Renewable linear alpha olefins by selective ethenolysis of decarboxylated unsaturated fatty acids
Abstract
A two-step concept for the production of linear alpha olefins from biomass is reported. As a starting material an internally unsaturated C17 alkene was used, which was obtained by the decarboxylation of oleic acid. Here, we report on the ethenolysis of this bio-based product, using commercially available metathesis catalysts. The desired alpha olefin products, 1-nonene and 1-decene, were obtained in excellent yield (96%) and selectivity (96%).
Practical applications: The two-step conversion described in this contribution, starting from unsaturated fatty acids, provides a method for the production of industrially important linear alpha olefins. These valuable products are widely used as starting materials for the production of surfactants and polymers such as linear low density polyethylene (LLDPE).
Abbreviations:
AcOH, acetic acid; DCM, dichloromethane; DMC, dimethyl carbonate; HG1, first generation Hoveyda–Grubbs catalyst; HG2, second generation Hoveyda–Grubbs catalyst; LAO, linear alpha olefins; MeOH, methanol
Introduction
Linear alpha olefins (LAO) are versatile and valuable petrochemical products which find a wide range of applications. While long chain LAO (C10–C14) play an important role in the production of surfactants and lubricants, short chain LAO (C4–C8) are used in substantial amounts as co-monomers in the production of high density polyethylene (HDPE) and linear low density polyethylene (LLDPE). Two main commercial processes to obtain LAO are oligomerisation of ethylene 1-7 and the Fischer–Tropsch process starting from syngas 8-12. However, these present some drawbacks such as lower yields in long chain LAO (C10–C14) compared to short chain LAO (C4–C8), coupled with a decrease in selectivity due to the formation of branched olefins and isomerisation reactions 5. The production of LAO with an odd number of carbons appears to be impossible by ethylene oligomerisation.
On the other hand it is nowadays widely recognised that the depletion of fossil feed stocks and its consequences (increasing oil prices, insecurity of supply, etc.), coupled with an increasing energy demand on one side, and universal concerns for environmental issues on the other, are forcing industries to look for alternative resources for transportation fuels and bulk chemicals 13-17. Hence, chemurgy has been the focus of an increasing number of research institutes and companies in the last decades. Carbohydrates, lignocellulose, glycerol, fatty acids and amino acids are nowadays feed stocks for a large number of fuels and chemicals, from laboratory scale experiments up to large-scale industrial products such as bioethanol or epichlorohydrin 18 for instance.
In recent years, it was shown that LAO can be produced from renewable resources via ethenolysis of unsaturated vegetable oils and FAME 19-26. As an example of the latter, Burdett et al. performed a fundamental mechanistic study of methyl oleate ethenolysis 19. A major drawback of this approach is that besides 1-decene, also methyl-9-decenoate is produced as a co-product in a 1:1 ratio. In the case of glycerol trioleate ethenolysis, the triglyceride of ω-decenoic acid is the co-product 27-32. Although these co-products are interesting bi- or tri-functional molecules with potentially valuable applications, the overall yield of alpha olefins is low: e.g. only 43% w/w of glycerol trioleate is converted into the desired high-value LAO.
As an alternative we suggest a two-step process for the conversion of an unsaturated fatty acid into two alpha olefin molecules (Fig. 1). The first step involves the decarboxylation of an unsaturated fatty acid (oleic acid), yielding an internally unsaturated C17 hydrocarbon. Subsequent ethenolysis of this substrate yields 1-nonene and 1-decene.
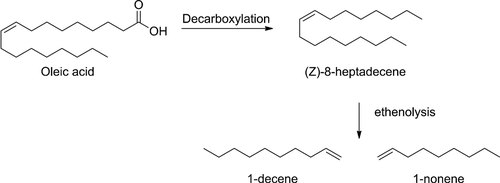
Two-step process for the production of two alpha olefins from oleic acid.
Decarboxylation of unsaturated fatty acid derivatives has been described via various routes, including biotechnological 33-35. Recently, we reported a semi-catalytic route to obtain these types of materials 36: decarboxylation of oleic acid derivatives leads to the selective formation of (Z)-8-heptadecene, which is not readily available from petrochemical resources. Given the increasing interest in catalytic decarboxylation of vegetable oils (for the production of renewable diesel) 37-42, it is expected that improved and greener synthetic methods will become available in the near future. Since in the first step only ‘green’ CO2 is removed from the molecule, and the ethylene used for the cross-metathesis reaction can be produced from bio-ethanol 43-46, the overall route is both highly atom-efficient and fully renewable. Here, we describe the second step; the selective ethenolysis of bio-derived (Z)-8-heptadecene to 1-nonene and 1-decene in high yield.
Materials and methods
General
(Z)-8-heptadecene was prepared by oxidative decarboxylation of oleic acid 36. The purity of the product is >90% and the main impurities are other alkanes derived from the other fatty acids present in the oleic acid starting material. Although purified by chromatography (silica gel), trace amounts of oxygenates might be present. The ruthenium-based catalysts were purchased from Sigma–Aldrich. All solvents were purchased from Acros, Aldrich and Merck in >98% purity, and were dried with the adequate drying agent and distilled under nitrogen before use. Ethene 3.5 (purity ≥99.95%) was supplied by Linde Gas Benelux and used as received. Silica gel 60 (0.040–0.063 mm) from Alfa Aesar was used for the removal of the catalysts after reaction.
Analytical equipment
GC-MS analyses were performed on an Interscience TraceGC Ultra GC with AS3000 II autosampler (He carrier gas, flow 1 mL/min, split flow 20 mL/min; Restek GC column Rxi-5 ms 30 m × 0.25 mm × 0.25 µm; GC program hold 3 min at 50°C, ramp 7.5 K/min, final temperature 330°C) connected to an Interscience TraceDSQ II XL quadrupole mass selective detector (EI, mass range 35–500 Da, 150 ms sample speed). Peak areas were integrated based on TIC. MS data is reported with the relative intensity of ions compared to the base peak (100%).
NMR spectra were recorded on a Bruker Avance III spectrometer operating at 400.17 MHz (1H) in CDCl3 (99.9 at% D, Aldrich).
Standard procedure for the ethenolysis of (Z)-8-heptadecene
All reactions were carried out in a 6 × 75 mL Parr Series 5000 Multiple Reactor System in glass liners. In a typical procedure, catalysts and magnetic stirring bars were introduced into the glass liners and placed in the reactors under a nitrogen atmosphere directly after weighing. Under a positive pressure of nitrogen, 10 mL of solvent and 300 µL of (Z)-8-heptadecene (1 mmol, 238 mg) were introduced into 75 mL pressure reactors. The reactors were closed and flushed three times with nitrogen followed by flushing three times with ethylene. After applying the desired ethylene pressure, stirring (500 rpm) was started and the reactors were heated to the desired temperature for 1–20 h. Next, the reaction mixtures were allowed to cool down to RT. After opening the reactors, the solutions were immediately filtered over a short plug of silica to remove the catalysts. Samples were analysed by GC-MS or, after removal of the solvent by using a rotary evaporator (40°C), by 1H NMR spectroscopy.
Analysis and identification of reaction products
For quantification by 1H NMR the following regions were compared after integration: LAOs: 1H NMR (400.17 MHz, CDCl3): δ(ppm) = 5.90–5.75 (1H, m, CH2CHCH2R), 4.85–5.05 (2H, m, CH2CHCH2R) and for the Internal Olefins (E/Z): 1H NMR (400.17 MHz, CDCl3): δ(ppm) = 5.45–5.30 (2H, m, RCHCHR).
The following retention times and MS patterns were found for the typical compounds in the reaction mixtures:
1-nonene: GC-MS: RT = 6.47 min. MS (GC-MS, 70 eV): m/z (%) = 126 (6) [M+], 98 (12%), 97 (33), 84 (26), 83 (32), 70 (55), 69 (69), 56 (100), 55 (87), 43 (70), 41 (68).
1-decene: GC-MS: RT = 8.67 min. MS (GC-MS, 70 eV): m/z (%) = 140 (5) [M+], 112 (11), 111 (19), 97 (35), 84 (30), 83 (49), 70 (96), 69 (81), 56 (100), 55 (97), 43 (57), 41 (81).
(Z)-8-hexadecene: GC-MS: RT = 19.63 min. MS (GC-MS, 70 eV): m/z (%) = 224 (10) [M+], 139 (4), 125 (13), 111 (38), 97 (73), 83 (89), 70 (66), 69 (100), 56 (60), 55 (95), 43 (57), 41 (49).
(E)-8-hexadecene: GC-MS: RT = 19.71 min. MS (GC-MS, 70 eV): m/z (%) = 224 (14) [M+], 125 (14), 111 (43), 97 (88), 83 (95), 70 (66), 69 (100), 56 (61), 55 (96), 43 (56), 41 (49).
(Z)-8-heptadecene (starting material): GC-MS: RT = 21.17 min. MS (GC-MS, 70 eV): m/z (%) = 238 (11) [M+], 210 (1), 168 (1), 139 (5), 125 (17), 111 (47), 97 (88), 83 (100), 69 (98), 57 (62), 55 (94), 43 (58), 41 (62).
(E)-8-heptadecene: GC-MS: RT = 21.26 min. MS (GC-MS, 70 eV): m/z (%) = 238 (13) [M+], 210 (1), 168 (2), 139 (6), 153 (3), 125 (17), 111 (46), 97 (88), 83 (100), 69 (98), 57 (62), 55 (94), 43 (58), 41 (62).
(Z)-9-octadecene: GC-MS: RT = 22.62 min. MS (GC-MS, 70 eV): m/z (%) = 252 (11) [M+], 139 (7), 125 (22), 111 (49), 97 (93), 83 (91), 69 (98), 57 (77), 55 (100), 43 (61), 41 (60).
(E)-9-octadecene: GC-MS: RT = 22.67 min. MS (GC-MS, 70 eV): m/z (%) = 252 (10) [M+], 139 (7), 125 (22), 111 (49), 97 (93), 83 (100), 69 (97), 57 (76), 55 (92), 43 (54), 41 (57).
Ethylene solubility tests
Each reactor, equipped with a glass liner and a stirring bar, was charged with 10 mL of one of the six following solvents: dichloromethane (DCM), methanol (MeOH), toluene, n-hexane, acetic acid (AcOH) and dimethyl carbonate (DMC). Without stirring, the reactors were purged with ethylene gas and an internal pressure of 5.38 bar was applied simultaneously to all the reactors. At RT (24°C) the stirring speed was set at 1000 rpm. After 10 min, the ethylene pressure was stable and a pressure reading was taken (Table 1). The temperature was then set to 40°C and the pressure was recorded after stabilisation (Table 1).
| DCM | MeOH | Toluene | n-Hexane | AcOH | DMC | |
|---|---|---|---|---|---|---|
| Pethylene (bar) at 24°C | 3.91 | 4.33 | 4.01 | 4.08 | 4.45 | 4.20 |
| Pethylene (bar) at 40°C | 4.65 | 4.87 | 4.36 | 4.64 | 4.86 | 4.60 |
Results and discussion
Initial screening of reaction conditions
Since the aim of our study was to investigate the proof of principle of our approach, we limited ourselves to the use of commercially available homogeneous catalysts, i.e. ruthenium-based (Hoveyda–)Grubbs catalysts (Fig. 2).
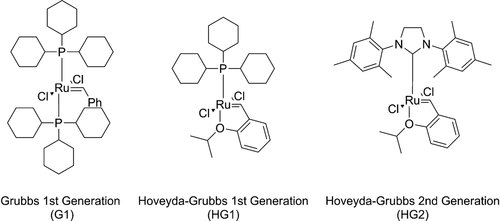
(Hoveyda–)Grubbs catalysts used in this study.
Initially, reactions were performed in DCM. At 1 bar ethylene pressure, all catalysts showed a high activity with conversions reaching 94% and higher. However, the second generation Hoveyda–Grubbs catalyst (HG2) displayed a slightly higher LAO yield than the first generation Hoveyda–Grubbs catalyst (HG1), and appeared more selective towards alpha olefins than the first generation Grubbs Catalyst (G1) (Table 2, entries 1–3). This result is surprising by comparison with literature, where phosphine-based ruthenium catalysts such as G1 and HG1 usually display better selectivity than their N-heterocyclic carbene-based analogues 20, 21. Besides the desired 1-nonene and 1-decene, the thermodynamic self-metathesis products (E,Z)-8-hexadecene, (E)-8-heptadecene and (E,Z)-9-octadecene were the only by-products observed (Fig. 3). Throughout all experiments, high conversion of the (Z)-8-heptadecene starting material was observed, independent of the selectivity towards the alpha olefins. The ratio of cis/trans for the internal olefins was ∼1:7 in all cases. For the self-metathesis products the statistically expected ratio of 1:2:1 was found for C16:C17:C18. The same observations are described in the literature for Ru-based catalysts, e.g. in the recent report by Bruneau and coworkers 47. Other types of catalysts, such as W- or Mo-based metathesis catalysts, lead to comparable results 48.
| Entry | Cat. | Loading (mol%) | Ethylene pressure (bar) | Conv. (%)b) | Select. to LAO (%)c) | Yield of LAO (%)d) |
|---|---|---|---|---|---|---|
| 1 | G1 | 4.9 | 1 | 94 | 44 | 41 |
| 2 | HG1 | 4.9 | 1 | 96 | 61 | 59 |
| 3 | HG2 | 3.4 | 1 | 97 | 63 | 61 |
| 4 | HG2 | 3.7 | 5 | 99 | 81 | 80 |
| 5 | HG2 | 3.4 | 10 | 98 | 74 | 73 |
- a) Reaction conditions: (Z)-8-heptadecene (238 mg, 1 mmol), 10 mL of dry DCM, 40°C, 20 h.
- b) Conversion of (Z)-8-heptadecene determined by GC-MS.
- c) Selectivity towards LAO = (total amount of 1-nonene and 1-decene)/(total amount of compounds − starting material) × 100%.
- d) Yield of LAO = (conv. × selectivity) in %.
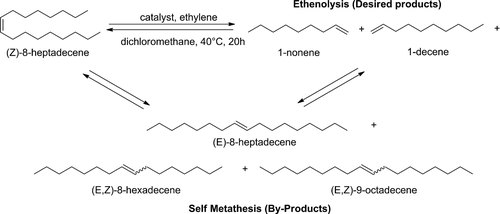
Products observed after the ethenolysis of (Z)-8-heptadecene.
For the HG2 catalyst, ethylene pressure was varied, since it was expected that this would influence the selectivity of the reaction 19, 21 (Table 2, entries 3–5). The best results were obtained at 5 bar ethylene pressure. At lower pressure the lack of available ethylene in the solvent favours the reaction of the catalyst with the internal olefins. At a pressure of 10 bar the selectivity towards the kinetic products slightly decreases.
Solvent screening
In order to find a more environmentally benign alternative to DCM, we screened six different non-chlorinated solvents, commonly used in this type of reactions 49, 50 (Table 3).
| Entry | Cat. loading (mol%) | Solvent | Conv. (%)b) | Select. to LAO (%)c) | Yield of LAO (%)d) |
|---|---|---|---|---|---|
| 1 | 3.7 | Dichloromethane | 99 | 81 | 80 |
| 2 | 3.5 | Methanol | 94 | 0 | 0 |
| 3 | 3.5 | Toluene | 99 | 72 | 71 |
| 4 | 3.5 | Hexane | 99 | 92 | 91 |
| 5 | 3.5 | Acetic acid | 95 | 34 | 32 |
| 6 | 3.4 | Ethyl acetate | 95 | 11 | 10 |
| 7 | 3.5 | Dimethyl carbonate | 98 | 21 | 21 |
- a) Reaction conditions: (Z)-8-heptadecene (238 mg, 1 mmol), HG2 catalyst, 10 mL of dry solvent, 40°C, 20 h, 5 bar of ethylene.
- b) Conversion of (Z)-8-heptadecene determined by GC-MS.
- c) Selectivity towards LAO = (total amount of 1-nonene and 1-decene)/(total amount of compounds − starting material) × 100%.
- d) Yield of LAO = (conv. × selectivity) in %.
All solvents showed high conversions. For the more polar solvents (MeOH, AcOH and ethyl acetate) the LAO selectivity dropped significantly compared to the results obtained with DCM. By applying the same ethylene pressure to various neat solvents, we observed that the solubility of ethylene in polar solvents was lower than in DCM, hexane or toluene (see Table 1). This could explain why the reaction equilibrium is shifted towards the internal olefins (self-metathesis products). The reaction performed in MeOH gave a two-phase system, and while no LAO formation was observed, 94% conversion of (Z)-8-heptadecene to the self-metathesis products was found. This is close to the maximum theoretical conversion when besides the self-metathesis products no other products are formed 51. These results (based on GC analysis) were verified by 1H NMR analysis of the isolated product after removal of the solvent (mass balance 88%), showing only the signals for the internal olefins (Fig. 4). In the case of DMC (Table 3, entry 7), a green solvent reported for metathesis reactions 52, 53, the formation of other by-products was observed: C8, C11, C14 and C15 olefins were found as well as internal C9 and C10 olefins. The formation of these products is most likely caused by deactivation of the catalyst, accompanied by the formation of ruthenium hydride species 54. These species can act as a double bond isomerisation catalyst and the remaining active HG2 catalyst can produce the short chain olefins via ethenolysis. The self-metathesis of these by-products in turn leads to the formation of new by-products. In recent years, the isomerisation of internal olefins has become a well-studied subject, e.g. the work of Fokou and Meier (and references herein) provides a nice overview of the phenomena playing a role in this process 55.
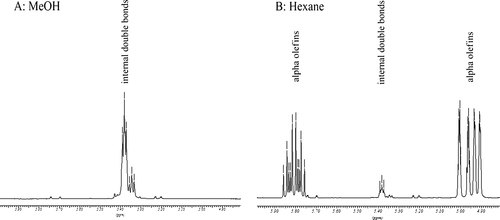
In contrast, non-polar solvents gave excellent results. While toluene was comparable to DCM, superior conversion and selectivity were obtained with n-hexane (Table 3, entries 1,3,4). Although the solubility of the catalyst in n-hexane is rather poor, both substrate and ethylene solubility are excellent. No by-products were observed and 1-nonene and 1-decene were the main products in the crude mixture. For entry 4 in Table 3, the GC-MS data were verified by 1H NMR analysis of the isolated products after removal of the solvent (mass balance 94%). According to 1H NMR analysis the product mixture consisted of 7% internal olefins and 93% alpha olefins (Fig. 4).
Optimisation of reaction conditions in n-hexane
As a next step the reaction in n-hexane was investigated in more detail by varying the reaction time, the catalyst loading and the ethylene pressure. At a catalyst loading of 3.4 mol% of HG2, the selectivity of the reaction towards the alpha olefins reached 82% within 1 h (Table 4, entry 1). Longer reaction time of 3 and 20 h results in an increase of the selectivity to 85 and 92%, respectively (Table 4, entry 2; Table 3, entry 4). Reducing the catalyst loading to 1 mol% does not affect the conversion but results in a decrease of selectivity towards LAO after 1 h. However, by increasing the reaction time to 20 h, the selectivity towards LAO is superior to that obtained with 3.4 mol% catalyst after 1 h. Although the catalyst does not completely dissolve in the hexane, an increased amount of catalyst gives a higher LAO yield. With 0.1 mol% catalyst, the reaction proceeds even slower given the yields after 1 h (Table 4, entry 5).
| Entry | Cat. loading (mol%) | T (°C) | Ethylene pressure (bar) | Time (h) | Conv. (%)b) | Select. to LAO (%)c) | Yield of LAO (%)d) |
|---|---|---|---|---|---|---|---|
| 1 | 3.4 | 40 | 5 | 1 | 99 | 82 | 81 |
| 2 | 3.4 | 40 | 5 | 3 | 99 | 85 | 84 |
| 3 | 1 | 40 | 5 | 1 | 98 | 67 | 66 |
| 4 | 1 | 40 | 5 | 20 | 99 | 88 | 87 |
| 5 | 0.1 | 40 | 5 | 1 | 96 | 31 | 30 |
| 6 | 3.6 | r.t. | 5 | 1 | 93 | 42 | 39 |
| 7 | 3.4 | r.t. | 5 | 3 | 95 | 34 | 32 |
| 8 | 3.4 | r.t. | 10 | 20 | 98 | 80 | 78 |
| 9 | 3.4 | r.t. | 5 | 20 | 100 | 96 | 96 |
| 10 | 3.4 | r.t. | 1 | 20 | 99 | 80 | 79 |
| 11 | 3.4 | 40 | 1 | 20 | 98 | 70 | 69 |
| 12e) | 0.1 | 40 | 5 | 20 | 94 | 27 | 25 |
| 13e),f) | 0.1 | r.t. | 5 | 20 | 93 | 29 | 27 |
- a) Reaction conditions: (Z)-8-heptadecene (238 mg, 1 mmol, except for entry 11), HG2 catalyst, 10 mL of n-hexane, 5 bar of ethylene.
- b) Conversion of (Z)-8-heptadecene determined by GC-MS.
- c) Selectivity towards LAO = (total amount of 1-nonene and 1-decene)/(total amount of compounds − starting material) × 100%.
- d) Yield of LAO = (conv. × selectivity) in %.
- e) Performed in neat condition (7 mL, 5.56 g, 23.4 mmol of (Z)-8-heptadecene).
- f) Ethylene pressure was maintained at 5 bar throughout the experiment.
When the reactions are performed at RT, it takes longer to attain equilibrium (compare Table 4, entries 1–3, 6, 9) although the equilibrium is shifted even more towards the LAO after 20 h. In the latter case a dramatic increase in selectivity towards 96% LAO is obtained at complete conversion of the starting material. Reducing the ethylene pressure from 5 to 1 bar results in a significant drop in LAO selectivity, both at RT (Table 4, entry 10) and 40°C (entry 11), the effect being even more pronounced in the latter case. Obviously, the ethylene solubility improves at lower temperatures, thus shifting the equilibrium towards the alpha olefins. However, increasing the ethylene pressure from 5 to 10 bar does not improve the selectivity towards LAO (Table 4, entry 8).
Reactions under neat conditions
Although the process thus far is highly atom efficient and can be run in non-chlorinated solvents, running it under solventless conditions would be even more desirable from both an economic as well as an ecological point of view 56. In principle, the products 1-nonene and 1-decene can be distilled from the self-metathesis products. The higher boiling point residue could serve as new starting material for a consecutive ethenolysis step.
So in order to verify the performance of the reaction under more industrially relevant conditions, solvent was omitted and the catalyst loading reduced to 0.1 mol% (Table 4, entry 12). After 20 h at 40°C, 27% of alpha olefins are obtained at 94% conversion of the starting material. This result is comparable to the yield obtained in hexane using the same catalyst loading (Table 4, entry 5), although in this case the reaction time was shorter. The amount of ethylene present in the reactor (around 10 mmol) was not sufficient for complete reaction with our substrate (23.4 mmol). However, even though the amount of ethylene is not sufficient for complete conversion, a significant conversion to LAO of 25% is achieved (maximum theoretical conversion to LAO: 10 mmol/23.4 mmol = 43%). Another reaction was performed under similar initial conditions except that the ethylene pressure was re-adjusted to 5 bar after 2 h of reaction (Table 4, entry 13). A second re-pressurisation was performed after another 2 h but then the pressure remained stable until the end of the experiment. The results show a slight improvement compared to the previous neat reaction (Table 4, entry 12) with a conversion of 93% and a selectivity for LAO of 27%. Despite the presence of an excess of ethylene, ethenolysis did not continue, probably due to catalyst deactivation over time.
Hence, we have demonstrated that reduction of the catalyst loading is feasible and that solvent is not required. Selectivity towards LAO in this case is modest. However, after isolation of the desired products (LAO) by distillation from the crude reaction mixture, the residue containing only self-metathesis products can be re-used as feed stock for further ethenolysis reactions as long as no double bond migration occurs. Further optimisation of the metathesis catalyst is currently in progress.
Conclusions
We have shown that decarboxylated unsaturated fatty acids are excellent starting materials for the production of alpha olefins via ethenolysis reactions. Virtually complete conversions and excellent selectivities up to 96% towards 1-nonene and 1-decene were achieved using a homogeneous HG2. Hence, we have proven that it is possible to convert unsaturated fatty acids, in a simple two-step process, into two high-value LAO in high yield and high selectivity.
Acknowledgements
The authors would like to thank Dr. Guus Frissen and Linda Gootjes BSc. for the NMR analyses, and Wouter Teunissen for his support with GC-MS analyses. This research has been performed within the framework of the CatchBio program. The authors gratefully acknowledge the support of the Smart Mix Program of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science.
The authors have declared no conflict of interest.




