Characteristic distributions of fatty acids in different lipids from Jack beans (Canavalia gladiata DC.)
Abstract
Extracted lipids obtained from Jack beans (white and red) were fractionated by TLC into nine subfractions. The major components were TAGs (TAG: 43.8–45.7 wt%) and phospholipids (PL: 46.7–47.0 wt%), while other components were also present in minor proportions (0.3–2.7 wt%). The principal fatty acids (FA) are generally palmitic (18.8–28.8%), stearic (0.7–6.8%), oleic (42.0–51.8%), linoleic (16.2–22.8%), and α-linolenic (3.0–8.2%) acids, the distribution of which differs according to these lipid classes. There were no significant differences (p>0.05) in the positional distribution of FA in the TAG; unsaturated FA (97.5%) were predominantly concentrated in the sn-2 position while saturated FA (33.3%) primarily occupied the sn-1 position or sn-3 position. However, significant differences (p<0.05) in FA distribution existed when the individual PL were compared between the white and red beans. Based on the FA composition of these lipids, it seems that the two cultivars of Jack beans are very similar to each other with a few exceptions. The results could be useful to both producers and consumers for our daily diet to improve value of the Japanese diet.
Practical applications: The lipid composition suggests that these beans could be a good source of nutraceuticals with providing heath benefits. The white and red beans may be well incorporated into our daily Japanese diets to improve nutritional value. The data obtained in this study provide valuable information for manufacturing functional drinks such as Jack bean tea in Japan.
Abbreviations:
DPG, diphosphatidyl glycerol; FA, fatty acids; FFA, free fatty acids; HC, hydrocarbons; PC, phoshatidyl choline; PE, phosphatidyl ethanolamine; PG, phosphatidyl glycerol; PI, phosphatidyl inositol; PL, phospholipids; SE, steryl esters
Introduction
Legumes are an important food source and play a significant role in traditional diets in many regions of the world 1. In Japan, pulses such as lentils (Lens culinaris Medikus), faba beans (Lathyrus sativus L.), broad beans (Vicia faba L.), chickpeas (Cicer arietinum L.), and peas (Pisum sativum L.) have been traditionally cultivated since ancient years. Among legume seeds, some are used as vegetables and others as supplementary sources of protein in animal diets 2. The widespread use of legumes makes this food group an important source of lipids and fatty acids (FA) in animal and human nutrition. Several publications dealing with the total lipids and FA compositions have been reviewed by several researchers 3, 4. Some beans are used as staple foods in many countries and are attracting increasing attention for protection against coronary heart disease 5. The Leguminase plant Canavalia (C.) gladiata DC. (Sword bean, Japanese name “natamame”) has been cultivated as a vegetable in Asian countries for feeding ruminants and widely studied for its nutritional value 6, 7. The seeds of C. gladiata have been known to exhibit anticmetic and demulcent effects and to stop hiccups in Chinese traditional medicine 8. As chemical constituents of this plant, gibberellins 9, an amino acid 10, and an enzyme 11 have been investigated. The composition of total FA is often the only information provided in studies on several bean lipids 7. The bean C. gladiata which is a leguminous plant is not eaten very frequently, but has a potential to become an important food source.
To the best of our knowledge no data have been reported on the lipid components and their FA distributions of Jack beans. Therefore, the focus of the present research was to investigate the FA composition of the separated different lipid classes in the white and red Jack beans-TAG, DAG, free fatty acids (FFA), phospholipids (PL), steryl esters (SE) in an attempt to examine the variability in their relative proportions and the FA percentages in each acyl lipid class.
Materials and methods
Jack beans
Commercially available mature Jack beans (C. gladiata DC.) used in this study were from white and red beans grown at Gifu Prefecture in Japan during the summer of 2010. These beans were selected for uniformity based on seed weight of 2090.0–2105.3 mg for white and 1849.6–1865.0 mg for red, respectively. The beans hand-selected to eliminate cracked or otherwise damaged seeds. These seeds were sealed in polyethylene bags under nitrogen gas and stored in a stainless steel container at −35°C until analysis.
Reagents and standards
All solvents and chemicals used were of analytical grade (Nacalai Tesque, Kyoto, Japan), but diethyl ether was further purified to remove peroxides. TLC plates (silica gel 60 G, 20 × 20 cm2, 0.25 mm thickness) were procured from Merck (Darmstadt, Germany). The TLC standard mixture, containing MAG, DAG, FFA, TAG, SE, and hydrocarbons (HC), was purchased from Nacalai Tesque. A PL kit from Serdary Research Laboratory (Missisauga, ON, Canada) was used as the PL standard for TLC. Lipase from porcine pancreas was obtained from Sigma–Aldrich Co. (St. Louis, MO, USA), and used after purification with acetone and then diethyl ether according to the previously described method 12. Glyceryl-sn-1,3-myristate-sn-2-oleate (Sigma Chemical Co.) was used as the TAG standard for enzymatic hydrolysis. FAME standards (F & OR mixture #3) were procured from Altech-Applied Science (State College, PA, USA). The internal standards, pentadecane and methyl pentadecanoate (C15:0, 100 mg; Merck), were purchased from Merck, and then 100 mg of each was dissolved in n-hexane (20 mL). Boron trifluoride (BF3) in methanol (14%; Wako Pure Chemical Inc., Osaka, Japan) was used to prepare the FAME.
Chemical analysis
AOAC 13 methods were used to determine the chemical composition of these beans. Samples were analyzed in triplicate for fat, protein and moisture contents according to the standard methods. Fat content was determined by solvent extraction (Method 991.36), protein content by a Kjeldahl method (Method 981.10) and moisture content by oven-drying to constant weight at 105°C (Method 925.40).
Extraction of lipids
In order to obtain fine flour, beans (200 g) were ground to pass through a 0.5-mm sieve, using a Maxim homogenizer (Nihonseiki Kaisha, Ltd., Tokyo, Japan) at high speed for 10 min at 0°C before extraction. Total lipids were extracted from 50 g of the flour in 300 mL chloroform/methanol (2:1, v/v) with vigorous shaking for 20 min at 0°C three times, following the Folch procedure 14. These solvents contained 0.01% butylated hydroxytoluene (BHT; Wako Pure Chemical Inc.) to inhibit oxidative degradation of lipids during analysis. Namely, the extraction was repeated thrice, and the individual extracts were vacuum-filtered through defatted filter paper on a Buchner funnel. These filtrates were combined and dried in a rotary evaporator at 35°C. The residue was dissolved in 100 mL of chloroform/methanol (2:1, v/v). Then, 20 mL aqueous KCl (0.75%) were added 14 and mixed vigorously. After phase separation, the chloroform layer was removed, dried over anhydrous Na2SO4, filtered, and the filtrate was concentrated under vacuum in a rotary evaporator at 35°C. The extracted lipids were weighed to determine the lipid content of the beans and then transferred to a 25-mL brown glass volumetric flask with chloroform/methanol (2:1, v/v) and kept under nitrogen at −35°C until further use.
Lipid analysis
According to the previously outlined procedure 15, total lipids were separated by TLC into nine sufractions with a solvent system of n-hexane/diethyl ether/acetic acid (80:20:1, v/v/v). Bands corresponding to HC, SE, TAG, unknown, FFA, 1,3-DAG, 1,2-DAG, MAG, and PL were scraped into separate test-tubes [105 × 16 mm2; poly(tetrafluoroethylene)-coated screw caps]. Methyl pentadecanoate (10–100 µg) from a standard solution (5 mg/mL) was added to each tube as the internal standard with a microsyringe (Hamilton Co., Reno, NV, USA) except that pentadecane (10 µg) was used as the internal standard for HC analysis. FAME were prepared from the isolated lipids by heating with silica-gel for 30 min at 80°C in BF3/methanol (3 mL) on an aluminum block 16. After cooling, 5 mL of n-hexane were added to each tube and washed several times with deionized water to remove the BF3 and silica-gel.
The n-hexane layer containing the FAME was recovered and dried over anhydrous Na2SO4. The solvent was then vaporized under a gentle stream of nitrogen, and the residue (FAME) was quantified by GC using Shimadzu Model-14B GC (Shimadzu, Kyoto, Japan) equipped with a hydrogen FID and a polar capillary column (ULBO HE-SS-10 for FAME fused silica WCOT [no. PSC5481], cyanopropyl silicone, 30 m × 0.32 mm i.d.; Shinwa Chem. Ind., Ltd., Kyoto, Japan). Helium was used as the carrier gas at a flow rate of 1.5 mL/min, and the GC was operated under a constant pressure of 180 kPa. The oven temperature was programmed from an initial temperature of 180°C (2 min held), and increased to 200°C at a rate of 2°C/min, and then held isothermally (200°C) for 15 min. Both injection and detector temperatures were set at 250°C. All samples were dissolved in n-hexane for injection and the aliquot (2–5 µL) was injected with a microsyringe. The component peaks were identified and compared against that of the standard FAME using an electronic integrator (Shimadzu C-R4A). The results are expressed as wt% of each FAME (>0.05) per total FAME. The other GC conditions were as previously described 17.
Samples of the extracted polar lipids, obtained as described above, were further separated by TLC into several fractions with chloroform/methanol/acetic acid/deionized water (170:30:20:7, v/v/v/v) as the mobile phase. PL classes were detected iodine vapor and were consistent with authentic standards. Bands corresponding to diphosphatidyl glycerol (DPG), phosphatidyl ethanolamine (PE), phosphatidyl glycerol (PG), phoshatidyl choline (PC), phosphatidyl inositol (PI), and others were carefully scraped into separate test-tube. Methyl pentadecanoate (10–25 µg) of a standard solution (5 mg/mL) was added to each tube as the internal standard with a microsyringe. Their FAME were prepared by the same method as described above and quantified by GC.
Enzymatic hydrolysis of TAG
TAG hydrolysis in vitro was carried out according to the previously described method 12. A 30 min reaction was selected based on the preliminary results using the standard TAG (glyceryl-sn-1,3-myristate-sn-2-oleate: Sigma Chemical Co.). After approximately 60% of the TAG was hydrolyzed, 0.5 mL of 6 M HCl and 1 mL ethanol were added to stop the reaction. In the preliminary experiments, no FA (oleic acid) at the sn-2 position of the standard TAG was transferred to the sn-1 or sn-3 position at 60% hydrolysis for 30 min. The reaction products were separated by TLC with n-hexane/diethyl ether/acetic acid (60:40:1, v/v/v) as previously described 17. The FFA and sn-2 MAG bands were carefully scraped into test-tubes, respectively, and then methylated 16. The procedure was checked by comparing the FA compositions of the original TAG and the TAG remaining after partial hydrolysis. The constituent FA were analyzed by GC described above.
Statistical analyses
All experiments were repeated at three times. The results were subjected to one-way ANOVA 18. Significant differences (p<0.05) were identified using multiple comparison tests, following a previously described method 19.
Results and discussion
Lipid compositions in the beans
The lipid content of the bean samples analyzed was 1.68% for white and 1.83% for red (data not shown). The percentage of lipid contents was higher in red bean than in white bean. As shown in Table 1, nine lipid components were detected in the beans and these different lipids were compared between the two cultivars. Predominant components were TAG (43.8–45.7 wt%) and PL (46.7–47.0 wt%), accompanied by very small amounts (0.3–2.7 wt%) of other lipid components. When comparing the nine lipid components between the two cultivars, with a few exceptions, no substantial differences (p>0.05) in the content of the lipid components could be observed using values estimated between the values estimated by combining TLC and GC, using the internal standard (C15:0). The results obtained in this work are similar to those described by previous researchers 20. Presumably, the minor components, such as FFA, 1,3- and 1,2-DAG, and MAG may be due to the partial enzymatic hydrolysis of reserve TAG during storage of the beans after their harvesting 21. The lipid components resulting from “fat by hydrolysis” in starch granules were examined, showing the presence of FFA with lysolecithin and lysoglycolipids 22.
| Lipid class | Cultivar | |
|---|---|---|
| White | Red | |
| Hydrocarbons | 13.4 ± 0.3b (0.4) | 11.0 ± 0.5a (0.3) |
| Steryl esters | 10.1 ± 0.2a (0.3) | 14.6 ± 0.4b (0.4) |
| TAG | 1468 ± 36a (43.8) | 1671 ± 42b (45.7) |
| Unknown | 87.2 ± 2.1a (2.6) | 91.2 ± 2.3a (2.5) |
| Free fatty acids | 90.5 ± 2.3b (2.7) | 54.8 ± 1.4a (1.5) |
| 1,3-DAG | 46.9 ± 1.2a (1.4) | 43.9 ± 1.1a (1.2) |
| 1,2-DAG | 40.2 ± 0.6a (1.2) | 40.2 ± 1.0a (1.1) |
| MAG | 30.2 ± 0.7b (0.9) | 11.0 ± 0.3a (0.3) |
| Phospholipids | 1566 ± 39a (46.7) | 1719 ± 42b (47.0) |
- a) Mean values ± standard error. Each value represents the average of three replicates, and is expressed as mg lipid per 200 g of beans. Values in parentheses are relative wt% contents of individual lipids in total lipids. Values in the same row with different superscripts are significantly different from these of individual cultivars.
FA compositions of the different lipids in the beans
Fatty acid compositions (expressed in terms of the esters by weight) of total lipids, TAG, FFA, 1,3-DAG, 1,2-DAG, and PL in the beans were compared between the two cultivars (Figs. 1 and 2). The principal FA components are generally palmitic (16:0), stearic (18:0), oleic (18:1n-9), linoleic (18:2n-6), and α-linolenic (18:3n-3) acids, the distribution of which varies according to these lipid classes. However, these FA distribution patterns were very similar to each other among the different lipids between the two cultivars. These lipids presented with high amounts of total unsaturated FA (which consisted mainly of oleic acid, followed by linoleic and α-linolenic acids), representing 77.4–78.7 wt% for total lipids, 76.1–76.4 wt% for PL, 75.6–76.1 wt% for TAG, 72.7–74.2 wt% for 1,3-DAG, 70.8–71.0 wt% for 1,2-DAG, and 63.3–65.0 wt% for FFA, respectively. Especially, oleic acid (18:1n-9) was predominantly (42.0–51.8 wt%) concentrated in all lipid classes.
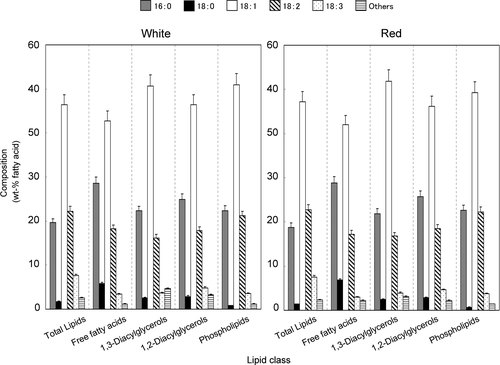
Fatty acid distribution of different lipids obtained from Jack beans. Each value is the average of three replicates, and vertical bars depict the mean and SD of the replicates. Others minor fatty acids include 14:0, 16:1, and 20:0.
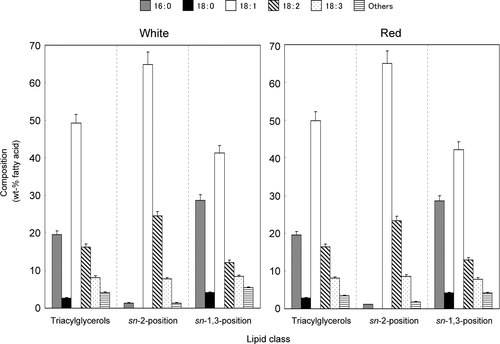
Composition and positional distribution of fatty acids of TAGs obtained from Jack beans. Each value is the average of three replicas, and vertical bars depict the mean and SD of the replicates. Others minor fatty acids include 14:0, 16:1, and 20:0.
However, some differences (p<0.05) in FA composition were noted when comparing among the five lipid classes (Fig. 1). With a few exceptions, the percentage of palmitic acid (16:0) was significantly (p<0.05) higher in the FFA, followed by 1,2- and 1,3-DAG or PL, while that of linoleic (18:2n-6) was higher (p<0.05) in the total lipids and PL than in the FFA, 1,3- and 1,2-DAG. On the other hand, there was no significant differences (p>0.05) in the percentage of palmitic (16:0) and linoleic (18:2n-6) acids in PL between the two cultivars. Furthermore, the percentage of α-linolenic (18:3n-3) acid was significantly (p<0.05) higher in total lipids and TAG (Fig. 2), while that of stearic (18:0) acid was significantly (p<0.05) higher in the FFA. The data for FA distribution of minor lipid components (SE and MAG) as shown in Table 1, were not included in Fig. 1 because these lipid components were too small to be reliable analyzed for the FA distributions. Therefore, we would like to consider these in our future work.
Positional distribution of FA within TAG in the beans
Our preliminary work on the positional distribution of FA on the glycerol backbone was done with pancreatic lipase 12. The characteristics of component and positional distribution of FA in the TAG were compared between the two cultivars (Fig. 2). The major component was palmitic (16:0; 19.5–19.6 wt%), oleic (18:1n-9; 49.2–49.8 wt%), and linoleic (18:2n-6; 16.2–16.4 wt%) acids, followed by stearic (18:0; 2.7–2.8 wt%) and α-linolenic (18:3n-3; 8.1–8.2 wt%) acids. Oleic (18:1n-9) was predominantly (64.9–65.1 wt%) concentrated in the sn-2 position of TAG molecules, while saturated FA such as palmitic (16:0) and stearic (18:0) acids were primarily sn-1 or sn-3 TAG molecules (28.6–28.7 and 4.1–4.2 wt%, respectively).
No significant difference (p>0.05) occurred in their FA distributions between the two cultivars. However, the positional distributions in the FA or TAG were significant (p<0.05) differences in the results obtained from other plant seed lipids such as kidney beans 23 and peas 24 because oleic (18:1n-9) is almost evenly distributed in the sn-1, 2, or 3 molecules, corroborating results of previous researchers 25.
FA distribution of major PL in the beans
To clarify the distribution of individual PL in Jack beans, further separation of the PL into several fractions (DPG, PE, PG, PC, PI, and unknown) was done by TLC in the presence of authentic standards, and the results were shown in Table 2. Comparison were made of the two cultivars for the profiles of DPG, PE, PG, PC, and PI. Regardless of cultivars, PC, PE, and PI were the principal PL in the these beans, and the highest content was observed for PC (43.2–47.5 wt%), followed by PI (24.3–26.5 wt%) and PE (18.2–22.5 wt%), with very small amounts (0.5–5.0 wt%) of other PL. Generally, the percentage of PC was significant (p<0.05) higher in white than that in red, while the percentage of PE and PI was significant (p<0.05) lower in white than that in red, respectively.
| Phospholipid | Cultivar | |
|---|---|---|
| White | Red | |
| Diphosphatidyl glycerol | 70.5 ± 3.2b (4.5) | 46.4 ± 1.5a (2.7) |
| Phosphatidyl ethanolamine | 285 ± 12a (18.2) | 387 ± 15b (22.5) |
| Phosphatidyl glycerol | 7.8 ± 0.2a (0.5) | 13.8 ± 0.4b (0.8) |
| Phosphatidyl choline | 744 ± 32a (47.5) | 742 ± 30b (43.2) |
| Phosphatidyl inositol | 381 ± 12a (24.3) | 456 ± 18b (26.5) |
| Unknown | 78.3 ± 3.0a (5.0) | 73.9 ± 2.8a (4.3) |
- a) Mean values ± standard error. Each value represents the average of three replicates, and is expressed as mg lipid per 200 g of beans. Values in parentheses are relative wt% contents of the individual lipids in total lipids.
Table 3 shows the distribution patterns of FA in the DPG, PE, PG, PC, and PI between the two cultivars. The major FA in the five PL were commonly palmitic (16:0), oleic (18:1n-9), and linoleic (18:2n-6) acids. With a few exceptions, these FA distributions were very similar to each other in the major individual PL between the two cultivars. When comparing FA compositions in the five PL between the two cultivars, the percentage of oleic (18:1n-9) acid was significantly (p<0.05) higher in PC (65.0–66.3 wt%) than in PE (53.0–53.4 wt%), while the percentage of palmitic (16:0) was significantly (p<0.05) higher in PE (24.2–24.5 wt%) than in PC (16.8–17.2 wt%). However, the percentage of linoleic (18:2n-6) was the same levels among DPG, PE and PI; 18.7–19.0, 18.2–18.3 and 16.5–17.0 wt%, respectively. However, PI was very unique in that it had the highest saturated FA contents (48.0–48.9%) among the five PL, although their patterns were very similar between the two cultivars. Especially, the percentage of palmitic (16:0) acid was significantly (p<0.05) higher in PI (44.8–45.6 wt%) and PG (34.9–35.2 wt%) than in PC (16.8–17.2 wt%) or PE (24.2–24.5 wt%) between the two cultivars. The FA distribution in PI differed significantly (p<0.05) from that of PE or PC among the two cultivars, presumably owing to differences in their biosynthetic pathway 26. The results reflect the differences in the PL compositions between white and red beans as shown in Table 3. However, their distribution patterns are not similar to the results observed in typical vegetable seeds, such as kidney beans 23 or broad beans 27.
| Lipid class | Cultivar | Fatty acid (wt%) | |||||
|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | Others | ||
| Diphosphatidyl glycerol | White | 24.2 ± 0.6a | 0.8 ± 0.1a | 52.2 ± 1.3a | 19.0 ± 0.4a | 2.4 ± 0.1a | 1.4 ± 0.1a |
| Red | 24.5 ± 0.6a | 1.2 ± 0.1b | 51.7 ± 1.3a | 18.7 ± 0.3a | 2.3 ± 0.1b | 1.6 ± 0.1b | |
| Phosphatidyl ethanolamine | White | 24.5 ± 0.7a | 0.8 ± 0.1a | 53.0 ± 1.3a | 18.2 ± 0.3a | 2.0 ± 0.1a | 1.5 ± 0.1a |
| Red | 24.2 ± 0.7a | 0.8 ± 0.1a | 53.4 ± 1.3a | 18.3 ± 0.3a | 2.1 ± 0.1a | 1.2 ± 0.1a | |
| Phosphatidyl glycerol | White | 35.2 ± 0.9a | 2.2 ± 0.1a | 34.7 ± 0.9a | 24.1 ± 0.6a | 3.1 ± 0.1b | 0.7 ± 0.1a |
| Red | 34.9 ± 0.7a | 2.3 ± 0.1a | 35.3 ± 1.3a | 23.4 ± 0.3a | 2.2 ± 0.1a | 1.9 ± 0.1b | |
| Phosphatidyl choline | White | 17.2 ± 0.4a | 0.6 ± 0.1a | 65.4 ± 1.5a | 14.0 ± 0.4a | 1.5 ± 0.1a | 1.3 ± 0.1a |
| Red | 16.8 ± 0.4a | 0.6 ± 0.1a | 66.3 ± 1.5a | 13.8 ± 0.4a | 1.3 ± 0.1a | 1.2 ± 0.1a | |
| Phosphatidyl inositol | White | 45.6 ± 1.1a | 3.0 ± 0.1a | 30.3 ± 0.7a | 16.5 ± 0.4a | 3.4 ± 0.1a | 1.2 ± 0.1a |
| Red | 44.8 ± 1.0a | 3.2 ± 0.1a | 30.2 ± 0.8a | 17.0 ± 0.4a | 3.5 ± 0.1a | 1.3 ± 0.1a | |
- a) Mean values ± standard error. Each value represents the average of three replicates, and is expressed as relative wt% contents of individual FA in each PL class. “Others” include minor FA such as 14:0, 16:1, and 20:0. Values in the same column followed by different letters are significantly different between individual cultivars (p<0.05).
Conclusions
The acyl lipids and their FA distributions in the oils prepared from Jack beans (C. gladiata DC.) was compared between two cultivars. Major lipid components in white and red beans were TAG (43.8–45.7%) and PL (46.7–47.0%), while other components were also present in minor proportions (0.3–2.7%). Especially, oleic (18:1n-9) was predominantly (41–51.8%) detected in all lipid classes. However, the lipid classes and FA distributions within the individual acyl lipids were not dependent on the two cultivars. To the best of the authors' knowledge this is the first report of the FA distribution profiles in the different lipids of Jack beans. The data obtained from this work provide useful information to both producers and consumers for manufacturing functional drinks such as Jack bean tea in Japan and elsewhere.
Acknowledgements
We thank Prof. Bruce Holub of the Department of Human Health and Nutritional Sciences, University of Guelph, Canada, for reviewing and commenting on this manuscript. A part of this study was financially supported by a grant-in-aid for Scientific Research (C) no. 20500730 (HY) from Japan Society for the Promotion of Science (JSPS).
The authors have declared no conflict of interest.




