Ethyl rhamnolipids as a renewable source to produce biopolyurethanes
Abstract
Responding to increasing environmental concerns, lipids are attractive feedstock to produce bio-based polyurethanes. In this study, ethyl rhamnolipids were used to synthesize polyurethanes by reacting with 1,6-hexane diisocyanate (HDI). Rhamnolipids were produced by microbial fermentation, providing a novel source for biopolyurethanes. FTIR and gel content analyses confirmed that the hydroxyl groups on rhamnose residues were reacted with isocyanates. DSC analysis revealed an adjustable glass transition temperature (Tg) from 19 to 50°C. Ethyl rhamnolipids are liquid at 50°C, presumably because rhamnose is connected with fatty acid at a mid-chain position and the unique structure decreases the tendency to crystallize. The liquid polyols were found to have good mixing and reaction properties. The study may inspire future synthesis of novel lipid-based polyols.
Practical applications: Use of vegetable oil-based polyols for polyurethane production had been extensively investigated. However, synthesis of these polyols mostly involved introduction of petroleum-based chemicals such as methanol and 1,2-propanediol. Rhamnolipids are natural glycolipids. Using ethyl rhamnolipids to prepare polyurethanes provides an alternative, more renewable route. In addition, it inspires synthesis of vegetable oil polyols by introducing natural sugars.
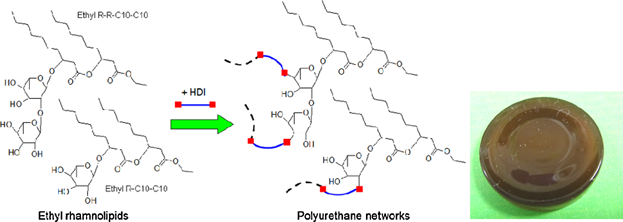
Bio-based polyurethanes were prepared from ethyl rhamnolipids, by reaction with 1,6-hexane diisocyanate (HDI). Rhamnolipids, obtained from fermentation, are an alternative renewable source of reactants for polyurethane production. The unique structure of rhamnolipids may also inspire future synthesis of novel lipid-based polyols.
Abbreviations
-
- HDI
-
- hexane diisocyanate
-
- MDI
-
- methylene diphenyl isocyanate
-
- TSB
-
- tryptic soy broth
1 Introduction
Because of depleting petroleum resources and increased environmental awareness, lipid-based polyurethane materials have attracted great attention 1-6. Polyurethanes are a class of important polymers with usage totaling 14 million tons in 2011, representing roughly 5% of the global polymer consumption 7. Polyurethanes are produced by reacting polyols with multi-isocyanate compounds. Lipids can be used to produce both multi-isocyanates and polyols, but lipid-based polyols have been more commonly used for polyurethane production 1-6.
Vegetable oils are the most extensively investigated lipids for polyurethane production. Vegetable oils are mainly composed of glycerol and fatty acids. Many reaction routes have been used for introducing hydroxyl groups into vegetable oils to make polyols 1, 2, 8. The common approach is by converting the double bonds in unsaturated fatty acids to epoxy groups, which are then ring-opened with agents such as methanol, 1,2-propanediol, 1-amino-2-propanol, and 2-mercaptoethanol 1-6, 9-12. However, these agents used to introduce hydroxyl groups are mostly derived from petroleum. Using these agents lowers the bio-based content of the final products. Also, only one or two hydroxyl groups can be introduced through one double bond, and side reactions tend to reduce the hydroxyl values to be lower than 300 mg KOH/g. These polyols are therefore less suitable for making rigid polyurethanes, which are widely used in heat insulation materials and account for about one third of the total polyurethane consumption 13-15.
Sugars are natural multi-hydroxyl compounds. However, they cannot be directly used as polyurethane polyols because their melting points are too high to mix homogeneously with multi-isocyanates. Sugars have been used to control the functionalities of petroleum-based polyols. For example, sucrose can initiate the polymerization of propylene oxide or ethylene oxide to develop polyols with eight hydroxyl groups, while sorbitol can form polyols with six hydroxyl groups 16. In view of the high functionalities of sugars, it is possible to introduce sugars to lipids for developing the lipid-based polyols. However, to our best knowledge, this synthetic route has seldom been used so far.
This study demonstrates that ethyl rhamnolipids, easily derivable from the fermentation-produced rhamnolipids, can be used to synthesize polyurethanes with sugar-containing backbones and lipid side chains. A series of polyurethanes were fabricated by reacting ethyl rhamnolipids with different amounts of 1,6-hexane diisocyanate (HDI). These polyurethanes were characterized by Fourier transform infrared spectroscopy (FTIR), gel content and differential scanning calorimetry (DSC) analyses. Obtained from a fermentation process, rhamnolipids provide a renewable source to produce bio-based polyurethanes. The work can motivate synthesis of novel sugar-lipid polyols, especially for producing rigid polyurethanes.
2 Materials and methods
2.1 Materials
Methanol, ethanol (200 proof), ethyl acetate, 1,6-hexane diisocyanate, sulfuric acid, and sodium methoxide methanol solution (25% w/w) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Water was Milli-Q water (Millipore, Molsheim, France; 18.2 MΩ · cm at 25°C). All other reagents were analytical grade.
2.2 Polyurethane characterization
Polyurethane samples synthesized in this study were subjected to hydroxyl value, gel content, FTIR, and DSC analyses according to previous methods 17. The method for gel content analysis was modified slightly. The sample was extracted with ethyl acetate instead of tetrahydrofuran and the extracted sample was dried for 24 h at room temperature and then for 8 h at 80°C.
2.3 Production and purification of rhamnolipids
Rhamnolipids used in this study were produced by Pseudomonas aeruginosa fermentation following procedures that have been established in this laboratory 18. A P. aeruginosa strain E03-40 isolated from soil samples taken near a biodiesel plant was used for the fermentation 19. Inoculum was prepared in a 30 g/L tryptic soy broth (TSB) medium. The inoculum was cultivated at 34°C for 8 h using a shaker operating at 250 rpm (Thermo Scientific MAXQ 5000). Ten milliliter inoculum was added to 90 mL TSB medium and similarly cultivated for 18 h to prepare the preculture. For rhamnolipid production, the preculture (100 mL) was added to a 2 L fermentor (BIOFLO 110, New Brunswick Scientific) containing 1 L medium and the inoculated broth was agitated at 600 rpm with two 6-blade turbines. The pH was maintained at 6.2 ± 0.1 by automatic addition of 1 N H2SO4 or NaOH. Dissolved oxygen concentration was kept at 10% (± 3%) by controlled bubbling of pure oxygen. Temperature was controlled at 32.0 ± 0.1°C. The fermentation was stopped after 5 days when the concentration of rhamnolipids was about 20 g/L.
The fermentation medium contained 100 g/L glycerol, 6 g/L KH2PO4, 5.73 g/L NH4Cl, 5 g/L yeast extract, 5 g/L peptone, 1.5 g/L NaCl, 0.9 g/L MgSO4 · 7H2O, 0.1 g/L FeSO4 · 7H2O, 0.03 g/L CaCl2 · 2H2O, 0.03 g/L MnCl2 · 4H2O, and 2 ml/L of a trace element solution. The trace element solution had 0.75 g/L MnSO4 · H2O, 0.75 g/L ZnSO4 · 7H2O, 0.15 g/L H3BO3, 0.08 g/L FeCl3 · 6H2O, 0.08 g/L CoCl2 · 6H2O, 0.075 g/L CuSO4 · 5H2O and 0.05 g/L Na2MoO4.
Rhamnolipids were collected from fermentation broth after 5-day fermentation. Six hundred milliliters of ethanol were added to 300 mL fermentation broth to precipitate extracellular biopolymers and cells. Supernatant was dried with a stream of filtered air. The sticky gel-like raw product obtained was re-dissolved in deionized water. The solution was adjusted with HCl to pH 2 to precipitate rhamnolipids. The supernatant was removed, and the precipitated rhamnolipids were again dissolved in deionized water. The solution pH was re-adjusted with HCl as before. This process was repeated several times until the supernatant was no longer turbid to naked eyes. The final rhamnolipid precipitate was collected for ethylation.
2.4 Ethylation of rhamnolipids
Three grams of rhamnolipids were put in a 500 mL glass reactor containing 150 mL ethanol. The reaction mixture was controlled at 0°C and magnetically stirred at 600 rpm. Then 6 mL H2SO4 was added slowly. After 24 h reaction, another 150 mL ethanol was added and the reaction mixture was neutralized with sodium methoxide. The mixture was centrifuged to remove salt; ethanol was vaporized by filtered air. The sample was re-dissolved in ethyl acetate and filtered through a 0.2 µm nylon membrane. After ethyl acetate was removed by vaporization under filter air, ethylated rhamnolipids were collected as the brownish product. Upon analysis the 1H NMR (300 MHz, chloroform-d, δ ppm) spectrum of the ethylated rhamnolipids obtained was as follows: 0.80–0.97 (m, CH3), 1.20–1.36 (m, CH2), 2.28–2.58 (m, CH2COO), 3.25–3.90 (m, rhamnose residue), 4.00–4.50 (m, CH3CH2OOC), 4.75–5.10 (m, CH[O]O, on rhamnose ring) and 5.15–5.30 (m, CH[OOC]-).
2.5 Preparation of ethyl rhamnolipid-based polyurethanes
One gram of ethyl rhamnolipids was put in a 25 mL glass vial. A preset amount of 1,6-hexane diisocyanate (0.85, 0.97 or 1.23 g) was then added. Reactants were mixed homogenously and centrifuged for 5 min at 4000 g to remove bubbles. Then, the vial was put in an oven for two 24 h curing steps: first at 80°C and then at 100°C. With those preset amounts of 1,6-hexane diisocyanate, the molar ratios of isocyanate groups to hydroxyl groups were set at 0.70:1, 0.80:1, and 1.05:1, respectively, and the corresponding polyurethane samples were coded as PU070, PU080, and PU105.
3 Results and discussion
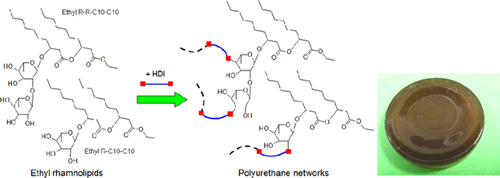
Molecular structures of ethyl rhamnolipids and the corresponding polyurethanes after cross-linking are shown in Fig. 1. Rhamnolipids have two main forms: mono-rhamnolipids and di-rhamnolipids. The former contain one rhamnose residue; the latter contain two connected rhamnose residues. The rhamnose moiety is linked to a chain of two β-hydroxyalka(e)noic acid residues, each most commonly with a chain length of ten carbons. Accordingly, these common mono- and di- rhamnolipids are hereafter referred to as R-C10-C10 and R-R-C10-C10, respectively 19, 20. Rhamnolipids in the present study was a mixture of about 40% w/w mono- and 60% w/w di-rhamnolipids (analyzed with silica chromatography, data not shown).
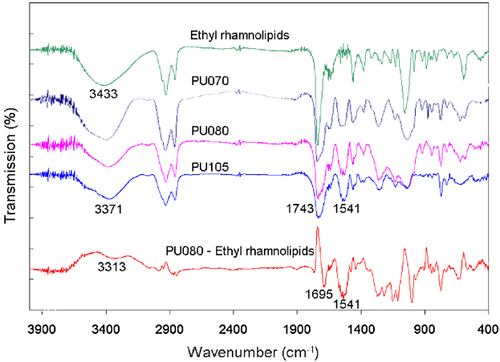
The FTIR spectra of synthesized polyurethanes are shown in Fig. 2. The signal at 1541 cm−1 corresponds to the N-H deformation in a urethane bond, indicating the polyurethane formation. The signal for CO vibration in the urethane bond, at 1695 cm−1, is a shoulder overlapping with the signal for ester group at 1745 cm−1, but it can be clearly seen after, e.g., the spectrum of PU080 is corrected for the ethyl rhamnolipid structure. The signal at 3371 cm−1, corresponding to stretching vibration of N-H, further confirms the formation of polyurethane.
According to the functionalities of ethyl rhamnolipids, the polyurethanes are assumed to have crosslinked rhamnose residues as the backbone and fatty acid resides as the side chains. This structure is different from the common soybean oil-based polyurethane networks where fatty acid residues are mostly involved in the backbone chains 21. Future study is warranted to more systematically examine how this basic structural difference may be used to impart unique/useful properties to the polyurethanes formed. Nonetheless, the polyurethanes synthesized in this work were rigid and shiny as shown in Fig. 1. The dark color observed was presumably caused by the presence of some pigment impurities that were not completely removed by the simple rhamnolipid collection procedures used in this study. P. aeruginosa is known to produce various pigments depending on the culture medium and conditions 22, 23.
The gel content of PU070, PU080, and PU105 was 35%, 92%, and 99%, respectively (Table 1). PU105 had the highest gel content, corresponding to the highest molar ratio of isocyanate-to-hydroxyl group. The low gel content of PU070 indicated that the molar ratio of isocyanate-to-hydroxyl group needed to be higher than 0.7:1 in order to get a more completely cross-linked polymeric network.
| Sample code | PU070 | PU080 | PU105 |
|---|---|---|---|
| Gel content (%) | 35 | 92 | 99 |
| Tg based on DSC (°C) | 19 | 40 | 50 |
The DSC results are shown in Table 1. PU070 showed a low Tg at 19°C, while PU080 and PU105 showed Tgs at 40 and 50°C, respectively. The Tg of polyurethanes depends on several aspects, such as polyol functionality, ratio of polyol to multi-isocyanate, and the structures of both polyol and multi-isocyanate used 24, 25. Tg usually increases with increasing isocyanate amount, as observed with the ethyl rhamnolipid-based polyurethanes synthesized in this study. There are not many commercial multi-isocyanates available for selection. Among them methylene diphenyl isocyanate (MDI) usually gives, at similar conditions, higher Tg's than others, including the 1,6-hexane diisocyanate used here 7. It is important to note that the soybean oil-based polyurethanes mostly exhibited Tg's of lower than 40°C even when MDI was used as the multi-isocyanate reactant 13-15. The relatively high Tg of 50°C obtained in this work for the ethyl rhamnolipid-based polyurethane PU105 is most likely due to the presence of rhamnose residues, which provide a high hydroxyl value (397 mg KOH/g) to afford stronger attraction between molecules.
In ethyl rhamnolipids, the rhamnose was attached to fatty acid at a rather unique mid-chain position. This structure is assumed to decrease the crystallization tendency of fatty acid and enable formation of the liquid ethyl rhamnolipid polyols observed in this study. The liquid polyols were found to be easy to mix and react with the multi-isocyanate reactant. Other synthetic sugar-lipids, such as sucrose fatty acid esters, have different sugar residues, fatty acid chains and connection patterns between sugar and fatty acid residues, and are rarely tested to synthesize polyurethanes 26. Future study is warranted to investigate the effects of this presumably better processing and reaction property of ethyl rhamnolipids against other synthetic sugar-lipid polyols.
4 Conclusion
Ethyl rhamnolipids are an excellent renewable source for producing polyurethanes. Instead of using petroleum-based reagents such as methanol, 1,2-propanediol, and 1-amino-2-propanol to introduce hydroxyl groups to fatty acid chains of vegetable oils, this work with ethyl rhamnolipids shows that rhamnolipid-based polyols are attractive alternatives for synthesis of lipid polyol-based polymers.
This work was supported by grants from the U.S. Department of Agriculture under the Biomass Research and Development Initiative (award # 2009-10001-05112) and from the US Department of Transportation, Office of the Secretary, Grant No. DTOS59-07-G-00050.
The authors have declared no conflict of interest.




