Sodium azide and metal chelator effects on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging compounds from methylene blue photosensitized lard
Abstract
Thermal oxidation of edible oils can generate 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging compounds from oxidized lipids (RSOLs). However, effects of photosensitization on the formation of RSOLs have not been reported yet. Methylene blue (MB) photosensitization and involvement of singlet oxygen and transition metals on the RSOL formations were determined in stripped lard oils. RSOLs were formed in lard containing MB and visible light irradiation only. Addition of sodium azide decreased RSOLs with concentration dependent manner, which implies singlet oxygen was involved on the RSOL formation. Ethylenediammetetraacetic acid (EDTA), a well known metal chelator, accelerated the formation of RSOLs through protecting the decomposition of MB photosensitizer. Results from p-anisidine values showed that RSOLs from photosensitization may not be formed from the same pathways compared to thermal oxidation.
Practical application: Understanding mechanisms of lipid oxidation can help extend the shelf-life of foods. Photosensitization plays important roles in accelerating the rates of lipid oxidation. The results of this study showed that foods containing photosensitizers can generate radical scavenging compounds from oxidized lipids (RSOLs) under visible light irradiation and singlet oxygen is involved in the formations of these compounds. However, these compounds may not share the same pathways with thermally oxidized lipids. Metal chelating agents accelerated the rates of lipid oxidation and formation of RSOLs which implies that metal chelators can act as prooxidant. Careful considerations are necessary on the addition of metal chelators because non-polar photosensitizers can act a prooxidant.
Abbreviations:
CDA, conjugated dienoic acid; DPPH, diphenyl-1-picrylhydrazyl; EDTA, ethylenediammetetraacetic acid; FRSs, free radical scavengers; MB, methylene blue; PUFA, polyunsaturated fatty acid; RSOL, radical scavenging compounds from oxidized lipid; TAG, triacylglycerol
Introduction
Lipid oxidation is one of key chemical reactions in foods containing lipids and oxidation products from lipids greatly deteriorate the nutritional values and flavor qualities in foods 1. Visible light irradiation can accelerates the rates of lipid oxidation in foods containing photosensitizers such as riboflavin and chlorophylls 2, 3. Upon irradiation, excited photosensitizers can abstract hydrogen or electrons through type I pathway or generate singlet oxygen by type II pathway and these reactive compounds are responsible for the acceleration of lipid oxidation. Effects of photosensitization have been reported in model systems of free fatty acids and triacylglycerols (TAGs) and food systems 4-6.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) is a stable free radical and has been extensively used to determine antioxidant properties of free radical scavengers (FRSs) in solvent extracts from natural resources 7, 8. Compounds or extract possessing high reducing ability to DPPH are considered as strong antioxidants. Our research group has shown that compounds with DPPH scavenging ability can be formed during thermal oxidation of lipids, which can be monitored by the absorbance changes of DPPH in isooctane solution. Therefore, FRSs as well as some compounds from oxidized lipids can react with DPPH radical and decrease the absorbance of DPPH 9, 10. These compounds are named as radical scavenging compounds from oxidized lipids (RSOLs) 11. The modified method using DPPH absorbance can give information on the changes of inherently or intentionally added FRSs including tocopherols, sesamol, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ) in thermally oxidized lipids 10, 11. Also, extraordinary high oxidative stability in sesame oil during thermal oxidation can be explained successfully by using the modified DPPH method 12. During thermal oxidation, continuous generation of sesamol from sesamolin in sesame oil was observed by monitoring the changes of DPPH absorbance 12.
Previous studies adapted thermally oxidized lard or vegetable oils ranging from 90 to 180°C 10, 11. However, whether RSOLs can be formed in photosensitized lipid has not been reported yet. Also, diverse factors related to RSOL formation under photosensitization should be elucidated to understand the formation mechanisms of RSOLs during lipid oxidation.
The objectives of this study were to determine whether photosensitization can induce RSOLs in bulk oils and to determine the involvement of singlet oxygen and transition metals on the formation of RSOLs in photosensitized bulk oils using sodium azide and ethylenediaminetetraacetic acid (EDTA), respectively. Also, oxidation products including RSOLs, conjugated dienoic acid (CDA) value, and p-anisidine value (p-AV) from methylene blue (MB) photosensitized bulk oils were compared to those from thermally oxidized bulk oils.
Materials and methods
Materials
Fat from a pig was purchased from a local grocery market (Seoul, Korea). DPPH was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Isooctane was purchased from Junsei Chemical Co. (Tokyo, Japan) and p-anisidine was purchased from Kanto Chemical Co. (Tokyo, Japan). Other chemicals were purchased from Daejung Chemical Co. (Seoul, Korea).
Stripped lard oil preparation
Thirty grams of pig fat was cut into small pieces and put into a 100-mL bottle sealed air-tightly with a Teflon-coated rubber septum and an aluminum cap. Headspace air in the bottle was replaced with nitrogen gas and put in a convection dry oven (Win Science, Seoul, Korea) at 80°C for 0.5 h to melt fat, and the lard oil was filtered through Whatman paper No.2. Lard oil was centrifuged at 6370 g for 10 min and collected in a 10-mL serum bottle wrapped with aluminum foil under nitrogen gas flow and stored −40°C until use.
Lard oil was stripped using a glass column packed with activated silicic acid (4 g), activated charcoal and celite mixture (4:1), powdered sugar and celite mixture (4:1), and activated silicic acid (4 g) from bottom to top order.
Methylene blue photosensitization and thermal oxidation
Stripped lard oil was mixed with MB to make 0.234 µmol/g oil and 0.5 g of sample was put in 10-mL serum bottles. Samples wrapped with aluminum foil were controls for the dark conditions. The treatments employed were without addition of MB and non-irradiation (OD), with addition of MB and non-irradiation (MBD), without addition of MB and with irradiation (OL), and with addition of MB and with irradiation (MBL). Samples were prepared in triplicate and placed in a hand-made light box, which had five tungsten light bulbs with 3800 Lux at 50°C. Light exposed lard oils were analyzed at 0, 4, 12, 24, and 60 h.
For the comparison of MB photosensitization with thermal oxidation, lard oil was heated at 180°C. Samples were prepared in triplicate and analyzed at 0, 15, 30, 45, and 60 min.
The involvement of singlet oxygen was tested using addition of sodium azide. Sodium azide was dissolved in acetone to make 160 mM and MBL was mixed with sodium azide solution to make final concentrations of 5, 10, and 20 mmol/g. Controls were samples without addition of sodium azide. Solvent in the samples was removed under nitrogen gas. Samples were prepared in triplicate and placed in the light box with the same conditions of oxidation. Samples were analyzed at 0, 12, 24, 36, and 60 h.
To test the effects of transition metals on the formation of RSOLs in photosensitized bulk oils, EDTA was dissolved in acetone and mixed with MBL to the final concentration of 0.5, 1, and 2 mmol/g. Controls were samples without addition of EDTA. Samples were prepared in triplicate and placed in the light box. Samples were analyzed at 0, 12, 24, and 36 h.
A modified DPPH method
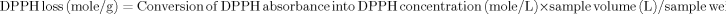
CDA and p-AV analysis
Conjugated dienoic acid can determine the amount of conjugated dienes, which are primary lipid oxidation products from polyunsaturated fatty acid (PUFA). CDA of samples was measured according to AOCS method Ti la-64 13. p-AV can be used to determine the content of 2-alkenals, which are typical secondary oxidation products from PUFA. p-AV of oxidized oils was determined according to an AOCS method Cd 18-90 14.
Statistical analysis
The data from DPPH methods, CDA values, and p-AV were analyzed statistically by ANOVA and Duncan's multiple range test using commercially available software package SPSS software program (SPSS Inc., Chicago, IL, USA). A p value <0.05 was considered significant.
Results
DPPH concentration, CDA, and p-AV analysis from methylene blue photosensitization
Effects of MB photosensitization on the changes of DPPH absorbance (Fig. 1A) and loss of DPPH concentration (Fig. 1B) in lard oils are shown in Fig. 1. The results of DPPH method have been expressed using the raw data of the DPPH absorbance Fig. 1A. Strong FRSs or RSOLs can decrease the DPPH absorbance below 0.1 of absorbance and the intensity of strong FRSs or RSOLs may not be expressed properly in current reporting systems. To solve this limitation, samples possessing high FRSs or RSOLs were properly diluted and the results of DPPH absorbance were expressed as the concentration of DPPH loss like Fig. 1B. Absorbance of DPPH was converted into concentration using a following equation, y = 13.031x − 0.0082, where x is DPPH absorbance and y is the concentration of DPPH in mmol/L. Samples of OD, MBD, and OL did not change the DPPH concentration significantly for 60 hr (p>0.05; Fig. 1). However, MBL decreased the concentration of DPPH significantly since 24 h irradiation (p<0.05). Therefore, RSOLs are definitely generated in MB photosensitized lipid oxidation only.
Effects of MB photosensitization on the changes of DPPH absorbance (A) and loss of DPPH concentration (B) in lard oils. Abbreviation of MBL, OL, MBD, and OD are samples with addition of MB and with irradiation, without addition of MB and with irradiation, with addition of MB and non-irradiation, and without addition of MB and non-irradiation, respectively.
Changes of CDA values and p-AV in lard oils with or without MB are shown in Table 1. As the light expose time increased to 60 h, CDA values and p-AV in MBL significantly higher than those in OL, MBD, or MBL (p<0.05). Results of CDA values and p-AV can be used to compare RSOL formation between photosensitization and thermal oxidation.
| Time (h) | CDA values | p-AV | ||||||
|---|---|---|---|---|---|---|---|---|
| MBLa) | OL | MBD | OD | MBL | OL | MBD | OD | |
| 0 | 0.343 ± 0.017b)Abc) | 0.313 ± 0.004 Aa | 0.325 ± 0.004 Aab | 0.328 ± 0.005 Aab | 6.392 ± 1.526 Aa | 4.983 ± 0.000 Aa | 5.648 ± 0.931 Aa | 5.267 ± 0.406 ABa |
| 4 | 0.380 ± 0.002 Bc | 0.356 ± 0.004 Bab | 0.353 ± 0.004 Ba | 0.373 ± 0.018 Cbc | 5.548 ± 0.169 Aa | 5.675 ± 0.296 Ba | 5.598 ± 0.598 Aa | 5.484 ± 0.162 Ba |
| 12 | 0.388 ± 0.015 Bb | 0.355 ± 0.002 Ba | 0.361 ± 0.002 Ca | 0.355 ± 0.005 Ba | 7.375 ± 1.221 Aa | 6.720 ± 0.431 Ca | 6.101 ± 0.499 Aa | 6.148 ± 0.417 Ca |
| 24 | 0.450 ± 0.009 Cc | 0.387 ± 0.002 Cb | 0.368 ± 0.006 Ca | 0.364 ± 0.003 BCa | 6.973 ± 0.947 Ab | 4.720 ± 0.292 Aa | 5.387 ± 0.593 Aa | 4.789 ± 0.158 Aa |
| 60 | 1.140 ± 0.005 Dc | 0.474 ± 0.004 Db | 0.398 ± 0.001 Da | 0.404 ± 0.002 Da | 14.159 ± 0.454 Bd | 7.073 ± 0.164 Cc | 6.216 ± 0.621 Ab | 5.178 ± 0.160 ABa |
- a) MBL, OL, MBD, and OD are samples with MB and light exposure, samples without MB and with light exposure, samples with MB and without light exposure, and samples without MB and without light exposure, respectively.
- b) Mean ± SD (n = 3).
- c) Different capital and small letters are significant in the same column and the same low, respectively at p<0.05.
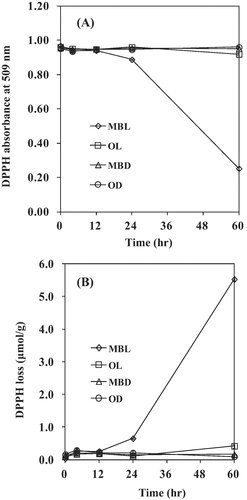
Comparison of DPPH absorbance, CDA values, and p-AV in thermally oxidized and MB photosensitized lard oils are shown in Fig. 2. For 60 min thermal oxidation, DPPH absorbance in thermally oxidized lard decreased by 0.56, whereas CDA and p-AV increased by 0.51 and 127.56, respectively. For 60 h MB photosensitized samples, DPPH absorbance decreased by 0.72, while CDA and p-AV increased by 0.80 and 7.77, respectively. At the similar CDA values and DPPH absorbance, p-AV in MB photosensitized samples are greatly lower than thermally treated samples (Fig. 2). This trend can be observed in other studies using different temperature of thermal oxidation. CDA values and p-AV in thermally oxidized lard at 150°C for 4 h were 1.06 and 173.1, respectively 10, whereas the value of p-AV was much higher than those in MB photosensitized samples.
Comparison of DPPH absorbance, CDA values, and p-AV in MB photosensitized (A) and thermally oxidized (B) lard. MBL and MTO are oils treated with photosensitization and thermal oxidation, respectively. Arrows indicate x-axis for each point.
Sodium azide effects
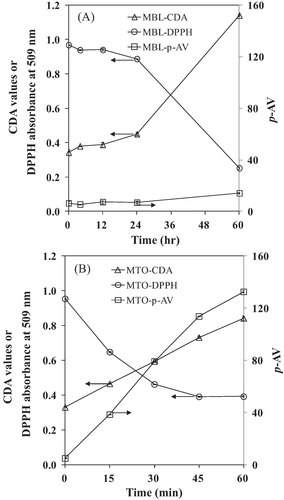
The previous results showed that the absence of MB photosensitizer and visible light induced no detectable products of lipid oxidation during the 60 h period. Therefore, the subsequent experiments were only done in the stripped lard oil containing MB and under visible light exposure. Changes of DPPH concentration (Fig. 3A), CDA values (Fig. 3B), and p-AV (Fig. 3C) in lard oils with MB photosensitization containing 0–20 mmol/g sodium azide are shown in Fig. 3. As the concentration of sodium azide increased from 5 to 20 mmol/g, loss of DPPH concentration decreased significantly with a concentration dependent manner (p<0.05; Fig. 3A), which implies that addition of sodium azide protected the loss of DPPH concentration. Also, addition of sodium azide inhibited the increase of CDA and p-AV (Fig. 2B and C), agreeing with the results of DPPH loss.
Changes of DPPH concentration (A), CDA values (B), and p-AV (C) in lard with MB photosensitization containing sodium azide (0–20 mmol/g). Different small letters in Figure 3A are significant at 60 hr treatment (p<0.05).
Transition metal effects
Effects of EDTA on the changes of DPPH concentration in lard oils with MB photosensitization (0–2 mmol/g) are shown in Fig. 4. Samples with 1 and 2 mmol/g EDTA had significantly high DPPH loss than samples with control and 0.1 mmol/g for 32 h MB photosensitized lard systems (p<0.05). Addition of EDTA accelerated the rates of lipid oxidation in bulk oil systems under photosensitization. MB in samples with 1 and 2 mmol/g EDTA remained higher contents than those in control and 0.1 mmol/g EDTA for 32 h (data not shown).
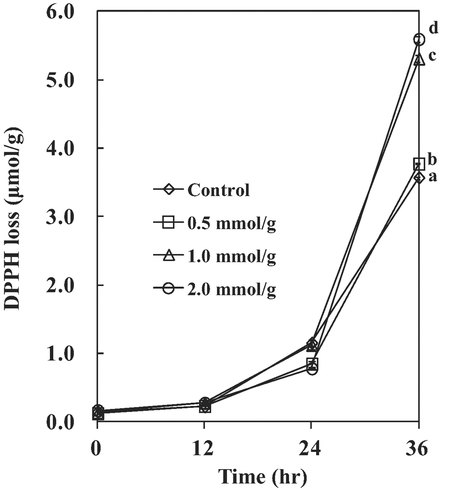
Effects of EDTA on the changes of DPPH concentration in lard with MB photosensitization (0–2 mmol/g). Different small letters are significant at 36 hr treatment (p<0.05).
Addition effects of EDTA on the changes of CDA and p-AV in lard oils under MB photosensitization are shown in Table 2. Like the results of DPPH loss in Fig. 4, higher concentration of EDTA accelerated the formation of CDA and p-AV in lipid oxidation.
| Time (h) | CDA values | p-AV | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 0.5 mmol/g | 1.0 mmol/g | 2.0 mmol/g | Control | 0.5 mmol/g | 1.0 mmol/g | 2.0 mmol/g | |
| 0 | 0.368 ± 0.004a) Abb) | 0.374 ± 0.001 Ab | 0.371 ± 0.004 Ab | 0.361 ± 0.004 Aa | 1.321 ± 0.158 Aab | 1.256 ± 0.000 Aa | 1.388 ± 0.146 Aab | 1.473 ± 0.000 Ab |
| 12 | 0.461 ± 0.006 Ba | 0.450 ± 0.009 Ba | 0.457 ± 0.006 Ba | 0.462 ± 0.008 Ba | 1.792 ± 0.283 ABb | 1.116 ± 0.000 Aa | 1.297 ± 0.155 Aa | 1.264 ± 0.168 Aa |
| 24 | 0.534 ± 0.004 Cc | 0.514 ± 0.002 Cb | 0.520 ± 0.001 Cb | 0.501 ± 0.006 Ca | 2.124 ± 0.334 Ba | 2.120 ± 0.276 Ba | 2.581 ± 0.267 Ba | 2.684 ± 0.908 Ba |
| 36 | 0.720 ± 0.004 Da | 0.730 ± 0.008 Da | 0.963 ± 0.006 Db | 1.010 ± 0.010 Dc | 3.737 ± 0.305 Ca | 3.830 ± 0.399 Ca | 6.478 ± 0.421 Cb | 7.058 ± 0.158 Cb |
- a) Mean ± SD (n = 3).
- b) Different capital and small letters are significant in the same column and the same low, respectively at p<0.05.
Discussion
General autoxidation pathway of lipid oxidation has initiation, propagation, and termination steps, which can be characterized as radical formations from unsaturated fatty acids, chain reactions of lipid radicals from triplet oxygen and unsaturated fatty acids, and non-radical formations among lipid radicals, respectively 1, 2. Most conventional assays for determining the degree of lipid oxidation analyze the consumption of substrates like headspace oxygen or unsaturated fatty acids or generation of oxidized products including lipid hydroperoxides, conjugated dienes, volatiles, and physical properties like dark color and viscosity 1, 7, 15-17. Monitoring RSOLs during lipid oxidation can provide useful information on the formation of oxidized products capable of reducing DPPH into DPPH-H. This method may detect a broad spectrum of oxidized compounds capable of reacting with DPPH radical, which is a clear difference from conventional assays determining specific target compounds.
In singlet oxygen oxidation, initiation step of lipid radical formation is skipped and hydroperoxides can be formed directly by ene reaction of singlet oxygen toward double bonds of unsaturated fatty acids 2, 3. Singlet oxygen can react with linoleic acid at the rate of 1.3 × 105 M−1 S−1, which is about 1450 times faster than that of triplet oxygen with linoleic acid 2. The fast formation of hydroperoxides by singlet oxygen oxidation may explain the increases of CDA values during MB photosensitization (Fig. 2). As shown in Fig. 2, p-AV from MB photosensitization are significantly lower than those from 180°C thermal oxidation. Current MB photosensitization was treated much low temperature (50°C) compared to thermal oxidation at 180°C. High thermal oxidation at 180°C accelerates the rates of initiation and propagation steps as well as the rates of β-scission of hydroperoxides into low molecular volatiles like aldehydes, ketones, and hydrocarbons 18, 19. In MB photosensitization, singlet oxygen can accelerate the formation of lipid hydroperoxides 2 whereas it may not play an important role in the β-scission of hydroperoxides, which can explain the low p-AV in MB photosensitized samples compared to thermally oxidized samples (Fig. 2). Preliminary study showed that aldehydes including pentanal, t-2-heptenal, and t-2-octenal are observed as members of RSOLs in thermally oxidized lipid systems while hydrocarbons and ketones are not (data not shown). Aldehydes are typical products of β-scission of hydroperoxides from unsaturated fatty acids 20. Not all 2-alkenal compounds which are responsible for the changes of p-AV in thermally oxidized lipids, may have ability to react with DPPH radical in isooctane.
Results from addition of sodium azide or EDTA showed that singlet oxygen and transition metals are closely involved on the formation of RSOLs under MB photosensitization. Sodium azide has been used as an indirect indicator for the involvement of singlet oxygen in various systems 4, 21. Addition study using sodium azide suggests that RSOLs can be formed by singlet oxygen oxidation in oil systems.
Transition metals like iron, especially ferrous (Fe+2) ion, are one of critical factors accelerating the rates of lipid oxidation in oil-in-water emulsion systems under thermal oxidation 22-24 and in riboflavin photosensitized oil-in-water emulsions 23. EDTA is a well-known transition metal chelator and addition of EDTA decreases the rates of lipid oxidation 20-23. However, chelating effects of EDTA in MB photosensitized bulk oil systems were opposite to the previous reports 22-25. It seems like that heavy metals ions such as iron (Fe2+) and copper (Cu2+) greatly decreases the stability of non-polar photosensitizer and EDTA-metal complex may protect the decomposition of photosensitizer. Oxidative stresses like singlet oxygen and superoxide anion induced by transition metals under light irradiation are major factors destabilize the non-polar chlorophylls 26. The stability of MB can be greatly enhanced by addition of EDTA and more remained MB generated more singlet oxygen by type II pathway, which may induce the increases of CDA, p-AV, and DPPH loss. Photosensitizers as well as transition metals both affect the oxidative stability of lipids and the generation of RSOLs in bulk oil systems.
In conclusion, RSOLs can be formed in lard oil by MB photosensitization and singlet oxygen is involved in the formation of RSOLs. Metal chelating by EDTA accelerated the generation of RSOLs, which may be due to the protection effects of EDTA–metal complex toward photosensitizers and generation of more ROS like singlet oxygen. p-AV in MB photosensitized samples was significantly lower than those of the thermal oxidized samples, implying compounds responsible for the changes of p-AV may not play an important roles in reaction with DPPH radical. The modified DPPH test can be used to monitor the degradation of oils subjected to visible light irradiation, which is a novel and easy way to be employed in shelf life studies. Further studies are needed to identify RSOLs and to elucidate the formation pathways of RSOLs in photosensitized lipids.
The authors have declared no conflict of interest.




