YTHDF1, YTHDF2, or YTHDF3 plasmids for 48 h. RIP assay showed that EBNA2 RNAs were specifically enriched by the anti-Flag antibody compared with IgG, suggesting all the three readers can bind to EBNA2 mRNA (shown in Figure 4C). We knocked down YTHDF1, YTHDF2, or YTHDF3 in Raji cells with lentivirus carrying shRNAs, respectively (shown in Figure 4D). The mRNA and protein levels of endogenous EBNA2 were decreased when YTHDF1 was knocked down but increased when YTHDF2 or YTHDF3 was knocked down in Raji cells (shown in Figure 4E,F). Meanwhile, exogenous expression of YTHDF1 increased the EBNA2 protein levels (Figure S4). These results implied that METTL3 and YTHDFs exert a different influence on EBNA2 expression, METTL3 and YTHDF1 increased EBNA2 expression, whereas YTHDF2 and YTHDF3 decreased its expression.
4 DISCUSSION
Recently, a study from Robertson's group that focused on the role of m6A modification in EBV latent and lytic phases was published.26 They found knockdown of METTL14 led to decreased expression of latent EBV transcripts and demonstrated that EBNA3C activated METTL14 transcription and increased its stability, contributing to EBV-mediated tumorigenesis. Their findings provided important resources for understanding m6A modification in EBV latent and lytic phases. However, little is known about the role of m6A modification during EBV de novo infection. In the current study, we employed a system of infecting B cells with EBV and investigated the virus–host interaction in the de novo infection phase from the perspective of m6A modification.
In de novo EBV-infected BJAB cells, the most apparent changes in host N6-methyladenosine epitranscriptome were the emerged m6A modifications preferentially distributed in the 3ʹ-UTR region of cellular transcripts, while the lost m6A modifications preferentially distributed in CDS (shown in Figure 1A). mRNA m6A modification located in the 3ʹ-UTR and CDS regions may result in different outcomes recognized by different m6A “readers.” For example, METTL3 and YTHDF1 preferentially recognize m6A residues on CPCP1 3ʹ-UTR and promote CDCP1 translation.27 Wu et al.28 found that JAK2 and SOSC3 have m6A modification at 3ʹ-UTR and demonstrated that YTHDF1 could bind m6A-modified mRNA of JAK2 to promote translation and protein expression, while YTHDF2 could target m6A-modified mRNA of SOCS3 to reduce the protein abundance. Mao et al.29 demonstrated that m6A in mRNA CDS regions promoted translation and removing CDS m6A results in a further decrease of translation. Li et al.30 found that methylated SOX2 transcripts, specifically the CDS regions, could be recognized by IGF2BP2 to prevent SOX2 mRNA degradation. These results implied that EBV infection might modulate host mRNA stability, translation or protein expression through altering the distribution pattern of m6A modification. Among the enriched pathways of m6A hypomethylated cellular genes, the endocytosis pathway ranked first (shown in Figure 1C). As receptor-mediated endocytosis is the main way for EBV entry into susceptible cells, this result may imply that m6A modification may be involved in the process of EBV entry into the host cells. Some molecule metabolic pathways were enriched in m6A hypomethylated cellular genes, including inositol phosphate metabolism and lysine degradation, suggesting m6A modification may be involved in the process of EBV infection-associated metabolism dysfunction. In the m6A hypermethylated cellular genes upon EBV infection, some important signaling pathways including apoptosis, ubiquitin-mediated proteolysis, and viral carcinogenesis were enriched (shown in Figure 1B). Considering the effect of m6A modification on gene expression, these results suggested that EBV infection may modulate some gene expression by altering their m6A modification. However, the specific mechanism needs to be further studied. One of these genes is FAS, an important gene of the apoptosis pathway.22 The stability and expression of FAS mRNA were enhanced by EBV infection (shown in Figure 2). It was reported that EBV LMP1 and LMP2A can induce FAS expression,31, 32 and de novo EBV infection also can increase FAS expression in T cells.33 Our observation suggested that besides LMP1 and LMP2A, EBV infection may upregulate the FAS expression at least partly by increasing its m6A modification levels (shown in Figure 2). Toll-like receptor (TLR) signaling is responsible for the primary recognition of infectious agents leading to the initiation of the innate and adaptive immune response. Among the TLRs, TLR9 senses unmethylated CpG double-stranded DNA (dsDNA) motifs.34, 35 It is conceivable that EBV is sensed by TLR9 in B cells during their de novo infection, because of the unmethylated dsDNA. However, EBV can suppress TLR9 expression to evade innate immune recognition and benefit the long-term survival of the virus.23, 36 Consistent with these studies, we found that EBV de novo infection suppressed the expression of TLR9. Given that the m6A modification and mRNA stability of TLR9 were significantly reduced after EBV infection (shown in Figure 2), m6A modification might enhance TLR9 mRNA stability. EBV may suppress TLR9 signaling by decreasing the m6A modification of the TLR9 transcript, and this might be a strategy employed by the virus to evade immune surveillance. Manipulating the m6A modification level of TLR9 may be a new therapeutic target.37, 38
In Robertson lab's study,26 they comprehensively defined much m6A modification of EBV latent and lytic transcripts. In the current study, we found that two EBV transcripts, EBNA2 and BHRF1, were m6A-modified when BJAB cells were infected with EBV for 24 h (shown in Figure 3A,B). These differences may be due to the different expression patterns of EBV genes in different life cycles. The background of our study is in the pre-latent phase during EBV de novo infection. It is reasonable that EBNA2 was m6A-modified during EBV de novo infection because as early as 6-h post-infection, EBNA2 transcripts were detectable in de novo-infected B lymphocytes.21 The lytic early gene BHRF1 was also m6A-modified in de novo-infected cells. The expression of BHRF1 might result from the epigenetically “naked” EBV genome that was not introduced to the repressive chromatin marks in the de novo-infected cells. In fact, Altmann et al.39 demonstrated that BHRF1 expression reached a high level at 24-h post-EBV infection and then declined rapidly. Our study also suggested the expression of BHRF1 at 24 h in EBV de novo infection. We chose EBNA2 for further study because of its essential role in B-cell proliferation, immortalization, activation of super-enhancers,40 and recently we reported EBNA2 can form liquid-like condensates through phase separation at super-enhancer sites of MYC and Runx3.41 Our results suggested EBNA2 was precisely regulated by the host m6A machinery. Figure 3E suggested that EBNA2 RNA was protected by m6A binding proteins; Figure 4A,B revealed that METTL3 increased the expression of EBNA2 in Raji cells. Previous reports suggested that YTHDF1, YTHDF2, and YTHDF3 could function cooperatively to promote efficient translation or degradation of specific m6A-containing mRNAs.42-44 In a further study, we also found that EBNA2 expression was regulated by these m6A “readers.” YTHDF1, YTHDF2, and YTHDF3 could bind m6A-modified EBNA2 mRNA. YTHDF1 increased EBNA2 expression, whereas YTHDF2 and YTHDF3 decreased its expression (shown in Figure 4E,F), which implied the importance of the reader's selection and binding. EBNA2 is one of the earliest expressed genes in EBV-infected B lymphocytes, the viral Cp and LMP1, LMP2A, and LMP2B promoters are strongly activated by EBNA2. Our study confirmed that the m6A machinery could regulate the expression of EBNA2, which may affect the function of EBNA2 as a transcriptional activator, and further affect the expression pattern of virus genes and the life cycle of the virus.
In this study, we report for the first time that during the pre-latency phase of EBV infection, EBNA2 is m6A-modified, and its expression can be modulated by m6A machinery. EBV infection can change the m6A abundances on multiple cellular genes such as FAS and TLR9, which are involved in apoptosis and immune response. We speculate that EBV and host may use m6A machinery to interfere with the virus–host interaction, and achieve long-term latency (such as modulating TLR9, FAS, and EBNA2 m6A levels). This study now provides a deeper understanding of EBV–B-cell interaction at the m6A modification level and suggests a critical role for m6A modification in EBV infection.
ACKNOWLEDGMENTS
We thank Drs. Ersheng Kuang, Ya Cao, and Pengfei Cao for providing plasmids and reagents. This study was supported by the National Natural Science Foundation of China (Nos. 81874170, 82073261, 82060042, 32000665); the China 111 Project (No. 111-2-12); and the Natural Science Foundation of Hunan Province, China (No. 2020JJ5480).
AUTHOR CONTRIBUTIONS
Xiang Zheng, Jia Wang, Qun Yan, and Jian Ma designed and conceived the experiments. Xiang Zheng, Jia Wang, Xiaoyue Zhang, Qiu Peng, Lingyu Wei, Zhengshuo Li, Can Liu, and Yangge Wu performed the experiments and analyzed the data. Jianhong Lu, Qun Yan, and Jian Ma analyzed data. Xiang Zheng, Jia Wang, Qun Yan, and Jian Ma wrote the paper.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
Collections and use of two health donors' blood samples were approved by the ethical review committees of Central South University and were in accordance with the Declaration of Helsinki. Written informed consent was obtained from the two donors.
Open Research
DATA AVAILABILITY STATEMENT
The raw sequencing data obtained from the MeRIP-seq reported in this study have been deposited in NCBI GEO under accession No. GSE133936.